9d07e8288bd8a9d39f1c53bd121af17a.ppt
- Количество слайдов: 43
 Molecular Techniques for the Identification of Pathogenic Fungi Wieland Meyer Ph. D Molecular Mycology Laboratory Westmead Hospital/The University of Sydney WM, Westmead Hospital, Sydney, Australia
Molecular Techniques for the Identification of Pathogenic Fungi Wieland Meyer Ph. D Molecular Mycology Laboratory Westmead Hospital/The University of Sydney WM, Westmead Hospital, Sydney, Australia
 Issues in Mycology Significant increase opportunistic fungal infections due to the increase in immunocompromised hosts Cosmopolitan environmental fungi have emerged as causes of potentially life-threatening diseases Many emerging pathogenic fungi are inherently resistant to antifungal drugs A number of pathogenic fungi are non-viable in tissue samples Traditional identification techniques lack sensitivity & specificity, are slow, labor-intensive and require skilled personnel Clinical and economic consequences WM, Westmead Hospital, Sydney, Australia
Issues in Mycology Significant increase opportunistic fungal infections due to the increase in immunocompromised hosts Cosmopolitan environmental fungi have emerged as causes of potentially life-threatening diseases Many emerging pathogenic fungi are inherently resistant to antifungal drugs A number of pathogenic fungi are non-viable in tissue samples Traditional identification techniques lack sensitivity & specificity, are slow, labor-intensive and require skilled personnel Clinical and economic consequences WM, Westmead Hospital, Sydney, Australia
 Conventional Fungal Identification Techniques Identification Method Concerns Culture ID - only in 26% positive if < 1 cfu/ml blood Serological Tests - limited value (e. g. Iatron Crypto Kit) - suboptimal sensitivity & specificity Germ-tube Formation - useful screening test only for C. albicans (does not differentiate C. dubliniensis) Carbohydrate Assimilation - limited species in the databases (e. g. Vitek & API strips) - can misidentify certain pathogenic species Morphological Characters - subjective measure, - high degree of skills required WM, Westmead Hospital, Sydney, Australia
Conventional Fungal Identification Techniques Identification Method Concerns Culture ID - only in 26% positive if < 1 cfu/ml blood Serological Tests - limited value (e. g. Iatron Crypto Kit) - suboptimal sensitivity & specificity Germ-tube Formation - useful screening test only for C. albicans (does not differentiate C. dubliniensis) Carbohydrate Assimilation - limited species in the databases (e. g. Vitek & API strips) - can misidentify certain pathogenic species Morphological Characters - subjective measure, - high degree of skills required WM, Westmead Hospital, Sydney, Australia
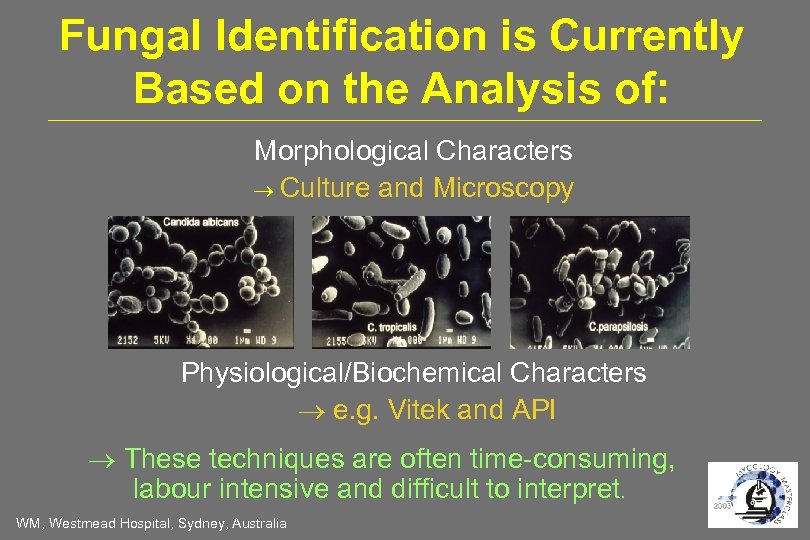 Fungal Identification is Currently Based on the Analysis of: Morphological Characters Culture and Microscopy Physiological/Biochemical Characters e. g. Vitek and API These techniques are often time-consuming, labour intensive and difficult to interpret. WM, Westmead Hospital, Sydney, Australia
Fungal Identification is Currently Based on the Analysis of: Morphological Characters Culture and Microscopy Physiological/Biochemical Characters e. g. Vitek and API These techniques are often time-consuming, labour intensive and difficult to interpret. WM, Westmead Hospital, Sydney, Australia
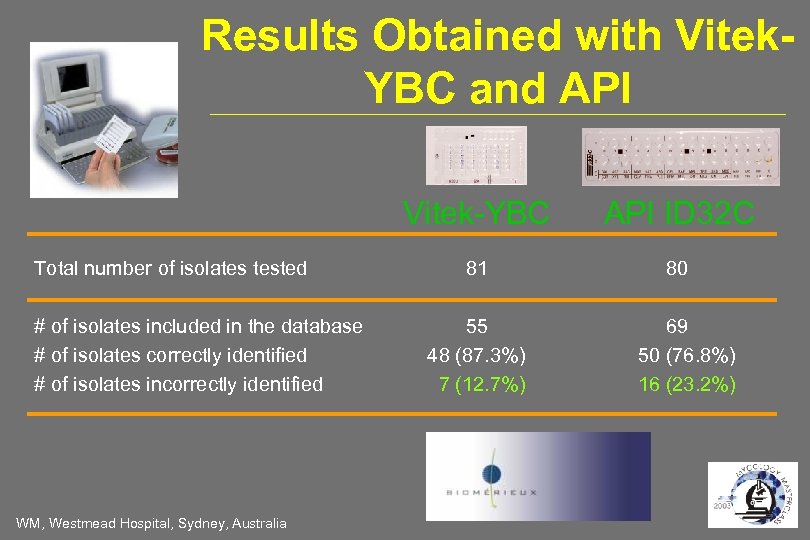 Results Obtained with Vitek. YBC and API Vitek-YBC Total number of isolates tested # of isolates included in the database # of isolates correctly identified # of isolates incorrectly identified WM, Westmead Hospital, Sydney, Australia API ID 32 C 81 80 55 48 (87. 3%) 7 (12. 7%) 69 50 (76. 8%) 16 (23. 2%)
Results Obtained with Vitek. YBC and API Vitek-YBC Total number of isolates tested # of isolates included in the database # of isolates correctly identified # of isolates incorrectly identified WM, Westmead Hospital, Sydney, Australia API ID 32 C 81 80 55 48 (87. 3%) 7 (12. 7%) 69 50 (76. 8%) 16 (23. 2%)
 Culture-ID Blood cultures positive in only 20 -58% of Invasive Candidaisis Only in 26% positive if < 1 cfu/ml blood, and 10% of Aspergillosis Serological Tests e. g. : Iatron Cryptococcus Kit 95% accurate with Cryptococcus neoformans Serotype specific antisera, problems AD strains Iatron Candida Check Kit 95% accurate with Candida specific antisera In general suboptimal sensitivity and specificity WM, Westmead Hospital, Sydney, Australia
Culture-ID Blood cultures positive in only 20 -58% of Invasive Candidaisis Only in 26% positive if < 1 cfu/ml blood, and 10% of Aspergillosis Serological Tests e. g. : Iatron Cryptococcus Kit 95% accurate with Cryptococcus neoformans Serotype specific antisera, problems AD strains Iatron Candida Check Kit 95% accurate with Candida specific antisera In general suboptimal sensitivity and specificity WM, Westmead Hospital, Sydney, Australia
 Ideal ID/Diagnostic Test - Sensitive and specific - High positive predictive value - High negative predictive value - Useful for monitoring - Simple, rapid and inexpensive WM, Westmead Hospital, Sydney, Australia
Ideal ID/Diagnostic Test - Sensitive and specific - High positive predictive value - High negative predictive value - Useful for monitoring - Simple, rapid and inexpensive WM, Westmead Hospital, Sydney, Australia
 Why ? Confirmation of medical diagnosis, choice of therapy, follow-up and prevention are critical to a successful infectious disease management. - Facilitate earlier diagnosis - Initiating earlier intervention with aggressive antifungal treatment to improve patient outcome - Reduce empiric use of antifungal agents How ? - Detection of fungal genomic sequences WM, Westmead Hospital, Sydney, Australia
Why ? Confirmation of medical diagnosis, choice of therapy, follow-up and prevention are critical to a successful infectious disease management. - Facilitate earlier diagnosis - Initiating earlier intervention with aggressive antifungal treatment to improve patient outcome - Reduce empiric use of antifungal agents How ? - Detection of fungal genomic sequences WM, Westmead Hospital, Sydney, Australia
 Why Molecular Methods? Phenotypic Characters are unstable and can change with environmental changes Identification methods based on Genotypic Characteristics would be advantageous and potentially more accurate, reproducible, simple and rapid WM, Westmead Hospital, Sydney, Australia
Why Molecular Methods? Phenotypic Characters are unstable and can change with environmental changes Identification methods based on Genotypic Characteristics would be advantageous and potentially more accurate, reproducible, simple and rapid WM, Westmead Hospital, Sydney, Australia
 Proposed Applications of DNA Protocols Organism detection in blood Organism detection in body fluids Organism detection in tissue Molecular ID Identification of the fungal agent Quantification of the fungal load Monitoring of antifungal treatment WM, Westmead Hospital, Sydney, Australia
Proposed Applications of DNA Protocols Organism detection in blood Organism detection in body fluids Organism detection in tissue Molecular ID Identification of the fungal agent Quantification of the fungal load Monitoring of antifungal treatment WM, Westmead Hospital, Sydney, Australia
 DNA Technology DNA probes – Southern Hybridization – In-situ Hybridization – Microarray – Macroarray – Reversed line blot Karyotyping WM, Westmead Hospital, Sydney, Australia PCR primers – SSCP – Genotyping – Panfungal PCR – Multiplex PCR – Nested PCR – Real Time PCR – Sequencing – PCR Fingerprinting – AFLP – PCR-RFLP
DNA Technology DNA probes – Southern Hybridization – In-situ Hybridization – Microarray – Macroarray – Reversed line blot Karyotyping WM, Westmead Hospital, Sydney, Australia PCR primers – SSCP – Genotyping – Panfungal PCR – Multiplex PCR – Nested PCR – Real Time PCR – Sequencing – PCR Fingerprinting – AFLP – PCR-RFLP
 Samples used: Blood: Should be collected in tubes containing EDTA (1 mg/ml) !! To not use heparin as anticoagulant !! Biopsy Tissue: !! Fresh tissue is always better !! Paraffin embedded tissue is not always amenable to PCR amplification because of DNA modification due to cross-linking induced by the fixative or fixation time! In addition time-dependent physical degradation of DNA in paraffinembedded tissue limits the length of the DNA fragment amplified. WM, Westmead Hospital, Sydney, Australia
Samples used: Blood: Should be collected in tubes containing EDTA (1 mg/ml) !! To not use heparin as anticoagulant !! Biopsy Tissue: !! Fresh tissue is always better !! Paraffin embedded tissue is not always amenable to PCR amplification because of DNA modification due to cross-linking induced by the fixative or fixation time! In addition time-dependent physical degradation of DNA in paraffinembedded tissue limits the length of the DNA fragment amplified. WM, Westmead Hospital, Sydney, Australia
 Targets for PCR, PCR-RFLP, Sequencing& In-situ Hybridization: Universal fungal primers for: - Multi-copy Genes r. DNA gene cluster 18 S, ITS 1/2, 5. 8 S, 28 S, 5 S, IGS - Single-copy Genes Actin, Alkaline Protease (ALP), Chitin Synthase, GP 43, Lanosterol - demethylase (LIA 1), URA 5, Secreted Aspartic Protease (SAP), Beta glucan synthetase (FKS), Histone, etc. Genus- or species-specific primers 18 S, ITS 1/2, 28 S r. DNA, Mitochondrial DNA, Histone e. g. Candida, Cryptococcus, Aspergillus WM, Westmead Hospital, Sydney, Australia
Targets for PCR, PCR-RFLP, Sequencing& In-situ Hybridization: Universal fungal primers for: - Multi-copy Genes r. DNA gene cluster 18 S, ITS 1/2, 5. 8 S, 28 S, 5 S, IGS - Single-copy Genes Actin, Alkaline Protease (ALP), Chitin Synthase, GP 43, Lanosterol - demethylase (LIA 1), URA 5, Secreted Aspartic Protease (SAP), Beta glucan synthetase (FKS), Histone, etc. Genus- or species-specific primers 18 S, ITS 1/2, 28 S r. DNA, Mitochondrial DNA, Histone e. g. Candida, Cryptococcus, Aspergillus WM, Westmead Hospital, Sydney, Australia
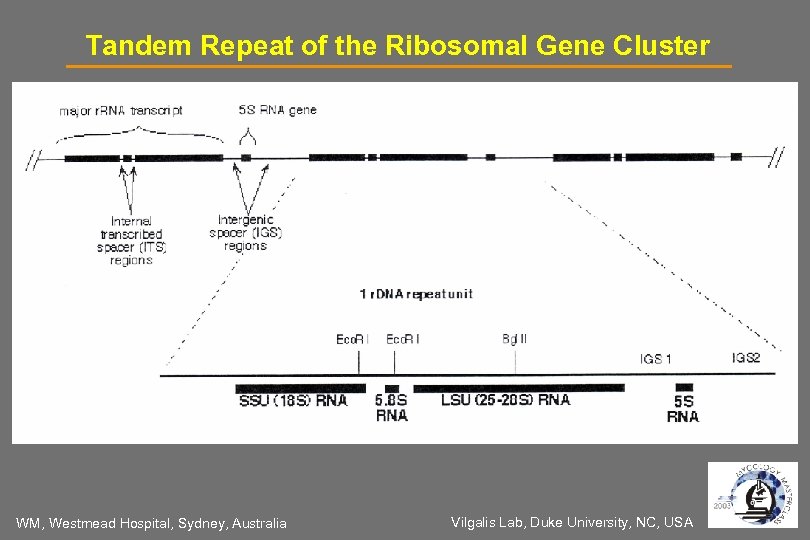 Tandem Repeat of the Ribosomal Gene Cluster WM, Westmead Hospital, Sydney, Australia Vilgalis Lab, Duke University, NC, USA
Tandem Repeat of the Ribosomal Gene Cluster WM, Westmead Hospital, Sydney, Australia Vilgalis Lab, Duke University, NC, USA
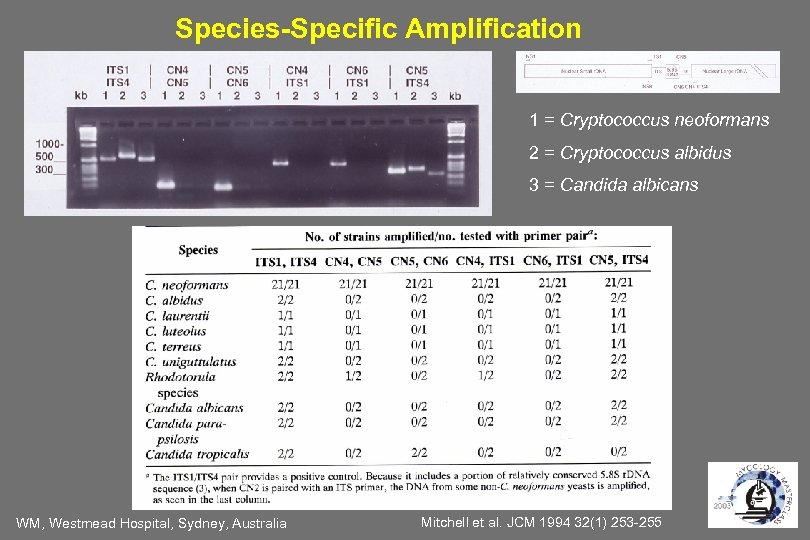 Species-Specific Amplification 1 = Cryptococcus neoformans 2 = Cryptococcus albidus 3 = Candida albicans WM, Westmead Hospital, Sydney, Australia Mitchell et al. JCM 1994 32(1) 253 -255
Species-Specific Amplification 1 = Cryptococcus neoformans 2 = Cryptococcus albidus 3 = Candida albicans WM, Westmead Hospital, Sydney, Australia Mitchell et al. JCM 1994 32(1) 253 -255
 PCR Amplification of Specific Genes Clinical specimen Direct PCR of a single colony form a primary isolation plate, tissue sample or clean culture Agarose gel electrophoresis ID via comparison with the data base Species Level ITS 1, 5. 8 S, ITS 2 region WM, Westmead Hospital, Sydney, Australia Nicolas Latouche
PCR Amplification of Specific Genes Clinical specimen Direct PCR of a single colony form a primary isolation plate, tissue sample or clean culture Agarose gel electrophoresis ID via comparison with the data base Species Level ITS 1, 5. 8 S, ITS 2 region WM, Westmead Hospital, Sydney, Australia Nicolas Latouche
 Commercial Fungal Molecular Identification Kits Fungal ID Kits for: - Blastomyces dermatitidis - Coccidioides immitis - Histoplasma capsulatum - Single-stranded DNA probe targeted to the ribosomal RNA - Selection reagent differentiates between non-hybridized and hybridized probe - Labelled DNA: RNA hybrids measured in a GEN-PROBE luminometer WM, Westmead Hospital, Sydney, Australia
Commercial Fungal Molecular Identification Kits Fungal ID Kits for: - Blastomyces dermatitidis - Coccidioides immitis - Histoplasma capsulatum - Single-stranded DNA probe targeted to the ribosomal RNA - Selection reagent differentiates between non-hybridized and hybridized probe - Labelled DNA: RNA hybrids measured in a GEN-PROBE luminometer WM, Westmead Hospital, Sydney, Australia
 Protocol of the Kits WM, Westmead Hospital, Sydney, Australia
Protocol of the Kits WM, Westmead Hospital, Sydney, Australia
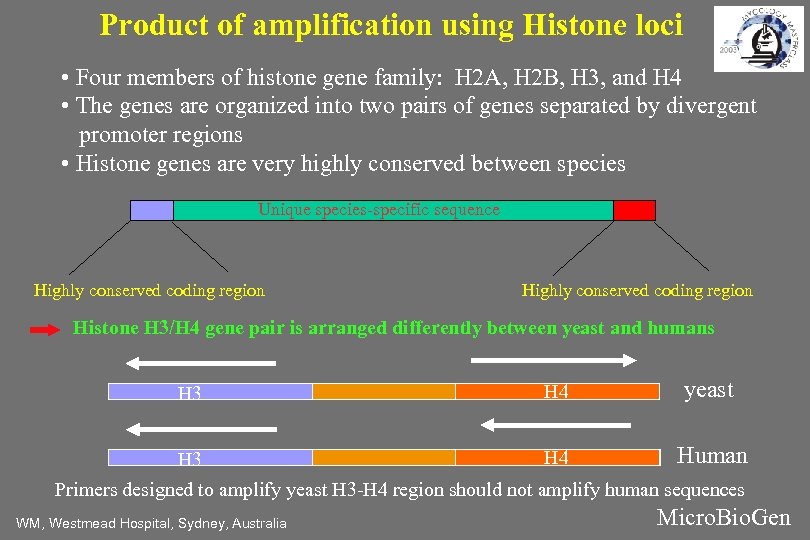 Product of amplification using Histone loci • Four members of histone gene family: H 2 A, H 2 B, H 3, and H 4 • The genes are organized into two pairs of genes separated by divergent promoter regions • Histone genes are very highly conserved between species Unique species-specific sequence Highly conserved coding region Histone H 3/H 4 gene pair is arranged differently between yeast and humans H 3 H 4 yeast Human H 4 H 3 Primers designed to amplify yeast H 3 -H 4 region should not amplify human sequences WM, Westmead Hospital, Sydney, Australia Micro. Bio. Gen
Product of amplification using Histone loci • Four members of histone gene family: H 2 A, H 2 B, H 3, and H 4 • The genes are organized into two pairs of genes separated by divergent promoter regions • Histone genes are very highly conserved between species Unique species-specific sequence Highly conserved coding region Histone H 3/H 4 gene pair is arranged differently between yeast and humans H 3 H 4 yeast Human H 4 H 3 Primers designed to amplify yeast H 3 -H 4 region should not amplify human sequences WM, Westmead Hospital, Sydney, Australia Micro. Bio. Gen
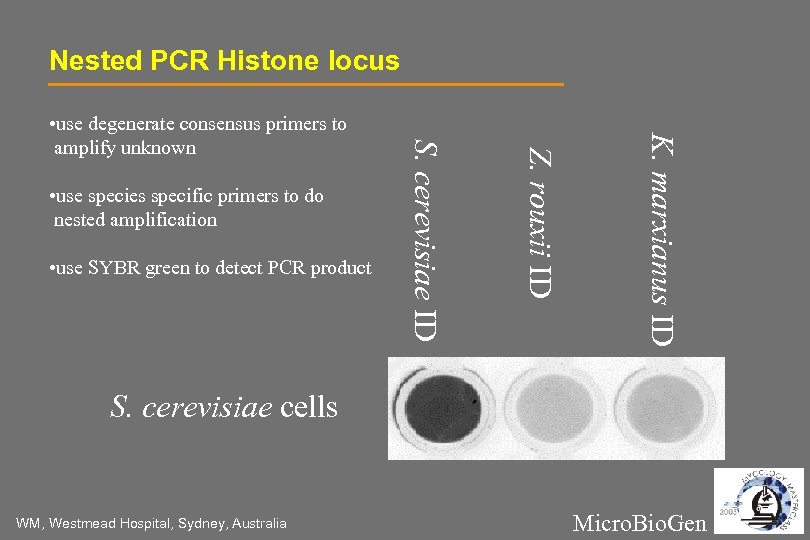 Nested PCR Histone locus K. marxianus ID • use SYBR green to detect PCR product Z. rouxii ID • use species specific primers to do nested amplification S. cerevisiae ID • use degenerate consensus primers to amplify unknown S. cerevisiae cells WM, Westmead Hospital, Sydney, Australia Micro. Bio. Gen
Nested PCR Histone locus K. marxianus ID • use SYBR green to detect PCR product Z. rouxii ID • use species specific primers to do nested amplification S. cerevisiae ID • use degenerate consensus primers to amplify unknown S. cerevisiae cells WM, Westmead Hospital, Sydney, Australia Micro. Bio. Gen
 PCR-RFLP (Restriction Fragment Length Polymorphism Analysis) Clinical specimen Direct PCR of a single colony form a primary isolation plate, tissue sample, or clean culture Agarose gel electrophoresis Digestion with restriction enzymes Agarose gel electrophoresis ID via comparison with the data base Species Level WM, Westmead Hospital, Sydney, Australia ITS 1, 5. 8 S, ITS 2 region Nicolas Latouche
PCR-RFLP (Restriction Fragment Length Polymorphism Analysis) Clinical specimen Direct PCR of a single colony form a primary isolation plate, tissue sample, or clean culture Agarose gel electrophoresis Digestion with restriction enzymes Agarose gel electrophoresis ID via comparison with the data base Species Level WM, Westmead Hospital, Sydney, Australia ITS 1, 5. 8 S, ITS 2 region Nicolas Latouche
 RFLP MAPS Candida albicans/dubliniensis Atlas of Clinical Fungi de Hoog et al 2000 RFLP maps available from: “Atlas of Clinical Fungi” 2 nd Edition 2000 by: GS de Hoog, J Guarro, J Gené & MJ Figueras Centraalbureau voor Schimmelcultures, Utrecht, The Netherlands Biolo. MICS at: www. cbs. knaw. nl by: V. Robert WM, Westmead Hospital, Sydney, Australia Centraalbureau voor Schimmelcultures, Utrecht, The Netherlands and Bio. Aware, Belgium
RFLP MAPS Candida albicans/dubliniensis Atlas of Clinical Fungi de Hoog et al 2000 RFLP maps available from: “Atlas of Clinical Fungi” 2 nd Edition 2000 by: GS de Hoog, J Guarro, J Gené & MJ Figueras Centraalbureau voor Schimmelcultures, Utrecht, The Netherlands Biolo. MICS at: www. cbs. knaw. nl by: V. Robert WM, Westmead Hospital, Sydney, Australia Centraalbureau voor Schimmelcultures, Utrecht, The Netherlands and Bio. Aware, Belgium
 Real Time PCR ABI 7700 System (Taq. Man) Reporter Dye and Quencher Probe Detection Quantitative DNA and Species Detection Roche Light. Cycler SYBR Green Detection Quantitative DNA Detection Diagnostics e. g. Candida sp. detection Guiver et al. J. Clin. Pathol. 2001 54: 362 -366 e. g. Pneumocystis carinii detection Kaiser et al. (2001) J. Microbiol. Meth. 45: 113 -118 Hybridization Probes (Donor Fluor and Acceptor Fluor) Detection Quantitative DNA and Species Detection e. g. C. albicans and A. fumigatus detection Loeffler et al. (2000) JCM 38: 586 -590 WM, Westmead Hospital, Sydney, Australia
Real Time PCR ABI 7700 System (Taq. Man) Reporter Dye and Quencher Probe Detection Quantitative DNA and Species Detection Roche Light. Cycler SYBR Green Detection Quantitative DNA Detection Diagnostics e. g. Candida sp. detection Guiver et al. J. Clin. Pathol. 2001 54: 362 -366 e. g. Pneumocystis carinii detection Kaiser et al. (2001) J. Microbiol. Meth. 45: 113 -118 Hybridization Probes (Donor Fluor and Acceptor Fluor) Detection Quantitative DNA and Species Detection e. g. C. albicans and A. fumigatus detection Loeffler et al. (2000) JCM 38: 586 -590 WM, Westmead Hospital, Sydney, Australia
 All necessary reagents for amplification and detection of Candida albicans Targets the ITS region Wide range of biological specimens including swabs, sputum, urine as well as blood cultures and isolated colonies Detect Candida albicans in less than 3 hours using the PCR workflow system Runs under a common thermal profile with other Microbiology specific kits eg. Enterococcus, Pseudomonas and Staphylococcus kits WM, Westmead Hospital, Sydney, Australia Diagnostics Light. Cycler Candida Kit MGRADE
All necessary reagents for amplification and detection of Candida albicans Targets the ITS region Wide range of biological specimens including swabs, sputum, urine as well as blood cultures and isolated colonies Detect Candida albicans in less than 3 hours using the PCR workflow system Runs under a common thermal profile with other Microbiology specific kits eg. Enterococcus, Pseudomonas and Staphylococcus kits WM, Westmead Hospital, Sydney, Australia Diagnostics Light. Cycler Candida Kit MGRADE
 Multilocus approaches: PCR-Fingerprinting RAPD (Random Amplified Polymorphic DNA) AFLP (Amplified Fragment Length Polymorphism) Clinical specimen Pure culture DNA extraction PCR amplification with Mini- or Microsatellite specific primers Agarose gel electrophoresis ID via comparison with the data bank Species and Strain Level Homology Primer M 13 (GACA)4 Intra-species 75 -95% 74 -94% Inter-species 5 -25% 6 -26% (Data obtained from 80 Candida species and 150 strains) Primer: M 13 WM, Westmead Hospital, Sydney, Australia Meyer et al. (1997) Electrophoresis, 18: 1548 -1559
Multilocus approaches: PCR-Fingerprinting RAPD (Random Amplified Polymorphic DNA) AFLP (Amplified Fragment Length Polymorphism) Clinical specimen Pure culture DNA extraction PCR amplification with Mini- or Microsatellite specific primers Agarose gel electrophoresis ID via comparison with the data bank Species and Strain Level Homology Primer M 13 (GACA)4 Intra-species 75 -95% 74 -94% Inter-species 5 -25% 6 -26% (Data obtained from 80 Candida species and 150 strains) Primer: M 13 WM, Westmead Hospital, Sydney, Australia Meyer et al. (1997) Electrophoresis, 18: 1548 -1559
 PCR Fingerprinting Database Development DNA extraction, PCR fingerprinting and gel running conditions standardized Reference profiles - 70 anamorph-teleomorph pairs - type cultures and clinical strains (>300 individual strains) Pattern analysis via Gelcompar. II and Integrated database/web access via Collaboration is welcome: WM, Westmead Hospital, Sydney, Australia w. meyer@usyd. edu. au Heide-Marie Daniel/Krystyna Marszewska/Vincent Robert
PCR Fingerprinting Database Development DNA extraction, PCR fingerprinting and gel running conditions standardized Reference profiles - 70 anamorph-teleomorph pairs - type cultures and clinical strains (>300 individual strains) Pattern analysis via Gelcompar. II and Integrated database/web access via Collaboration is welcome: WM, Westmead Hospital, Sydney, Australia w. meyer@usyd. edu. au Heide-Marie Daniel/Krystyna Marszewska/Vincent Robert
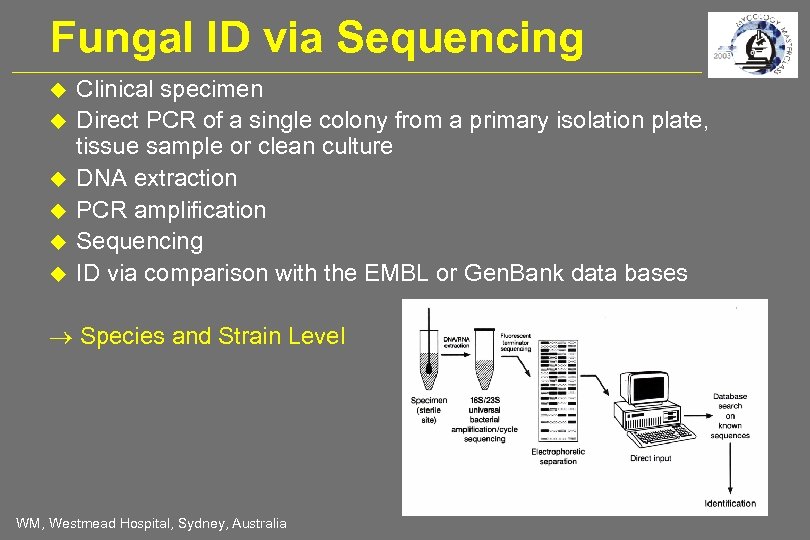 Fungal ID via Sequencing Clinical specimen Direct PCR of a single colony from a primary isolation plate, tissue sample or clean culture DNA extraction PCR amplification Sequencing ID via comparison with the EMBL or Gen. Bank data bases Species and Strain Level WM, Westmead Hospital, Sydney, Australia
Fungal ID via Sequencing Clinical specimen Direct PCR of a single colony from a primary isolation plate, tissue sample or clean culture DNA extraction PCR amplification Sequencing ID via comparison with the EMBL or Gen. Bank data bases Species and Strain Level WM, Westmead Hospital, Sydney, Australia
 r. DNA gene cluster Heide-Marie Daniel/Wieland Meyer ITS 1 Sequence variability ITS 2 ITS 1 Discriminatory power ITS 2 LSU 5. 8 S LSU SSU WM, Westmead Hospital, Sydney, Australia
r. DNA gene cluster Heide-Marie Daniel/Wieland Meyer ITS 1 Sequence variability ITS 2 ITS 1 Discriminatory power ITS 2 LSU 5. 8 S LSU SSU WM, Westmead Hospital, Sydney, Australia
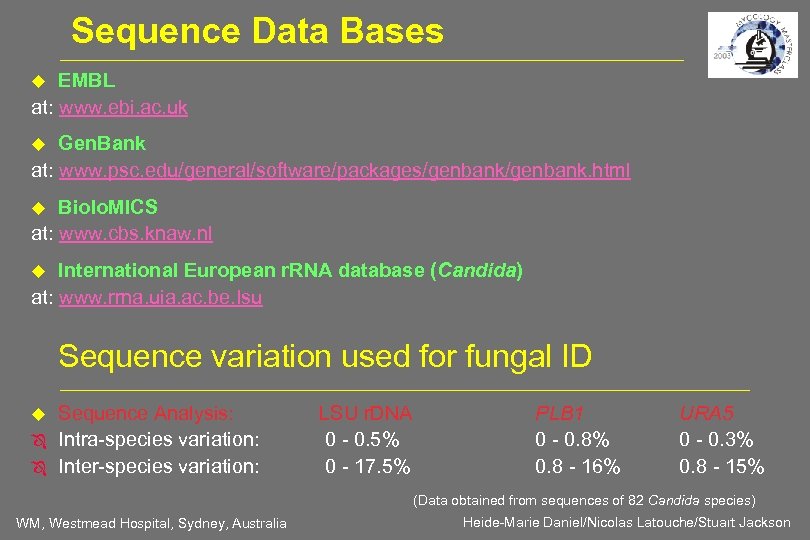 Sequence Data Bases EMBL at: www. ebi. ac. uk Gen. Bank at: www. psc. edu/general/software/packages/genbank. html Biolo. MICS at: www. cbs. knaw. nl International European r. RNA database (Candida) at: www. rrna. uia. ac. be. Isu Sequence variation used for fungal ID Ô Ô Sequence Analysis: Intra-species variation: Inter-species variation: LSU r. DNA 0 - 0. 5% 0 - 17. 5% PLB 1 0 - 0. 8% 0. 8 - 16% URA 5 0 - 0. 3% 0. 8 - 15% (Data obtained from sequences of 82 Candida species) WM, Westmead Hospital, Sydney, Australia Heide-Marie Daniel/Nicolas Latouche/Stuart Jackson
Sequence Data Bases EMBL at: www. ebi. ac. uk Gen. Bank at: www. psc. edu/general/software/packages/genbank. html Biolo. MICS at: www. cbs. knaw. nl International European r. RNA database (Candida) at: www. rrna. uia. ac. be. Isu Sequence variation used for fungal ID Ô Ô Sequence Analysis: Intra-species variation: Inter-species variation: LSU r. DNA 0 - 0. 5% 0 - 17. 5% PLB 1 0 - 0. 8% 0. 8 - 16% URA 5 0 - 0. 3% 0. 8 - 15% (Data obtained from sequences of 82 Candida species) WM, Westmead Hospital, Sydney, Australia Heide-Marie Daniel/Nicolas Latouche/Stuart Jackson
 Not all fungal species are sequenced!!! e. g. Gen. Bank 1. 10. 2002 WM, Westmead Hospital, Sydney, Australia
Not all fungal species are sequenced!!! e. g. Gen. Bank 1. 10. 2002 WM, Westmead Hospital, Sydney, Australia
 ® Workflow Micro. Seq or Sample from colony or pure culture Prep. Man Ultra DNA isolation < 30 minutes Micro. Seq PCR module (1 reaction) < 2 hours Micro. Seq Cycle sequencing module (2 reactions) < 2 hours Micro. Seq analysis software and r. DNA database < 30 minutes Final identification report WM, Westmead Hospital, Sydney, Australia
® Workflow Micro. Seq or Sample from colony or pure culture Prep. Man Ultra DNA isolation < 30 minutes Micro. Seq PCR module (1 reaction) < 2 hours Micro. Seq Cycle sequencing module (2 reactions) < 2 hours Micro. Seq analysis software and r. DNA database < 30 minutes Final identification report WM, Westmead Hospital, Sydney, Australia
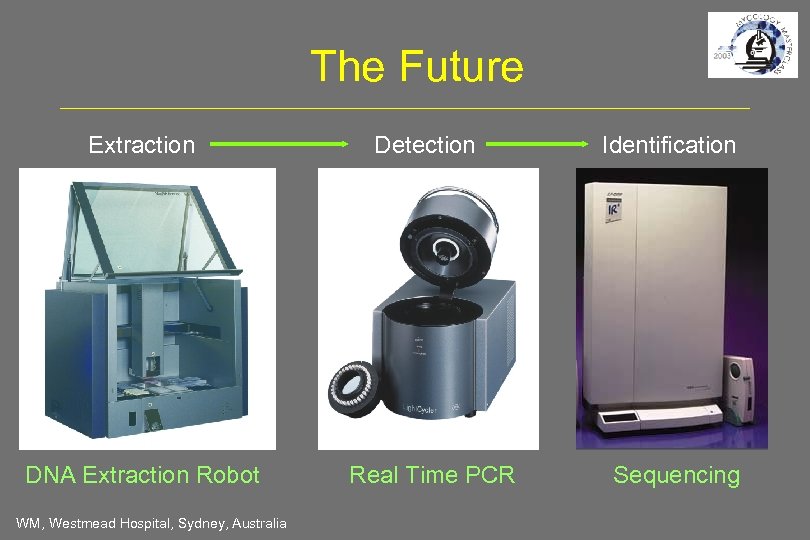 The Future Extraction DNA Extraction Robot WM, Westmead Hospital, Sydney, Australia Detection Real Time PCR Identification Sequencing
The Future Extraction DNA Extraction Robot WM, Westmead Hospital, Sydney, Australia Detection Real Time PCR Identification Sequencing
 DNA Array Technology Small glass or silicon matrices on which potentially thousands of short oligonucleotides can be immobilized e. g. - species-specific DNA probes - antifungal resistance genes - virulence genes C. g. C. p. C. l. C. k. C. n. C. li. C. m. C. t. C. u. Macroarray C. glabrata C. parapsilopsis C. lipolytica C. krusei C. norvegensis C. lusitaniae C. multigemmis C. tropicalis C. utilis WM, Westmead Hospital, Sydney, Australia Microarray
DNA Array Technology Small glass or silicon matrices on which potentially thousands of short oligonucleotides can be immobilized e. g. - species-specific DNA probes - antifungal resistance genes - virulence genes C. g. C. p. C. l. C. k. C. n. C. li. C. m. C. t. C. u. Macroarray C. glabrata C. parapsilopsis C. lipolytica C. krusei C. norvegensis C. lusitaniae C. multigemmis C. tropicalis C. utilis WM, Westmead Hospital, Sydney, Australia Microarray
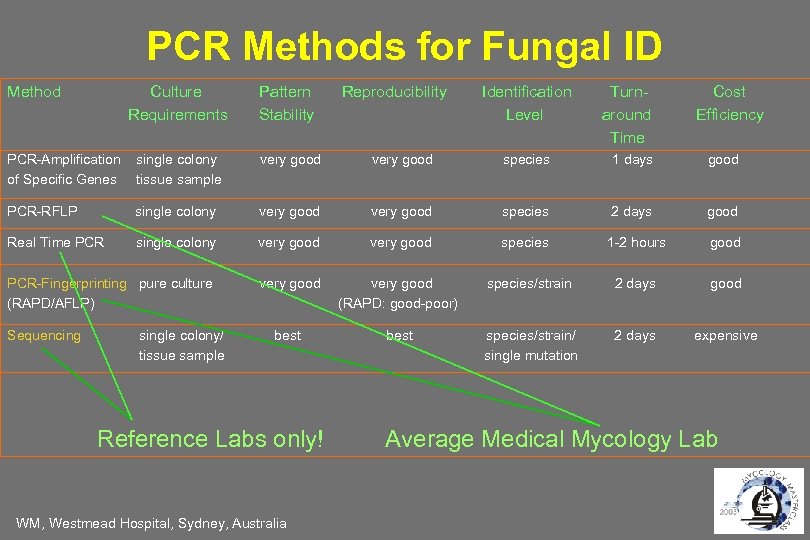 PCR Methods for Fungal ID Method Culture Requirements Pattern Stability PCR-Amplification of Specific Genes single colony tissue sample very good species 1 days good PCR-RFLP single colony very good species 2 days good Real Time PCR single colony very good species PCR-Fingerprinting pure culture (RAPD/AFLP) very good (RAPD: good-poor) best Sequencing single colony/ tissue sample Reference Labs only! WM, Westmead Hospital, Sydney, Australia Reproducibility Identification Level Turnaround Time Cost Efficiency 1 -2 hours good species/strain 2 days good species/strain/ single mutation 2 days expensive Average Medical Mycology Lab
PCR Methods for Fungal ID Method Culture Requirements Pattern Stability PCR-Amplification of Specific Genes single colony tissue sample very good species 1 days good PCR-RFLP single colony very good species 2 days good Real Time PCR single colony very good species PCR-Fingerprinting pure culture (RAPD/AFLP) very good (RAPD: good-poor) best Sequencing single colony/ tissue sample Reference Labs only! WM, Westmead Hospital, Sydney, Australia Reproducibility Identification Level Turnaround Time Cost Efficiency 1 -2 hours good species/strain 2 days good species/strain/ single mutation 2 days expensive Average Medical Mycology Lab
 PCR Assay Sensitivity Multi-copy e. g. Genes: 18 S r. RNA ITS Single-copy e. g. 1 -5 CFU 10 CFU/ml blood Genes: Actin 1. 4 - lanosterol demethylase Heat-shock protein 90 WM, Westmead Hospital, Sydney, Australia 1 -10 CFU 10 -100 CFU/ml blood 10 -100 CFU
PCR Assay Sensitivity Multi-copy e. g. Genes: 18 S r. RNA ITS Single-copy e. g. 1 -5 CFU 10 CFU/ml blood Genes: Actin 1. 4 - lanosterol demethylase Heat-shock protein 90 WM, Westmead Hospital, Sydney, Australia 1 -10 CFU 10 -100 CFU/ml blood 10 -100 CFU
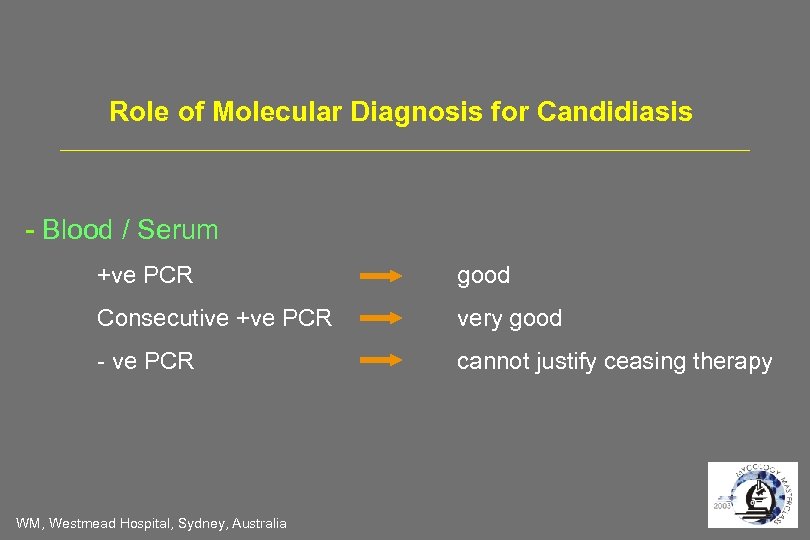 Role of Molecular Diagnosis for Candidiasis - Blood / Serum +ve PCR good Consecutive +ve PCR very good - ve PCR cannot justify ceasing therapy WM, Westmead Hospital, Sydney, Australia
Role of Molecular Diagnosis for Candidiasis - Blood / Serum +ve PCR good Consecutive +ve PCR very good - ve PCR cannot justify ceasing therapy WM, Westmead Hospital, Sydney, Australia
 Precautions for clinical PCR - Standard approaches for the three major phases of clinical PCR: Sample preparation Target and probe selection PCR and post-PCR analysis Positive and negative controls PCR contamination!!! WM, Westmead Hospital, Sydney, Australia
Precautions for clinical PCR - Standard approaches for the three major phases of clinical PCR: Sample preparation Target and probe selection PCR and post-PCR analysis Positive and negative controls PCR contamination!!! WM, Westmead Hospital, Sydney, Australia
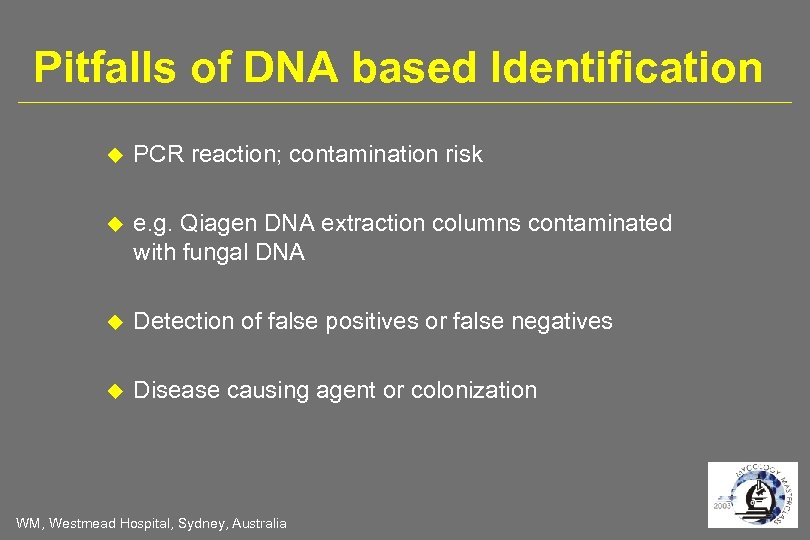 Pitfalls of DNA based Identification PCR reaction; contamination risk e. g. Qiagen DNA extraction columns contaminated with fungal DNA Detection of false positives or false negatives Disease causing agent or colonization WM, Westmead Hospital, Sydney, Australia
Pitfalls of DNA based Identification PCR reaction; contamination risk e. g. Qiagen DNA extraction columns contaminated with fungal DNA Detection of false positives or false negatives Disease causing agent or colonization WM, Westmead Hospital, Sydney, Australia
 Sources of Contamination Pre-PCR Contamination No routine sample collection methods have been established for PCRdiagnosis Contaminating DNA can originate from: Any persons skin, hair, door handles, surfaces in the laboratory Clinical equipment (maybe sterile but not DNA free) Reagents Use only PCR grade!! Taq polymerase (can contain procaryotic or eucaryotic DNA) PCR components (e. g: gelatine, BSA) PCR products from previous PCR reactions (e. g. if diagnostic PCR is repeatedly performed) WM, Westmead Hospital, Sydney, Australia
Sources of Contamination Pre-PCR Contamination No routine sample collection methods have been established for PCRdiagnosis Contaminating DNA can originate from: Any persons skin, hair, door handles, surfaces in the laboratory Clinical equipment (maybe sterile but not DNA free) Reagents Use only PCR grade!! Taq polymerase (can contain procaryotic or eucaryotic DNA) PCR components (e. g: gelatine, BSA) PCR products from previous PCR reactions (e. g. if diagnostic PCR is repeatedly performed) WM, Westmead Hospital, Sydney, Australia
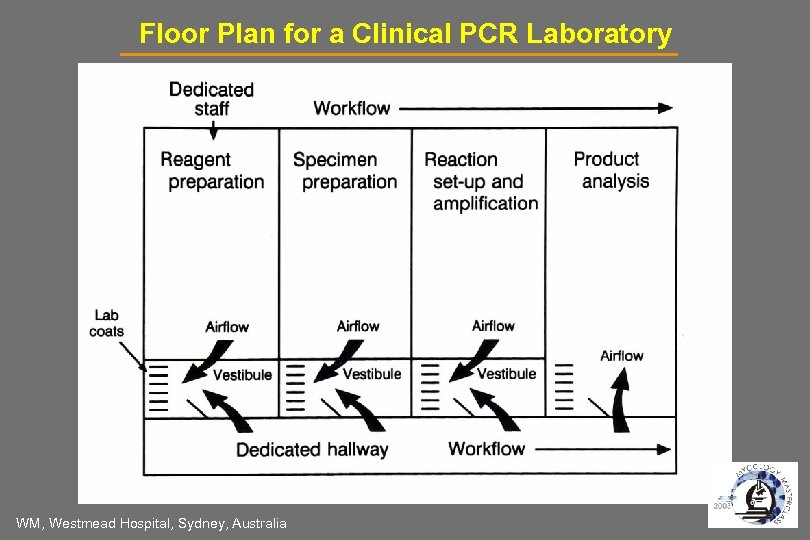 Floor Plan for a Clinical PCR Laboratory WM, Westmead Hospital, Sydney, Australia
Floor Plan for a Clinical PCR Laboratory WM, Westmead Hospital, Sydney, Australia
 In summary, consistently reliable, universally applicable and standardized methods for fungal ID are still to be established. Many in-house DNA based fungal ID techniques exist: Problem all of them lack standardization There is a lack of commercial interest to develop DNA based fungal ID systems, because of: - the limited market - limited antifungal spectrum available - high development costs Commercially available DNA based ID kits exist only for: - Blastomyces dermatitidis, Histoplasma capsulatum, Coccidioides immitis (Accu. Probe Kits, Gen-Probe, USA) - Candida albicans (Roche) - Universal Fungal ID via Sequencing Micro. Seq (Applied Biosystems) WM, Westmead Hospital, Sydney, Australia
In summary, consistently reliable, universally applicable and standardized methods for fungal ID are still to be established. Many in-house DNA based fungal ID techniques exist: Problem all of them lack standardization There is a lack of commercial interest to develop DNA based fungal ID systems, because of: - the limited market - limited antifungal spectrum available - high development costs Commercially available DNA based ID kits exist only for: - Blastomyces dermatitidis, Histoplasma capsulatum, Coccidioides immitis (Accu. Probe Kits, Gen-Probe, USA) - Candida albicans (Roche) - Universal Fungal ID via Sequencing Micro. Seq (Applied Biosystems) WM, Westmead Hospital, Sydney, Australia
 Acknowledgements Molecular Mycology Laboratory Microbiogen/Macquarie University Krystyna Marszewska Dr. Phillip Bell Mathew Huynh Sarah Kidd CBS, The Netherlands Dr. Nicolas G. Latouche Dr. Vincent Robert Dr. Heide-Marie Daniel David Yarrow Dr. Catriona L. Halliday WM, Westmead Hospital, Sydney, Australia
Acknowledgements Molecular Mycology Laboratory Microbiogen/Macquarie University Krystyna Marszewska Dr. Phillip Bell Mathew Huynh Sarah Kidd CBS, The Netherlands Dr. Nicolas G. Latouche Dr. Vincent Robert Dr. Heide-Marie Daniel David Yarrow Dr. Catriona L. Halliday WM, Westmead Hospital, Sydney, Australia
 Molecular Mycology Reference Laboratory Samples Should be Directed to: Westmead Hospital ICPMR Darcy Road Westmead, NSW 2145 Contact Persons: Marked: Molecular Mycology Laboratory Dr. Wieland Meyer Ph. : 61 -2 -98456895 Fax: 61 -2 -98915317 E-mail: w. meyer@usyd. edu. au More Info soon at: www. usyd. edu. au/~cidm WM, Westmead Hospital, Sydney, Australia
Molecular Mycology Reference Laboratory Samples Should be Directed to: Westmead Hospital ICPMR Darcy Road Westmead, NSW 2145 Contact Persons: Marked: Molecular Mycology Laboratory Dr. Wieland Meyer Ph. : 61 -2 -98456895 Fax: 61 -2 -98915317 E-mail: w. meyer@usyd. edu. au More Info soon at: www. usyd. edu. au/~cidm WM, Westmead Hospital, Sydney, Australia


