529d063841fa6f4ca60da1ca59bf2045.ppt
- Количество слайдов: 27
 Motif Discovery: Algorithm and Application Dan Scanfeld Hong Xue Sumeet Gupta Varun Aggarwal
Motif Discovery: Algorithm and Application Dan Scanfeld Hong Xue Sumeet Gupta Varun Aggarwal
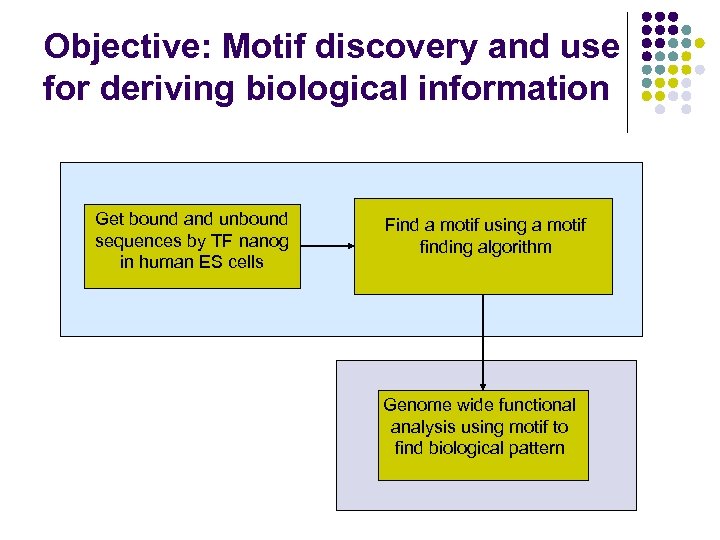 Objective: Motif discovery and use for deriving biological information Get bound and unbound sequences by TF nanog in human ES cells Find a motif using a motif finding algorithm Genome wide functional analysis using motif to find biological pattern
Objective: Motif discovery and use for deriving biological information Get bound and unbound sequences by TF nanog in human ES cells Find a motif using a motif finding algorithm Genome wide functional analysis using motif to find biological pattern
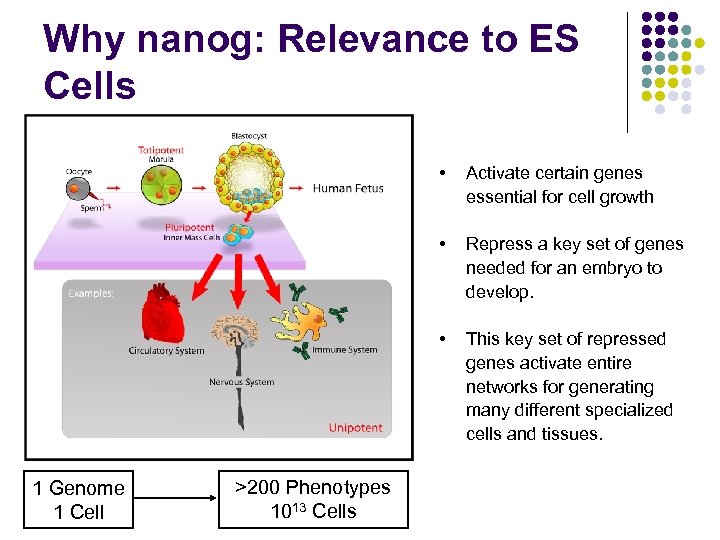 Why nanog: Relevance to ES Cells • • >200 Phenotypes 1013 Cells Repress a key set of genes needed for an embryo to develop. • 1 Genome 1 Cell Activate certain genes essential for cell growth This key set of repressed genes activate entire networks for generating many different specialized cells and tissues.
Why nanog: Relevance to ES Cells • • >200 Phenotypes 1013 Cells Repress a key set of genes needed for an embryo to develop. • 1 Genome 1 Cell Activate certain genes essential for cell growth This key set of repressed genes activate entire networks for generating many different specialized cells and tissues.
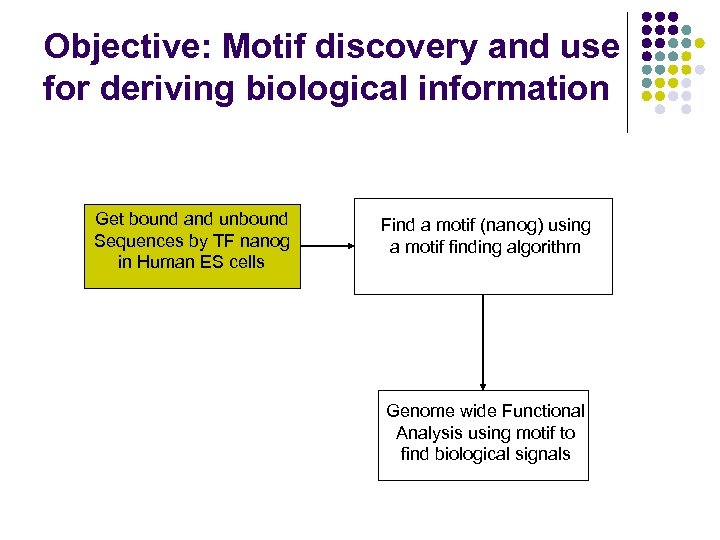 Objective: Motif discovery and use for deriving biological information Get bound and unbound Sequences by TF nanog in Human ES cells Find a motif (nanog) using a motif finding algorithm Genome wide Functional Analysis using motif to find biological signals
Objective: Motif discovery and use for deriving biological information Get bound and unbound Sequences by TF nanog in Human ES cells Find a motif (nanog) using a motif finding algorithm Genome wide Functional Analysis using motif to find biological signals
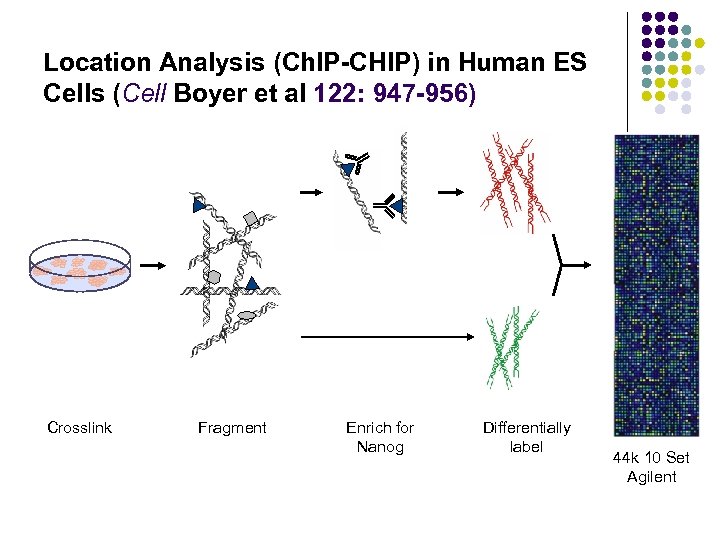 Location Analysis (Ch. IP-CHIP) in Human ES Cells (Cell Boyer et al 122: 947 -956) Crosslink Fragment Enrich for Nanog Differentially label 44 k 10 Set Agilent
Location Analysis (Ch. IP-CHIP) in Human ES Cells (Cell Boyer et al 122: 947 -956) Crosslink Fragment Enrich for Nanog Differentially label 44 k 10 Set Agilent
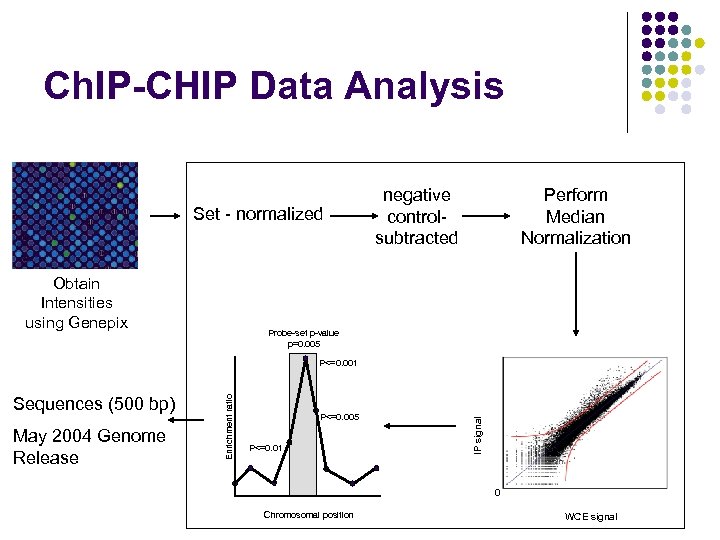 Ch. IP-CHIP Data Analysis Set - normalized Obtain Intensities using Genepix negative controlsubtracted Perform Median Normalization Probe-set p-value p=0. 005 May 2004 Genome Release P<=0. 005 P<=0. 01 IP signal Sequences (500 bp) Enrichment ratio P<=0. 001 0 Chromosomal position WCE signal
Ch. IP-CHIP Data Analysis Set - normalized Obtain Intensities using Genepix negative controlsubtracted Perform Median Normalization Probe-set p-value p=0. 005 May 2004 Genome Release P<=0. 005 P<=0. 01 IP signal Sequences (500 bp) Enrichment ratio P<=0. 001 0 Chromosomal position WCE signal
 Objective: Motif discovery and use for deriving biological information Get bound and unbound Sequences by TF nanog in Human ES cells Find a motif (nanog) using a motif finding algorithm (State-of-the-art) Genome wide functional analysis using motif to find biological pattern
Objective: Motif discovery and use for deriving biological information Get bound and unbound Sequences by TF nanog in Human ES cells Find a motif (nanog) using a motif finding algorithm (State-of-the-art) Genome wide functional analysis using motif to find biological pattern
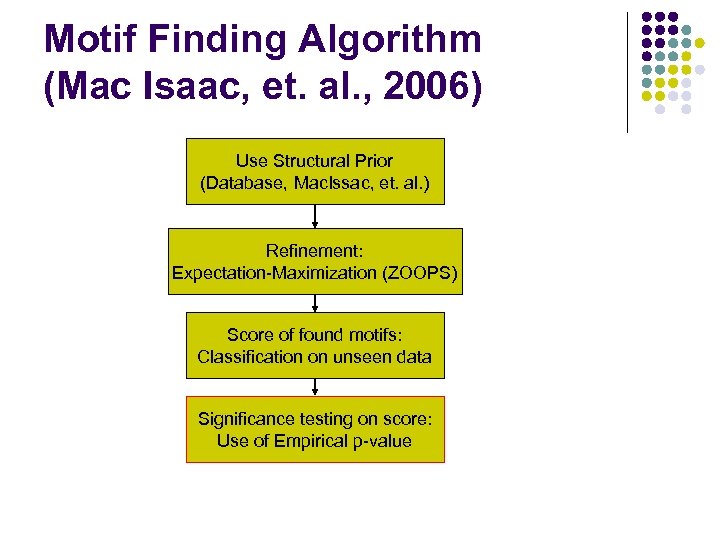 Motif Finding Algorithm (Mac Isaac, et. al. , 2006) Use Structural Prior (Database, Mac. Issac, et. al. ) Refinement: Expectation-Maximization (ZOOPS) Score of found motifs: Classification on unseen data Significance testing on score: Use of Empirical p-value
Motif Finding Algorithm (Mac Isaac, et. al. , 2006) Use Structural Prior (Database, Mac. Issac, et. al. ) Refinement: Expectation-Maximization (ZOOPS) Score of found motifs: Classification on unseen data Significance testing on score: Use of Empirical p-value
 Refinement: Expectation-Maximization Differences from EM in Lab 1 l Use of structural prior (beta = Strength of prior) l ZOOPS (Zero or One per sequence) model l 5 th order Markov Model for background trained over unbound sequences l SVM for hypothesis testing
Refinement: Expectation-Maximization Differences from EM in Lab 1 l Use of structural prior (beta = Strength of prior) l ZOOPS (Zero or One per sequence) model l 5 th order Markov Model for background trained over unbound sequences l SVM for hypothesis testing
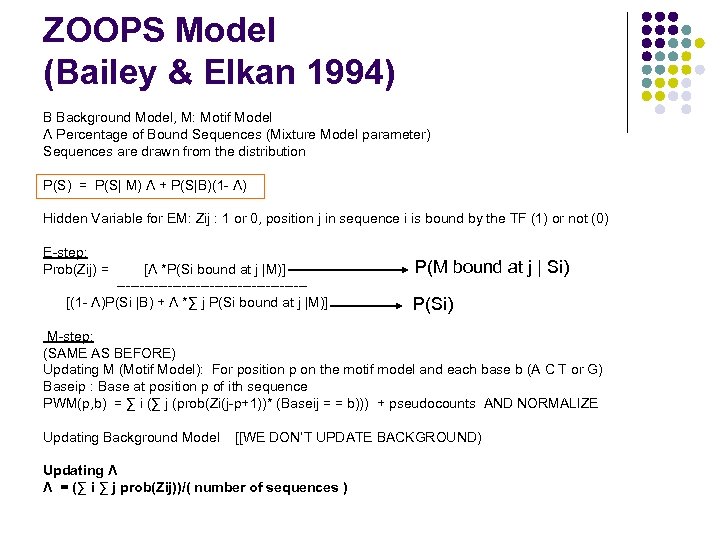 ZOOPS Model (Bailey & Elkan 1994) B Background Model, M: Motif Model Λ Percentage of Bound Sequences (Mixture Model parameter) Sequences are drawn from the distribution P(S) = P(S| M) Λ + P(S|B)(1 - Λ) Hidden Variable for EM: Zij : 1 or 0, position j in sequence i is bound by the TF (1) or not (0) E-step: Prob(Zij) = [Λ *P(Si bound at j |M)] --------------------[(1 - Λ)P(Si |B) + Λ *∑ j P(Si bound at j |M)] P(M bound at j | Si) P(Si) M-step: (SAME AS BEFORE) Updating M (Motif Model): For position p on the motif model and each base b (A C T or G) Baseip : Base at position p of ith sequence PWM(p, b) = ∑ i (∑ j (prob(Zi(j-p+1))* (Baseij = = b))) + pseudocounts AND NORMALIZE Updating Background Model [[WE DON’T UPDATE BACKGROUND) Updating Λ Λ = (∑ i ∑ j prob(Zij))/( number of sequences )
ZOOPS Model (Bailey & Elkan 1994) B Background Model, M: Motif Model Λ Percentage of Bound Sequences (Mixture Model parameter) Sequences are drawn from the distribution P(S) = P(S| M) Λ + P(S|B)(1 - Λ) Hidden Variable for EM: Zij : 1 or 0, position j in sequence i is bound by the TF (1) or not (0) E-step: Prob(Zij) = [Λ *P(Si bound at j |M)] --------------------[(1 - Λ)P(Si |B) + Λ *∑ j P(Si bound at j |M)] P(M bound at j | Si) P(Si) M-step: (SAME AS BEFORE) Updating M (Motif Model): For position p on the motif model and each base b (A C T or G) Baseip : Base at position p of ith sequence PWM(p, b) = ∑ i (∑ j (prob(Zi(j-p+1))* (Baseij = = b))) + pseudocounts AND NORMALIZE Updating Background Model [[WE DON’T UPDATE BACKGROUND) Updating Λ Λ = (∑ i ∑ j prob(Zij))/( number of sequences )
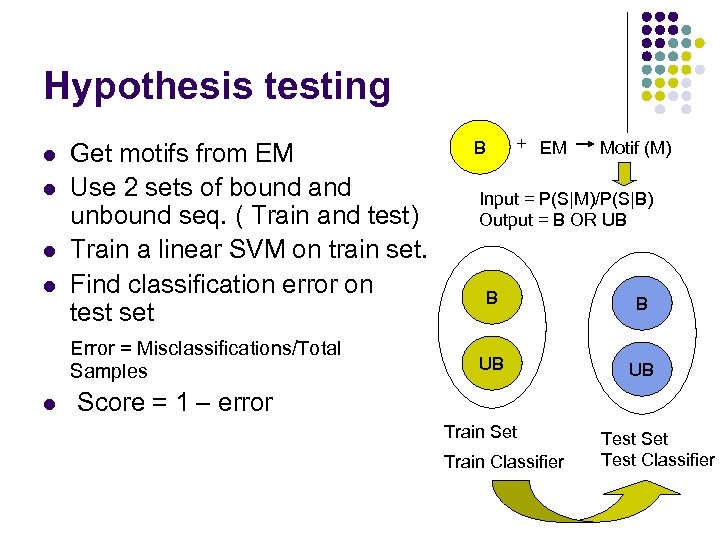 Hypothesis testing l l Get motifs from EM Use 2 sets of bound and unbound seq. ( Train and test) Train a linear SVM on train set. Find classification error on test set Error = Misclassifications/Total Samples l + EM B Motif (M) Input = P(S|M)/P(S|B) Output = B OR UB B B UB UB Score = 1 – error Train Set Train Classifier Test Set Test Classifier
Hypothesis testing l l Get motifs from EM Use 2 sets of bound and unbound seq. ( Train and test) Train a linear SVM on train set. Find classification error on test set Error = Misclassifications/Total Samples l + EM B Motif (M) Input = P(S|M)/P(S|B) Output = B OR UB B B UB UB Score = 1 – error Train Set Train Classifier Test Set Test Classifier
 Expectation-Maximization When to stop? Will it overtrain? l Rules of thumb (When likelihood increases very slowly) l Second derivative is negative for given number of times l Euclidean distance is less than given value l Over-train to given sequences l Maximizes likelihood of motif in given sequences. Disregards their likelihood in unbound sequences l Find test classification error at each EM step using SVMs.
Expectation-Maximization When to stop? Will it overtrain? l Rules of thumb (When likelihood increases very slowly) l Second derivative is negative for given number of times l Euclidean distance is less than given value l Over-train to given sequences l Maximizes likelihood of motif in given sequences. Disregards their likelihood in unbound sequences l Find test classification error at each EM step using SVMs.
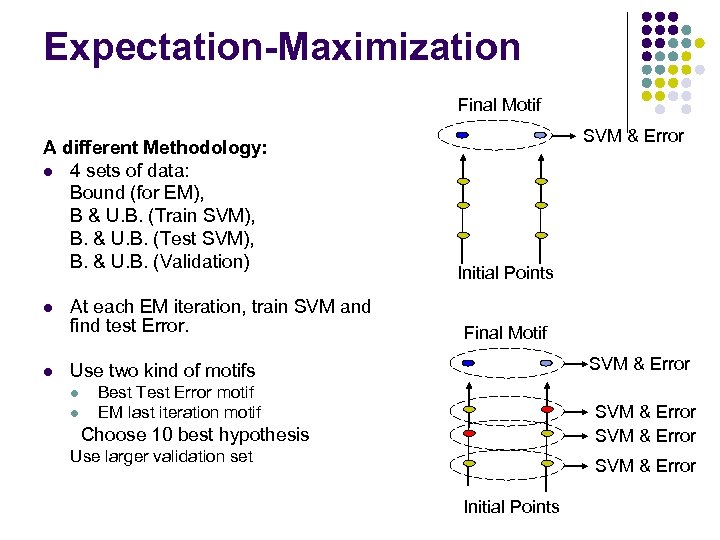 Expectation-Maximization Final Motif A different Methodology: l 4 sets of data: Bound (for EM), B & U. B. (Train SVM), B. & U. B. (Test SVM), B. & U. B. (Validation) l l At each EM iteration, train SVM and find test Error. SVM & Error Initial Points Final Motif SVM & Error Use two kind of motifs l l Best Test Error motif EM last iteration motif SVM & Error Choose 10 best hypothesis Use larger validation set SVM & Error Initial Points
Expectation-Maximization Final Motif A different Methodology: l 4 sets of data: Bound (for EM), B & U. B. (Train SVM), B. & U. B. (Test SVM), B. & U. B. (Validation) l l At each EM iteration, train SVM and find test Error. SVM & Error Initial Points Final Motif SVM & Error Use two kind of motifs l l Best Test Error motif EM last iteration motif SVM & Error Choose 10 best hypothesis Use larger validation set SVM & Error Initial Points
 Expectation-Maximization Details of RUN l Transfactor: Nanog l Beta = [0 0. 2 0. 35 0. 6 0. 7 1] (Strength of prior) l 5 motifs per beta by masking motifs l Motif Length : 8 l 25 bound seqs for EM l 500 base pairs in each seq. l 150 total train seq (SVM) [Low: Noisy] l 150 total test seq (SVM) [Low: Noisy] l 500 total Validation seq. l c = [1 e-3, 0. 05, 100. 0] (SVM: Budget for misclassifications) l EM for minimum 60 iterations, Second derivative is negative for five iterations
Expectation-Maximization Details of RUN l Transfactor: Nanog l Beta = [0 0. 2 0. 35 0. 6 0. 7 1] (Strength of prior) l 5 motifs per beta by masking motifs l Motif Length : 8 l 25 bound seqs for EM l 500 base pairs in each seq. l 150 total train seq (SVM) [Low: Noisy] l 150 total test seq (SVM) [Low: Noisy] l 500 total Validation seq. l c = [1 e-3, 0. 05, 100. 0] (SVM: Budget for misclassifications) l EM for minimum 60 iterations, Second derivative is negative for five iterations
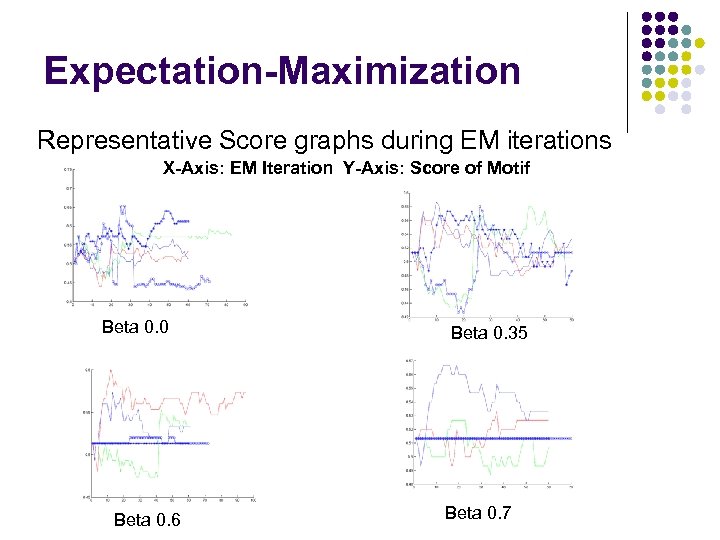 Expectation-Maximization Representative Score graphs during EM iterations X-Axis: EM Iteration Y-Axis: Score of Motif Beta 0. 0 Beta 0. 6 Beta 0. 35 Beta 0. 7
Expectation-Maximization Representative Score graphs during EM iterations X-Axis: EM Iteration Y-Axis: Score of Motif Beta 0. 0 Beta 0. 6 Beta 0. 35 Beta 0. 7
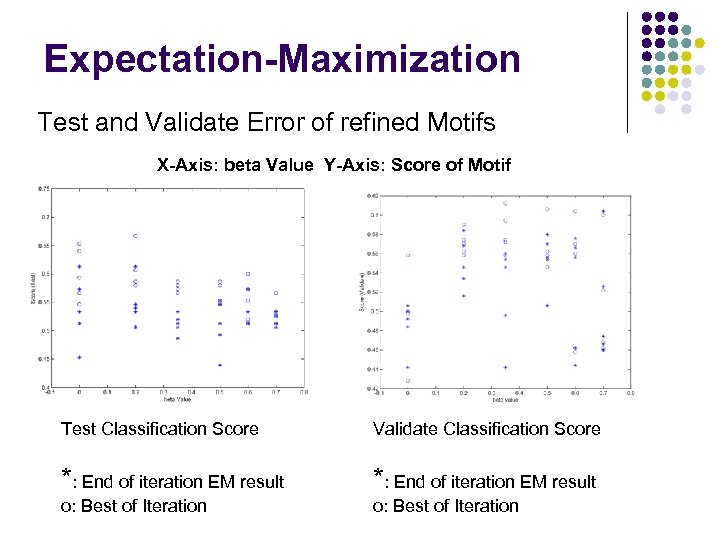 Expectation-Maximization Test and Validate Error of refined Motifs X-Axis: beta Value Y-Axis: Score of Motif Test Classification Score Validate Classification Score *: End of iteration EM result o: Best of Iteration
Expectation-Maximization Test and Validate Error of refined Motifs X-Axis: beta Value Y-Axis: Score of Motif Test Classification Score Validate Classification Score *: End of iteration EM result o: Best of Iteration
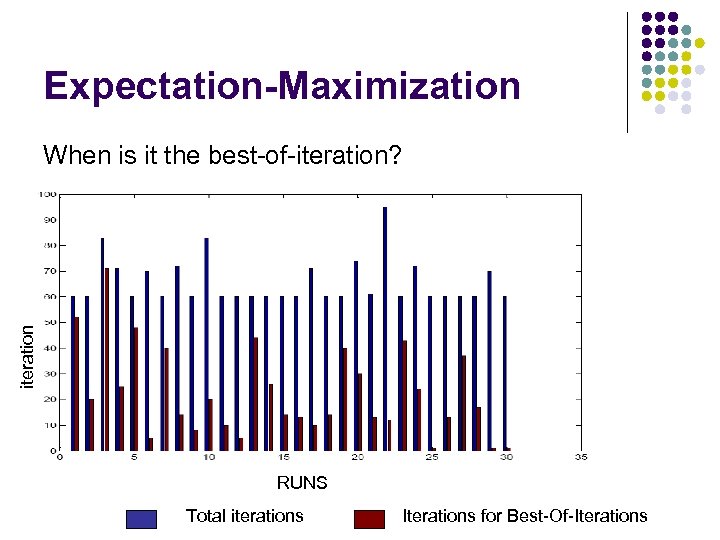 Expectation-Maximization iteration When is it the best-of-iteration? RUNS Total iterations Iterations for Best-Of-Iterations
Expectation-Maximization iteration When is it the best-of-iteration? RUNS Total iterations Iterations for Best-Of-Iterations
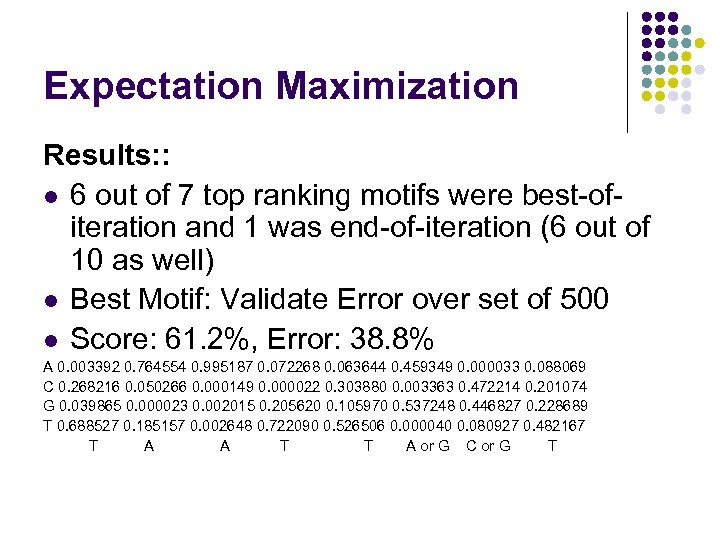 Expectation Maximization Results: : l 6 out of 7 top ranking motifs were best-ofiteration and 1 was end-of-iteration (6 out of 10 as well) l Best Motif: Validate Error over set of 500 l Score: 61. 2%, Error: 38. 8% A 0. 003392 0. 764554 0. 995187 0. 072268 0. 063644 0. 459349 0. 000033 0. 088069 C 0. 268216 0. 050266 0. 000149 0. 000022 0. 303880 0. 003363 0. 472214 0. 201074 G 0. 039865 0. 000023 0. 002015 0. 205620 0. 105970 0. 537248 0. 446827 0. 228689 T 0. 688527 0. 185157 0. 002648 0. 722090 0. 526506 0. 000040 0. 080927 0. 482167 T A A T T A or G C or G T
Expectation Maximization Results: : l 6 out of 7 top ranking motifs were best-ofiteration and 1 was end-of-iteration (6 out of 10 as well) l Best Motif: Validate Error over set of 500 l Score: 61. 2%, Error: 38. 8% A 0. 003392 0. 764554 0. 995187 0. 072268 0. 063644 0. 459349 0. 000033 0. 088069 C 0. 268216 0. 050266 0. 000149 0. 000022 0. 303880 0. 003363 0. 472214 0. 201074 G 0. 039865 0. 000023 0. 002015 0. 205620 0. 105970 0. 537248 0. 446827 0. 228689 T 0. 688527 0. 185157 0. 002648 0. 722090 0. 526506 0. 000040 0. 080927 0. 482167 T A A T T A or G C or G T
 Assumptions and Caveats l Random baseline: End-of-run motif in EM l Low number of sequences for test error l Bound sets may actually not be bound. Better to use highly probable sequences as bound. l All runs (inc. beta=0) used starting point as the structural prior.
Assumptions and Caveats l Random baseline: End-of-run motif in EM l Low number of sequences for test error l Bound sets may actually not be bound. Better to use highly probable sequences as bound. l All runs (inc. beta=0) used starting point as the structural prior.
 Objective: Motif discovery and use for deriving biological information Get bound and unbound Sequences by TF nanog in Human ES cells Find a motif (nanog) using a motif finding algorithm Genome wide functional analysis using motif to find biological pattern
Objective: Motif discovery and use for deriving biological information Get bound and unbound Sequences by TF nanog in Human ES cells Find a motif (nanog) using a motif finding algorithm Genome wide functional analysis using motif to find biological pattern
 GSEA (Subramanian et al 2005) l Gene Set Enrichment Analysis (GSEA) determines whether an a priori defined set of genes shows statistically significant differences between two biological states.
GSEA (Subramanian et al 2005) l Gene Set Enrichment Analysis (GSEA) determines whether an a priori defined set of genes shows statistically significant differences between two biological states.
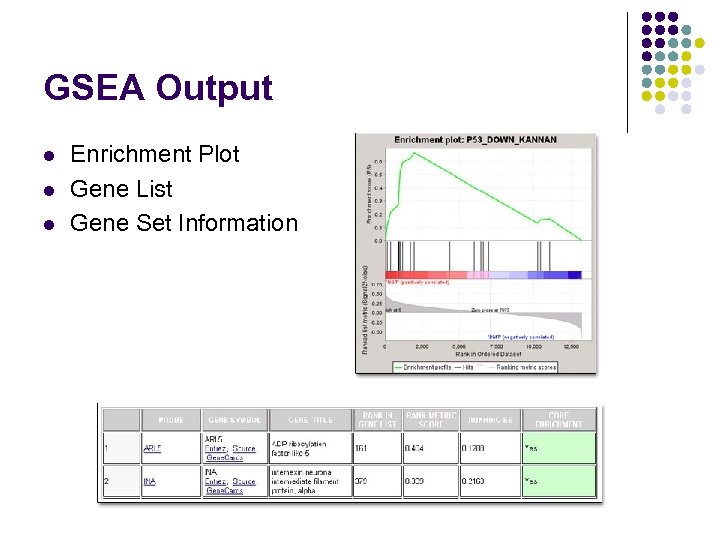 GSEA Output l l l Enrichment Plot Gene List Gene Set Information
GSEA Output l l l Enrichment Plot Gene List Gene Set Information
 GSEA Ranked List l l l Set of promoter sequences for every human gene. 2000 bp upstream and 200 bp downstream of Transcription initiation site. Score each promoter for likelihood of the motif. Input this ranked list into GSEA. Search for gene sets enriched in the ranked list.
GSEA Ranked List l l l Set of promoter sequences for every human gene. 2000 bp upstream and 200 bp downstream of Transcription initiation site. Score each promoter for likelihood of the motif. Input this ranked list into GSEA. Search for gene sets enriched in the ranked list.
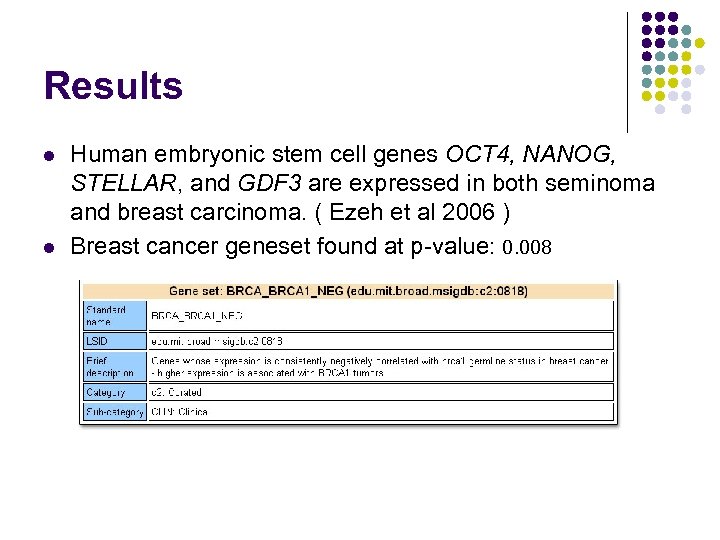 Results l l Human embryonic stem cell genes OCT 4, NANOG, STELLAR, and GDF 3 are expressed in both seminoma and breast carcinoma. ( Ezeh et al 2006 ) Breast cancer geneset found at p-value: 0. 008
Results l l Human embryonic stem cell genes OCT 4, NANOG, STELLAR, and GDF 3 are expressed in both seminoma and breast carcinoma. ( Ezeh et al 2006 ) Breast cancer geneset found at p-value: 0. 008
 Implementation Details l Young Lab Error model for ch. IP-chip data Analysis l Motif finding Algorithm in MATLAB l l l Implemented Markov Model Implemented ZOOPS Model Integrated SVM Toolbox ( by S. R. Gunn. ) with code l Used structural prior from Mac. Isaac, et. al. 2006 l Used software for GSEA for Functional Analysis.
Implementation Details l Young Lab Error model for ch. IP-chip data Analysis l Motif finding Algorithm in MATLAB l l l Implemented Markov Model Implemented ZOOPS Model Integrated SVM Toolbox ( by S. R. Gunn. ) with code l Used structural prior from Mac. Isaac, et. al. 2006 l Used software for GSEA for Functional Analysis.
 Future Directions l Algorithm l l l Better use of classification error. Maximize Likelihood in Bound + Minimizes Likelihood in Unbound (Multi-objective Optimization using GAs) Biological Information: Distance from transcription site, Conservation Integrating expression data Cross-species Motif search and functional analysis, maybe using GO Terms l l Scoring Sequence length
Future Directions l Algorithm l l l Better use of classification error. Maximize Likelihood in Bound + Minimizes Likelihood in Unbound (Multi-objective Optimization using GAs) Biological Information: Distance from transcription site, Conservation Integrating expression data Cross-species Motif search and functional analysis, maybe using GO Terms l l Scoring Sequence length
 Acknowledgments l l l Fraenkel Lab Young Lab Kenzie D. Mac. Isaac Dr. David Gifford (CSAIL) Dr. Richard Young (WIBR) Dr. Tommi Jaakkola (CSAIL)
Acknowledgments l l l Fraenkel Lab Young Lab Kenzie D. Mac. Isaac Dr. David Gifford (CSAIL) Dr. Richard Young (WIBR) Dr. Tommi Jaakkola (CSAIL)


