fc343cdda707d004c26d64045ce9518a.ppt
- Количество слайдов: 48

Molecular Predictors in Clinical Decision Making for Breast Cancer George W. Sledge, Jr. M. D. Indiana University Simon Cancer Center
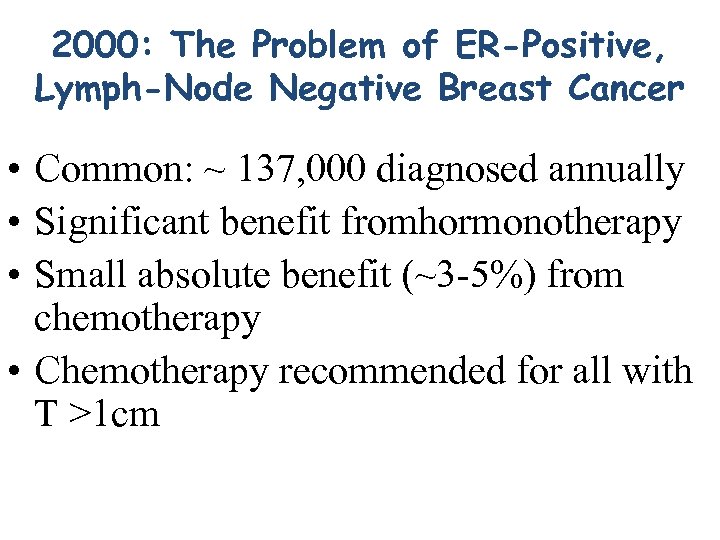
2000: The Problem of ER-Positive, Lymph-Node Negative Breast Cancer • Common: ~ 137, 000 diagnosed annually • Significant benefit fromhormonotherapy • Small absolute benefit (~3 -5%) from chemotherapy • Chemotherapy recommended for all with T >1 cm
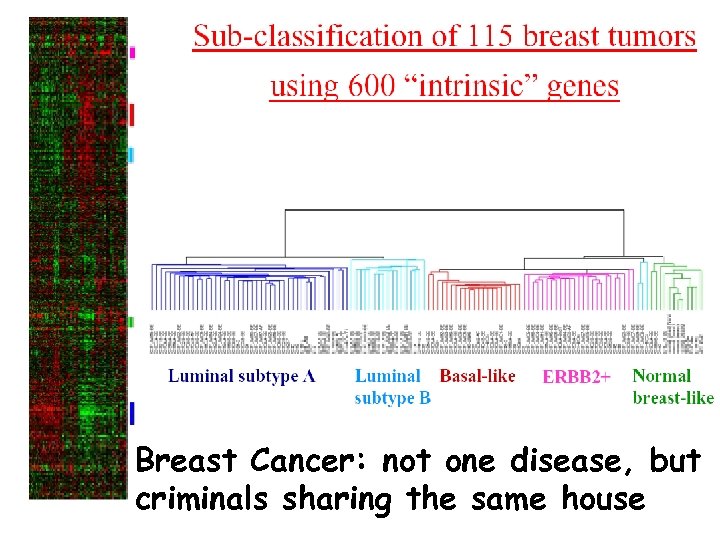
Breast Cancer: not one disease, but criminals sharing the same house
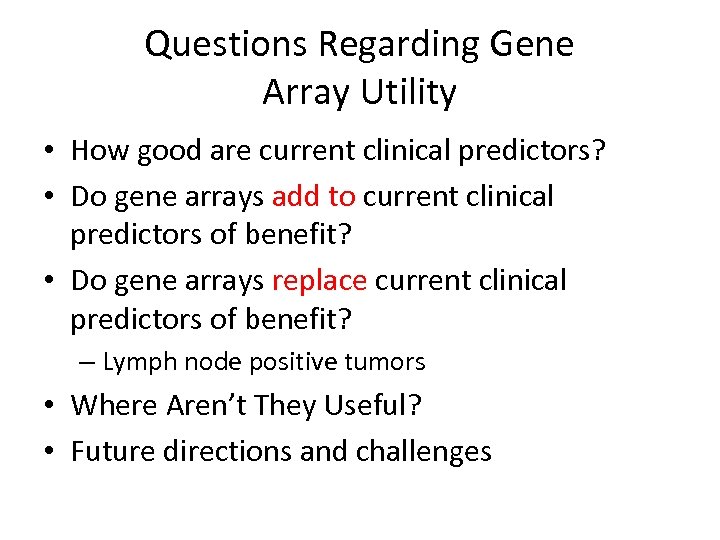
Questions Regarding Gene Array Utility • How good are current clinical predictors? • Do gene arrays add to current clinical predictors of benefit? • Do gene arrays replace current clinical predictors of benefit? – Lymph node positive tumors • Where Aren’t They Useful? • Future directions and challenges

How Good Are Current Clinical Predictors?
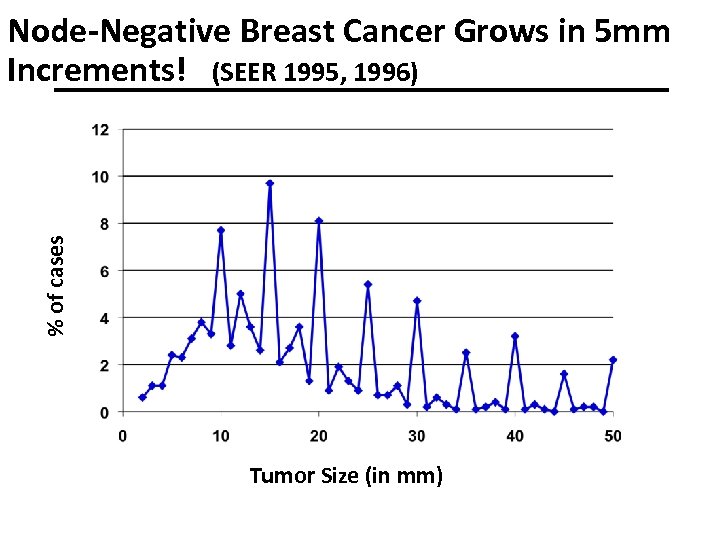
% of cases Node-Negative Breast Cancer Grows in 5 mm Increments! (SEER 1995, 1996) Tumor Size (in mm)
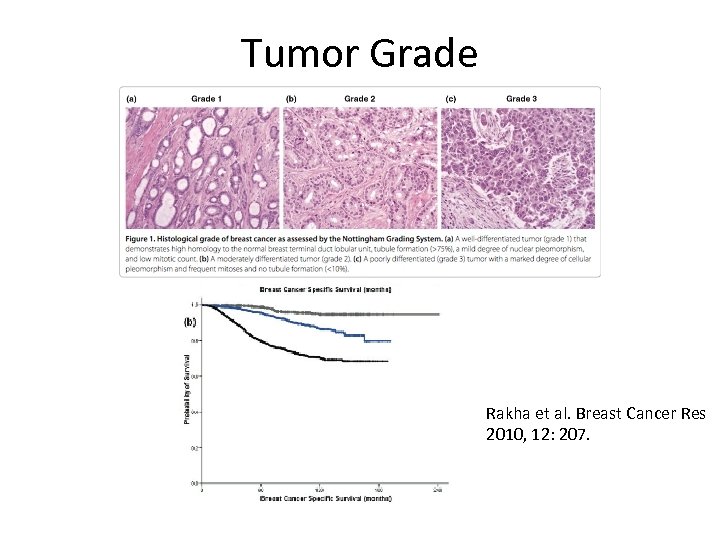
Tumor Grade Rakha et al. Breast Cancer Res 2010, 12: 207.
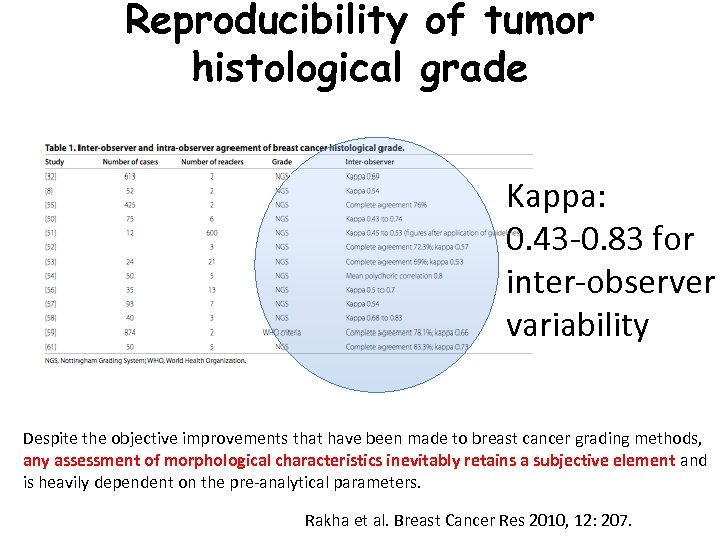
Reproducibility of tumor histological grade Kappa: 0. 43 -0. 83 for inter-observer variability Despite the objective improvements that have been made to breast cancer grading methods, any assessment of morphological characteristics inevitably retains a subjective element and is heavily dependent on the pre-analytical parameters. Rakha et al. Breast Cancer Res 2010, 12: 207.

How Good are Current Clinical Predictors of Benefit? • They aren’t useless (i. e. , stage and grade mean something) • But they lack reproducibility • And they aren’t perfect predictors

Do gene arrays add to current clinical predictors of benefit?
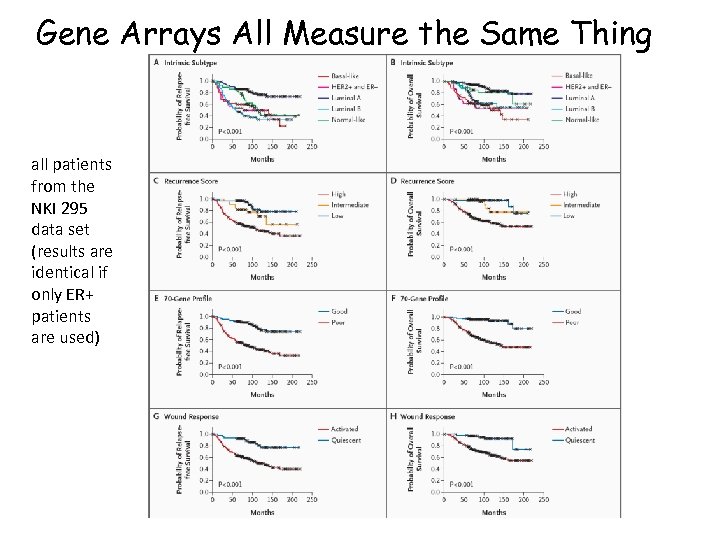
Gene Arrays All Measure the Same Thing all patients from the NKI 295 data set (results are identical if only ER+ patients are used)
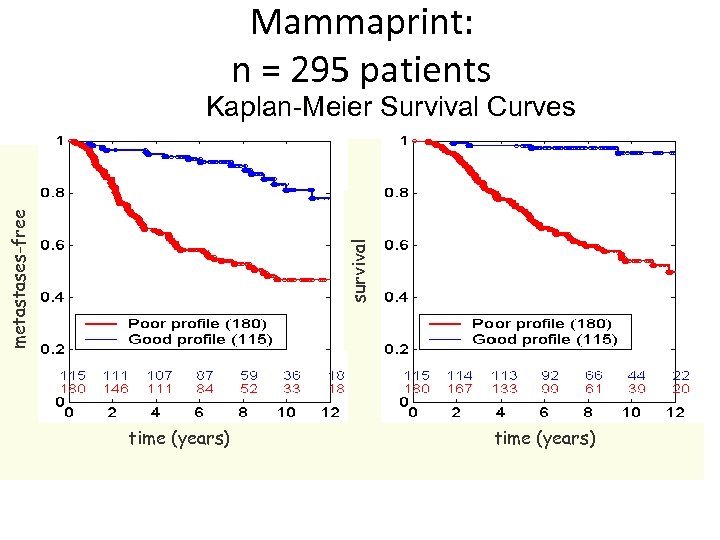
Mammaprint: n = 295 patients survival metastases-free Kaplan-Meier Survival Curves time (years)
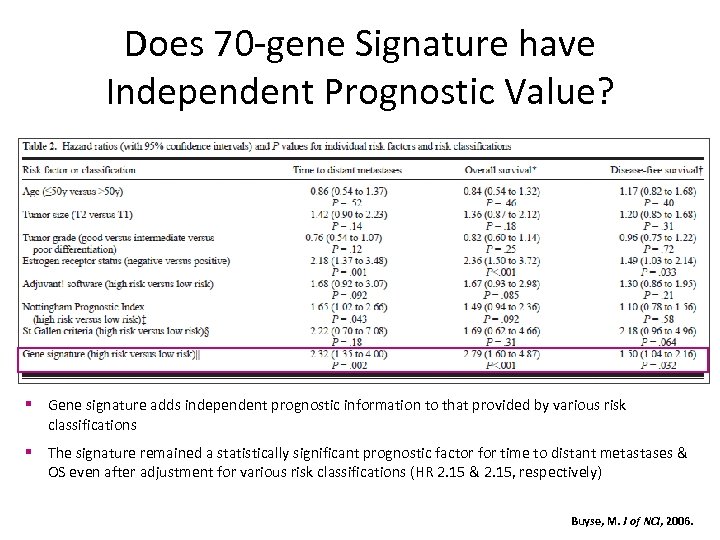
Does 70 -gene Signature have Independent Prognostic Value? § Gene signature adds independent prognostic information to that provided by various risk classifications § The signature remained a statistically significant prognostic factor for time to distant metastases & OS even after adjustment for various risk classifications (HR 2. 15 & 2. 15, respectively) Buyse, M. J of NCI, 2006.
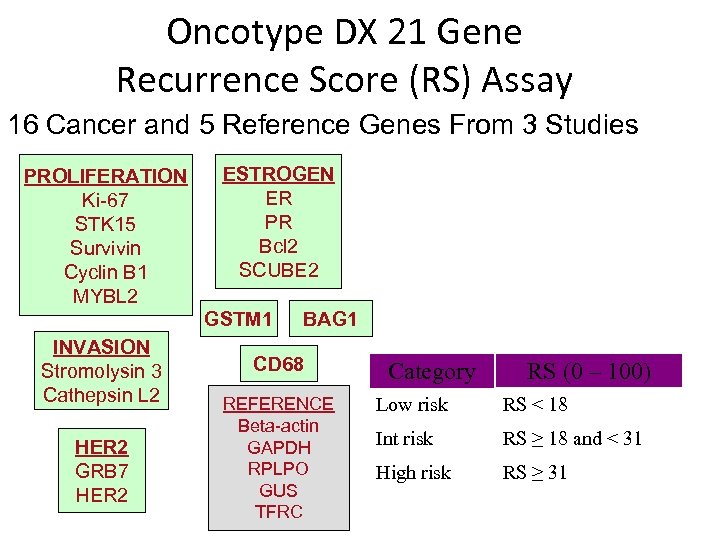
Oncotype DX 21 Gene Recurrence Score (RS) Assay 16 Cancer and 5 Reference Genes From 3 Studies PROLIFERATION Ki-67 STK 15 Survivin Cyclin B 1 MYBL 2 ESTROGEN ER PR Bcl 2 SCUBE 2 GSTM 1 INVASION Stromolysin 3 Cathepsin L 2 HER 2 GRB 7 HER 2 BAG 1 CD 68 REFERENCE Beta-actin GAPDH RPLPO GUS TFRC Category RS (0 – 100) Low risk RS < 18 Int risk RS ≥ 18 and < 31 High risk RS ≥ 31
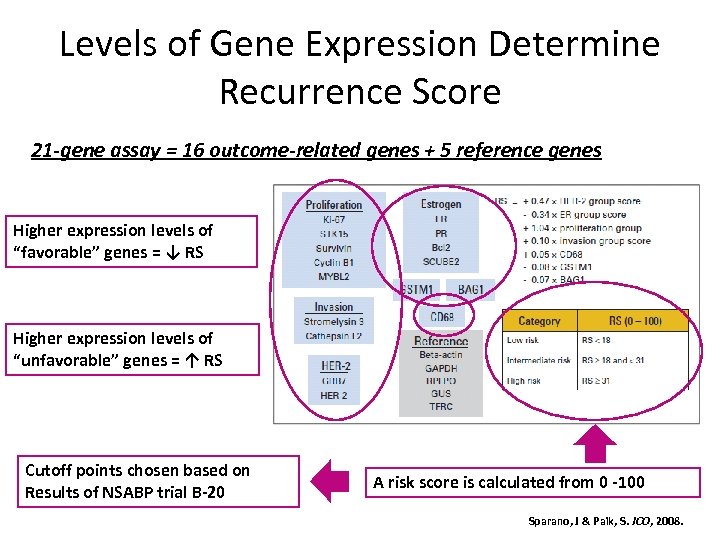
Levels of Gene Expression Determine Recurrence Score 21 -gene assay = 16 outcome-related genes + 5 reference genes Higher expression levels of “favorable” genes = ↓ RS Higher expression levels of “unfavorable” genes = ↑ RS Cutoff points chosen based on Results of NSABP trial B-20 A risk score is calculated from 0 -100 Sparano, J & Paik, S. JCO, 2008.
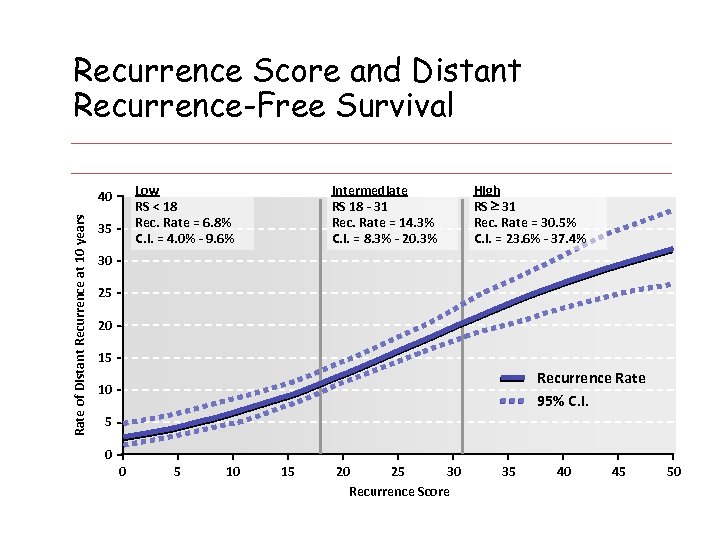
Recurrence Score and Distant Recurrence-Free Survival Low RS < 18 Rec. Rate = 6. 8% C. I. = 4. 0% - 9. 6% Rate of Distant Recurrence at 10 years 40 35 Intermediate RS 18 - 31 Rec. Rate = 14. 3% C. I. = 8. 3% - 20. 3% High RS 31 Rec. Rate = 30. 5% C. I. = 23. 6% - 37. 4% 30 25 20 15 Recurrence Rate 95% C. I. 10 5 0 0 5 10 15 20 25 30 Recurrence Score 35 40 45 50 Paik. S. et al. N Engl J Med 2004; 351: 2817 -26
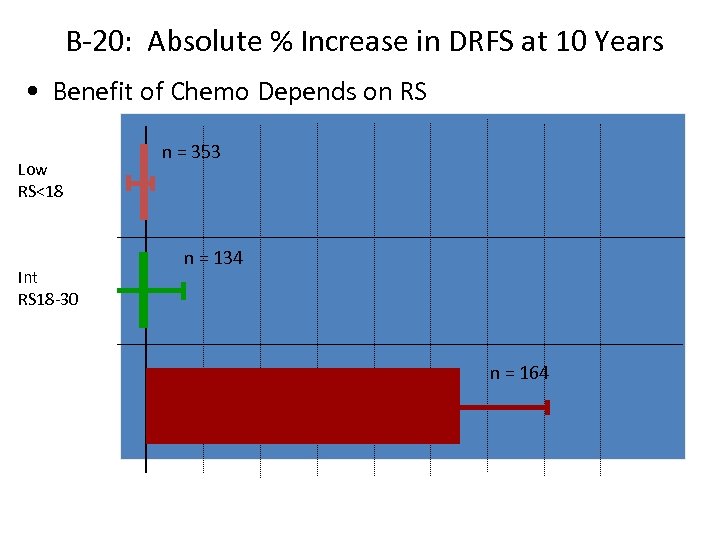
B-20: Absolute % Increase in DRFS at 10 Years • Benefit of Chemo Depends on RS n = 353 Low RS<18 n = 134 Int RS 18 -30 n = 164 High RS≥ 31 0 10% 20% 30% 40% % Increase in DRFS at 10 Yrs (mean ± SE)
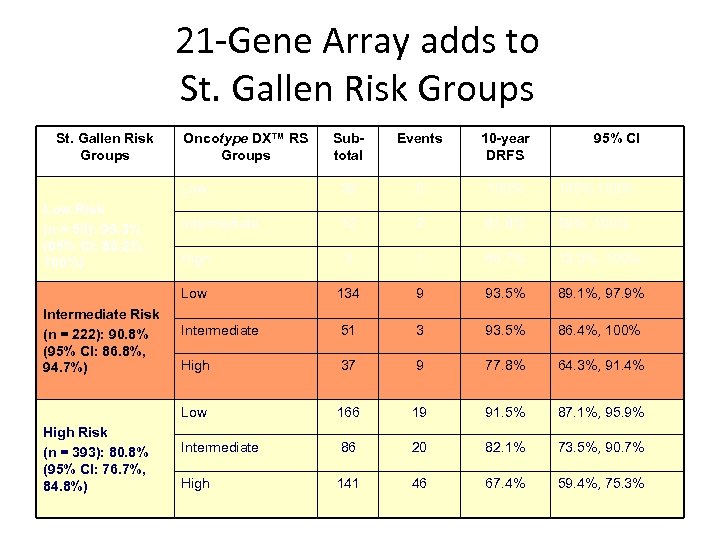
21 -Gene Array adds to St. Gallen Risk Groups 10 -year DRFS 38 0 100%, 100% Intermediate 12 2 81. 8% 59%, 100% High 3 1 66. 7% 13. 3%, 100% 134 9 93. 5% 89. 1%, 97. 9% Intermediate 51 3 93. 5% 86. 4%, 100% High 37 9 77. 8% 64. 3%, 91. 4% Low High Risk (n = 393): 80. 8% (95% CI: 76. 7%, 84. 8%) Events Low Intermediate Risk (n = 222): 90. 8% (95% CI: 86. 8%, 94. 7%) Subtotal Low Risk (n = 53): 95. 3% (95% CI: 86. 2%, 100%) Oncotype DX™ RS Groups 95% CI 166 19 91. 5% 87. 1%, 95. 9% Intermediate 86 20 82. 1% 73. 5%, 90. 7% High 141 46 67. 4% 59. 4%, 75. 3%
Do Gene Arrays Add To Standard Predictors? • YES • Highly reproducible, robust assays • Among ER-positive node-neg. patients, they: – are prognostic for recurrence – are predictive of chemotherapy benefit

Do gene arrays replace current clinical predictors of benefit?
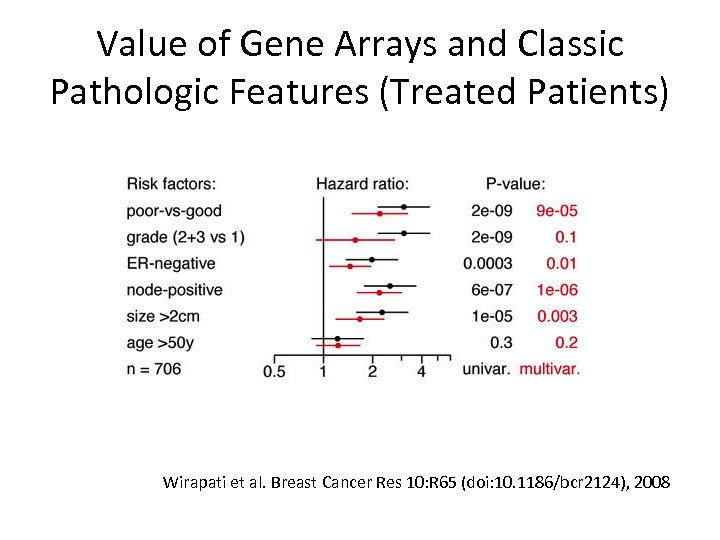
Value of Gene Arrays and Classic Pathologic Features (Treated Patients) Wirapati et al. Breast Cancer Res 10: R 65 (doi: 10. 1186/bcr 2124), 2008
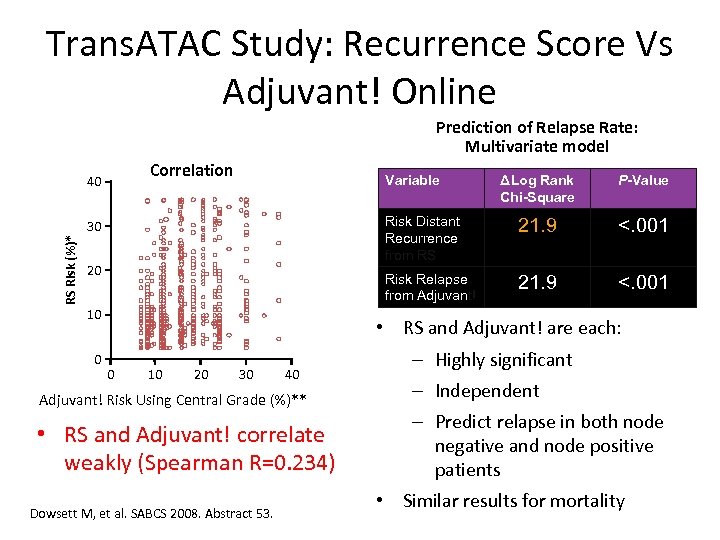
Trans. ATAC Study: Recurrence Score Vs Adjuvant! Online Prediction of Relapse Rate: Multivariate model Correlation Variable RS Risk (%)* 20 10 0 P-Value 21. 9 <. 001 Risk Relapse from Adjuvant! 30 ΔLog Rank Chi-Square Risk Distant Recurrence from RS 40 21. 9 <. 001 • RS and Adjuvant! are each: 0 10 20 30 40 Adjuvant! Risk Using Central Grade (%)** • RS and Adjuvant! correlate weakly (Spearman R=0. 234) Dowsett M, et al. SABCS 2008. Abstract 53. – Highly significant – Independent – Predict relapse in both node negative and node positive patients • Similar results for mortality
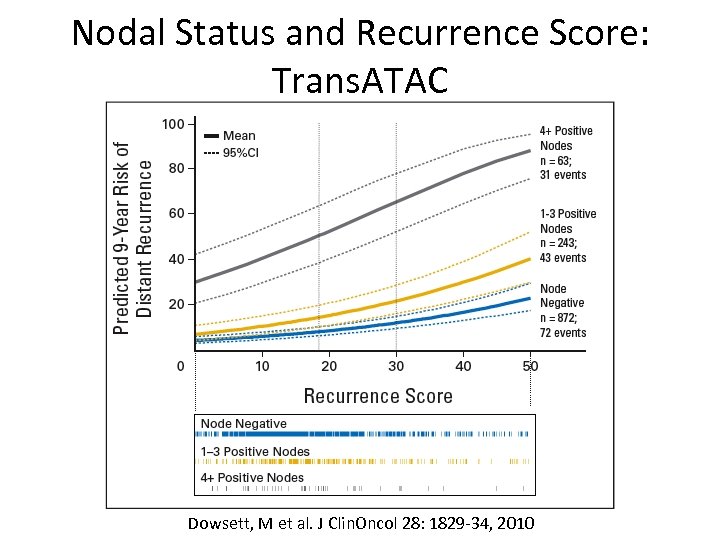
Nodal Status and Recurrence Score: Trans. ATAC Dowsett, M et al. J Clin. Oncol 28: 1829 -34, 2010
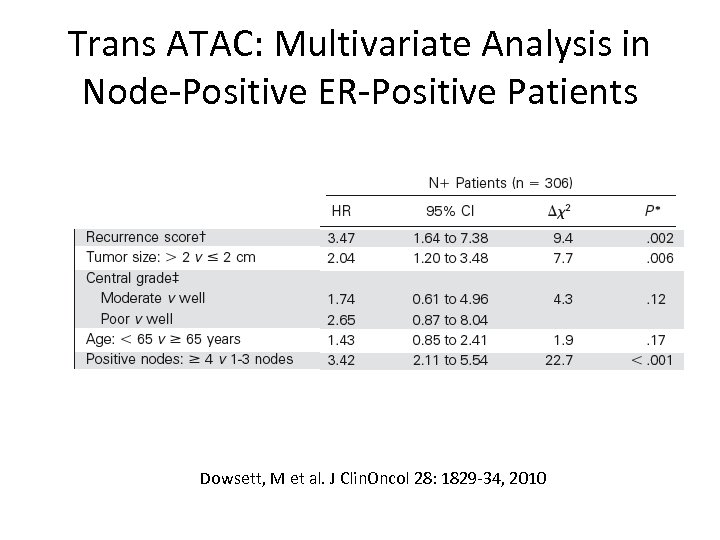
Trans ATAC: Multivariate Analysis in Node-Positive ER-Positive Patients Dowsett, M et al. J Clin. Oncol 28: 1829 -34, 2010
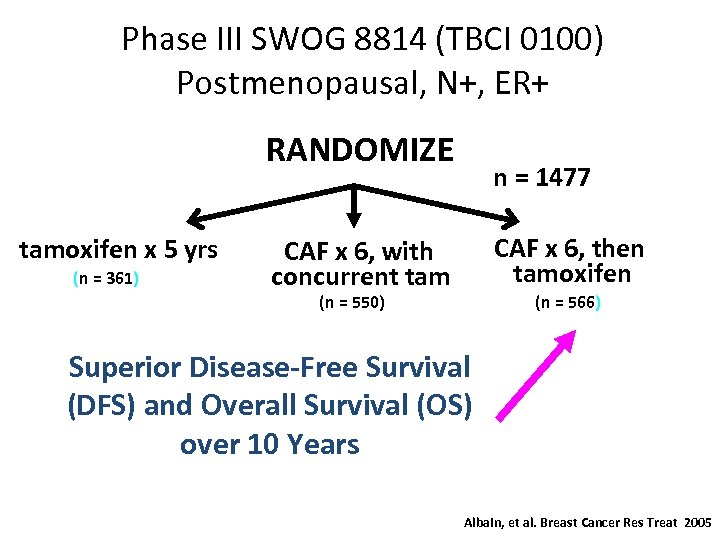
Phase III SWOG 8814 (TBCI 0100) Postmenopausal, N+, ER+ RANDOMIZE tamoxifen x 5 yrs (n = 361) n = 1477 CAF x 6, then tamoxifen CAF x 6, with concurrent tam (n = 550) (n = 566) Superior Disease-Free Survival (DFS) and Overall Survival (OS) over 10 Years Albain, et al. Breast Cancer Res Treat 2005
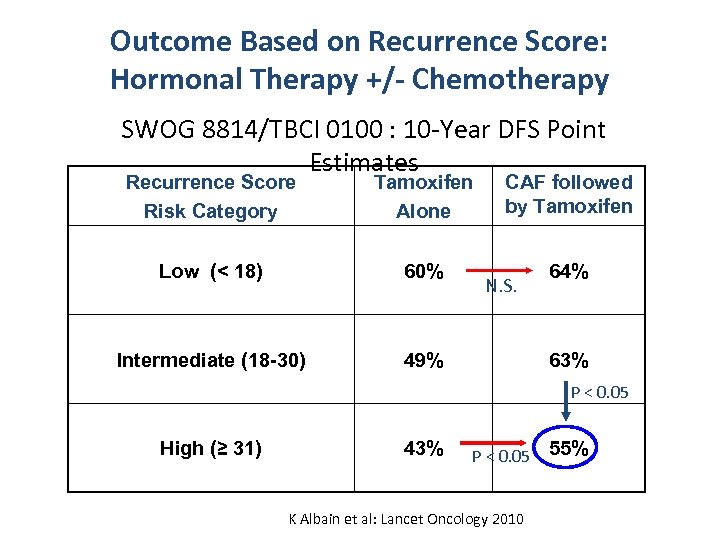
Outcome Based on Recurrence Score: Hormonal Therapy +/- Chemotherapy SWOG 8814/TBCI 0100 : 10 -Year DFS Point Estimates Recurrence Score Risk Category Tamoxifen Alone CAF followed by Tamoxifen Low (< 18) 60% 64% Intermediate (18 -30) 49% N. S. 63% P < 0. 05 High (≥ 31) 43% P < 0. 05 K Albain et al: Lancet Oncology 2010 55%
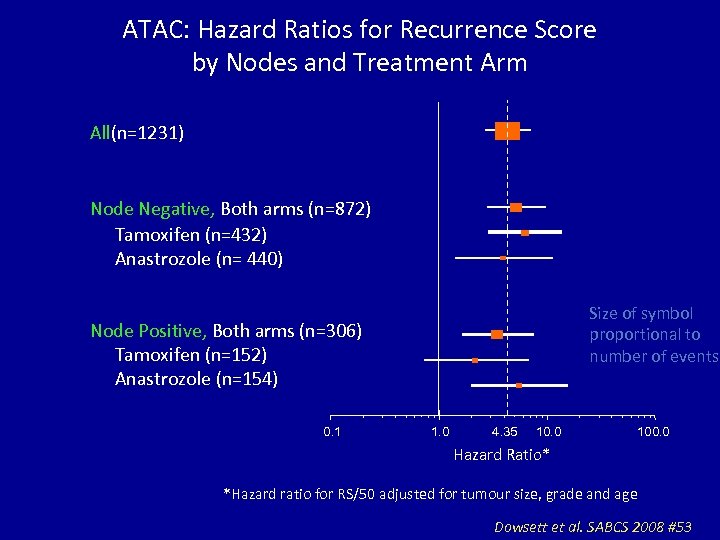
ATAC: Hazard Ratios for Recurrence Score by Nodes and Treatment Arm All(n=1231) Node Negative, Both arms (n=872) Tamoxifen (n=432) Anastrozole (n= 440) Size of symbol proportional to number of events Node Positive, Both arms (n=306) Tamoxifen (n=152) Anastrozole (n=154) 0. 1 1. 0 4. 35 10. 0 100. 0 Hazard Ratio* *Hazard ratio for RS/50 adjusted for tumour size, grade and age Dowsett et al. SABCS 2008 #53
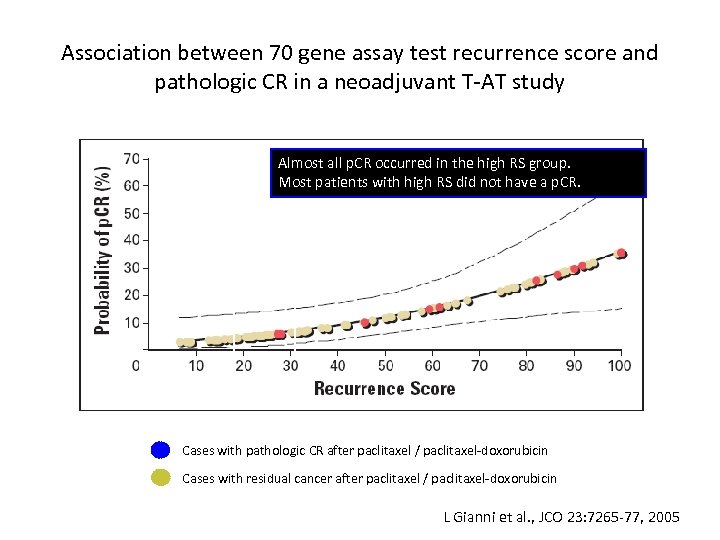
Association between 70 gene assay test recurrence score and pathologic CR in a neoadjuvant T-AT study Almost all p. CR occurred in the high RS group. Most patients with high RS did not have a p. CR. <18 low RS >31 high RS Cases with pathologic CR after paclitaxel / paclitaxel-doxorubicin Cases with residual cancer after paclitaxel / paclitaxel-doxorubicin L Gianni et al. , JCO 23: 7265 -77, 2005
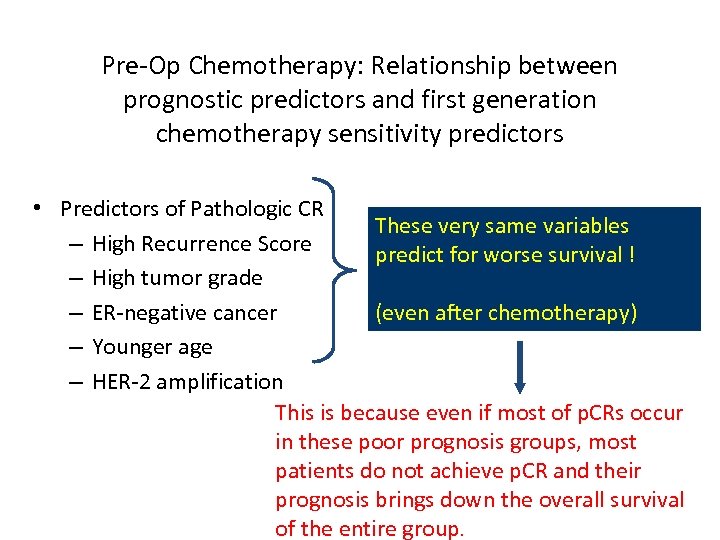
Pre-Op Chemotherapy: Relationship between prognostic predictors and first generation chemotherapy sensitivity predictors • Predictors of Pathologic CR These very same variables – High Recurrence Score predict for worse survival ! – High tumor grade – ER-negative cancer (even after chemotherapy) – Younger age – HER-2 amplification This is because even if most of p. CRs occur in these poor prognosis groups, most patients do not achieve p. CR and their prognosis brings down the overall survival of the entire group.
An inconvenient truth about biomarkers • Survival of individual patients with stage I-III breast cancer depends on – Baseline prognosis – Efficacy of chemotherapy – Efficacy of endocrine therapy Many known biomarkers interact separately with each of these outcome variables!
Gene Arrays in Node-Positive ERPositive Patients • Size and lymph node status matter: big tumors and multiple nodes kill • Gene arrays define a high-risk population that does not benefit from chemotherapy • Gene arrays DO NOT replace standard predictors
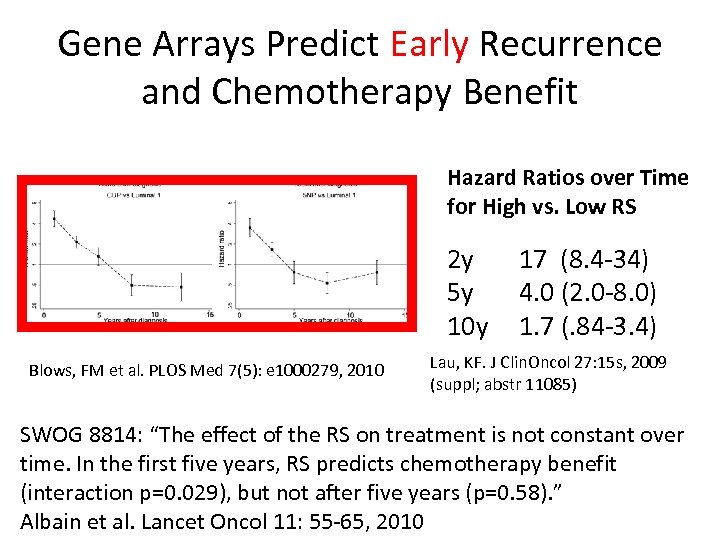
Gene Arrays Predict Early Recurrence and Chemotherapy Benefit Hazard Ratios over Time for High vs. Low RS 2 y 5 y 10 y Blows, FM et al. PLOS Med 7(5): e 1000279, 2010 17 (8. 4 -34) 4. 0 (2. 0 -8. 0) 1. 7 (. 84 -3. 4) Lau, KF. J Clin. Oncol 27: 15 s, 2009 (suppl; abstr 11085) SWOG 8814: “The effect of the RS on treatment is not constant over time. In the first five years, RS predicts chemotherapy benefit (interaction p=0. 029), but not after five years (p=0. 58). ” Albain et al. Lancet Oncol 11: 55 -65, 2010
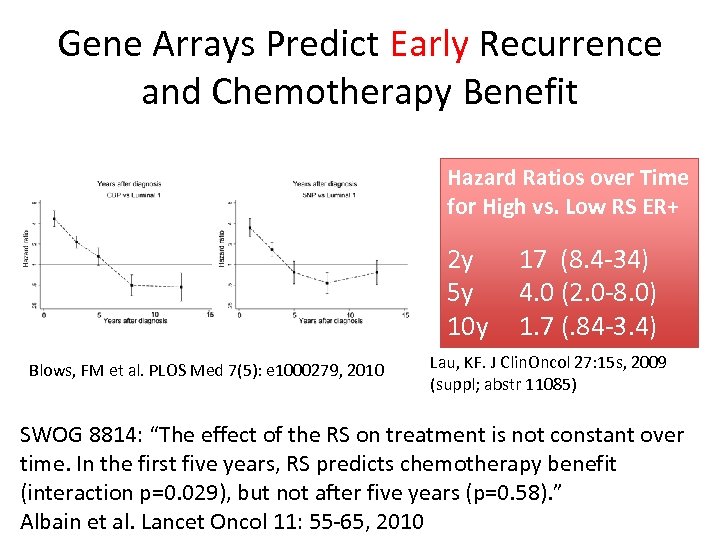
Gene Arrays Predict Early Recurrence and Chemotherapy Benefit Hazard Ratios over Time for High vs. Low RS ER+ 2 y 5 y 10 y Blows, FM et al. PLOS Med 7(5): e 1000279, 2010 17 (8. 4 -34) 4. 0 (2. 0 -8. 0) 1. 7 (. 84 -3. 4) Lau, KF. J Clin. Oncol 27: 15 s, 2009 (suppl; abstr 11085) SWOG 8814: “The effect of the RS on treatment is not constant over time. In the first five years, RS predicts chemotherapy benefit (interaction p=0. 029), but not after five years (p=0. 58). ” Albain et al. Lancet Oncol 11: 55 -65, 2010
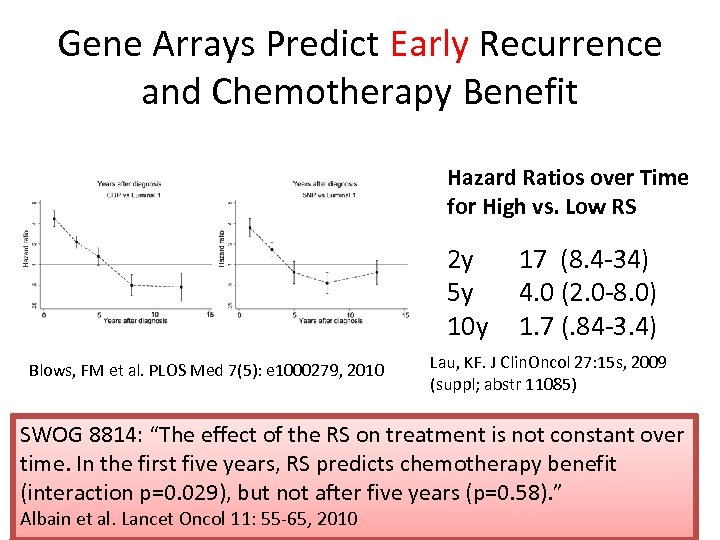
Gene Arrays Predict Early Recurrence and Chemotherapy Benefit Hazard Ratios over Time for High vs. Low RS 2 y 5 y 10 y Blows, FM et al. PLOS Med 7(5): e 1000279, 2010 17 (8. 4 -34) 4. 0 (2. 0 -8. 0) 1. 7 (. 84 -3. 4) Lau, KF. J Clin. Oncol 27: 15 s, 2009 (suppl; abstr 11085) SWOG 8814: “The effect of the RS on treatment is not constant over time. In the first five years, RS predicts chemotherapy benefit (interaction p=0. 029), but not after five years (p=0. 58). ” Albain et al. Lancet Oncol 11: 55 -65, 2010
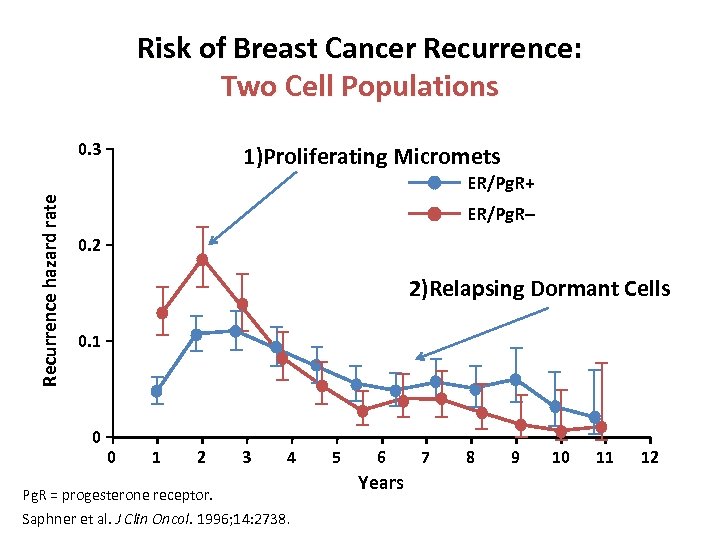
Risk of Breast Cancer Recurrence: Two Cell Populations Recurrence hazard rate 0. 3 1)Proliferating Micromets ER/Pg. R+ ER/Pg. R– 0. 2 2)Relapsing Dormant Cells 0. 1 0 0 1 2 3 4 Pg. R = progesterone receptor. Saphner et al. J Clin Oncol. 1996; 14: 2738. 5 6 Years 7 8 9 10 11 12
Future Directions: Integration of Gene Arrays and Standard Predictors • Tang et al. (ASCO 2010 Abstract 509) combined RS with pathologic and clinical information (RSPC Index) • RSPC risk index is superior to RS alone at predicting 10 -year distant recurrence rates • RSPC risk index reduces patients in the intermediate category (18% vs 26%, p = 0. 001)

Where Aren’t They Useful? • “Special Type” Cancers – Most have excellent prognosis – Some have specific mutational events • ER-Negative and HER 2 -Positive cancers – Most require treatment • Inflammatory cancer
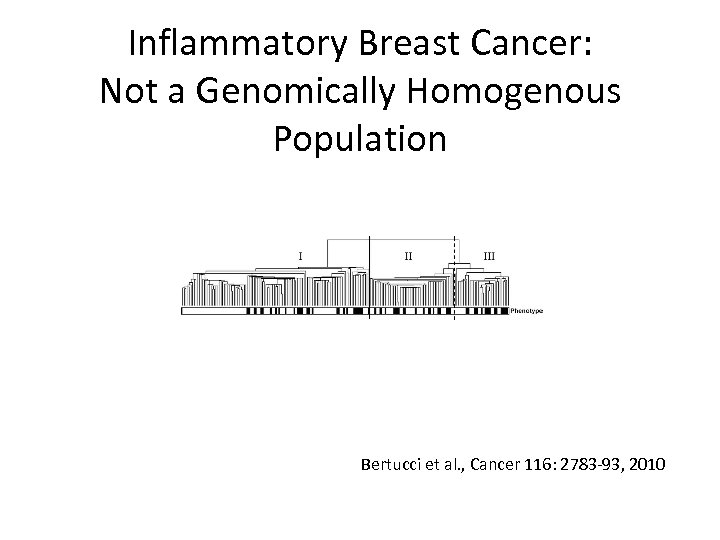
Inflammatory Breast Cancer: Not a Genomically Homogenous Population Bertucci et al. , Cancer 116: 2783 -93, 2010

Future Directions
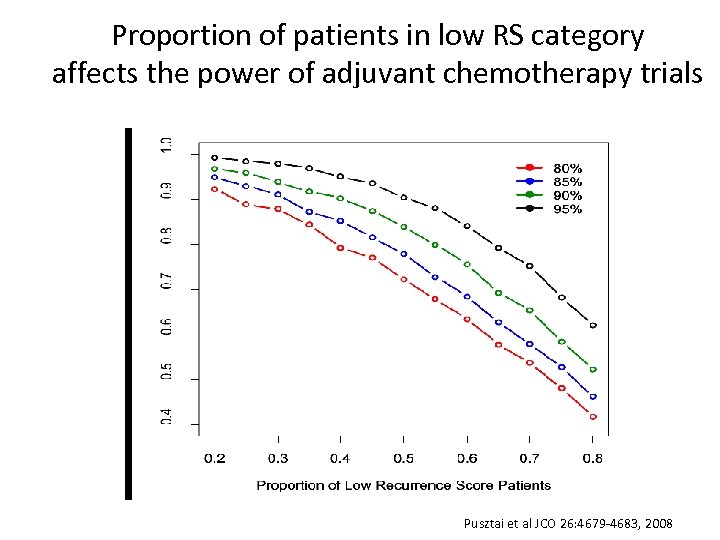
Proportion of patients in low RS category affects the power of adjuvant chemotherapy trials in ER-positive breast cancer Base power Pusztai et al JCO 26: 4679 -4683, 2008
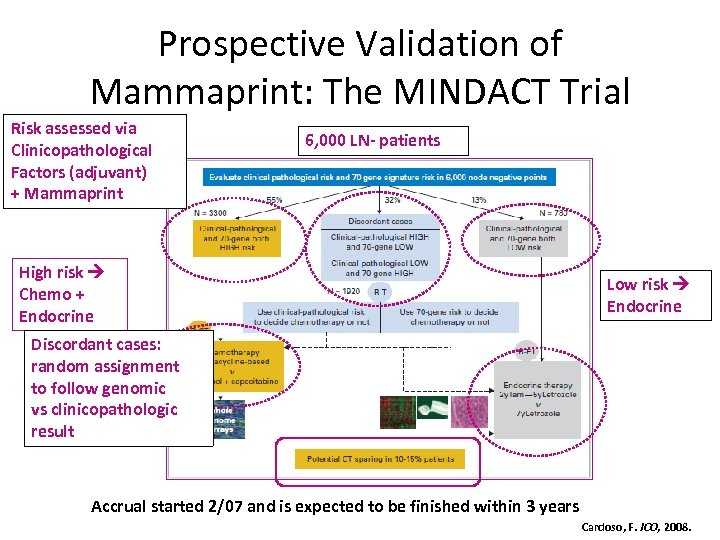
Prospective Validation of Mammaprint: The MINDACT Trial Risk assessed via Clinicopathological Factors (adjuvant) + Mammaprint 6, 000 LN- patients High risk Chemo + Endocrine Low risk Endocrine Discordant cases: random assignment to follow genomic vs clinicopathologic result Accrual started 2/07 and is expected to be finished within 3 years Cardoso, F. JCO, 2008.
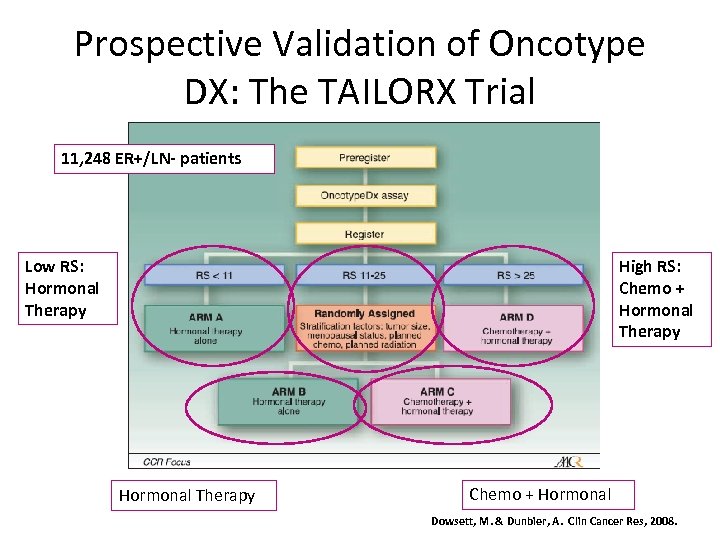
Prospective Validation of Oncotype DX: The TAILORX Trial 11, 248 ER+/LN- patients Low RS: Hormonal Therapy High RS: Chemo + Hormonal Therapy Chemo + Hormonal Dowsett, M. & Dunbier, A. Clin Cancer Res, 2008.
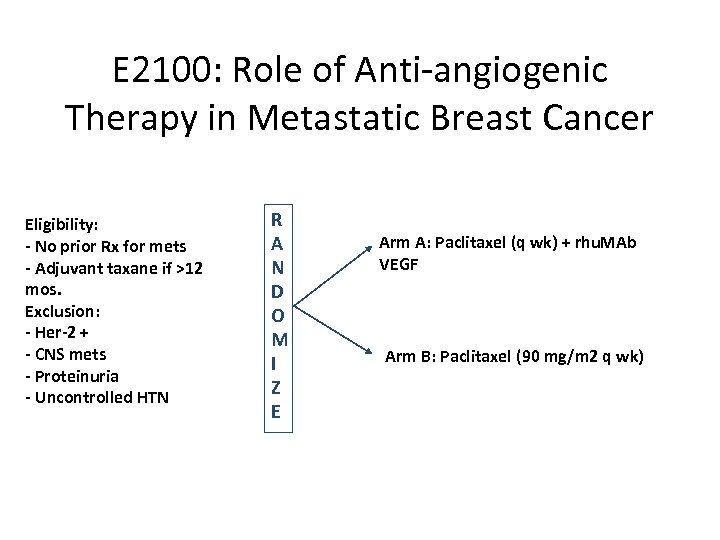
E 2100: Role of Anti-angiogenic Therapy in Metastatic Breast Cancer Eligibility: - No prior Rx for mets - Adjuvant taxane if >12 mos. Exclusion: - Her-2 + - CNS mets - Proteinuria - Uncontrolled HTN R A N D O M I Z E Arm A: Paclitaxel (q wk) + rhu. MAb VEGF Arm B: Paclitaxel (90 mg/m 2 q wk)
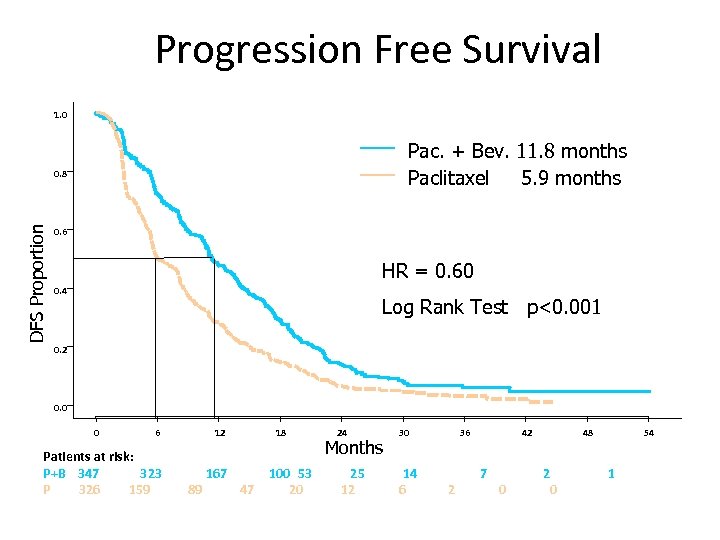
Progression Free Survival 1. 0 Pac. + Bev. 11. 8 months Paclitaxel 5. 9 months DFS Proportion 0. 8 0. 6 HR = 0. 60 0. 4 Log Rank Test p<0. 001 0. 2 0. 0 0 6 12 Patients at risk: P+B 347 323 P 326 159 167 89 18 47 100 53 20 24 Months 25 12 30 14 6 36 2 42 7 0 48 2 0 54 1
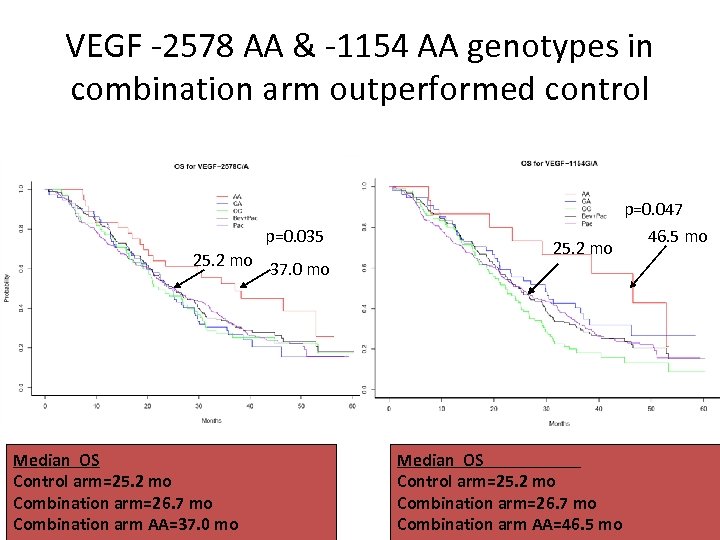
VEGF -2578 AA & -1154 AA genotypes in combination arm outperformed control p=0. 047 p=0. 035 25. 2 mo 37. 0 mo Median OS Control arm=25. 2 mo Combination arm=26. 7 mo Combination arm AA=37. 0 mo 25. 2 mo Median OS Control arm=25. 2 mo Combination arm=26. 7 mo Combination arm AA=46. 5 mo
Challenges for Gene Arrays • T 1 a, b ER-positive tumors: how low do you go? • ER-negative, node-negative tumors: who doesn’t need chemotherapy? • Prediction of late relapse in ER-positive patients (tumor dormancy) • Prediction of benefit for specific drugs or regimens

Conclusions • Multi-gene assays provide consistent prognostic results and probably measure the same biology • Arrays add to but do not replace current clinical predictors • Much work remains .

Thank You!
fc343cdda707d004c26d64045ce9518a.ppt