bc0522fb2d7644e8d0d3954699b35e31.ppt
- Количество слайдов: 44
 Monitoring the Sterilization Process THETA CHAPTER May 09
Monitoring the Sterilization Process THETA CHAPTER May 09
 Quality Assurance Each Health Care Facility should have a system in place to provide quality patient care through the provision of sterile equipment and medical devices.
Quality Assurance Each Health Care Facility should have a system in place to provide quality patient care through the provision of sterile equipment and medical devices.
 Quality Assurance Program Should include: • Administrative Controls • Chemical Indicator Monitoring • Biological Indicator Monitoring • Mechanical Indicators • Continuing Education
Quality Assurance Program Should include: • Administrative Controls • Chemical Indicator Monitoring • Biological Indicator Monitoring • Mechanical Indicators • Continuing Education
 Quality Assurance CPD - Administrative Controls – policies and procedures – continuous education, training and observation of employees – maintain updated knowledge about guidelines, current research and recommended practices – CPD designed to facilitate efficient processing of patient care items
Quality Assurance CPD - Administrative Controls – policies and procedures – continuous education, training and observation of employees – maintain updated knowledge about guidelines, current research and recommended practices – CPD designed to facilitate efficient processing of patient care items
 Accepted Practice Guidelines u CSA Canadian Standards Association International u AAMI Association for the Advancement of Medical Instrumentation u ASHCSP American Society for Healthcare Central Service Professionals u AORN Association of Operating Room Nurses u ORNAC Operating Room Nurses Association of Canada u CDC Centers for Disease Control and Prevention u LCDC Laboratory Centre for Disease Control
Accepted Practice Guidelines u CSA Canadian Standards Association International u AAMI Association for the Advancement of Medical Instrumentation u ASHCSP American Society for Healthcare Central Service Professionals u AORN Association of Operating Room Nurses u ORNAC Operating Room Nurses Association of Canada u CDC Centers for Disease Control and Prevention u LCDC Laboratory Centre for Disease Control
 Objectives of Monitoring the Sterilization Process • Assure high probability of absence of microbes on processed items • Detect failures as soon as possible • Remove medical devices involved in failures before patient use • Improve patient outcomes • Control costs • Peace of mind October 21, 1998
Objectives of Monitoring the Sterilization Process • Assure high probability of absence of microbes on processed items • Detect failures as soon as possible • Remove medical devices involved in failures before patient use • Improve patient outcomes • Control costs • Peace of mind October 21, 1998
 Methods of Monitoring 1. Mechanical Indicators Equipment control 2. Chemical Indicators Exposure/Process control Pack control 3. Biological Indicators Load control October 21, 1998
Methods of Monitoring 1. Mechanical Indicators Equipment control 2. Chemical Indicators Exposure/Process control Pack control 3. Biological Indicators Load control October 21, 1998
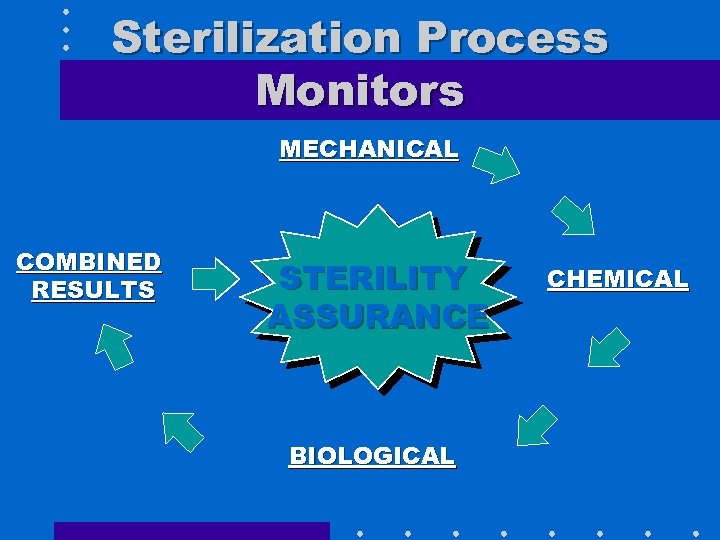 Sterilization Process Monitors MECHANICAL COMBINED RESULTS STERILITY ASSURANCE BIOLOGICAL CHEMICAL
Sterilization Process Monitors MECHANICAL COMBINED RESULTS STERILITY ASSURANCE BIOLOGICAL CHEMICAL
 Sterilization Process Monitors Equipment Control Mechanical Indicators show: • • • what is happening in the chamber whether conditions are being met cycle, time, temperature and pressure October 21, 1998
Sterilization Process Monitors Equipment Control Mechanical Indicators show: • • • what is happening in the chamber whether conditions are being met cycle, time, temperature and pressure October 21, 1998
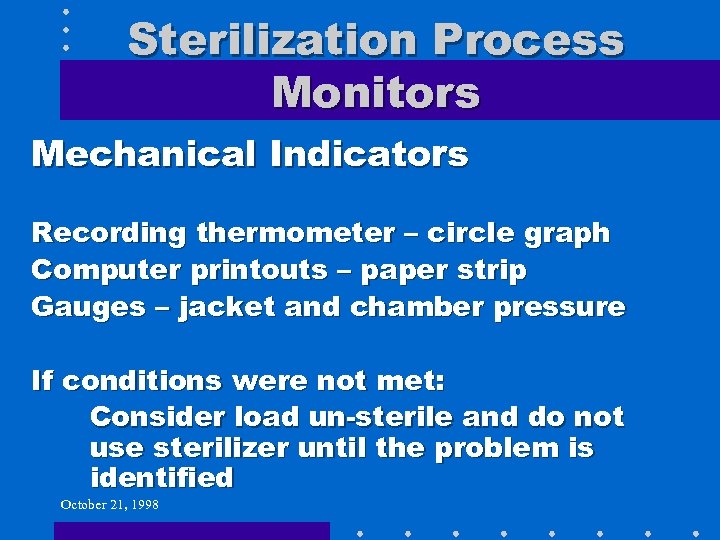 Sterilization Process Monitors Mechanical Indicators Recording thermometer – circle graph Computer printouts – paper strip Gauges – jacket and chamber pressure If conditions were not met: Consider load un-sterile and do not use sterilizer until the problem is identified October 21, 1998
Sterilization Process Monitors Mechanical Indicators Recording thermometer – circle graph Computer printouts – paper strip Gauges – jacket and chamber pressure If conditions were not met: Consider load un-sterile and do not use sterilizer until the problem is identified October 21, 1998
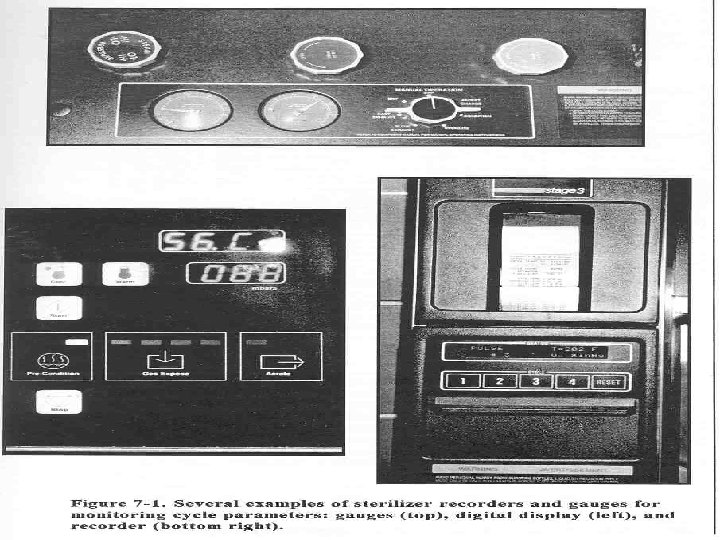
 Sterilization Process Monitors Equipment Control Mechanical Indicators • monitor one location in sterilizer • do not monitor each pack or tray • do not indicate sterility October 21, 1998
Sterilization Process Monitors Equipment Control Mechanical Indicators • monitor one location in sterilizer • do not monitor each pack or tray • do not indicate sterility October 21, 1998
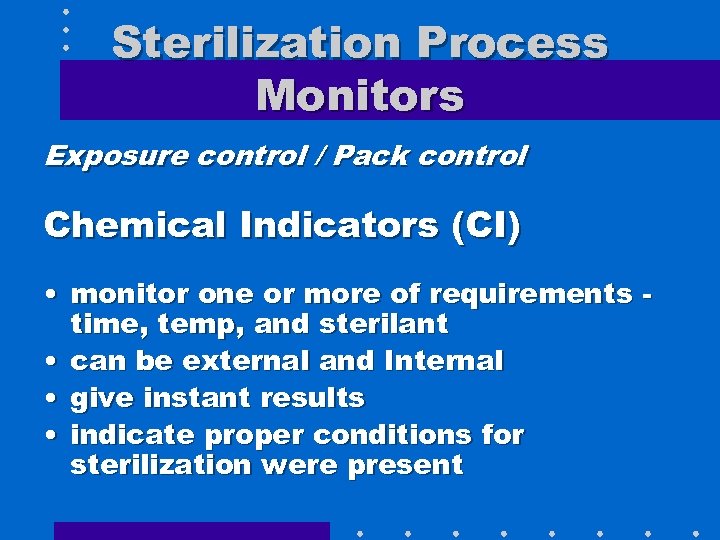 Sterilization Process Monitors Exposure control / Pack control Chemical Indicators (CI) • monitor one or more of requirements time, temp, and sterilant • can be external and Internal • give instant results • indicate proper conditions for sterilization were present
Sterilization Process Monitors Exposure control / Pack control Chemical Indicators (CI) • monitor one or more of requirements time, temp, and sterilant • can be external and Internal • give instant results • indicate proper conditions for sterilization were present
 Sterilization Process Monitors Exposure Control External Chemical Indicator • process indicator - autoclave tape • distinguishes processed from unprocessed medical devices • secures pack • labels pack If indicator did not change, do not use October 21, 1998
Sterilization Process Monitors Exposure Control External Chemical Indicator • process indicator - autoclave tape • distinguishes processed from unprocessed medical devices • secures pack • labels pack If indicator did not change, do not use October 21, 1998
 Sterilization Process Monitors Pack Control Internal Chemical Indicator • inside each package, tray or container • paper strips or cards • validates sterilant penetration • colour change strip or moving front format • can measure all process parameters (Integrators) October 21, 1998
Sterilization Process Monitors Pack Control Internal Chemical Indicator • inside each package, tray or container • paper strips or cards • validates sterilant penetration • colour change strip or moving front format • can measure all process parameters (Integrators) October 21, 1998
 Chemical Indicators
Chemical Indicators
 Sterilization Process Monitors Pack Control Internal Chemical Indicator Pack Control CI - advantages • detects incorrect packaging • incorrect loading • malfunction of sterilizer • easy to retrieve and read October 21, 1998
Sterilization Process Monitors Pack Control Internal Chemical Indicator Pack Control CI - advantages • detects incorrect packaging • incorrect loading • malfunction of sterilizer • easy to retrieve and read October 21, 1998
 Sterilization Process Monitors Chemical Indicators • Do not tell you that spores are killed • Do not tell you that item is sterile October 21, 1998
Sterilization Process Monitors Chemical Indicators • Do not tell you that spores are killed • Do not tell you that item is sterile October 21, 1998
 Chemical Indicators cannot replace Biological Indicators based on accepted practice guidelines and current scientific knowledge October 21, 1998
Chemical Indicators cannot replace Biological Indicators based on accepted practice guidelines and current scientific knowledge October 21, 1998
 Sterilization Process Monitoring All recommended practices state that both biological and chemical indicators shall be used to monitor the sterilization process. October 21, 1998
Sterilization Process Monitoring All recommended practices state that both biological and chemical indicators shall be used to monitor the sterilization process. October 21, 1998
 Sterilization Process Monitors Load Control Biological Indicators Confirm the ability of the sterilization process to kill microbial spores
Sterilization Process Monitors Load Control Biological Indicators Confirm the ability of the sterilization process to kill microbial spores
 Sterilization Process Monitors Load Control Biological Indicators • large number of spores • Integrate all the parameters of the sterilization process • Most critical test of the sterilization process • CSA requires routine monitoring daily
Sterilization Process Monitors Load Control Biological Indicators • large number of spores • Integrate all the parameters of the sterilization process • Most critical test of the sterilization process • CSA requires routine monitoring daily
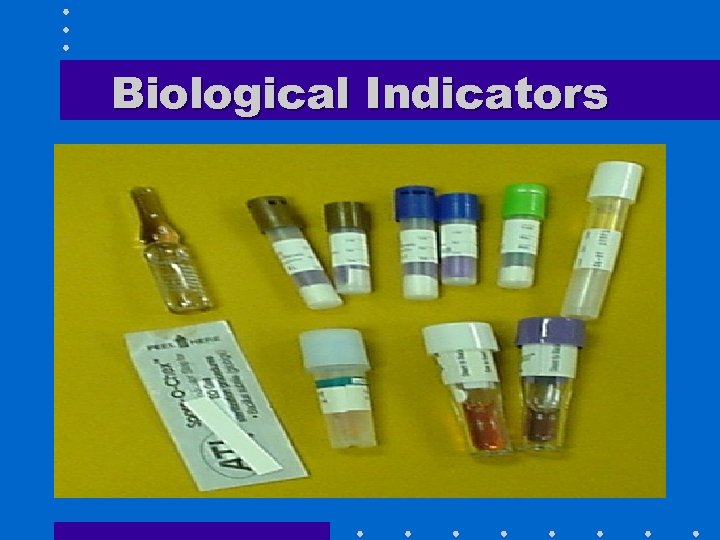 Biological Indicators
Biological Indicators
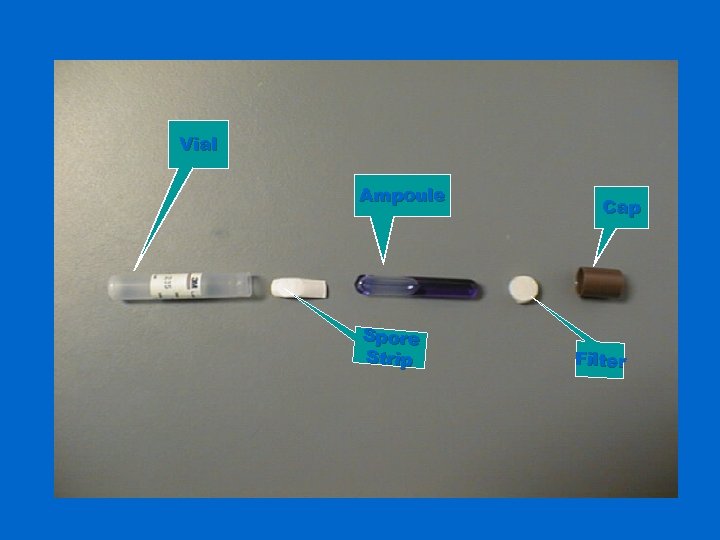 Vial Ampoule Spore Strip Cap Filter
Vial Ampoule Spore Strip Cap Filter
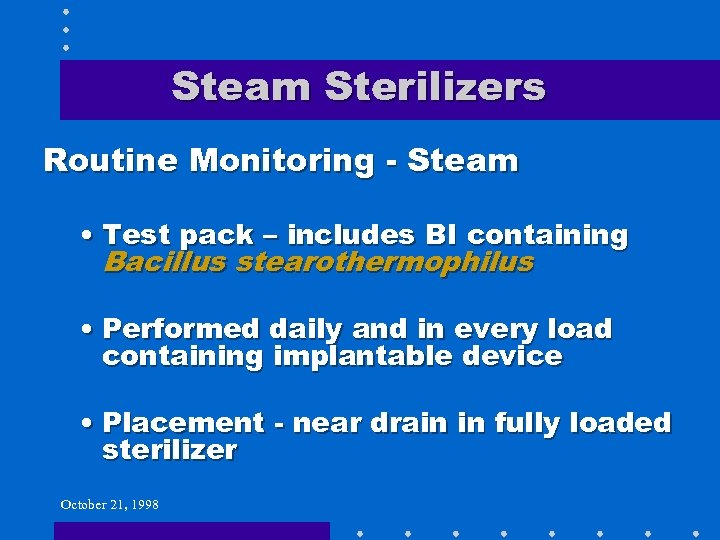 Steam Sterilizers Routine Monitoring - Steam • Test pack – includes BI containing Bacillus stearothermophilus • Performed daily and in every load containing implantable device • Placement - near drain in fully loaded sterilizer October 21, 1998
Steam Sterilizers Routine Monitoring - Steam • Test pack – includes BI containing Bacillus stearothermophilus • Performed daily and in every load containing implantable device • Placement - near drain in fully loaded sterilizer October 21, 1998
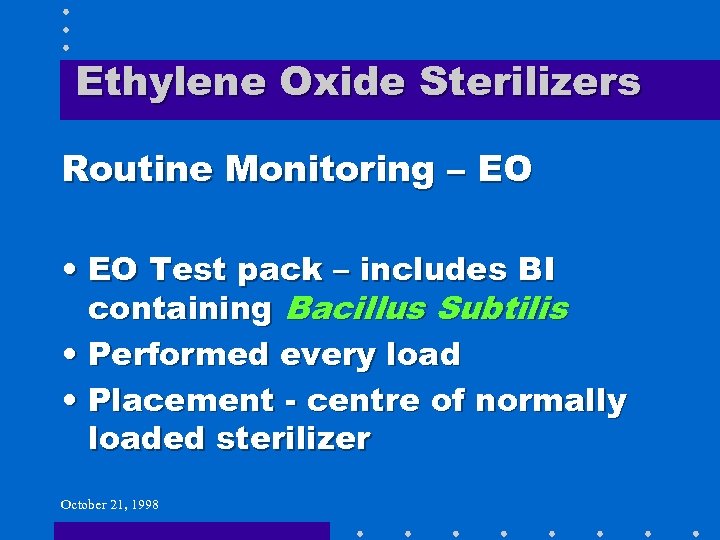 Ethylene Oxide Sterilizers Routine Monitoring – EO • EO Test pack – includes BI containing Bacillus Subtilis • Performed every load • Placement - centre of normally loaded sterilizer October 21, 1998
Ethylene Oxide Sterilizers Routine Monitoring – EO • EO Test pack – includes BI containing Bacillus Subtilis • Performed every load • Placement - centre of normally loaded sterilizer October 21, 1998
 Biological Indicator Test Packs
Biological Indicator Test Packs
 Sterilization Process Monitors Bowie Dick Type Tests • Detects entrapped air in Vacuumassisted sterilizers, not for Gravity • Measures steam penetration • Run daily • Test packs – can be in-house or commercially prepared
Sterilization Process Monitors Bowie Dick Type Tests • Detects entrapped air in Vacuumassisted sterilizers, not for Gravity • Measures steam penetration • Run daily • Test packs – can be in-house or commercially prepared
 Sterilization Process Monitors Bowie Dick Test • Run a warm-up cycle first • Place test pack in an empty sterilizer over the drain • 132 C (270 F) for 3. 5 - 4 minutes • Uniform colour change • Retain in records
Sterilization Process Monitors Bowie Dick Test • Run a warm-up cycle first • Place test pack in an empty sterilizer over the drain • 132 C (270 F) for 3. 5 - 4 minutes • Uniform colour change • Retain in records
 Sterilization Process Monitors Bowie Dick Test results If colour change not uniform • Repeat test • Shut down • Call repair person • Retest If uniform colour change • Use sterilizer
Sterilization Process Monitors Bowie Dick Test results If colour change not uniform • Repeat test • Shut down • Call repair person • Retest If uniform colour change • Use sterilizer
 Bowie Dick Test Unprocessed Processed
Bowie Dick Test Unprocessed Processed
 Bowie Dick Test Packs
Bowie Dick Test Packs
 “It is a dangerous practice simply to conclude, without investigation, that indicator giving warning is incorrect. ” AAMI TIR
“It is a dangerous practice simply to conclude, without investigation, that indicator giving warning is incorrect. ” AAMI TIR
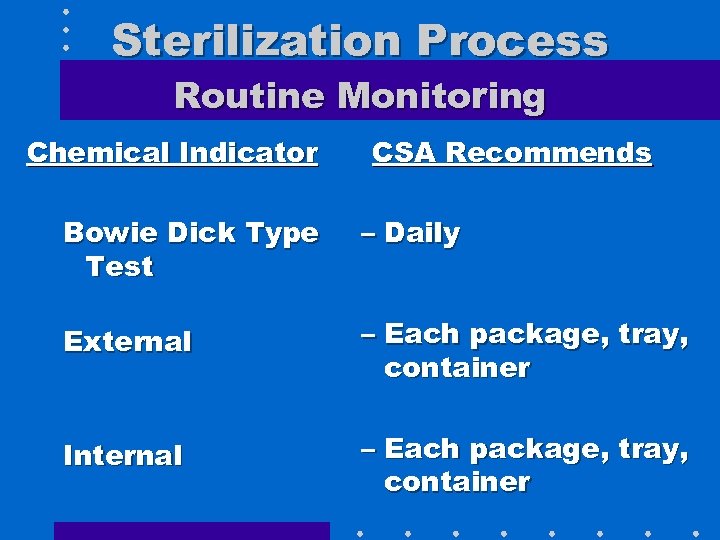 Sterilization Process Routine Monitoring Chemical Indicator CSA Recommends Bowie Dick Type Test – Daily External – Each package, tray, container Internal – Each package, tray, container
Sterilization Process Routine Monitoring Chemical Indicator CSA Recommends Bowie Dick Type Test – Daily External – Each package, tray, container Internal – Each package, tray, container
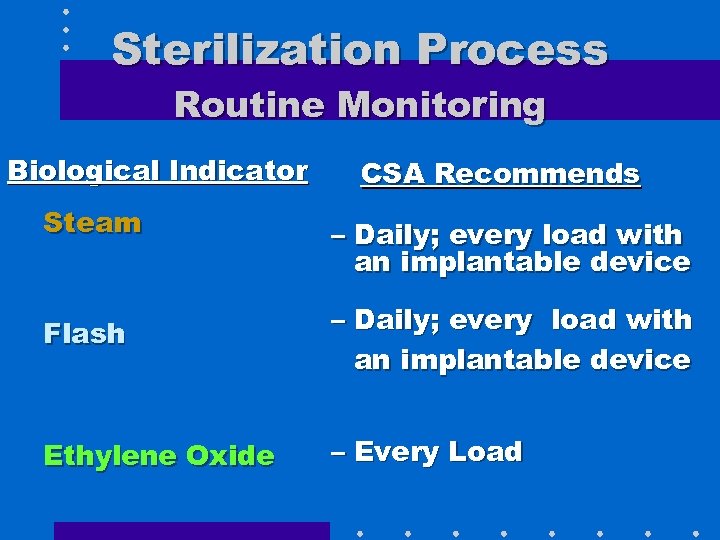 Sterilization Process Routine Monitoring Biological Indicator Steam CSA Recommends – Daily; every load with an implantable device Flash – Daily; every load with an implantable device Ethylene Oxide – Every Load
Sterilization Process Routine Monitoring Biological Indicator Steam CSA Recommends – Daily; every load with an implantable device Flash – Daily; every load with an implantable device Ethylene Oxide – Every Load
 Installation & Repair Testing Performed: – – – before sterilizer released for use after major repairs or relocation after unexplained sterility failures after changes in sterilant supply annually 3 cycles using BI test pack – yielding 3 negative results If vacuum – 3 cycles with Bowie-Dick test pack October 21, 1998
Installation & Repair Testing Performed: – – – before sterilizer released for use after major repairs or relocation after unexplained sterility failures after changes in sterilant supply annually 3 cycles using BI test pack – yielding 3 negative results If vacuum – 3 cycles with Bowie-Dick test pack October 21, 1998
 Sterilization Process Monitors Record Keeping • Document all materials that have been processed and the results of the sterilization process monitoring
Sterilization Process Monitors Record Keeping • Document all materials that have been processed and the results of the sterilization process monitoring
 Record Keeping Product Labeling • • • lot or load control number processing date sterilizer number cycle number Expiration statement • event-related • time-related October 21, 1998
Record Keeping Product Labeling • • • lot or load control number processing date sterilizer number cycle number Expiration statement • event-related • time-related October 21, 1998
 Record Keeping Load Records • • • date and time of all cycles exposure time and temperature load contents initials of operator BI results, CI results Records of sterilizer maintenance, calibration, and repair October 21, 1998
Record Keeping Load Records • • • date and time of all cycles exposure time and temperature load contents initials of operator BI results, CI results Records of sterilizer maintenance, calibration, and repair October 21, 1998
 Product Recall Procedure If positive BI: • • • review record, quarantine load notify maintenance personnel identify microorganism on + BI If contamination occurred, and record is OK, release load October 21, 1998
Product Recall Procedure If positive BI: • • • review record, quarantine load notify maintenance personnel identify microorganism on + BI If contamination occurred, and record is OK, release load October 21, 1998
 Product Recall If microorganism is the spore, do further testing • Follow hospital policy • Initiate recall and request sterilizer service as needed • Written recall order • Written report October 21, 1998
Product Recall If microorganism is the spore, do further testing • Follow hospital policy • Initiate recall and request sterilizer service as needed • Written recall order • Written report October 21, 1998
 Continuing Education Ø Ø Ø Quality patient care Review CSA standards Know your hospital policies Ask questions; Keep learning Apply what you learn into practice October 21, 1998
Continuing Education Ø Ø Ø Quality patient care Review CSA standards Know your hospital policies Ask questions; Keep learning Apply what you learn into practice October 21, 1998
 Reference CSA Standards 4 CAN/CSA-Z 11140 -1 -98 Sterilization of Health Care Products - Chemical Indicators - Part 1: General Requirements (Adopted ISO 111401: 1995) 4 CAN/CSA-Z 314. 2 -01 Effective Sterilization in Health Care Facilities by the Ethylene Oxide Process 4 CAN/CSA-Z 314. 3 -01 Effective Sterilization in Health Care Facilities by the Steam Process October 21, 1998
Reference CSA Standards 4 CAN/CSA-Z 11140 -1 -98 Sterilization of Health Care Products - Chemical Indicators - Part 1: General Requirements (Adopted ISO 111401: 1995) 4 CAN/CSA-Z 314. 2 -01 Effective Sterilization in Health Care Facilities by the Ethylene Oxide Process 4 CAN/CSA-Z 314. 3 -01 Effective Sterilization in Health Care Facilities by the Steam Process October 21, 1998


