8d41225f33d786c0091fd00e2de84e87.ppt
- Количество слайдов: 19

MRC SUPREMO (BIG 2 -04) Selective Use of Postoperative Radiotherapy aft. Er Mastect. Omy Phase III randomised trial of chest wall RT in intermediate- risk breast cancer Kunkler I, Canney P, Price A, Anderson N, Dixon J, Sainsbury R, Aird E, Thomas G, Bowman A, Thomas J, Bartlett J, Devine I, Denvir M, Mc. Donagh T, Russell N, Cairns J, Boon Chua, Karlsson P, Northridge D, van Tienhoven G, Velikova G, Walker A, Macdonald E 1

Agenda • • • Protocol V 29 Eligibility Guide – Non Neo-adjuvant & Neo-adjuvant Informed Consent Procedures SAE Guidelines Supremo Team Recruitment 2

Protocol V 29 • Implemented on the 14 th February 2011 • 108 out of 157 sites confirmed approval for V 29 Ø UK: 104 out of 117 Ø International: 4 out of 9 (5 China sites will continue to work to V 27) Ø EORTC: 0 out of 31. New protocol approved in April 2011 and is due to be implemented by Summer 2011. 3

4

5

Patient Consent Procedures 1 Patient Information • Patients should have a verbal explanation of the trial and be given most recent MREC-approved Patient Information Sheet (PIS) for the main SUPREMO trial/TRANS-SUPREMO and Quality of Life/Health Economics sub-studies (version 29) 6

Patient Consent Procedures 2 Consent Form Completion • Consent forms must be printed on hospital headed paper & accompanied by centre’s standard radiotherapy information sheets • All participating patients must sign the most recent version of consent forms (separate consent forms for Main trial/TRANSSUPREMO, Quality of Life/Health Economics substudies). • Patients must initial, not tick boxes • PI to countersign forms, but PI can delegate responsibility as appropriate (must be clearly documented in delegation log). • No trial procedures can be performed prior to the patient giving informed consent. 7

Patient Consent Procedures 3 Consent Form Copies 4 copies of signed consent forms required • Original copy should be kept in site folder • Copy given to patient • Copy sent to ISD CCTT • Copy kept in patient hospital notes 8

Serious Adverse Events 1 SAE Definition A SAE is defined as an untoward occurrence that: • Results in death • Is life threatening • Requires hospitalisation or prolongation of existing hospitalisation • Results in persistent disability or incapacity • Is a congenital anomaly/birth defect • Is otherwise considered medically significant by the investigator 9

Serious Adverse Events 2 Expected Radiotherapy SAEs For SUPREMO patients receiving radiotherapy the potential expected adverse events/reactions include: • Skin reactions leading to chest wall tenderness and itching • Chest wall pain • Pneumonitis (inflammation of the lung) causing shortness of breath • Osteitis (inflammation of the ribs) causing the ribs to fracture • Late cardiac damage If any of the above expected reactions fall under the definition of SAE they should be listed on SAE/SUSARs form 10

Serious Adverse Events 3 Chemotherapy SAEs Chemotherapy related SAEs that require reporting 1. 2. 3. 4. Wound infections Necrosis of the mastectomy skin flaps Any cardiac event Development of any other serious medical condition between date of consent and planned start of radiotherapy (or equivalent period for those patients randomised to not receive radiotherapy) Chemotherapy related SAEs that do not require reporting on SAE/ SUSAR form (unless they impact on delivery of the randomised treatment) Hospitalisation due to: 1. Neutropenia 2. Febrile neutropenia 3. Diarrhoea 4. Infections, including those to Hickman line, catheter. 5. Pyrexia 6. Sore throat 7. Nausea or vomiting 8. Cellulitis 11

Serious Adverse Events 4 Reporting At the centre: • Use SAE/SUSAR form to report all SAEs (even if the SAE is chemotherapy related) • Fax copy to ISD Trials Unit within 24 hours (from site staff becoming aware) • Do not delay because of missing information and/or signatures • Local PI to assess whether unexpected, severity and/or related to radiotherapy • Provide missing information (and outcome information) as soon as it is known 12

Serious Adverse Events 5 Processing ISD Trials Unit will: • Allocate an SAE number • Forward initial report to CI immediately if local PI assesses event as unexpected • Advise the PI if SAE is evaluated as a SUSAR • Comply with NRES guidelines on reporting of SAEs • Report all SAEs to DMEC/MREC on an annual basis 13

SUPREMO Team Eve Macdonald (eve. macdonald@nhs. net) Senior Trial Coordinator Julian Lipscombe (jlipscombe@nhs. net) Trial Coordinator Leigh Fell (leigh. fell@nhs. net) Trial Coordinator Ann Moncrieff (ann. moncrieff@nhs. net) Data Manager ISD Cancer Clinical Trials Team Edinburgh nss. isdsupremo@nhs. net 14

Trial Co-ordination Provided by ISD Trials Unit • Randomisation service • Advice on protocol and eligibility • Investigator Site Files (ISFs) • Regular newsflashes & website reports (www. supremo-trial. com) • 10% Source data monitoring 15
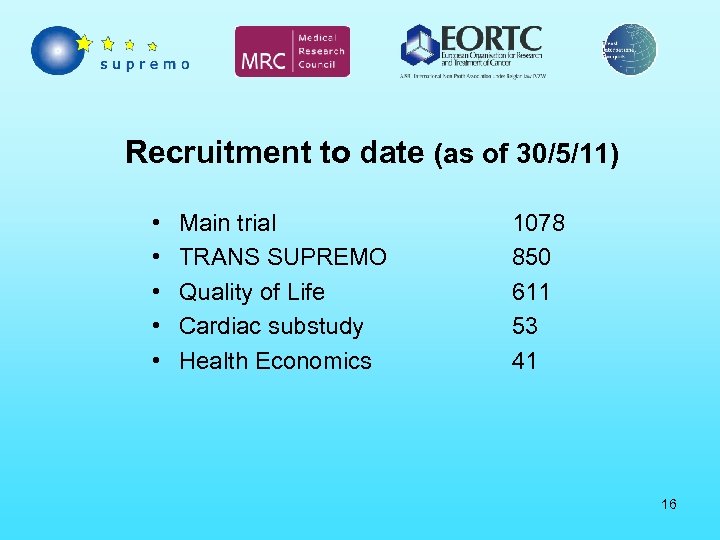
Recruitment to date (as of 30/5/11) • • • Main trial TRANS SUPREMO Quality of Life Cardiac substudy Health Economics 1078 850 611 53 41 16
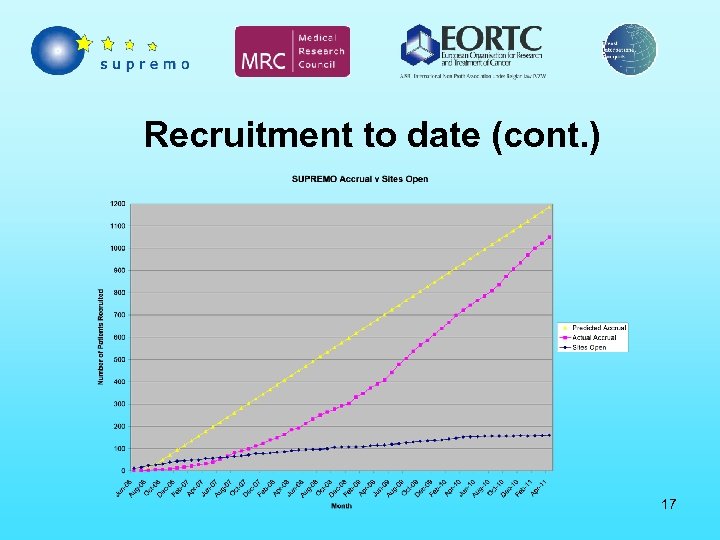
Recruitment to date (cont. ) 17

Thank you for your time. Questions? 18

ISD CANCER CLINICAL TRIALS TEAM 19
8d41225f33d786c0091fd00e2de84e87.ppt