b0780dd5ba5903f5a82a515dfd9fec1d.ppt
- Количество слайдов: 25
 Molecular Mechanisms and Signaling Pathways in Muscle Atrophy in Immobilization and Aging Marina Bar- Shai Abraham Z. Reznick Department of Anatomy and Cell Biology The Bruce Rappaport Faculty of Medicine Technion – Israel Institute of Technology Haifa, Israel
Molecular Mechanisms and Signaling Pathways in Muscle Atrophy in Immobilization and Aging Marina Bar- Shai Abraham Z. Reznick Department of Anatomy and Cell Biology The Bruce Rappaport Faculty of Medicine Technion – Israel Institute of Technology Haifa, Israel
 Summary of the main topics 1. Introduction to aging and muscle protein degradation 2. In vivo model of immobilization and the stages of skeletal muscle breakdown 3. In vitro model of the involvement of RNS in activation of NF- κB in muscle cells
Summary of the main topics 1. Introduction to aging and muscle protein degradation 2. In vivo model of immobilization and the stages of skeletal muscle breakdown 3. In vitro model of the involvement of RNS in activation of NF- κB in muscle cells
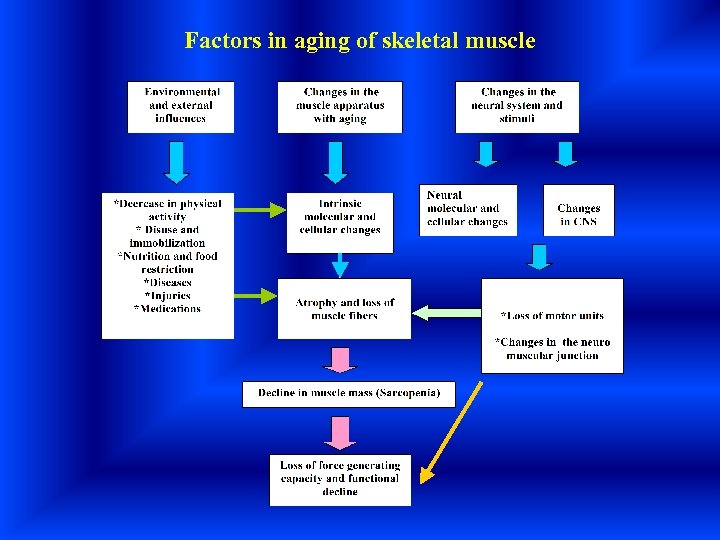 Factors in aging of skeletal muscle
Factors in aging of skeletal muscle
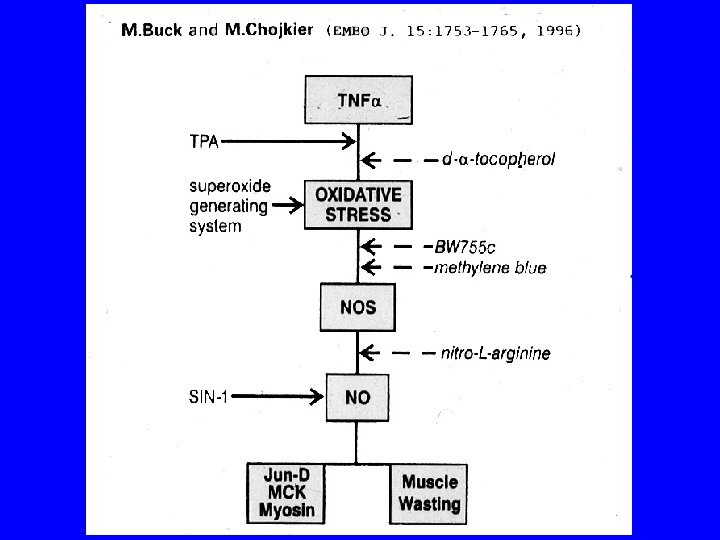
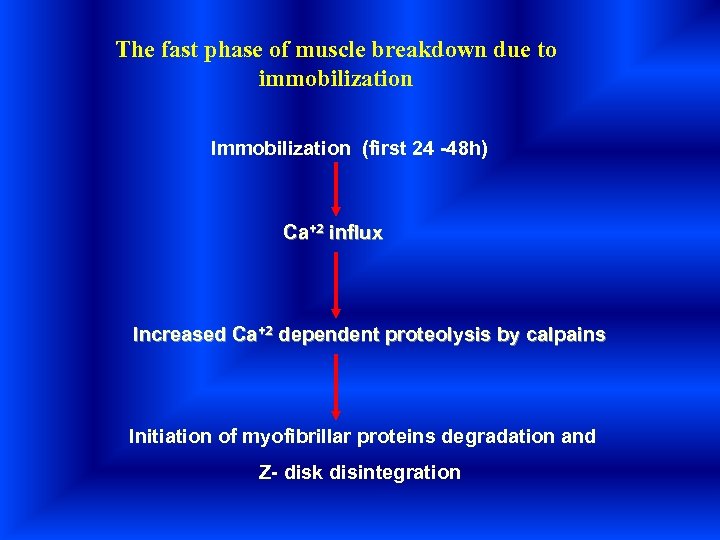 The fast phase of muscle breakdown due to immobilization Immobilization (first 24 -48 h) Ca+2 influx Increased Ca+2 dependent proteolysis by calpains Initiation of myofibrillar proteins degradation and Z- disk disintegration
The fast phase of muscle breakdown due to immobilization Immobilization (first 24 -48 h) Ca+2 influx Increased Ca+2 dependent proteolysis by calpains Initiation of myofibrillar proteins degradation and Z- disk disintegration
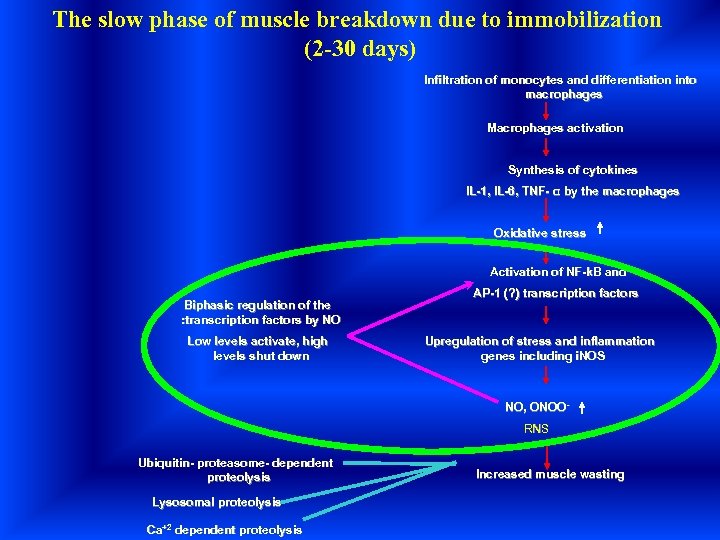
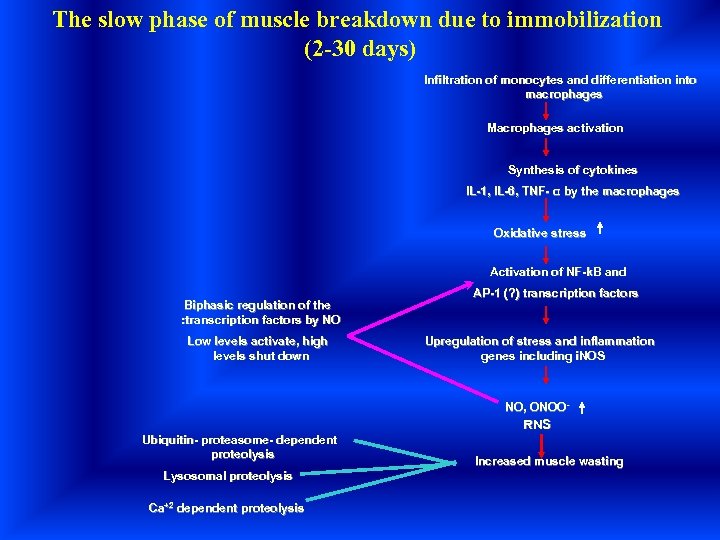 The slow phase of muscle breakdown due to immobilization (2 -30 days) Infiltration of monocytes and differentiation into macrophages Macrophages activation Synthesis of cytokines IL-1, IL-6, TNF- α by the macrophages Oxidative stress Activation of NF-k. B and Biphasic regulation of the : transcription factors by NO Low levels activate, high levels shut down AP-1 (? ) transcription factors Upregulation of stress and inflammation genes including i. NOS NO, ONOO- RNS Ubiquitin- proteasome- dependent proteolysis Lysosomal proteolysis Ca+2 dependent proteolysis Increased muscle wasting
The slow phase of muscle breakdown due to immobilization (2 -30 days) Infiltration of monocytes and differentiation into macrophages Macrophages activation Synthesis of cytokines IL-1, IL-6, TNF- α by the macrophages Oxidative stress Activation of NF-k. B and Biphasic regulation of the : transcription factors by NO Low levels activate, high levels shut down AP-1 (? ) transcription factors Upregulation of stress and inflammation genes including i. NOS NO, ONOO- RNS Ubiquitin- proteasome- dependent proteolysis Lysosomal proteolysis Ca+2 dependent proteolysis Increased muscle wasting
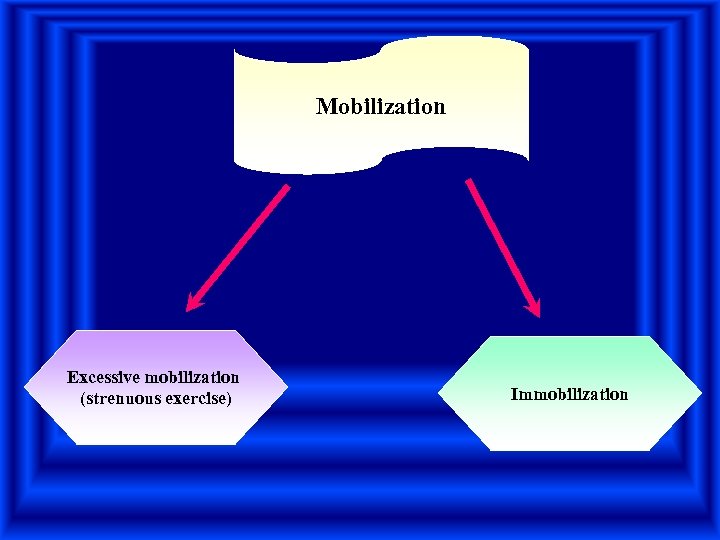 Mobilization Excessive mobilization (strenuous exercise) Immobilization
Mobilization Excessive mobilization (strenuous exercise) Immobilization
 In vivo model: Immobilized young and old rats
In vivo model: Immobilized young and old rats
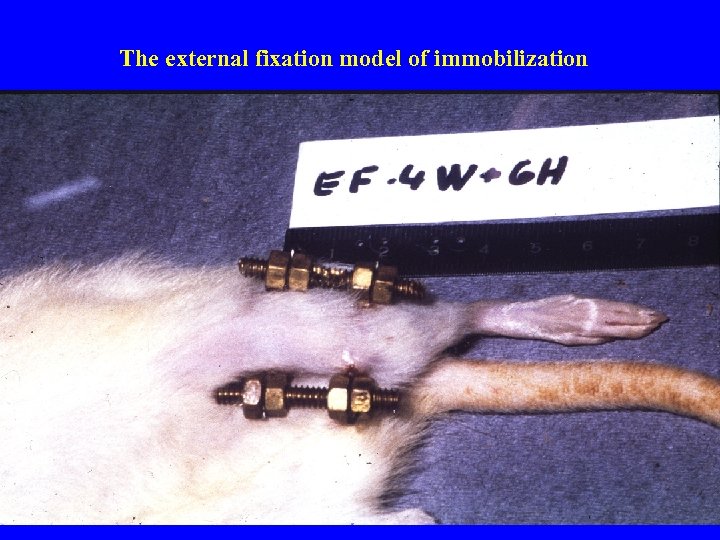 The external fixation model of immobilization
The external fixation model of immobilization
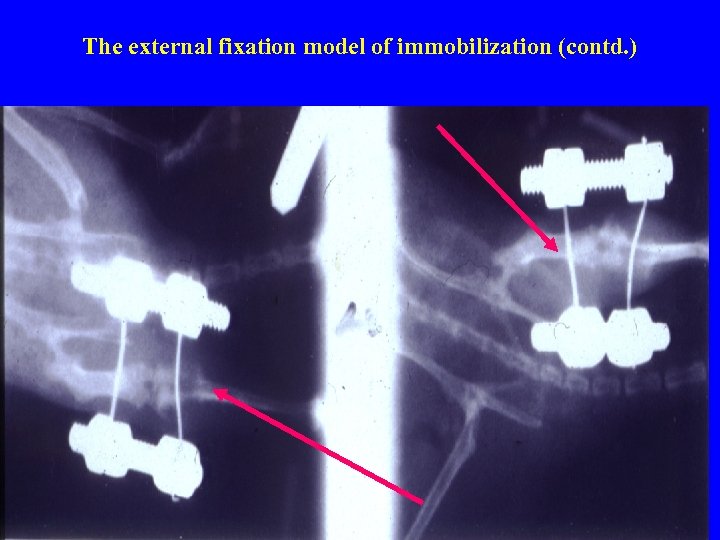 The external fixation model of immobilization (contd. )
The external fixation model of immobilization (contd. )
 Experimental design v 6 -8 months old female Wistar rats (250 -300 gr) and 24 months old female Wistar rats (300 -350 gr) v Immobilization periods : one, two, three and four weeks v Right limbs were immobilized, left limbs served as controls v At the end of each immobilization period the muscles were removed for biochemical and histological studies
Experimental design v 6 -8 months old female Wistar rats (250 -300 gr) and 24 months old female Wistar rats (300 -350 gr) v Immobilization periods : one, two, three and four weeks v Right limbs were immobilized, left limbs served as controls v At the end of each immobilization period the muscles were removed for biochemical and histological studies
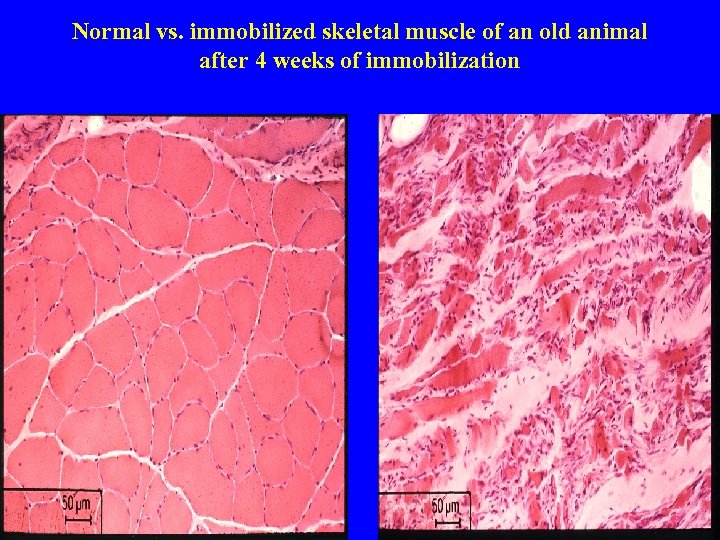 Normal vs. immobilized skeletal muscle of an old animal after 4 weeks of immobilization
Normal vs. immobilized skeletal muscle of an old animal after 4 weeks of immobilization
 The activation of various muscle protein degradation systems in immobilized animals
The activation of various muscle protein degradation systems in immobilized animals
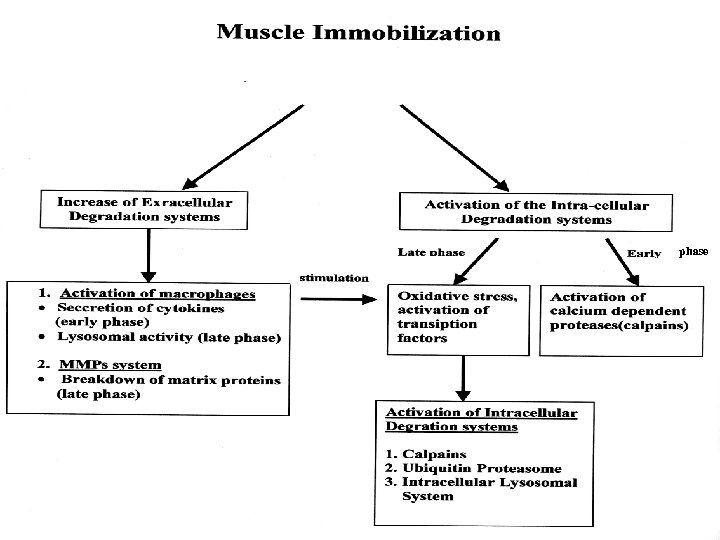
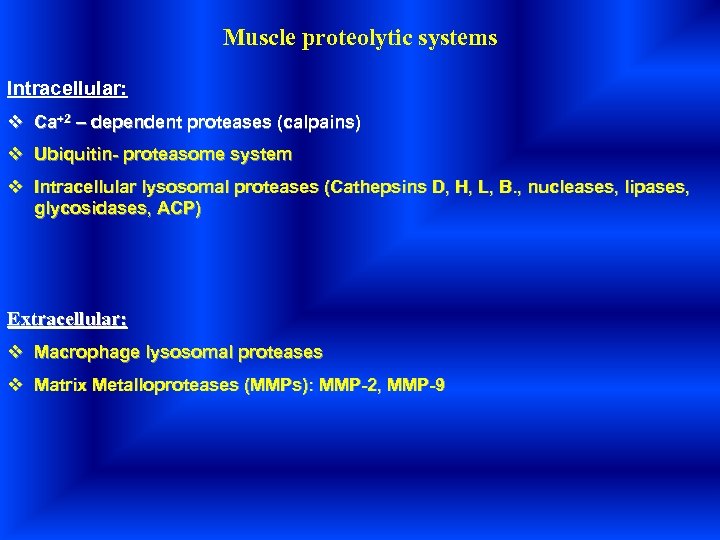 Muscle proteolytic systems Intracellular: v Ca+2 – dependent proteases (calpains) v Ubiquitin- proteasome system v Intracellular lysosomal proteases (Cathepsins D, H, L, B. , nucleases, lipases, glycosidases, ACP) Extracellular: v Macrophage lysosomal proteases v Matrix Metalloproteases (MMPs): MMP-2, MMP-9
Muscle proteolytic systems Intracellular: v Ca+2 – dependent proteases (calpains) v Ubiquitin- proteasome system v Intracellular lysosomal proteases (Cathepsins D, H, L, B. , nucleases, lipases, glycosidases, ACP) Extracellular: v Macrophage lysosomal proteases v Matrix Metalloproteases (MMPs): MMP-2, MMP-9
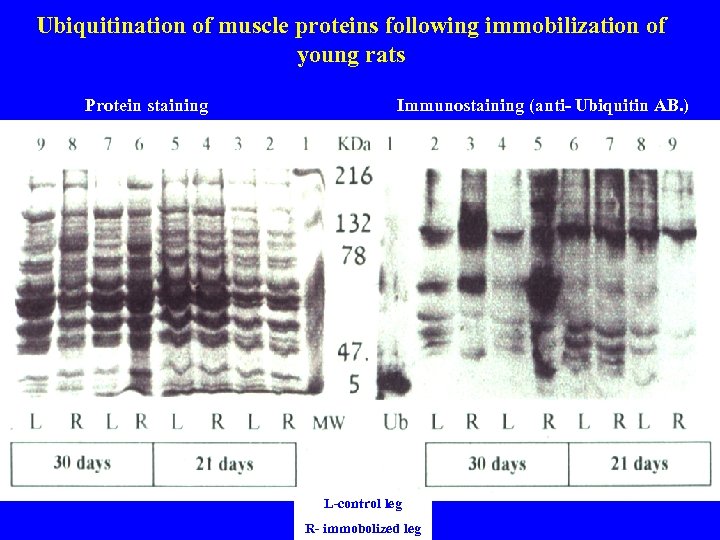 Ubiquitination of muscle proteins following immobilization of young rats Protein staining Immunostaining (anti- Ubiquitin AB. ) L-control leg R- immobolized leg
Ubiquitination of muscle proteins following immobilization of young rats Protein staining Immunostaining (anti- Ubiquitin AB. ) L-control leg R- immobolized leg
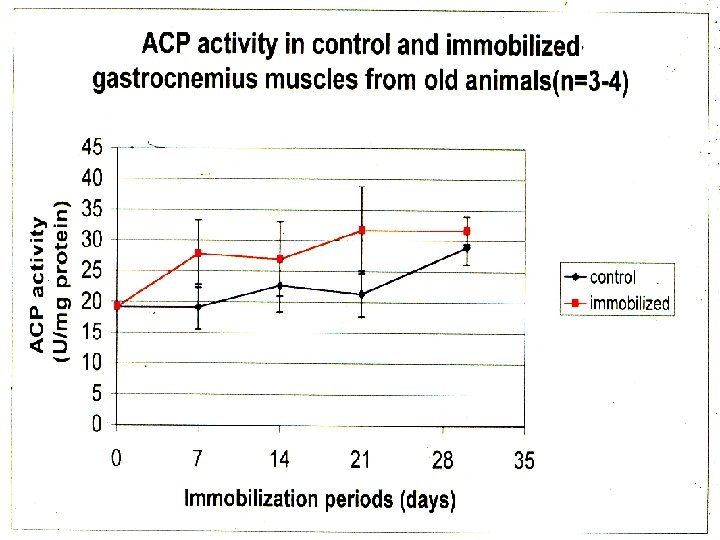
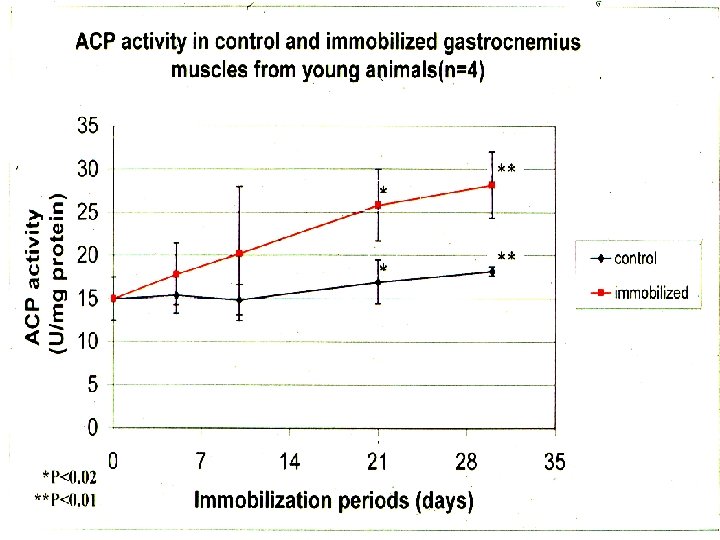 *
*
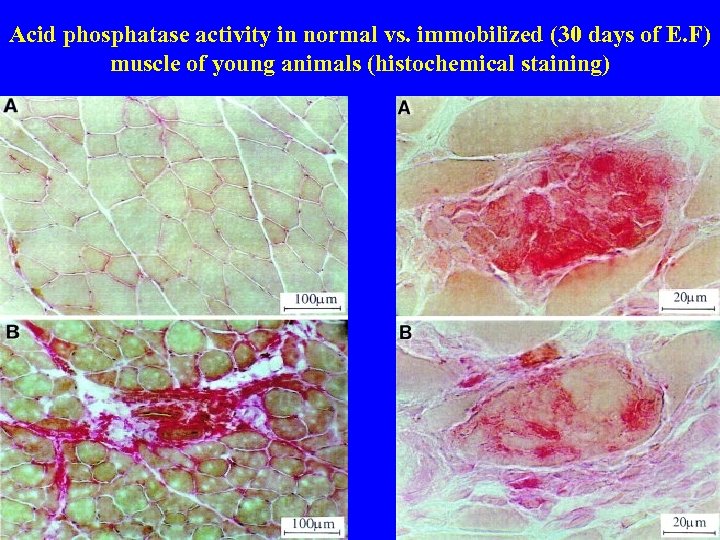 Acid phosphatase activity in normal vs. immobilized (30 days of E. F) muscle of young animals (histochemical staining)
Acid phosphatase activity in normal vs. immobilized (30 days of E. F) muscle of young animals (histochemical staining)
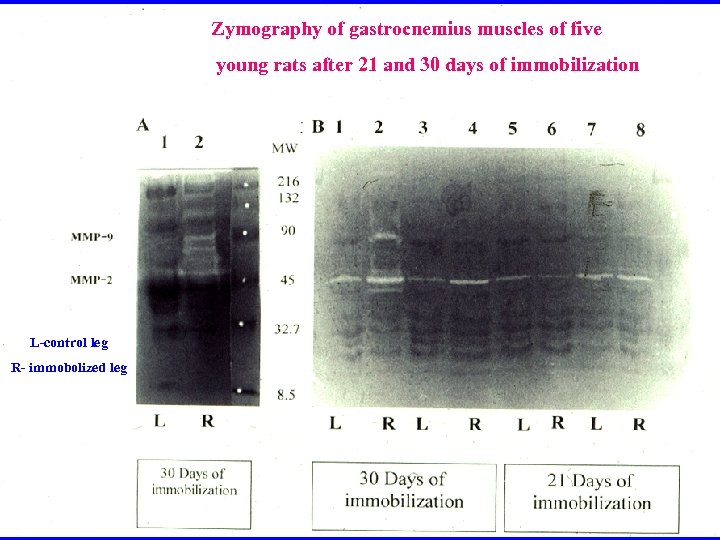 Zymography of gastrocnemius muscles of five young rats after 21 and 30 days of immobilization L-control leg R- immobolized leg
Zymography of gastrocnemius muscles of five young rats after 21 and 30 days of immobilization L-control leg R- immobolized leg
 Observations In the slow phase of muscle atrophy due to limb immobilization, the kinetics of activation of the extracellular and the intracellular degradation systems are very similar.
Observations In the slow phase of muscle atrophy due to limb immobilization, the kinetics of activation of the extracellular and the intracellular degradation systems are very similar.
 Conclusion There appears to be a link between the activation of the extracellular and intracellular proteolytic systems
Conclusion There appears to be a link between the activation of the extracellular and intracellular proteolytic systems
 phase
phase
 The slow phase of muscle breakdown due to immobilization (2 -30 days) Infiltration of monocytes and differentiation into macrophages Macrophages activation Synthesis of cytokines IL-1, IL-6, TNF- α by the macrophages Oxidative stress Activation of NF-k. B and Biphasic regulation of the : transcription factors by NO Low levels activate, high levels shut down AP-1 (? ) transcription factors Upregulation of stress and inflammation genes including i. NOS NO, ONOORNS Ubiquitin- proteasome- dependent proteolysis Lysosomal proteolysis Ca+2 dependent proteolysis Increased muscle wasting
The slow phase of muscle breakdown due to immobilization (2 -30 days) Infiltration of monocytes and differentiation into macrophages Macrophages activation Synthesis of cytokines IL-1, IL-6, TNF- α by the macrophages Oxidative stress Activation of NF-k. B and Biphasic regulation of the : transcription factors by NO Low levels activate, high levels shut down AP-1 (? ) transcription factors Upregulation of stress and inflammation genes including i. NOS NO, ONOORNS Ubiquitin- proteasome- dependent proteolysis Lysosomal proteolysis Ca+2 dependent proteolysis Increased muscle wasting
 9 th Annual Meeting of The Oxygen Society San Antonio , TX, U. S. A Nov. 20 -24, 2002
9 th Annual Meeting of The Oxygen Society San Antonio , TX, U. S. A Nov. 20 -24, 2002
 Acknowledgements • • • Eli Carmeli, Ph. D Raymond Coleman, Ph. D Ophir Menashe, MSc Marina Bar Shai, BSc Erez Hasnis, BSc Pessia Shantzer Bilha Pinkhasi Shoshan Perek Yotam Shkedi
Acknowledgements • • • Eli Carmeli, Ph. D Raymond Coleman, Ph. D Ophir Menashe, MSc Marina Bar Shai, BSc Erez Hasnis, BSc Pessia Shantzer Bilha Pinkhasi Shoshan Perek Yotam Shkedi


