b054875520062eae8fcc1d45768bfde9.ppt
- Количество слайдов: 24

Month __, 20__

TOPICAL SKIN ADHESIVES DERMABOND® Topical Skin Adhesive Product 1 Product 2

DERMABOND® TOPICAL SKIN ADHESIVE INTRODUCTION DERMABOND® Adhesive is a strong and highly flexible topical skin adhesive with a chemical composition that gives it flexibility and strength superior to those of competitors’ products. 1

DERMABOND® TOPICAL SKIN ADHESIVE INTRODUCTION DERMABOND® Adhesive may be used in place of sutures or staples to close wounds in conjunction with deep dermal stitches (you will learn more about this later in the tutorial). It may be used to close wounds of any length (DERMABOND® Adhesive has been clinically shown to be effectively close skin on wounds of up to 69 cm), with excellent cosmetic outcomes.

DERMABOND® TOPICAL SKIN ADHESIVE INTRODUCTION When DERMABOND® Adhesive is used in addition to skin sutures or staples it functions as a microbial barrier to protect wounds from bacteria that can cause infection.

DERMABOND® TOPICAL SKIN ADHESIVE FEATURES AND BENEFITS DERMABOND® Adhesive is a highly viscous, sterile, nonabsorbable, single-use liquid made from the polymer 2 -octyl cyanoacrylate (you will learn more about why this is important in Tutorial 2). It is dyed with D&C Violet #2 for easier visualization during application. 2

DERMABOND® TOPICAL SKIN ADHESIVE FEATURES AND BENEFITS DERMABOND® Adhesive: • 7 days of wound-healing strength in 3 minutes 4 • Faster and as strong as 4 -0 suture 5, 6 • Acts as a microbial barrier and as its own dressing 7, 8 • Has been clinically proven to close skin effectively in both short and long incisions (up to 69 cm)9 • Can save the surgeon significant amounts of time in some procedures 5

DERMABOND® TOPICAL SKIN ADHESIVE FEATURES AND BENEFITS DERMABOND® Adhesive: • Is water-resistant enough for a patient to shower a few minutes after application 10 • Holds securely to the wound and sloughs off as the skin re-epithelializes at around 5 to 10 days 2 • Does not require a return visit for removal • Can be stored at room temperature for up to 24 months 11 Throughout the tutorial, we will learn more about the many features and benefits of DERMABOND® Adhesive.

DERMABOND® TOPICAL SKIN ADHESIVE PRODUCTS DERMABOND® Adhesive is supplied in the following 3 packaging options: • DHV 12: DERMABOND® Adhesive High Viscosity, in 0. 5 m. L ampoule form • DPP 6: DERMABOND® Adhesive Pro. Pen, containing 0. 5 m. L • DPPXL 6: DERMABOND® Adhesive Pro. Pen XL, containing 0. 75 m. L We will be learning more about each of these later in this tutorial. First, let’s take a look at how DERMABOND® Adhesive is applied to a wound using the DERMABOND® Adhesive Pro. Pen XL.

DERMABOND® TOPICAL SKIN ADHESIVE PRODUCTS DERMABOND® Adhesive is supplied in ampoule form And in DERMABOND® Adhesive Pro. Pen

DERMABOND® TOPICAL SKIN ADHESIVE PREPARING A WOUND When applying DERMABOND® Adhesive to a wound, the wound should be clean and dry, and adequate hemostasis should be achieved. 2 In the following frames, we will look at how DERMABOND ® Adhesive is used in conjunction with sutures to close deeper wounds that extend below the skin.
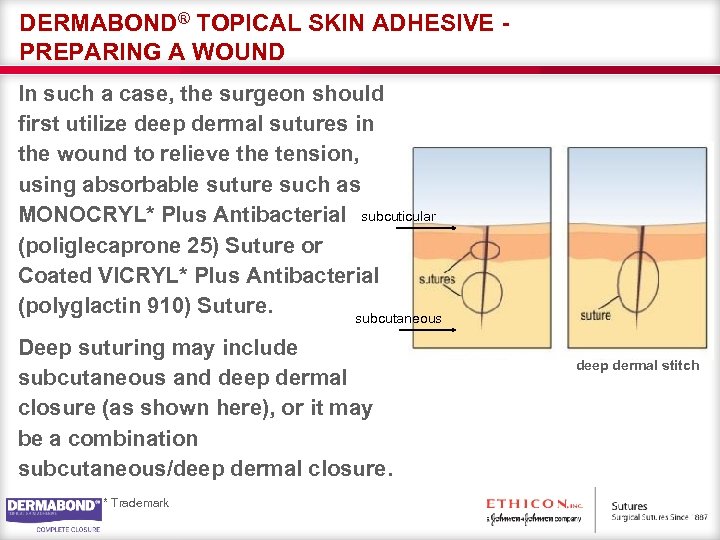
DERMABOND® TOPICAL SKIN ADHESIVE PREPARING A WOUND In such a case, the surgeon should first utilize deep dermal sutures in the wound to relieve the tension, using absorbable suture such as MONOCRYL* Plus Antibacterial subcuticular (poliglecaprone 25) Suture or Coated VICRYL* Plus Antibacterial (polyglactin 910) Suture. subcutaneous Deep suturing may include subcutaneous and deep dermal closure (as shown here), or it may be a combination subcutaneous/deep dermal closure. * Trademark deep dermal stitch
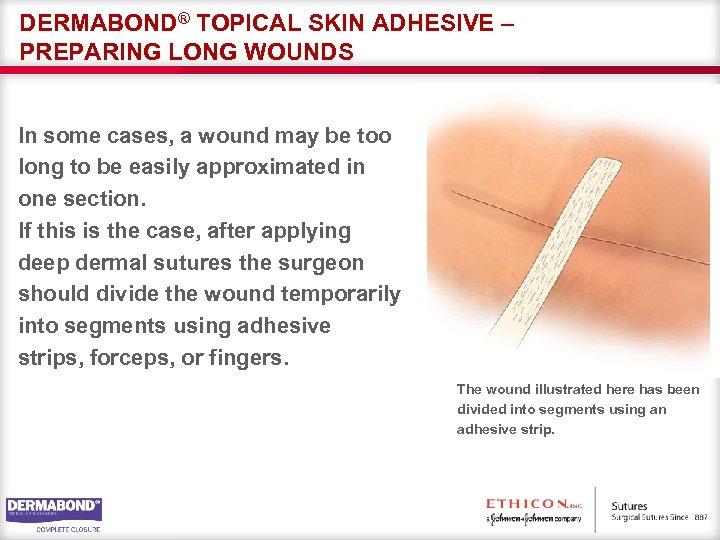
DERMABOND® TOPICAL SKIN ADHESIVE – PREPARING LONG WOUNDS In some cases, a wound may be too long to be easily approximated in one section. If this is the case, after applying deep dermal sutures the surgeon should divide the wound temporarily into segments using adhesive strips, forceps, or fingers. The wound illustrated here has been divided into segments using an adhesive strip.

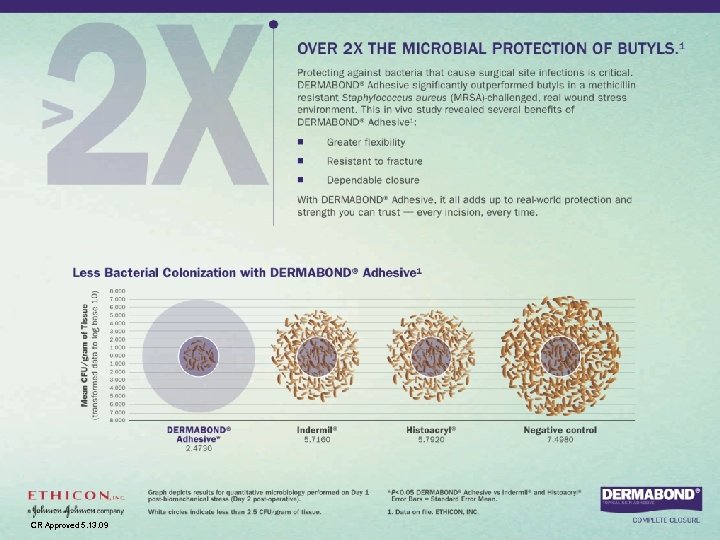
DERMABOND® TOPICAL SKIN ADHESIVE – THE PATIENT EXPERIENCE Once in place, DERMABOND® Adhesive provides an extremely flexible microbial barrier that helps prevents some of the most common infection-causing bacteria from entering a wound. 1, 12
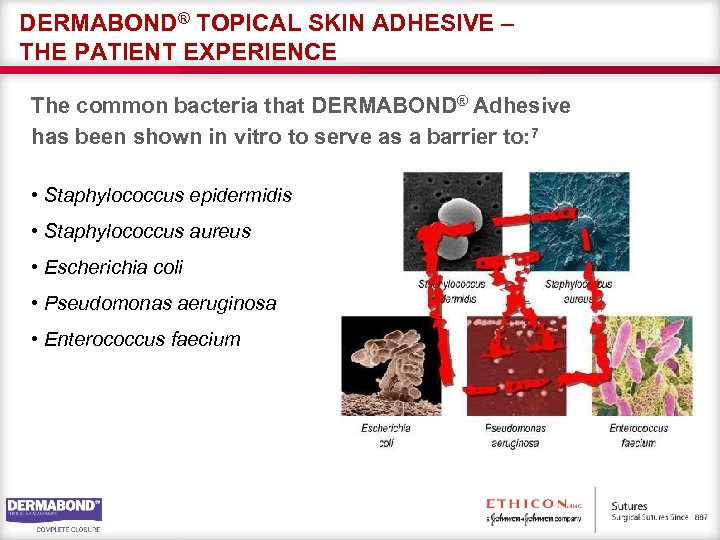
DERMABOND® TOPICAL SKIN ADHESIVE – THE PATIENT EXPERIENCE The common bacteria that DERMABOND® Adhesive has been shown in vitro to serve as a barrier to: 7 • Staphylococcus epidermidis • Staphylococcus aureus • Escherichia coli • Pseudomonas aeruginosa • Enterococcus faecium

DERMABOND® TOPICAL SKIN ADHESIVE – THE PATIENT EXPERIENCE A wound to which DERMABOND® Adhesive has been applied needs no other dressing and the patient, if mobile, can shower immediately after application. 7 DERMABOND® Adhesive remains on the skin until after healing has occurred. Ultimately, it will slough off as a result of natural epithelialization. 2
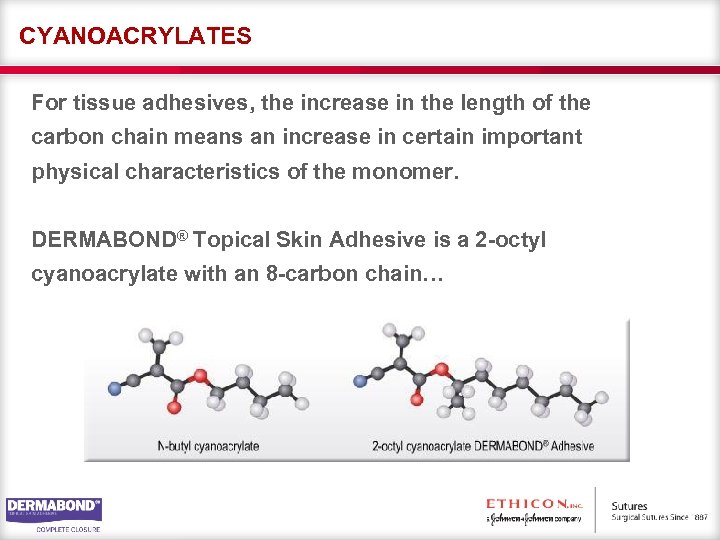
CYANOACRYLATES For tissue adhesives, the increase in the length of the carbon chain means an increase in certain important physical characteristics of the monomer. DERMABOND® Topical Skin Adhesive is a 2 -octyl cyanoacrylate with an 8 -carbon chain…
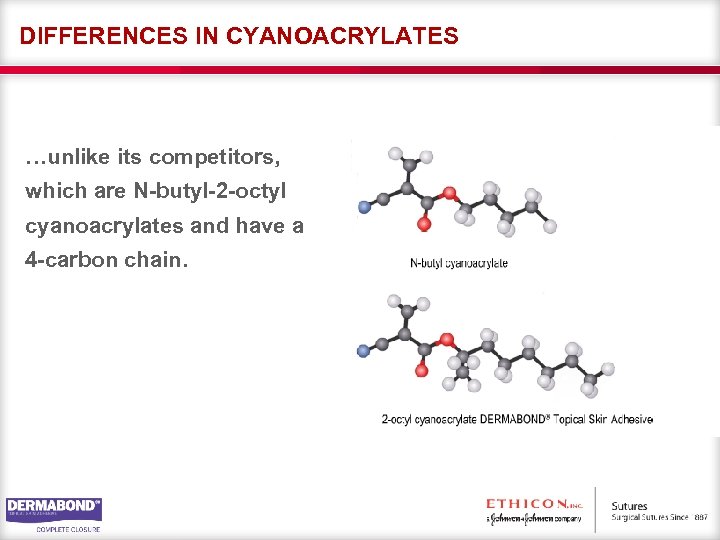
DIFFERENCES IN CYANOACRYLATES …unlike its competitors, which are N-butyl-2 -octyl cyanoacrylates and have a 4 -carbon chain.
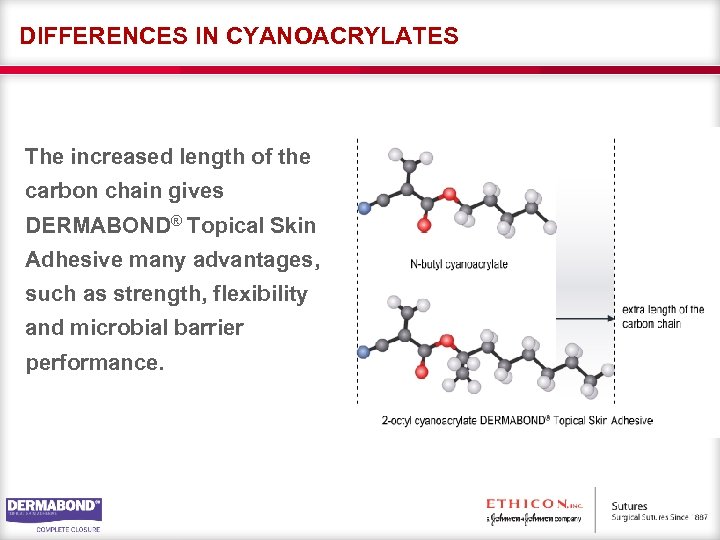
DIFFERENCES IN CYANOACRYLATES The increased length of the carbon chain gives DERMABOND® Topical Skin Adhesive many advantages, such as strength, flexibility and microbial barrier performance.
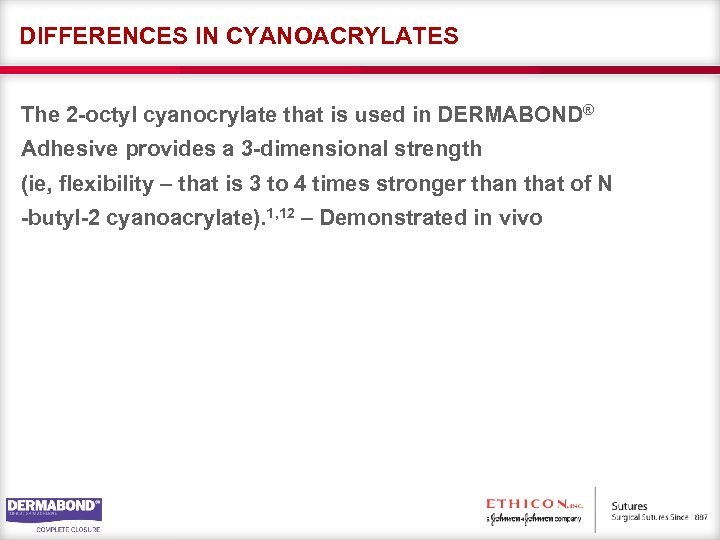
DIFFERENCES IN CYANOACRYLATES The 2 -octyl cyanocrylate that is used in DERMABOND® Adhesive provides a 3 -dimensional strength (ie, flexibility – that is 3 to 4 times stronger than that of N -butyl-2 cyanoacrylate). 1, 12 – Demonstrated in vivo

PLASTICIZERS In addition to using different base formulas, manufacturers can vary the physical characteristics of cyanoacrylates by adding components, such as plasticizers and elasticizers. The plasticizers used in DERMABOND® Adhesive produce a stronger, more pliable tissue-compatible end product that flexes with the skin and remains in place for longer. DERMABOND® Adhesive provides more dependable closure than butyl-based skin closure products, which become brittle and weak. 1, 12, 13 – Demonstrated in vivo.

REFERENCES 1 Data on file. Ethicon, Inc. 2 DERMABOND® Adhesive Package Insert. 3 INDERMIL® Tissue Adhesive (package insert). Norwalk, CT: United States Surgical/Syneture; 2002. 4 Quinn J, Wells G, Sutcliffe T, et al. A randomized trial comparing octylcyanoacrylate tissue adhesive and sutures in the management of lacerations. JAMA. 1997; 277: 1527 -1530. 5 Toriumi DM, O’Grady K, Desai D, Bagal A. Use of octyl-2 cyanocrylate for skin closure in facial plastic surgery. Plast Reconst Surg. 1998; 102: 2209 -2219. 6 Shapiro AJ, Dinsmore RC, North JH. Tensile strength of would closure with cyanoacrylate glue. Am Surg. 2001; 67; 1113 -1115. 7 Bhende S, Rothenberger S, Spangler DJ, Dito M. In vitro assessment of microbial barrier properties of Dermabond Topical Skin Adhesive. Surg Infect. 2002; 3: 251 -257. 8 Kannon GA, Garrett AB. Moist wound healing with occlusive dressings: a clinical review. Dermatol Surg. 1995: 21: 583 -590. 9 Blondeel MD. Closure of long surgical incisions with a new formulation of 2 -octylcyanoacrylate tissue adhesive versus commercially available methods. Am. J Surg. 2004; 186; 307 -313.
REFERENCES 10 Rubio PA. Use of semiocclusive, transparent film dressings for surgical wound protection: experience in 3637 cases. Int Surg. 1991; 7: 253 -254. 11 Laura Mc. Crum, Final Report – Marketing Claims Support: Wound Length Coverage for High Viscosity DERMABOND Products. 12 Singer AJ, Zimmerman T, Rooney J, Cameau P, Rudomen G, Mc. Clain SA. Comparison of woundbursting strengths and surface characteristics of FDA-approved tissue adhesive for skin closure. J Adhes Sci Technol. 2004; 18: 19 -27. 13 Perry L. C. An evaluation of acute incisional strength with tramaseal surgical tissue adhesive wound closure. Findings by: Dimensional Analysis Systems, Inc. Leonia, NJ. 1995. 14 Hall, LT, MD, Bailes, JE, MD. Using DERMABOND for wound closure in lumbar and cervical neurosurgical procedures. Abstract taken from Neurosurgery. 56(1) Operative Neurosurgery Suppliment 1: 147 -150. Viewed January 2005. http: //www. neurosurgeryonline. com/pt/re/neurosurg/abstract. 00006123 -200501001 -00018. htm. Accessed 4/10/2007.
CR Approved 5. 13. 09
b054875520062eae8fcc1d45768bfde9.ppt