f3eda289b7ff26178b16a98d8a9601a1.ppt
- Количество слайдов: 28
 Multicenter Trial of the Orqis® Medical Cancion System for the ENhanced Treatment of CHF Unresponsive to Medical Therapy Barry Greenberg, Barbara Czerska, Reynolds M. Delgado, Robert Bourge, Michael R. Zile, Marc Silver, Marc Klapholz, Ernest Haeusslein, Paul Mather, William T. Abraham, Mandeep Mehra, James D. Neaton, B. Scott Brown, Irene C. Parker, Marvin A. Konstam, for MOMENTUM Investigators & Coordinators
Multicenter Trial of the Orqis® Medical Cancion System for the ENhanced Treatment of CHF Unresponsive to Medical Therapy Barry Greenberg, Barbara Czerska, Reynolds M. Delgado, Robert Bourge, Michael R. Zile, Marc Silver, Marc Klapholz, Ernest Haeusslein, Paul Mather, William T. Abraham, Mandeep Mehra, James D. Neaton, B. Scott Brown, Irene C. Parker, Marvin A. Konstam, for MOMENTUM Investigators & Coordinators
 Presenter Disclosure Information The following relationships exist related to this presentation: Barry Greenberg: Orqis: grants/contracts, consultant (significant) Barbara Czerska: Orqis: grants/contracts, consultant (significant) Reynolds M. Delgado: Orqis: grants/contracts, consultant (significant) Robert Bourge: Orqis: grants/contracts, consultant (significant) Michael R. Zile: Orqis: grants/contracts, consultant (significant) Marc Silver: Orqis: grants/contracts, consultant (significant) Marc Klapholz: Orqis: grants/contracts, consultant (significant) Ernest Haeusslein: Orqis: grants/contracts, consultant (significant) Paul Mather: Orqis: grants/contracts, consultant (significant) William T. Abraham: Orqis: grants/contracts, consultant (significant) Mandeep Mehra: Orqis: grants/contracts, consultant (significant) Irene C. Parker: Orqis: salary, stock options (significant) M. A. Konstam: Orqis: salary, stock options (significant) James D. Neaton: Orqis: consultant (significant) B. Scott Brown: Orqis: consultant (significant)
Presenter Disclosure Information The following relationships exist related to this presentation: Barry Greenberg: Orqis: grants/contracts, consultant (significant) Barbara Czerska: Orqis: grants/contracts, consultant (significant) Reynolds M. Delgado: Orqis: grants/contracts, consultant (significant) Robert Bourge: Orqis: grants/contracts, consultant (significant) Michael R. Zile: Orqis: grants/contracts, consultant (significant) Marc Silver: Orqis: grants/contracts, consultant (significant) Marc Klapholz: Orqis: grants/contracts, consultant (significant) Ernest Haeusslein: Orqis: grants/contracts, consultant (significant) Paul Mather: Orqis: grants/contracts, consultant (significant) William T. Abraham: Orqis: grants/contracts, consultant (significant) Mandeep Mehra: Orqis: grants/contracts, consultant (significant) Irene C. Parker: Orqis: salary, stock options (significant) M. A. Konstam: Orqis: salary, stock options (significant) James D. Neaton: Orqis: consultant (significant) B. Scott Brown: Orqis: consultant (significant)
 MOMENTUM Committees Steering Committee Barry Greenberg, M. D. - CHAIR Barbara Czerska, M. D. William Abraham, M. D. Data Safety Monitoring Committee Barry Uretsky, M. D. - CHAIR Thomas D. Cook, Ph. D. Barry Massie, M. D. Richard J. Shemin, M. D. Lynne Warner-Stevenson, M. D. Advisory Committee Robert C. Bourge, MD Reynolds Delgado, M. D. Paul Hauptman, M. D. Paul Mather, M. D. Mandeep R. Mehra, M. D. Hani N. Sabbah, Ph. D. Lynne Wagoner, M. D. Core Laboratories Hemodynamics : Michael Zile, M. D. Medical University of South Carolina Neurohormonal: John Burnett, M. D. Mayo Clinical Events Committee Alan Miller, M. D. - CHAIR Peter E. Carson, M. D. Walter P. Dembitsky, M. D. Steven Goldsmith M. D. Howard Kirshner, M. D. Ronald M. Lazar, M. D.
MOMENTUM Committees Steering Committee Barry Greenberg, M. D. - CHAIR Barbara Czerska, M. D. William Abraham, M. D. Data Safety Monitoring Committee Barry Uretsky, M. D. - CHAIR Thomas D. Cook, Ph. D. Barry Massie, M. D. Richard J. Shemin, M. D. Lynne Warner-Stevenson, M. D. Advisory Committee Robert C. Bourge, MD Reynolds Delgado, M. D. Paul Hauptman, M. D. Paul Mather, M. D. Mandeep R. Mehra, M. D. Hani N. Sabbah, Ph. D. Lynne Wagoner, M. D. Core Laboratories Hemodynamics : Michael Zile, M. D. Medical University of South Carolina Neurohormonal: John Burnett, M. D. Mayo Clinical Events Committee Alan Miller, M. D. - CHAIR Peter E. Carson, M. D. Walter P. Dembitsky, M. D. Steven Goldsmith M. D. Howard Kirshner, M. D. Ronald M. Lazar, M. D.
 Participating Investigators and their Hospitals Marc Silver, M. D. Barbara Czerska, M. D. Paul Mather, M. D. Marc Klapholz, M. D. Advocate Christ Medical Center Henry Ford Thomas Jefferson Univ. of Medicine and Dentistry NJ Mohamad El-Zaru, M. D. Adrian Van Bakel, M. D. Barry Rayburn, M. D. Leway Chen, M. D. Albany Associated Medical Univ of South Carolina Univ. of Alabama University of Rochester Darshak Karia, M. D. Guillermo Torre- Amione, M. D. Lynne Wagoner, M. D. Tayo Addo, M. D. Albert Einstein Health Network Methodist Houston Univ. of Cincinnati Univ. of Texas Southwestern Srinivas Murali, M. D. Paul Vaitkus, M. D. Barry Cabuay, M. D. Utpal Pandya Allegheny General Hospital Mid. West Heart Foundation Univ. of Iowa University of Toledo H. Edward Garrett, M. D. Simon Maybaum, M. D. Mandeep Mehra, M. D. Edward M. Gilbert, M. D. Baptist Memorial Hospital Montefiore Medical Center Univ. of Maryland Univ. of Utah Medical Center Robert F. Tranbaugh, M. D. William Cotts, M. D. Theo Meyer, M. D. Mark Cunningham, M. D. Beth Israel Medical Center Northwestern University Univ. of Massachusetts USC Health Sciences Cntr Ernest A Haeusslein, M. D. Nirav Y. Raval, M. D. Keith D. Aaronson, M. D. James Revenaugh, M. D. California Pacific Medical Center St. Joseph's Research Inst. Univ. of Michigan Utah - Later Day Saints Randall C. Starling, M. D. Paul Hauptman, M. D. Thomas Cappola, M. D. Cleveland Clinic Foundation St. Louis Univ Hospital Univ. of Pennsylvania Donna Mancini, M. D. Reynolds Delgado, M. D. Barry H. Greenberg, M. D. Columbia University Texas Heart Institute Univ. CA at SD Howard Eisen, M. D. Michael Kwan, M. D. Andreas Brieke, M. D. Drexel University Texas Transplant Univ. of CO Health Sciences Ctr Wendy Book, M. D. James H. Leggett, M. D. Emory University Hospital The Hope Heart Institute Garrie Haas, M. D. The Ohio State University
Participating Investigators and their Hospitals Marc Silver, M. D. Barbara Czerska, M. D. Paul Mather, M. D. Marc Klapholz, M. D. Advocate Christ Medical Center Henry Ford Thomas Jefferson Univ. of Medicine and Dentistry NJ Mohamad El-Zaru, M. D. Adrian Van Bakel, M. D. Barry Rayburn, M. D. Leway Chen, M. D. Albany Associated Medical Univ of South Carolina Univ. of Alabama University of Rochester Darshak Karia, M. D. Guillermo Torre- Amione, M. D. Lynne Wagoner, M. D. Tayo Addo, M. D. Albert Einstein Health Network Methodist Houston Univ. of Cincinnati Univ. of Texas Southwestern Srinivas Murali, M. D. Paul Vaitkus, M. D. Barry Cabuay, M. D. Utpal Pandya Allegheny General Hospital Mid. West Heart Foundation Univ. of Iowa University of Toledo H. Edward Garrett, M. D. Simon Maybaum, M. D. Mandeep Mehra, M. D. Edward M. Gilbert, M. D. Baptist Memorial Hospital Montefiore Medical Center Univ. of Maryland Univ. of Utah Medical Center Robert F. Tranbaugh, M. D. William Cotts, M. D. Theo Meyer, M. D. Mark Cunningham, M. D. Beth Israel Medical Center Northwestern University Univ. of Massachusetts USC Health Sciences Cntr Ernest A Haeusslein, M. D. Nirav Y. Raval, M. D. Keith D. Aaronson, M. D. James Revenaugh, M. D. California Pacific Medical Center St. Joseph's Research Inst. Univ. of Michigan Utah - Later Day Saints Randall C. Starling, M. D. Paul Hauptman, M. D. Thomas Cappola, M. D. Cleveland Clinic Foundation St. Louis Univ Hospital Univ. of Pennsylvania Donna Mancini, M. D. Reynolds Delgado, M. D. Barry H. Greenberg, M. D. Columbia University Texas Heart Institute Univ. CA at SD Howard Eisen, M. D. Michael Kwan, M. D. Andreas Brieke, M. D. Drexel University Texas Transplant Univ. of CO Health Sciences Ctr Wendy Book, M. D. James H. Leggett, M. D. Emory University Hospital The Hope Heart Institute Garrie Haas, M. D. The Ohio State University

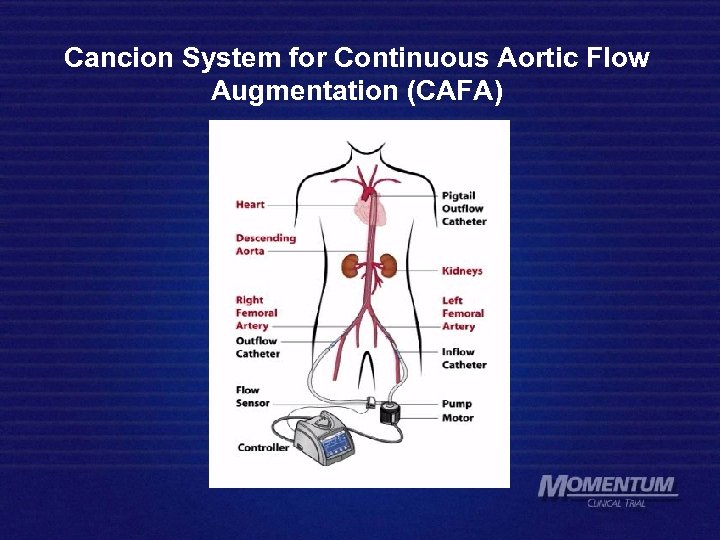 Cancion System for Continuous Aortic Flow Augmentation (CAFA)
Cancion System for Continuous Aortic Flow Augmentation (CAFA)
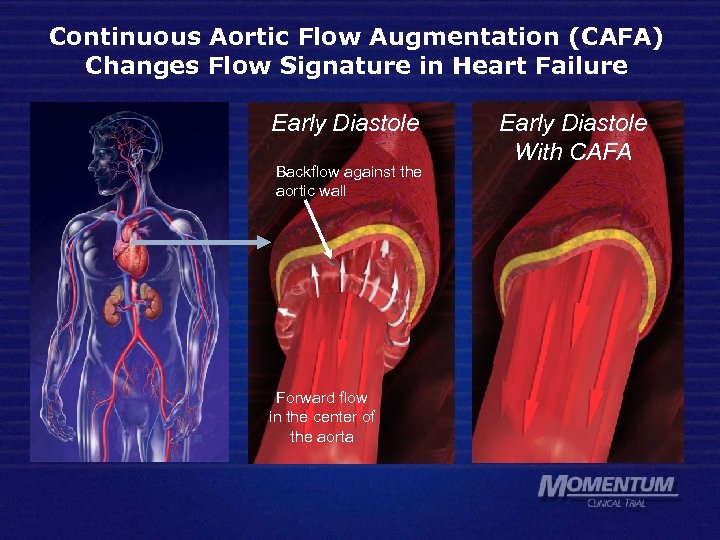 Continuous Aortic Flow Augmentation (CAFA) Changes Flow Signature in Heart Failure Early Diastole Backflow against the aortic wall Forward flow in the center of the aorta Early Diastole With CAFA
Continuous Aortic Flow Augmentation (CAFA) Changes Flow Signature in Heart Failure Early Diastole Backflow against the aortic wall Forward flow in the center of the aorta Early Diastole With CAFA
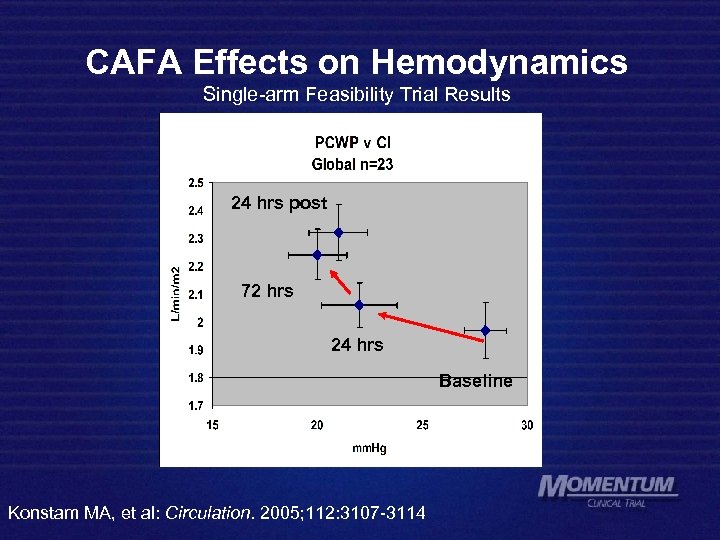 CAFA Effects on Hemodynamics Single-arm Feasibility Trial Results 24 hrs post 72 hrs 24 hrs Baseline Konstam MA, et al: Circulation. 2005; 112: 3107 -3114
CAFA Effects on Hemodynamics Single-arm Feasibility Trial Results 24 hrs post 72 hrs 24 hrs Baseline Konstam MA, et al: Circulation. 2005; 112: 3107 -3114
 • Entry Criteria – Chronic HF with acute decompensation; not adequately responding to IV inotrope and/or vasodilator and diuretic therapy. – PCWP > 20 mm. Hg – CI < 2. 4 L/min/m 2 – Creatinine > 1. 2 mg/d. L or > 120 mg/day IV furosemide • Treatment – Randomized to CAFA + med Rx or med Rx alone for 4 days – 1: 1 Randomization changed to 2: 1 after 49 patients enrolled to increase device experience
• Entry Criteria – Chronic HF with acute decompensation; not adequately responding to IV inotrope and/or vasodilator and diuretic therapy. – PCWP > 20 mm. Hg – CI < 2. 4 L/min/m 2 – Creatinine > 1. 2 mg/d. L or > 120 mg/day IV furosemide • Treatment – Randomized to CAFA + med Rx or med Rx alone for 4 days – 1: 1 Randomization changed to 2: 1 after 49 patients enrolled to increase device experience
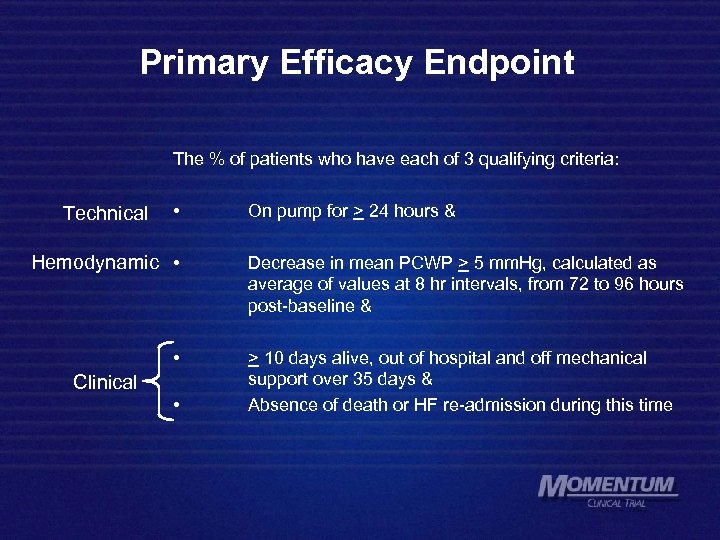 Primary Efficacy Endpoint The % of patients who have each of 3 qualifying criteria: Technical • Hemodynamic • • Clinical • On pump for > 24 hours & Decrease in mean PCWP > 5 mm. Hg, calculated as average of values at 8 hr intervals, from 72 to 96 hours post-baseline & > 10 days alive, out of hospital and off mechanical support over 35 days & Absence of death or HF re-admission during this time
Primary Efficacy Endpoint The % of patients who have each of 3 qualifying criteria: Technical • Hemodynamic • • Clinical • On pump for > 24 hours & Decrease in mean PCWP > 5 mm. Hg, calculated as average of values at 8 hr intervals, from 72 to 96 hours post-baseline & > 10 days alive, out of hospital and off mechanical support over 35 days & Absence of death or HF re-admission during this time
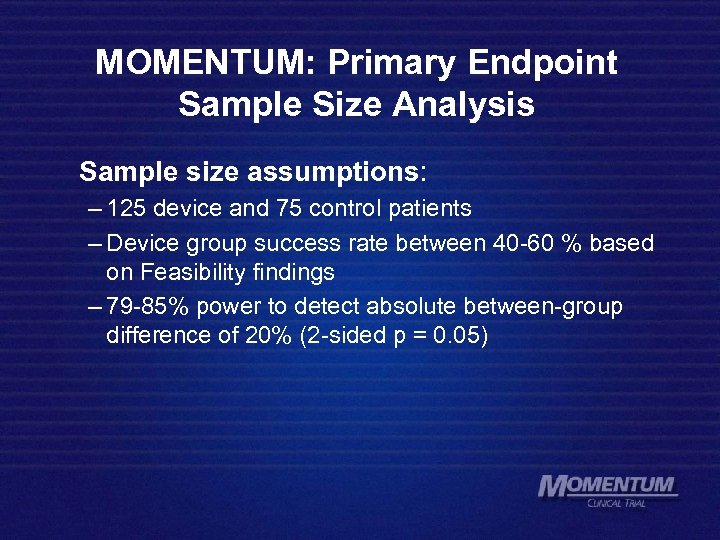 MOMENTUM: Primary Endpoint Sample Size Analysis Sample size assumptions: – 125 device and 75 control patients – Device group success rate between 40 -60 % based on Feasibility findings – 79 -85% power to detect absolute between-group difference of 20% (2 -sided p = 0. 05)
MOMENTUM: Primary Endpoint Sample Size Analysis Sample size assumptions: – 125 device and 75 control patients – Device group success rate between 40 -60 % based on Feasibility findings – 79 -85% power to detect absolute between-group difference of 20% (2 -sided p = 0. 05)
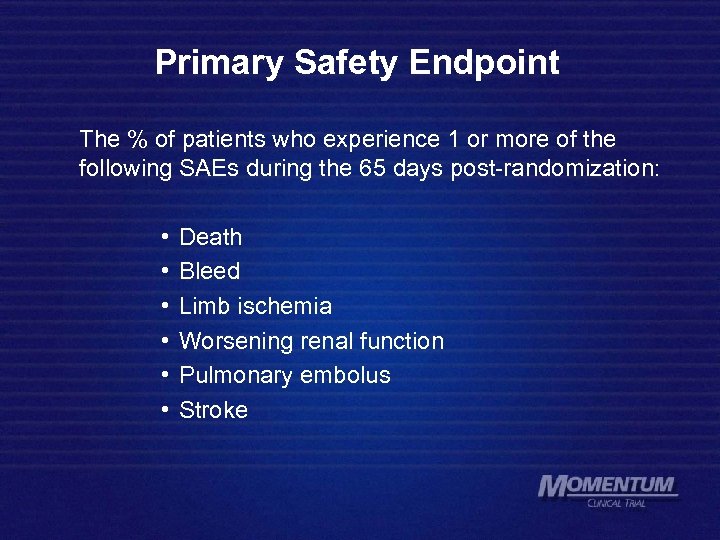 Primary Safety Endpoint The % of patients who experience 1 or more of the following SAEs during the 65 days post-randomization: • • • Death Bleed Limb ischemia Worsening renal function Pulmonary embolus Stroke
Primary Safety Endpoint The % of patients who experience 1 or more of the following SAEs during the 65 days post-randomization: • • • Death Bleed Limb ischemia Worsening renal function Pulmonary embolus Stroke
 DSMB Recommendation Study enrollment was truncated at 168 when the DSMB concluded that “this device did not show worse results in the composite safety primary endpoint than medical therapy alone, ” but “it is also very unlikely that the device group will show positive results for the composite endpoint or any of its components, ” and “continuation…would put future patients assigned to the device group at excess risk…of bleeding”
DSMB Recommendation Study enrollment was truncated at 168 when the DSMB concluded that “this device did not show worse results in the composite safety primary endpoint than medical therapy alone, ” but “it is also very unlikely that the device group will show positive results for the composite endpoint or any of its components, ” and “continuation…would put future patients assigned to the device group at excess risk…of bleeding”
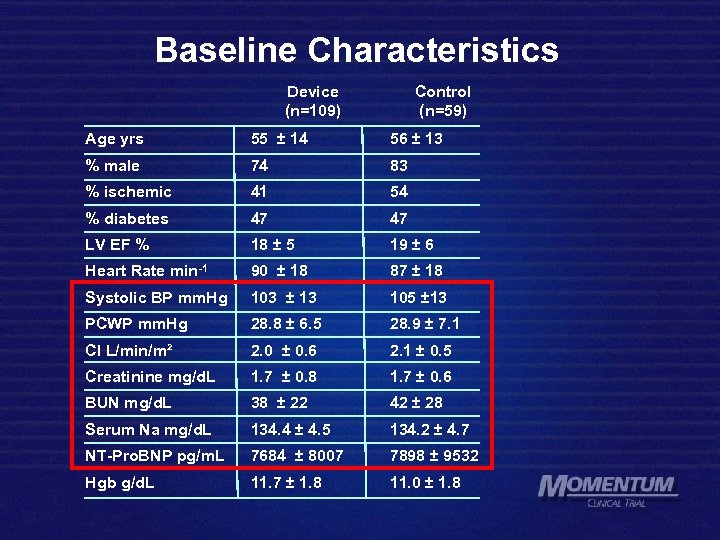 Baseline Characteristics Device (n=109) Control (n=59) Age yrs 55 ± 14 56 ± 13 % male 74 83 % ischemic 41 54 % diabetes 47 47 LV EF % 18 ± 5 19 ± 6 Heart Rate min-1 90 ± 18 87 ± 18 Systolic BP mm. Hg 103 ± 13 105 ± 13 PCWP mm. Hg 28. 8 ± 6. 5 28. 9 ± 7. 1 CI L/min/m² 2. 0 ± 0. 6 2. 1 ± 0. 5 Creatinine mg/d. L 1. 7 ± 0. 8 1. 7 ± 0. 6 BUN mg/d. L 38 ± 22 42 ± 28 Serum Na mg/d. L 134. 4 ± 4. 5 134. 2 ± 4. 7 NT-Pro. BNP pg/m. L 7684 ± 8007 7898 ± 9532 Hgb g/d. L 11. 7 ± 1. 8 11. 0 ± 1. 8
Baseline Characteristics Device (n=109) Control (n=59) Age yrs 55 ± 14 56 ± 13 % male 74 83 % ischemic 41 54 % diabetes 47 47 LV EF % 18 ± 5 19 ± 6 Heart Rate min-1 90 ± 18 87 ± 18 Systolic BP mm. Hg 103 ± 13 105 ± 13 PCWP mm. Hg 28. 8 ± 6. 5 28. 9 ± 7. 1 CI L/min/m² 2. 0 ± 0. 6 2. 1 ± 0. 5 Creatinine mg/d. L 1. 7 ± 0. 8 1. 7 ± 0. 6 BUN mg/d. L 38 ± 22 42 ± 28 Serum Na mg/d. L 134. 4 ± 4. 5 134. 2 ± 4. 7 NT-Pro. BNP pg/m. L 7684 ± 8007 7898 ± 9532 Hgb g/d. L 11. 7 ± 1. 8 11. 0 ± 1. 8
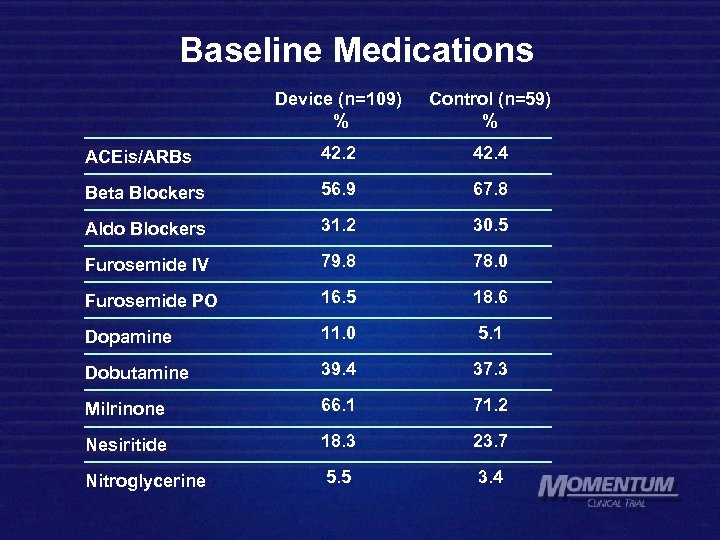 Baseline Medications Device (n=109) % Control (n=59) % ACEis/ARBs 42. 2 42. 4 Beta Blockers 56. 9 67. 8 Aldo Blockers 31. 2 30. 5 Furosemide IV 79. 8 78. 0 Furosemide PO 16. 5 18. 6 Dopamine 11. 0 5. 1 Dobutamine 39. 4 37. 3 Milrinone 66. 1 71. 2 Nesiritide 18. 3 23. 7 Nitroglycerine 5. 5 3. 4
Baseline Medications Device (n=109) % Control (n=59) % ACEis/ARBs 42. 2 42. 4 Beta Blockers 56. 9 67. 8 Aldo Blockers 31. 2 30. 5 Furosemide IV 79. 8 78. 0 Furosemide PO 16. 5 18. 6 Dopamine 11. 0 5. 1 Dobutamine 39. 4 37. 3 Milrinone 66. 1 71. 2 Nesiritide 18. 3 23. 7 Nitroglycerine 5. 5 3. 4
 Results
Results
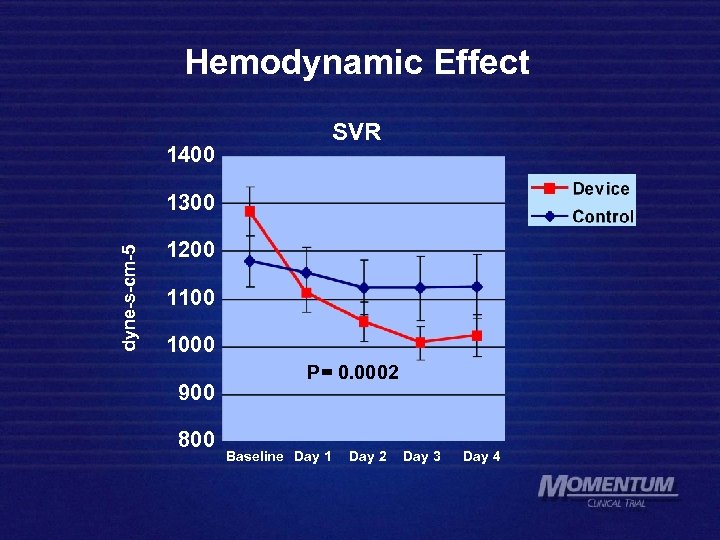 Hemodynamic Effect 1400 SVR dyne-s-cm-5 1300 1200 1100 1000 900 800 P= 0. 0002 Baseline Day 1 Day 2 Day 3 Day 4
Hemodynamic Effect 1400 SVR dyne-s-cm-5 1300 1200 1100 1000 900 800 P= 0. 0002 Baseline Day 1 Day 2 Day 3 Day 4
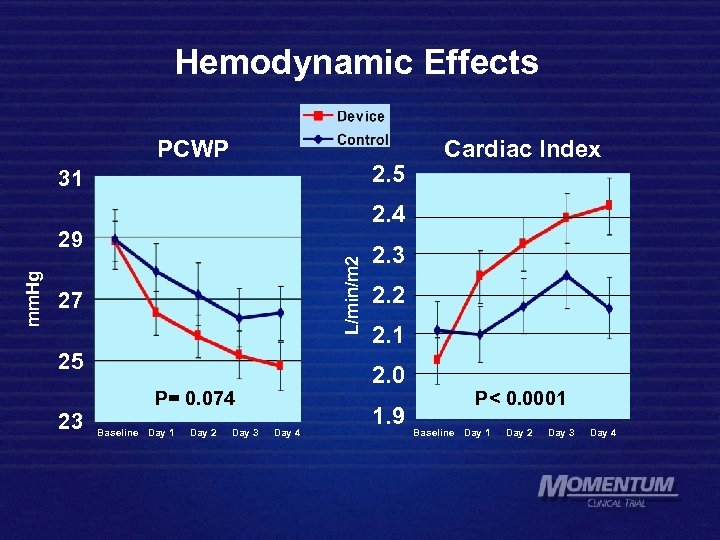 Hemodynamic Effects PCWP 2. 5 31 2. 4 L/min/m 2 mm. Hg 29 27 25 Baseline Day 1 Day 2 Day 3 2. 2 2. 1 2. 0 P= 0. 074 23 Cardiac Index Day 4 1. 9 P< 0. 0001 Baseline Day 1 Day 2 Day 3 Day 4
Hemodynamic Effects PCWP 2. 5 31 2. 4 L/min/m 2 mm. Hg 29 27 25 Baseline Day 1 Day 2 Day 3 2. 2 2. 1 2. 0 P= 0. 074 23 Cardiac Index Day 4 1. 9 P< 0. 0001 Baseline Day 1 Day 2 Day 3 Day 4
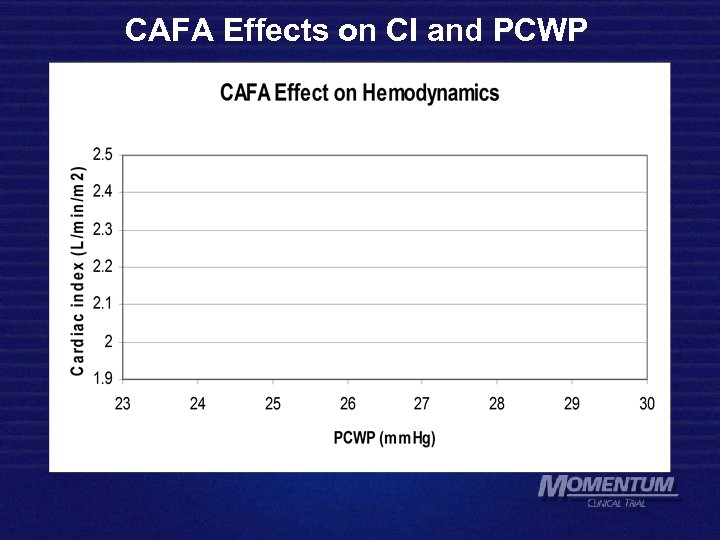 CAFA Effects on CI and PCWP 72 hr 96 hr 48 hr 24 hr 48 hr 72 hr 96 hr base 24 hr base
CAFA Effects on CI and PCWP 72 hr 96 hr 48 hr 24 hr 48 hr 72 hr 96 hr base 24 hr base
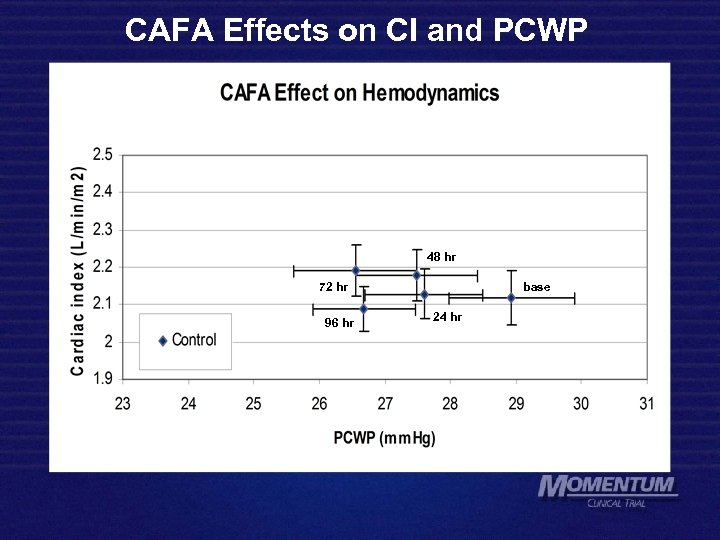 CAFA Effects on CI and PCWP 72 hr 96 hr 48 hr 24 hr 48 hr 72 hr 96 hr base 24 hr base
CAFA Effects on CI and PCWP 72 hr 96 hr 48 hr 24 hr 48 hr 72 hr 96 hr base 24 hr base
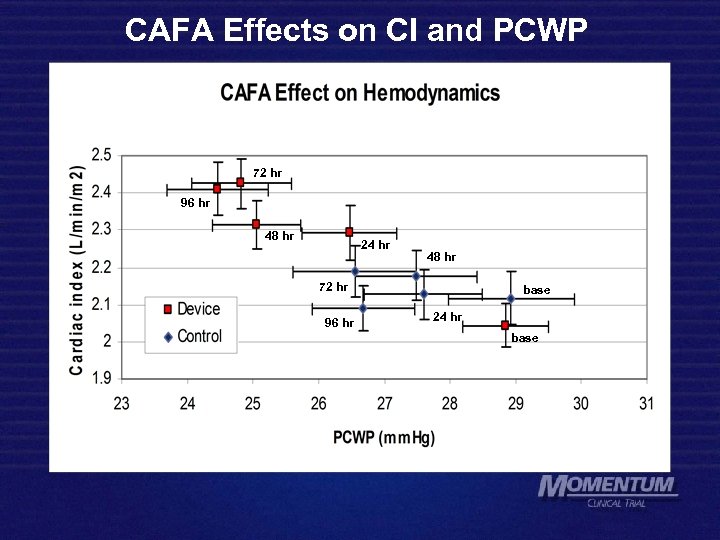 CAFA Effects on CI and PCWP 72 hr 96 hr 48 hr 24 hr 48 hr 72 hr 96 hr base 24 hr base
CAFA Effects on CI and PCWP 72 hr 96 hr 48 hr 24 hr 48 hr 72 hr 96 hr base 24 hr base
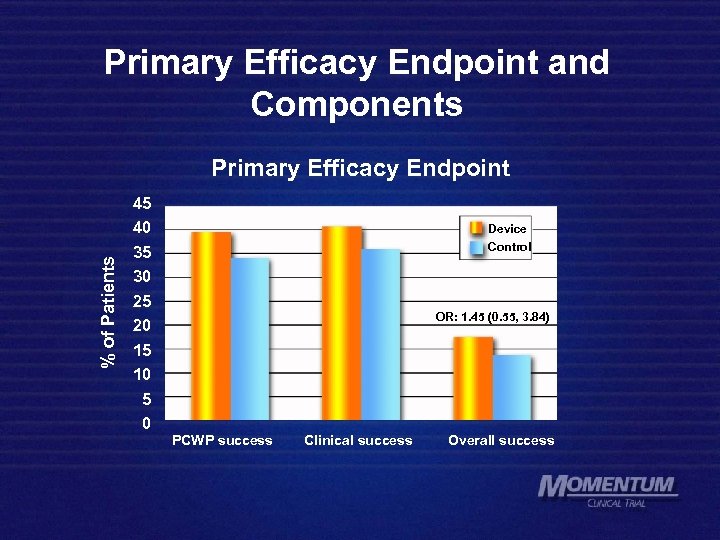 Primary Efficacy Endpoint and Components % of Patients Primary Efficacy Endpoint 45 40 35 30 25 20 15 10 5 0 Device Control OR: 1. 45 (0. 55, 3. 84) PCWP success Clinical success Overall success
Primary Efficacy Endpoint and Components % of Patients Primary Efficacy Endpoint 45 40 35 30 25 20 15 10 5 0 Device Control OR: 1. 45 (0. 55, 3. 84) PCWP success Clinical success Overall success
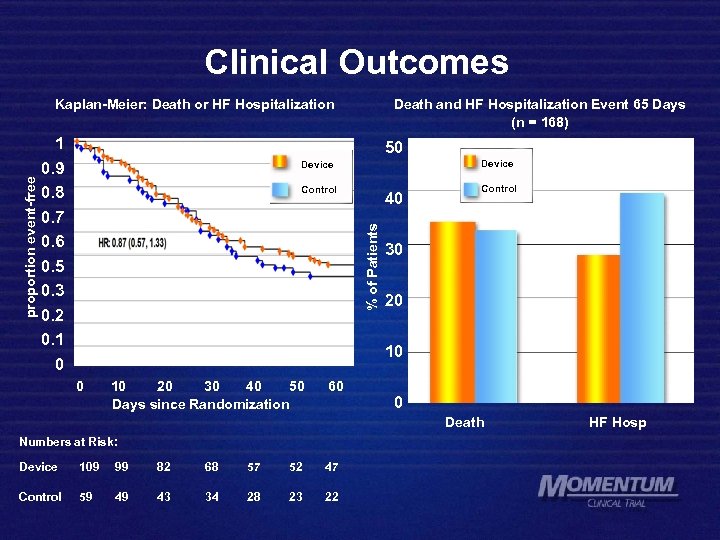 Clinical Outcomes 1 0. 9 0. 8 0. 7 0. 6 0. 5 0. 3 0. 2 0. 1 0 Death and HF Hospitalization Event 65 Days (n = 168) 50 Device Control 40 % of Patients proportion event-free Kaplan-Meier: Death or HF Hospitalization Control 30 20 10 20 30 40 50 Days since Randomization 60 0 Death Numbers at Risk: Device 109 99 82 68 57 52 47 Control 59 49 43 34 28 23 22 HF Hosp
Clinical Outcomes 1 0. 9 0. 8 0. 7 0. 6 0. 5 0. 3 0. 2 0. 1 0 Death and HF Hospitalization Event 65 Days (n = 168) 50 Device Control 40 % of Patients proportion event-free Kaplan-Meier: Death or HF Hospitalization Control 30 20 10 20 30 40 50 Days since Randomization 60 0 Death Numbers at Risk: Device 109 99 82 68 57 52 47 Control 59 49 43 34 28 23 22 HF Hosp
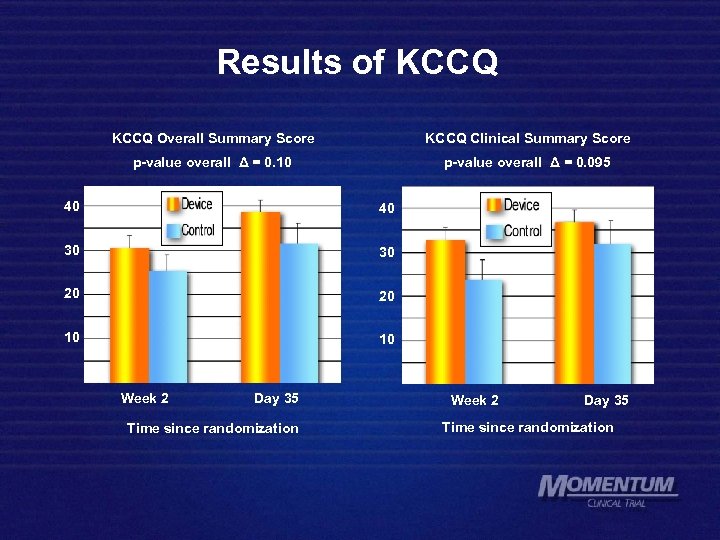 Results of KCCQ Overall Summary Score KCCQ Clinical Summary Score p-value overall Δ = 0. 10 p-value overall Δ = 0. 095 40 40 30 30 20 20 10 10 Week 2 Day 35 Time since randomization
Results of KCCQ Overall Summary Score KCCQ Clinical Summary Score p-value overall Δ = 0. 10 p-value overall Δ = 0. 095 40 40 30 30 20 20 10 10 Week 2 Day 35 Time since randomization
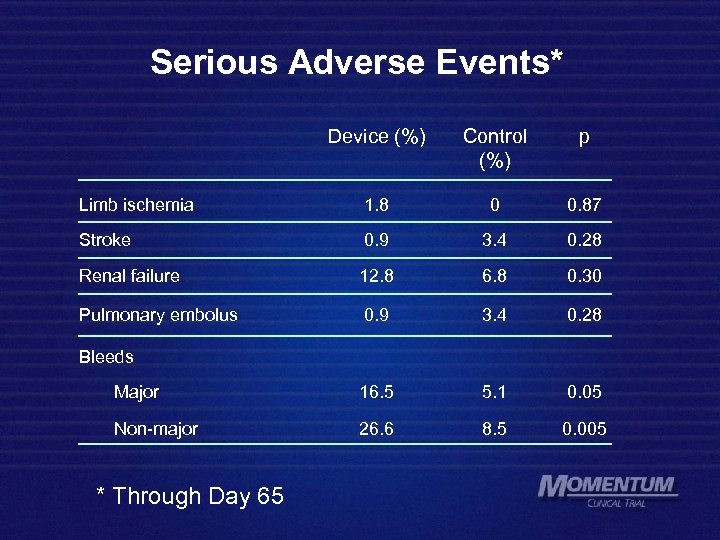 Serious Adverse Events* Device (%) Control (%) p Limb ischemia 1. 8 0 0. 87 Stroke 0. 9 3. 4 0. 28 Renal failure 12. 8 6. 8 0. 30 Pulmonary embolus 0. 9 3. 4 0. 28 Major 16. 5 5. 1 0. 05 Non-major 26. 6 8. 5 0. 005 Bleeds * Through Day 65
Serious Adverse Events* Device (%) Control (%) p Limb ischemia 1. 8 0 0. 87 Stroke 0. 9 3. 4 0. 28 Renal failure 12. 8 6. 8 0. 30 Pulmonary embolus 0. 9 3. 4 0. 28 Major 16. 5 5. 1 0. 05 Non-major 26. 6 8. 5 0. 005 Bleeds * Through Day 65
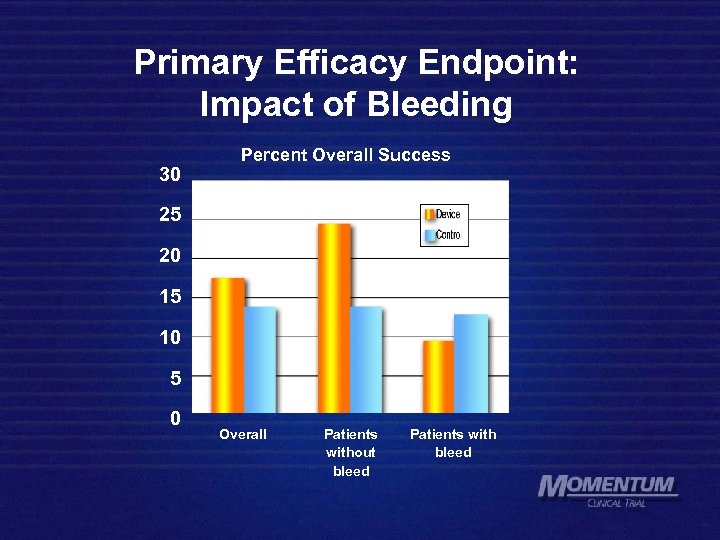 Primary Efficacy Endpoint: Impact of Bleeding 30 Percent Overall Success 25 20 15 10 5 0 Overall Patients without bleed Patients with bleed
Primary Efficacy Endpoint: Impact of Bleeding 30 Percent Overall Success 25 20 15 10 5 0 Overall Patients without bleed Patients with bleed
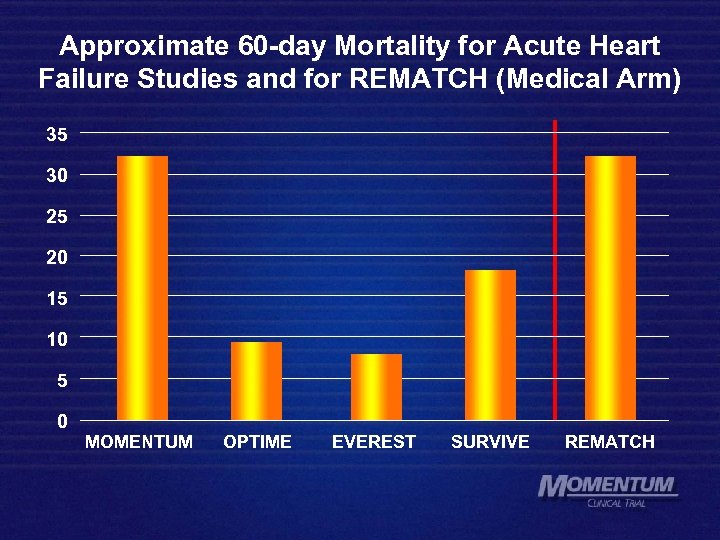 Approximate 60 -day Mortality for Acute Heart Failure Studies and for REMATCH (Medical Arm) 35 30 25 20 15 10 5 0 MOMENTUM OPTIME EVEREST SURVIVE REMATCH
Approximate 60 -day Mortality for Acute Heart Failure Studies and for REMATCH (Medical Arm) 35 30 25 20 15 10 5 0 MOMENTUM OPTIME EVEREST SURVIVE REMATCH
 Conclusions Ø MOMENTUM enrolled, perhaps, the sickest acute heart failure population ever studied in a randomized controlled trial. Ø Continuous aortic flow augmentation (CAFA) for 4 days improved left ventricular performance with increased CI, while PCWP decreased. Ø CAFA for 4 days showed no significant benefit on the primary composite efficacy endpoint. Ø Observed favorable trends provide guidance toward future investigation, with a focus on reducing the bleeding incidence and potentially targeting a less-sick population.
Conclusions Ø MOMENTUM enrolled, perhaps, the sickest acute heart failure population ever studied in a randomized controlled trial. Ø Continuous aortic flow augmentation (CAFA) for 4 days improved left ventricular performance with increased CI, while PCWP decreased. Ø CAFA for 4 days showed no significant benefit on the primary composite efficacy endpoint. Ø Observed favorable trends provide guidance toward future investigation, with a focus on reducing the bleeding incidence and potentially targeting a less-sick population.


