1bd03241bfd4bf0db6096b8da3a040f3.ppt
- Количество слайдов: 85
Molecular Geometry and Bonding Theories

Two Simple Theories of Covalent Bonding n Valence Shell Electron Pair Repulsion Theory ¨ VSEPR ¨ R. n J. Gillespie - 1950’s Valence Bond Theory ¨ Hybridized orbitals ¨ L. Pauling - 1930’s & 40’s
Stereochemistry n Study of the 3 dimensional shapes of molecules ¨ TWO MODELS ¨ VSEPR Theory ¨ Valence Bond Theory n Some questions to examine: Why are we interested in shapes? What role does molecular shape play in life? How do we determine molecular shapes? How do we predict molecular shapes?
Determining Molecular Structure n Draw the Lewis dot structure identify central atom Count # of regions of high electron density on central atom n VSEPR tells the geometry around central atom n
Determining Molecular Structure Identify lone pair effect on ideal molecular geometry n Repeat procedure for more than one central atom n Determine polarity from entire molecular geometry n ¨ electronegativity differences

VSEPR Theory regions of high electron density around the central atom go as far apart as possible to minimize repulsions n five basic shapes n ¨ based n on # of regions of high electron density several modifications of these five basic shapes will also be examined
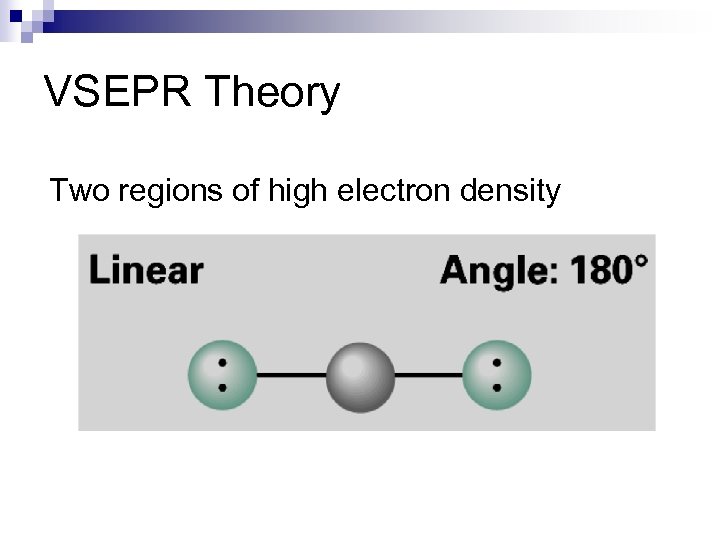
VSEPR Theory Two regions of high electron density
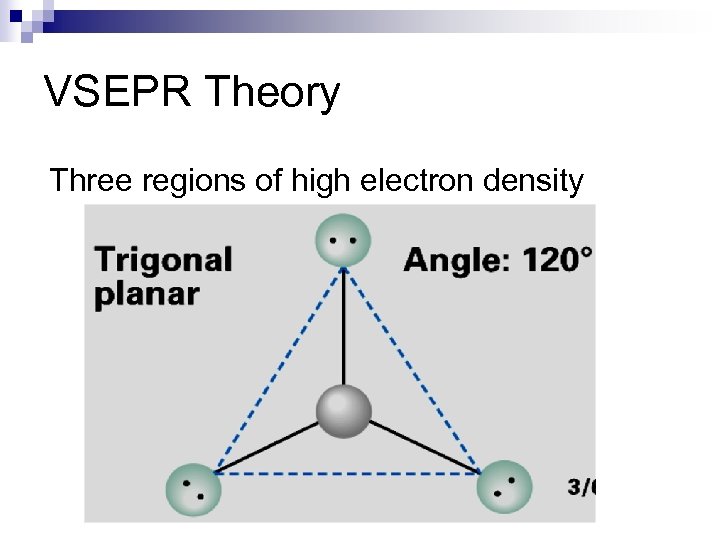
VSEPR Theory Three regions of high electron density
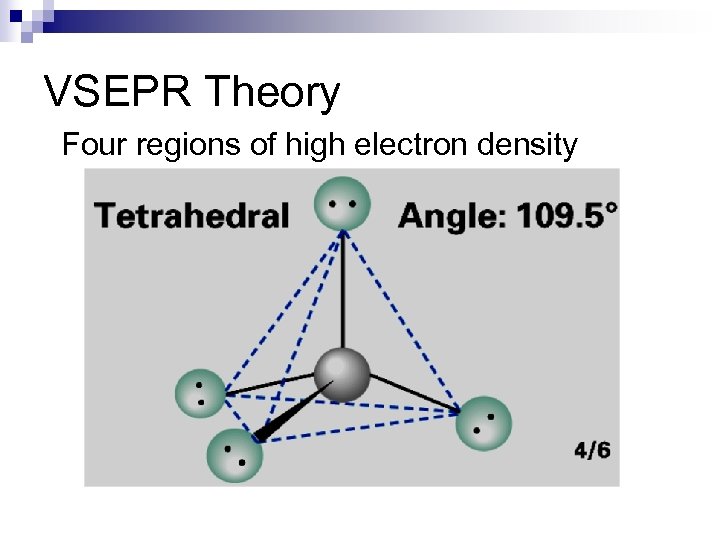
VSEPR Theory Four regions of high electron density
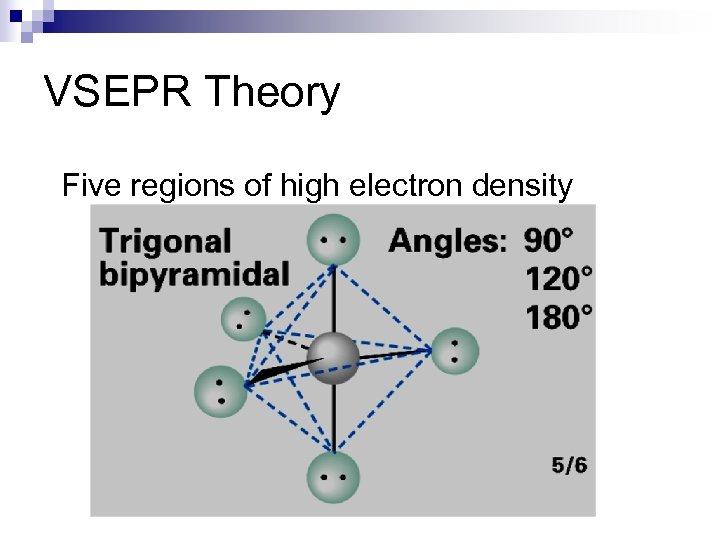
VSEPR Theory Five regions of high electron density
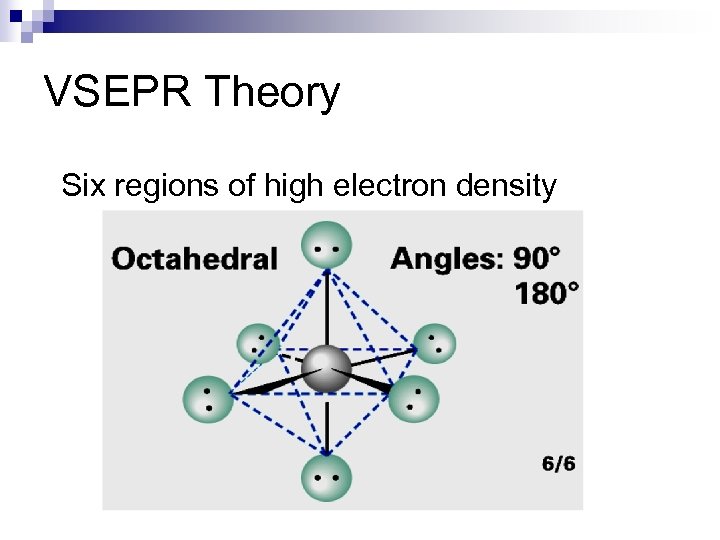
VSEPR Theory Six regions of high electron density
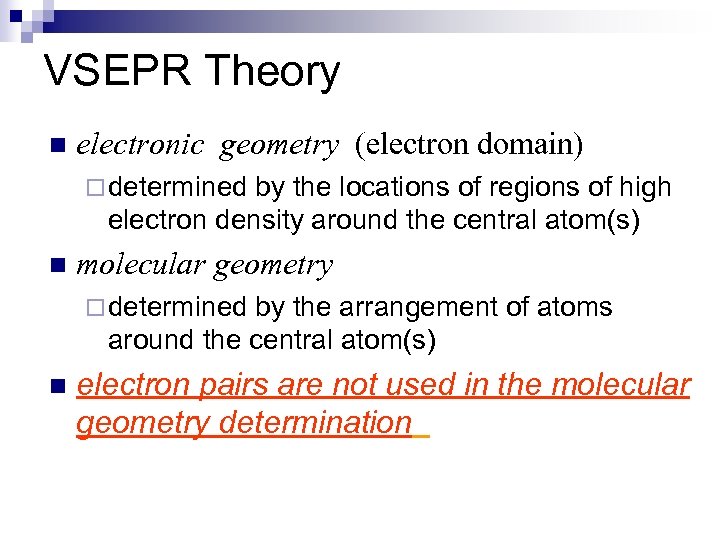
VSEPR Theory n electronic geometry (electron domain) ¨ determined by the locations of regions of high electron density around the central atom(s) n molecular geometry ¨ determined by the arrangement of atoms around the central atom(s) n electron pairs are not used in the molecular geometry determination
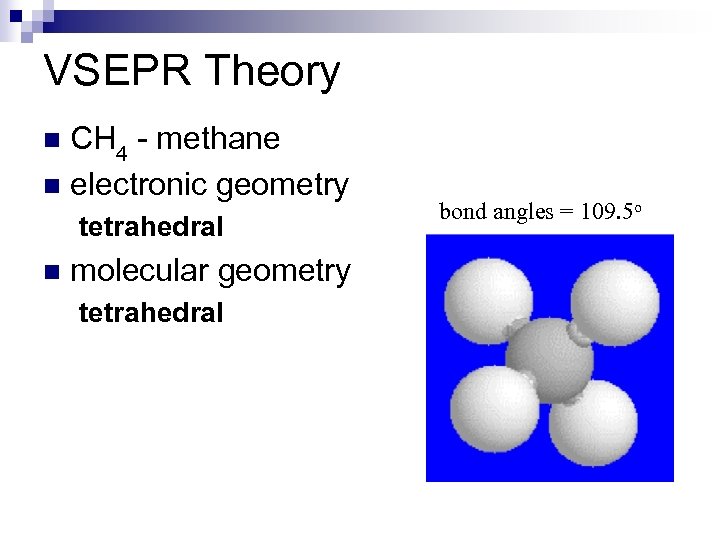
VSEPR Theory CH 4 - methane n electronic geometry n tetrahedral n molecular geometry tetrahedral bond angles = 109. 5 o
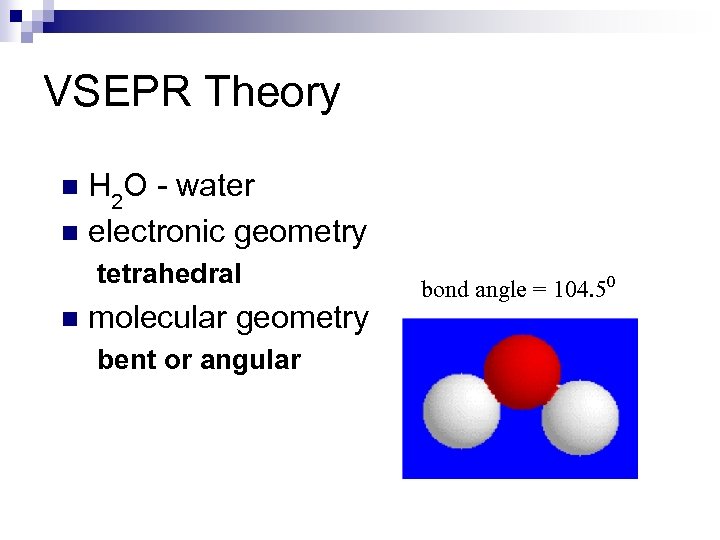
VSEPR Theory H 2 O - water n electronic geometry n tetrahedral n molecular geometry bent or angular bond angle = 104. 50

VSEPR Theory lone pairs of electrons (unshared pairs) require more volume than shared pairs n there is an ordering of repulsions of electrons around central atom n

VSEPR Theory 1 2 3 n lone pair to lone pair repulsion is strongest lone pair to bonding pair repulsion is intermediate bonding pair to bonding pair repulsion is weakest mnemonic for repulsion strengths lp/lp > lp/bp > bp/bp n lone pair to lone pair repulsion is why bond angles in water are less than 109. 50
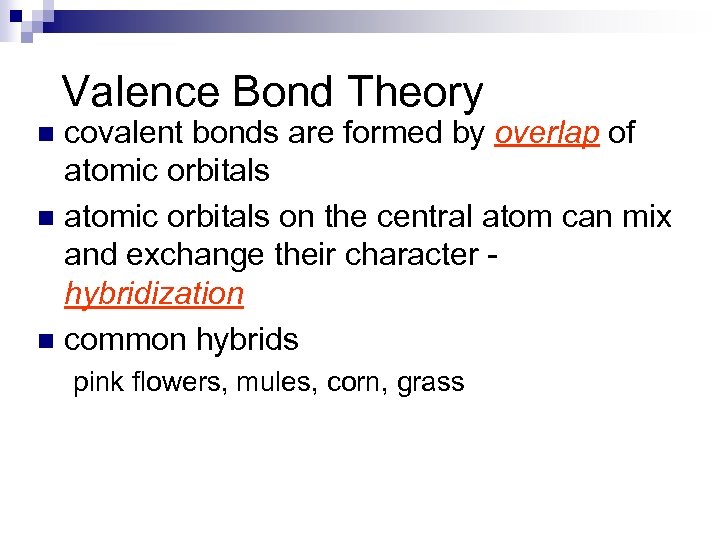
Valence Bond Theory covalent bonds are formed by overlap of atomic orbitals n atomic orbitals on the central atom can mix and exchange their character hybridization n common hybrids n pink flowers, mules, corn, grass
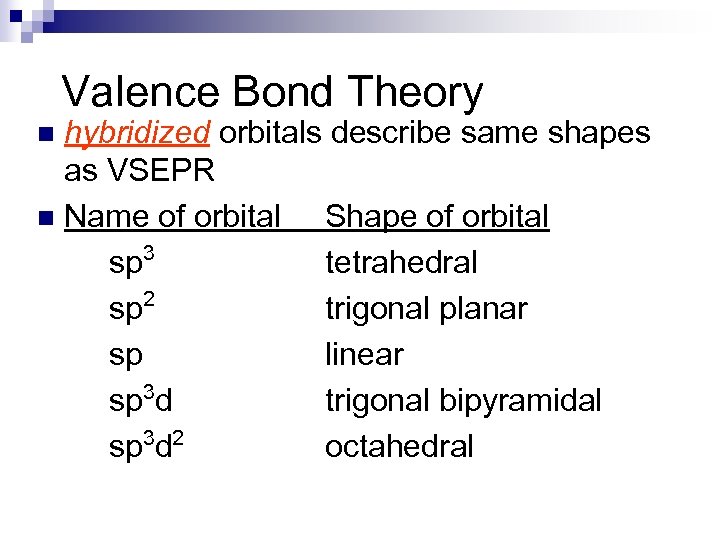
Valence Bond Theory hybridized orbitals describe same shapes as VSEPR n Name of orbital Shape of orbital sp 3 tetrahedral sp 2 trigonal planar sp linear sp 3 d trigonal bipyramidal sp 3 d 2 octahedral n
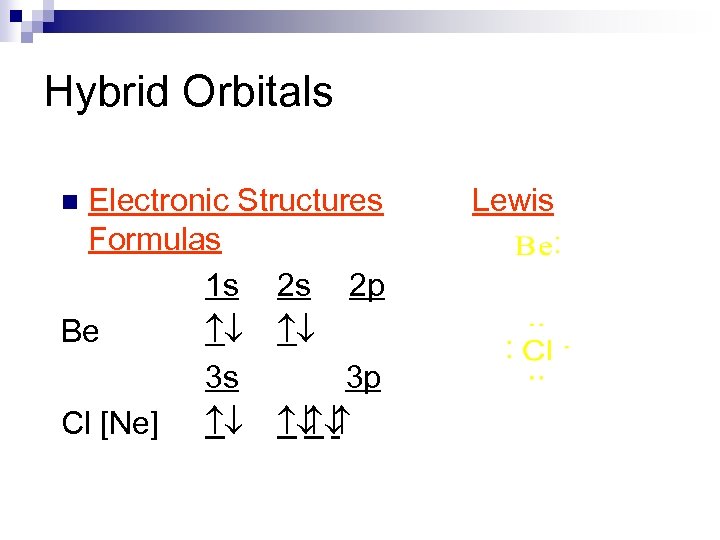
Hybrid Orbitals Electronic Structures Formulas 1 s 2 s 2 p Be ¯ ¯ 3 s 3 p Cl [Ne] ¯ ¯ ¯ n Lewis
Hybrid Orbitals n Dot Formula Electronic Geometry
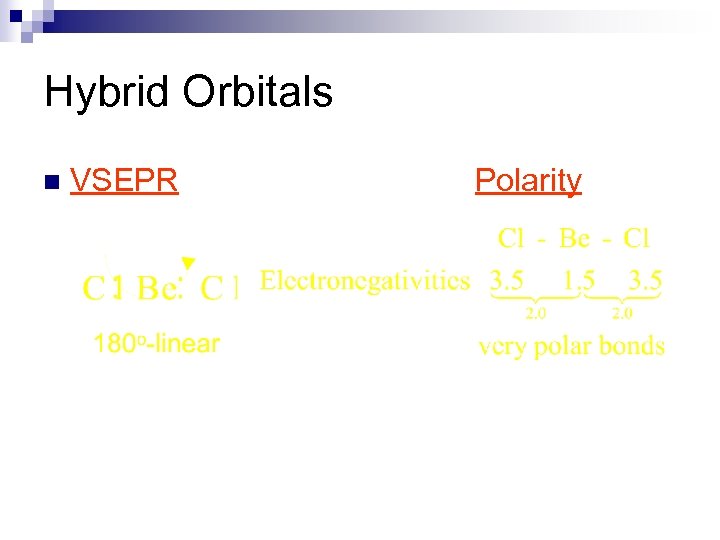
Hybrid Orbitals n VSEPR Polarity
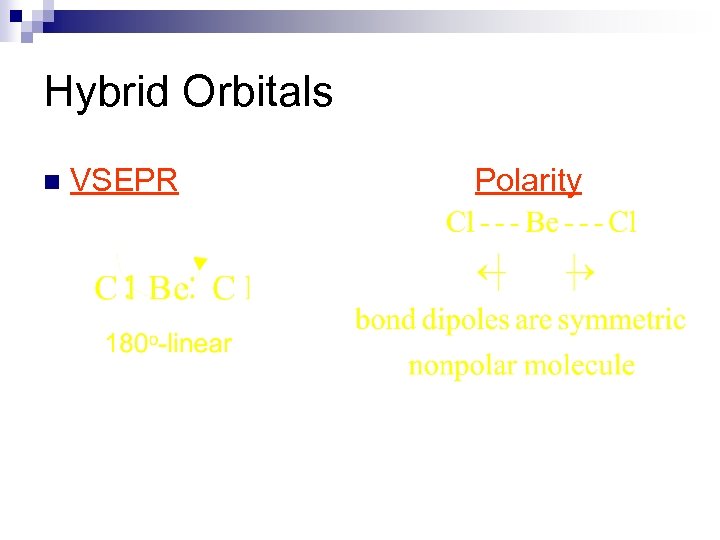
Hybrid Orbitals n VSEPR Polarity
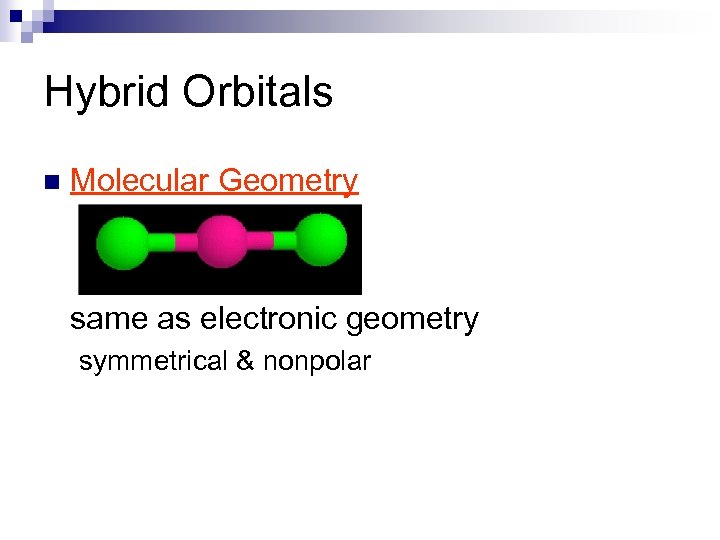
Hybrid Orbitals n Molecular Geometry same as electronic geometry symmetrical & nonpolar
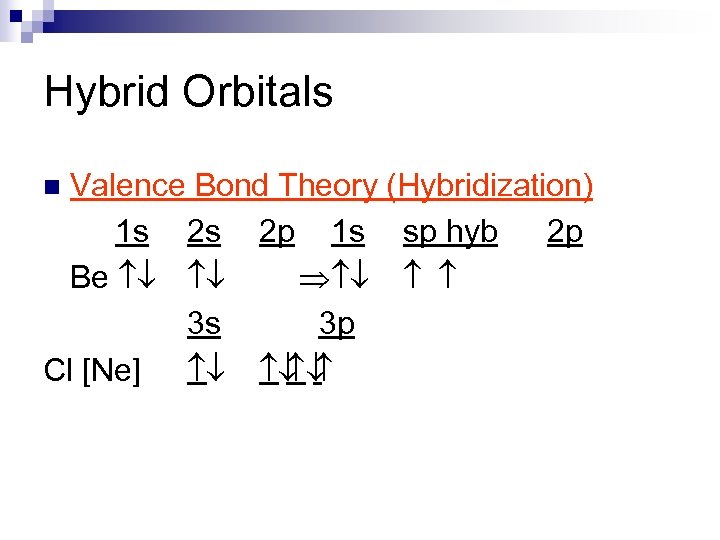
Hybrid Orbitals Valence Bond Theory (Hybridization) 1 s 2 s 2 p 1 s sp hyb 2 p Be ¯ ¯ Þ ¯ 3 s 3 p Cl [Ne] ¯ ¯ ¯ n
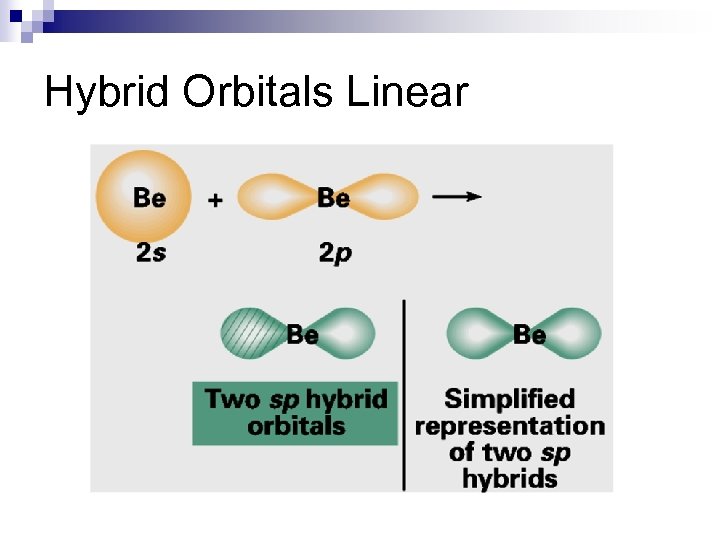
Hybrid Orbitals Linear
Hybrid Orbitals n examples BF 3, BCl 3 n all are trigonal planar, nonpolar molecules
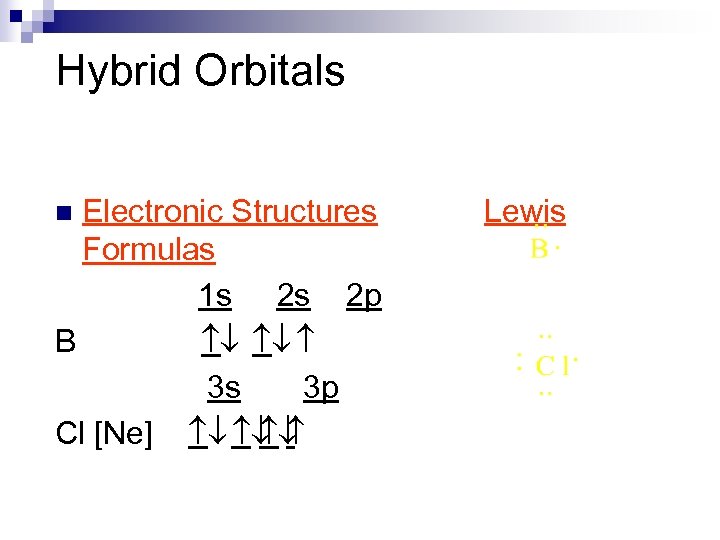
Hybrid Orbitals Electronic Structures Formulas 1 s 2 s 2 p B ¯ ¯ 3 s 3 p Cl [Ne] ¯ ¯ ¯ n Lewis
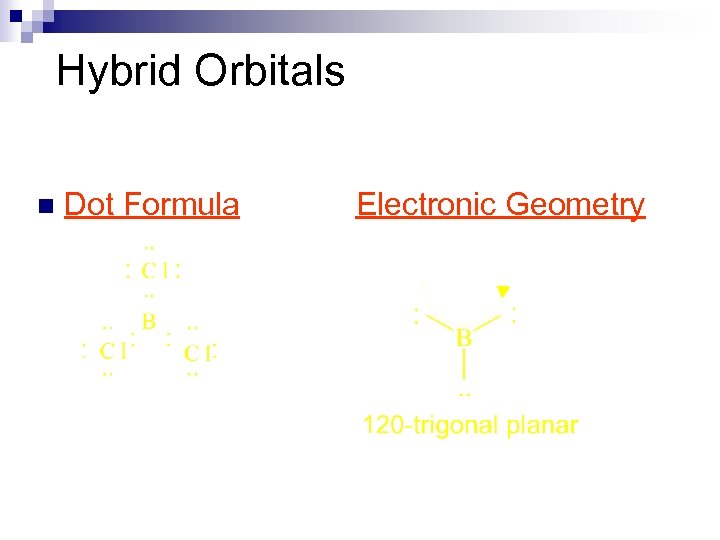
Hybrid Orbitals n Dot Formula Electronic Geometry
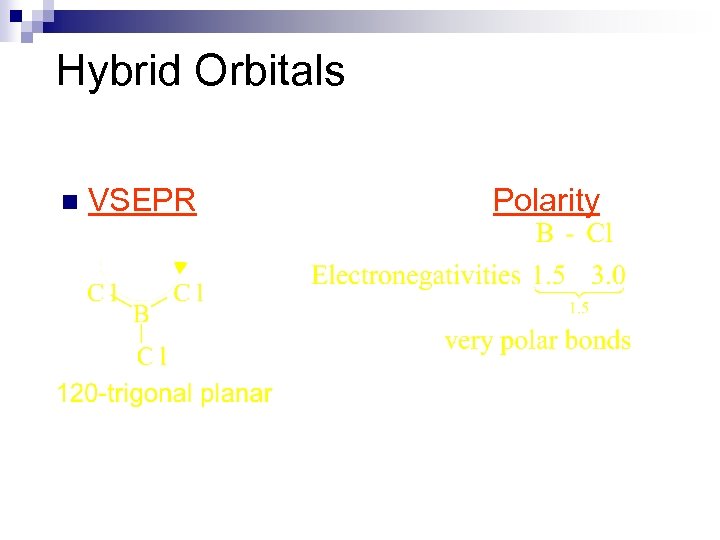
Hybrid Orbitals n VSEPR Polarity
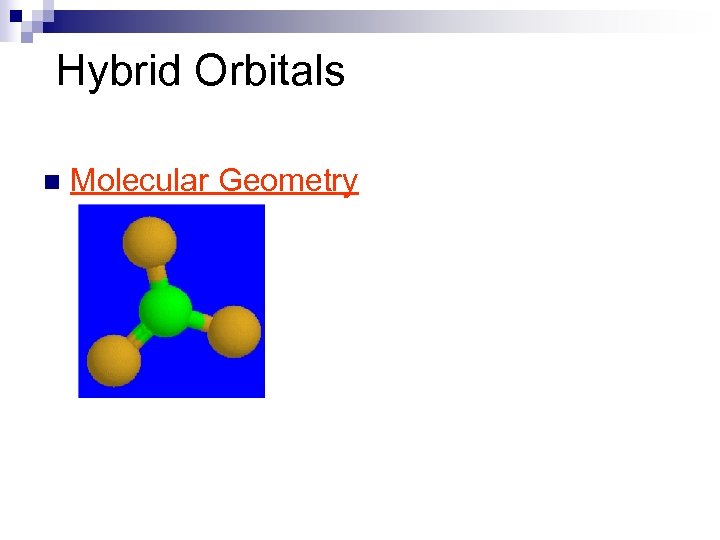
Hybrid Orbitals n Molecular Geometry
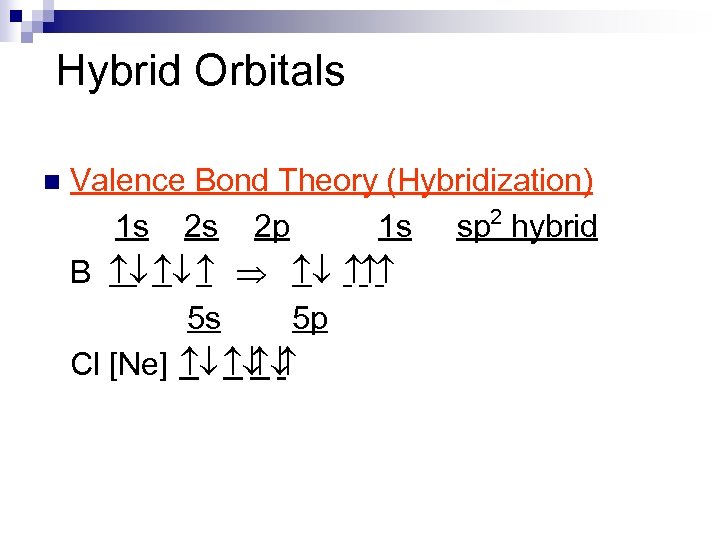
Hybrid Orbitals n Valence Bond Theory (Hybridization) 2 1 s 2 s 2 p 1 s sp hybrid B ¯ ¯ Þ ¯ 5 s 5 p Cl [Ne] ¯ ¯ ¯
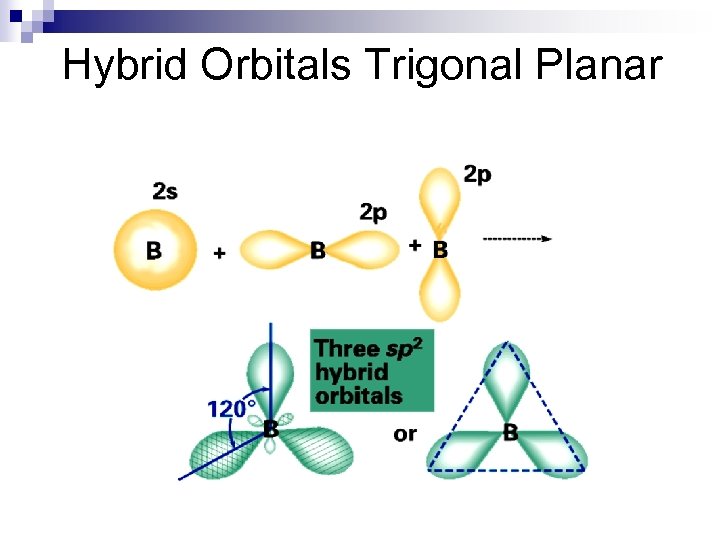
Hybrid Orbitals Trigonal Planar
Hybrid Orbitals n examples CH 4, CF 4, CCl 4, Si. H 4, Si. F 4 n all are tetrahedral, nonpolar molecules ¨ as long as they have the same 4 substituents
![Hybrid Orbitals n Electronic Structures C [He] 2 s 2 p ¯ Lewis Formulas Hybrid Orbitals n Electronic Structures C [He] 2 s 2 p ¯ Lewis Formulas](https://present5.com/presentation/1bd03241bfd4bf0db6096b8da3a040f3/image-34.jpg)
Hybrid Orbitals n Electronic Structures C [He] 2 s 2 p ¯ Lewis Formulas
![Hybrid Orbitals n Electronic Structures C [He] H 2 s 2 p ¯ 1 Hybrid Orbitals n Electronic Structures C [He] H 2 s 2 p ¯ 1](https://present5.com/presentation/1bd03241bfd4bf0db6096b8da3a040f3/image-35.jpg)
Hybrid Orbitals n Electronic Structures C [He] H 2 s 2 p ¯ 1 s Lewis Formulas
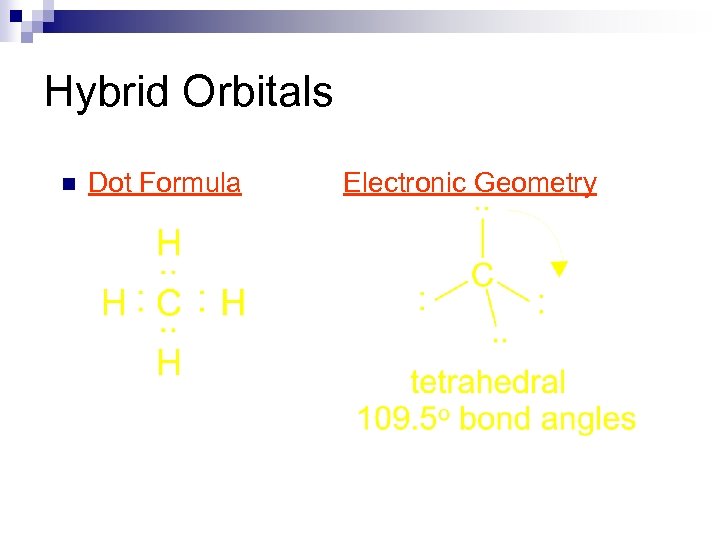
Hybrid Orbitals n Dot Formula Electronic Geometry
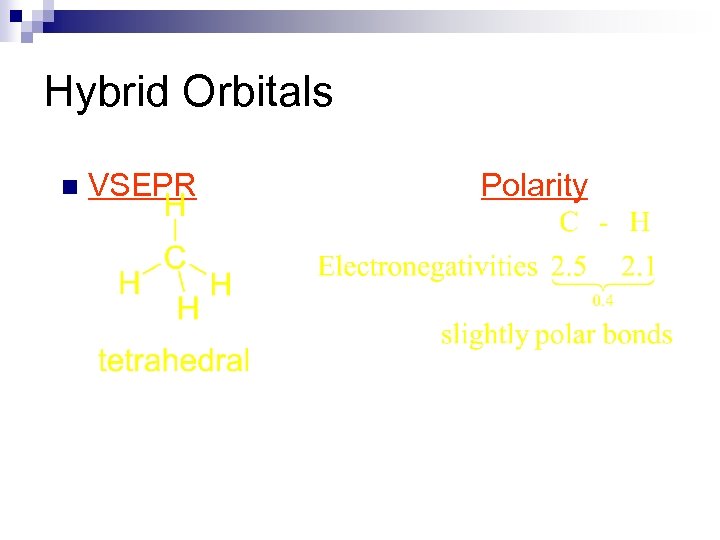
Hybrid Orbitals n VSEPR Polarity
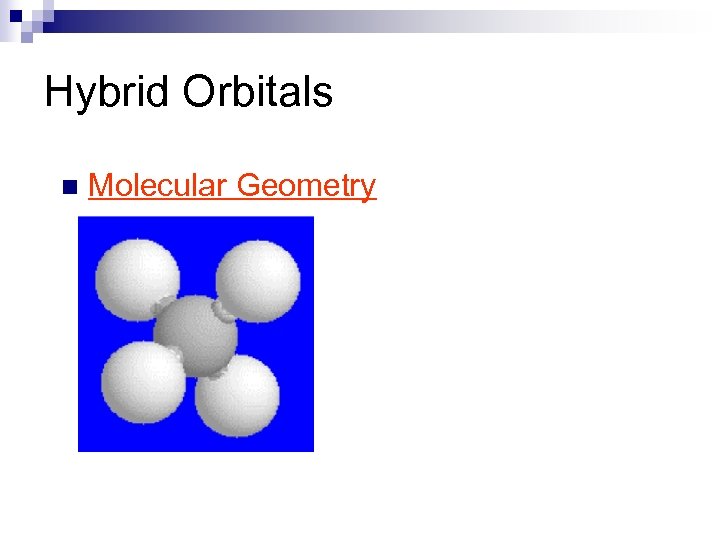
Hybrid Orbitals n Molecular Geometry
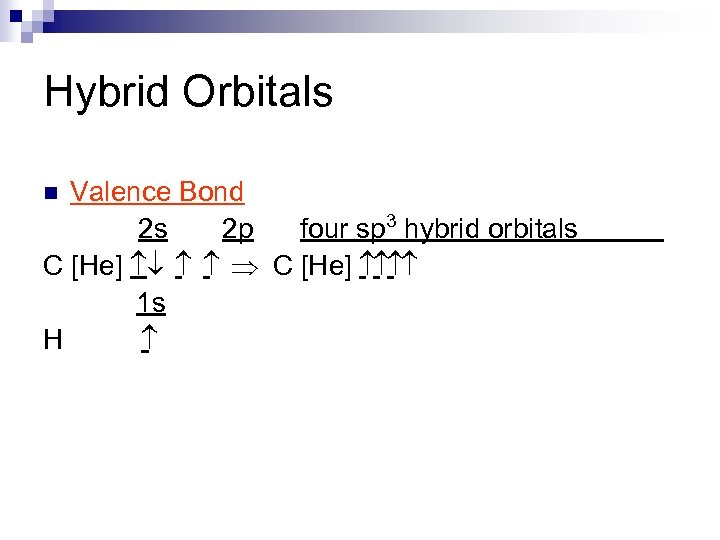
Hybrid Orbitals Valence Bond 2 s 2 p four sp 3 hybrid orbitals C [He] ¯ Þ C [He] 1 s H n
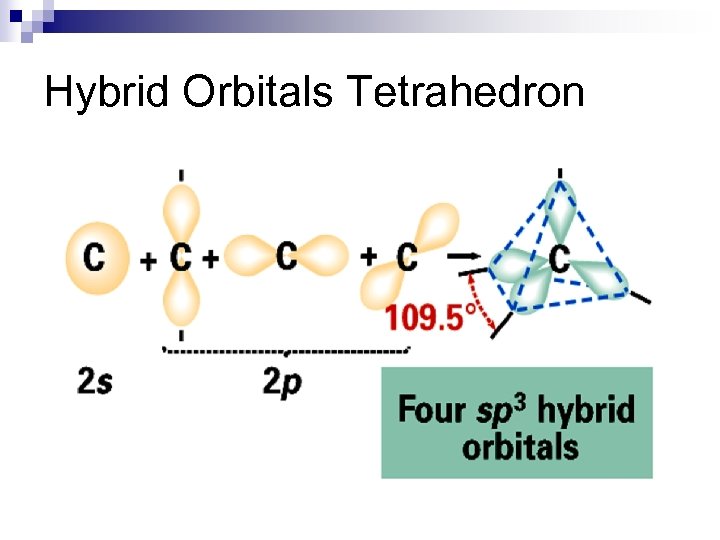
Hybrid Orbitals Tetrahedron
Hybrid Orbitals n Examples PF 5, As. F 5, PCl 5, etc. n All are trigonal bipyramidal, nonpolar molecules.
![Hybrid Orbitals n Electronic Structures 4 s 4 p As [Ar] 3 d 10 Hybrid Orbitals n Electronic Structures 4 s 4 p As [Ar] 3 d 10](https://present5.com/presentation/1bd03241bfd4bf0db6096b8da3a040f3/image-42.jpg)
Hybrid Orbitals n Electronic Structures 4 s 4 p As [Ar] 3 d 10 ¯ 2 s 2 p F [He] ¯ ¯ ¯ Lewis Formulas
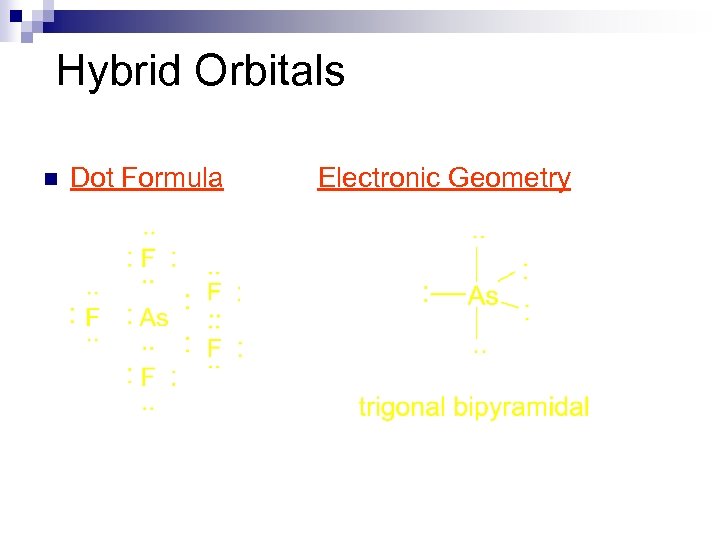
Hybrid Orbitals n Dot Formula Electronic Geometry
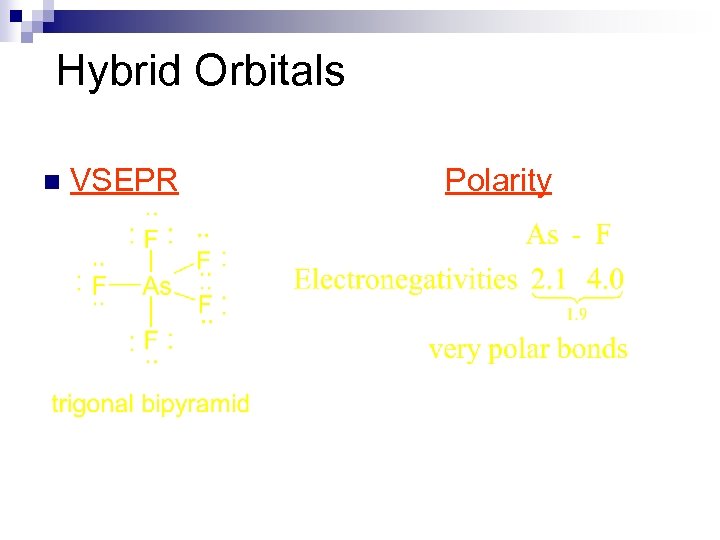
Hybrid Orbitals n VSEPR Polarity
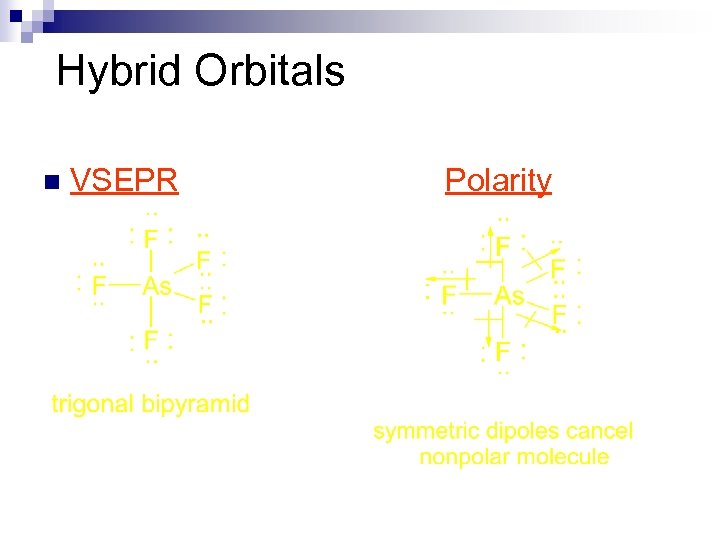
Hybrid Orbitals n VSEPR Polarity
Hybrid Orbitals n Molecular Geometry
![Hybrid Orbitals n Valence Bond (Hybridization) As [Ar] 3 d 10 4 s 4 Hybrid Orbitals n Valence Bond (Hybridization) As [Ar] 3 d 10 4 s 4](https://present5.com/presentation/1bd03241bfd4bf0db6096b8da3a040f3/image-47.jpg)
Hybrid Orbitals n Valence Bond (Hybridization) As [Ar] 3 d 10 4 s 4 p 4 d ¯ ___ ___ ___ ß five sp 3 d hybrids
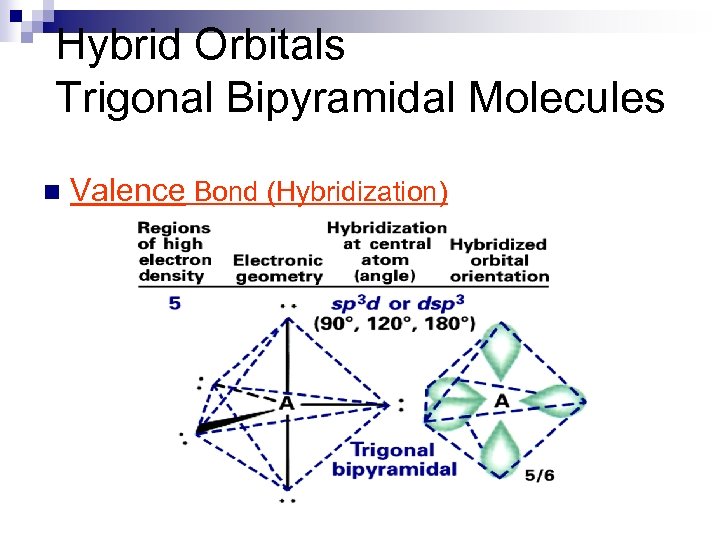
Hybrid Orbitals Trigonal Bipyramidal Molecules n Valence Bond (Hybridization)
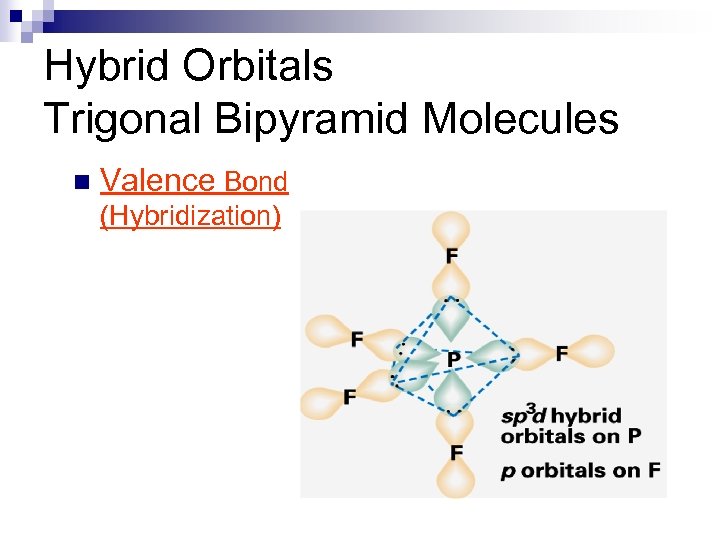
Hybrid Orbitals Trigonal Bipyramid Molecules n Valence Bond (Hybridization)
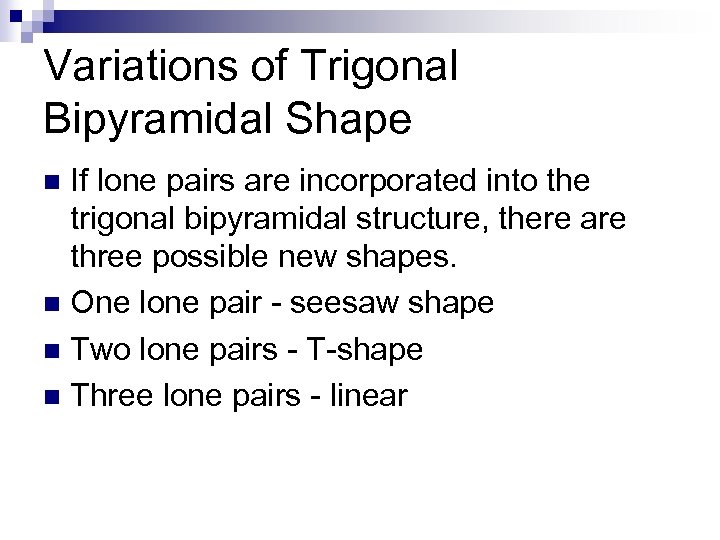
Variations of Trigonal Bipyramidal Shape If lone pairs are incorporated into the trigonal bipyramidal structure, there are three possible new shapes. n One lone pair - seesaw shape n Two lone pairs - T-shape n Three lone pairs - linear n
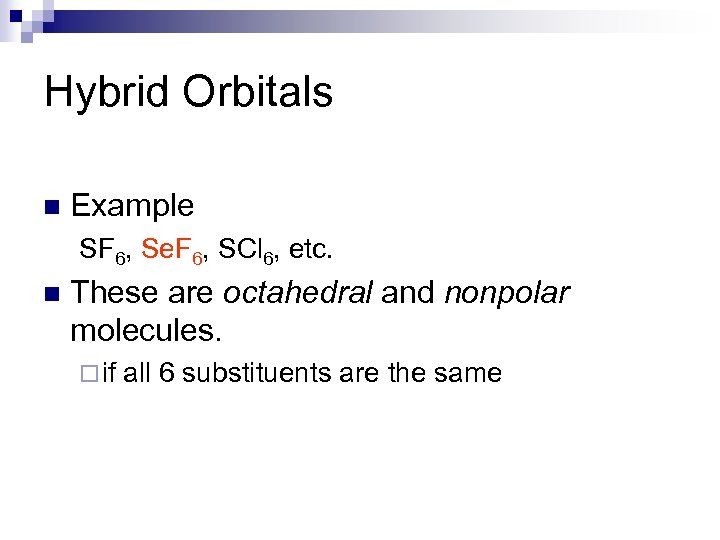
Hybrid Orbitals n Example SF 6, Se. F 6, SCl 6, etc. n These are octahedral and nonpolar molecules. ¨ if all 6 substituents are the same
![Hybrid Orbitals n Electronic Structures 4 s 4 p Se [Ar] 3 d 10 Hybrid Orbitals n Electronic Structures 4 s 4 p Se [Ar] 3 d 10](https://present5.com/presentation/1bd03241bfd4bf0db6096b8da3a040f3/image-52.jpg)
Hybrid Orbitals n Electronic Structures 4 s 4 p Se [Ar] 3 d 10 ¯ ¯ 2 s 2 p F [He] ¯ ¯ ¯ Lewis Formulas
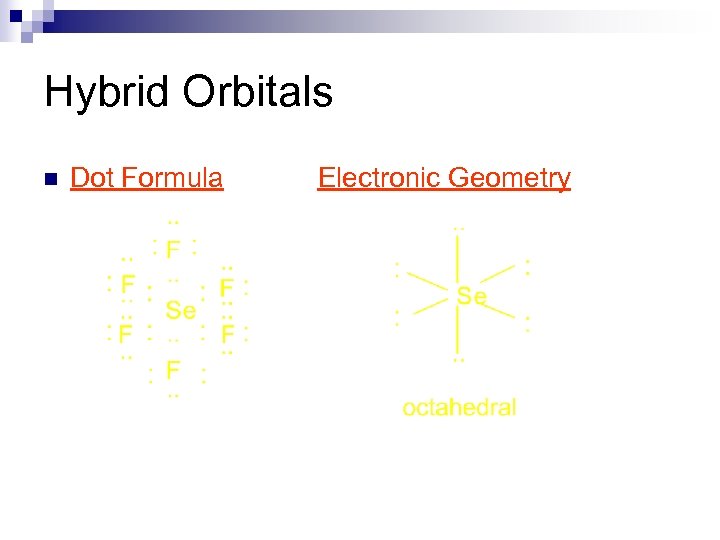
Hybrid Orbitals n Dot Formula Electronic Geometry
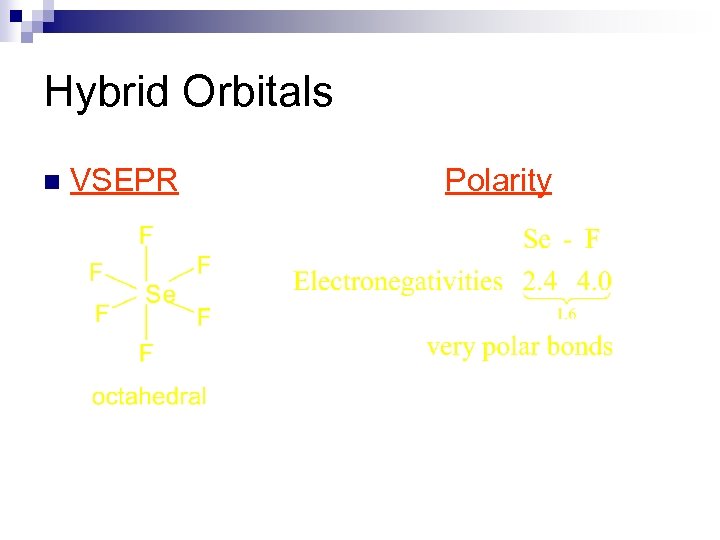
Hybrid Orbitals n VSEPR Polarity
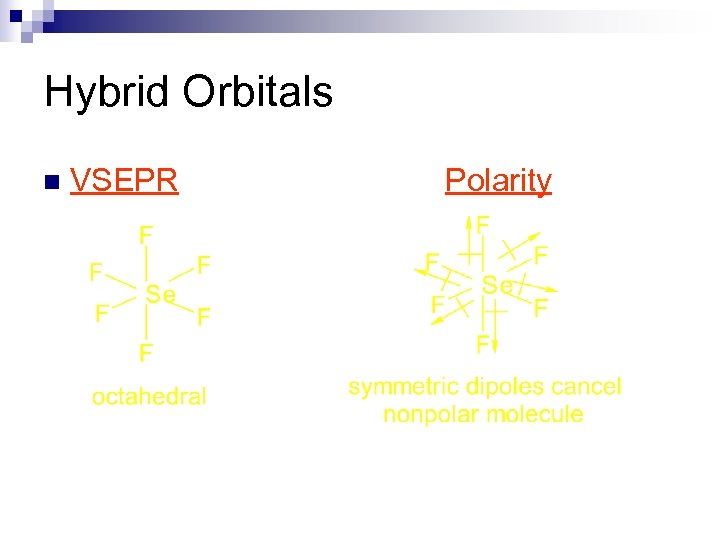
Hybrid Orbitals n VSEPR Polarity
Hybrid Orbitals n Molecular Geometry
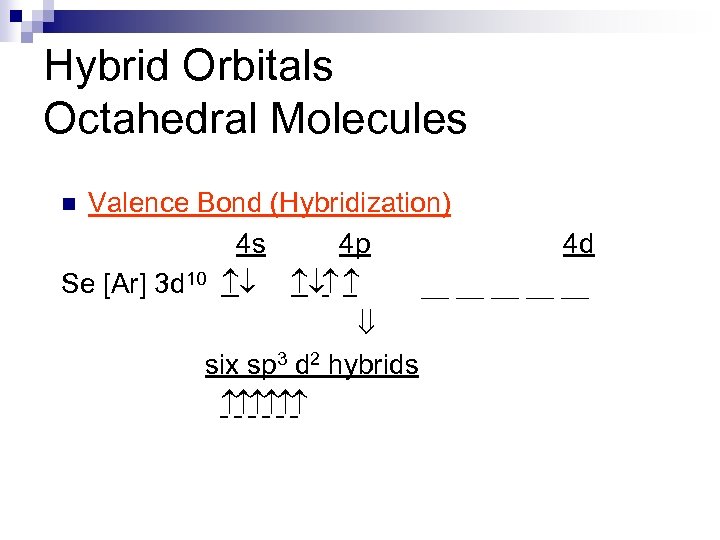
Hybrid Orbitals Octahedral Molecules Valence Bond (Hybridization) 4 s 4 p 4 d Se [Ar] 3 d 10 ¯ ¯ __ __ __ ß six sp 3 d 2 hybrids n
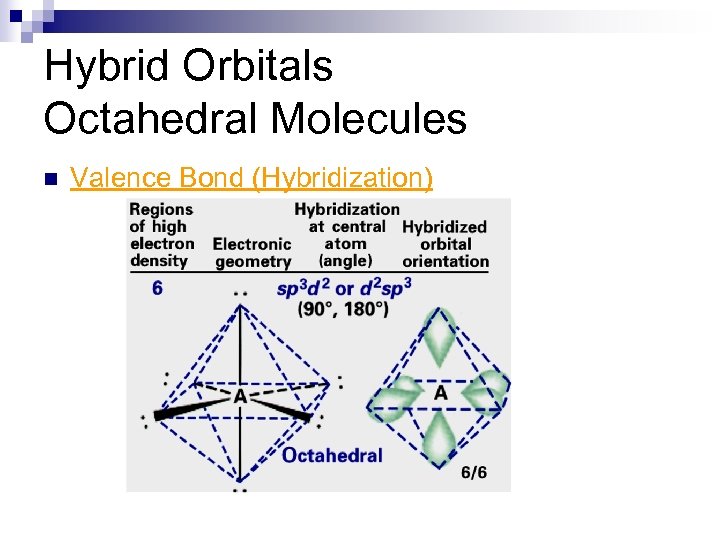
Hybrid Orbitals Octahedral Molecules n Valence Bond (Hybridization)
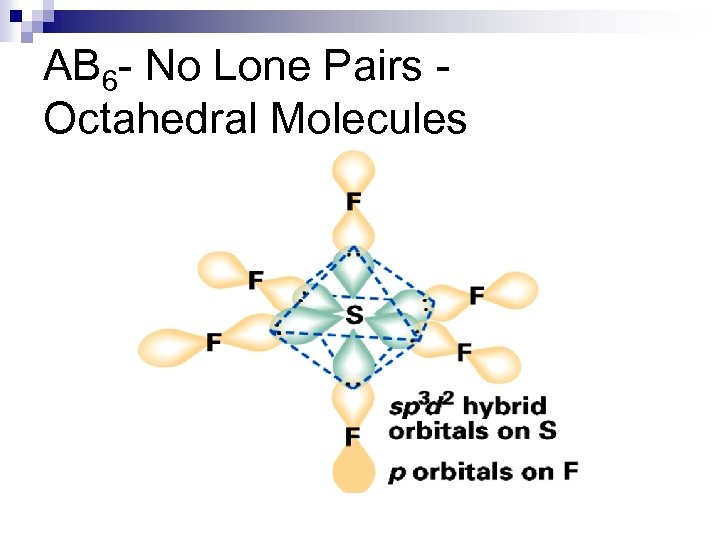
AB 6 - No Lone Pairs Octahedral Molecules
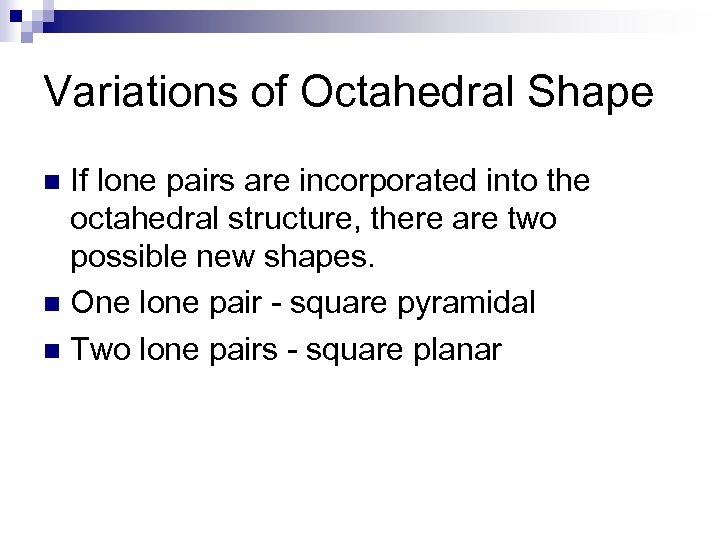
Variations of Octahedral Shape If lone pairs are incorporated into the octahedral structure, there are two possible new shapes. n One lone pair - square pyramidal n Two lone pairs - square planar n
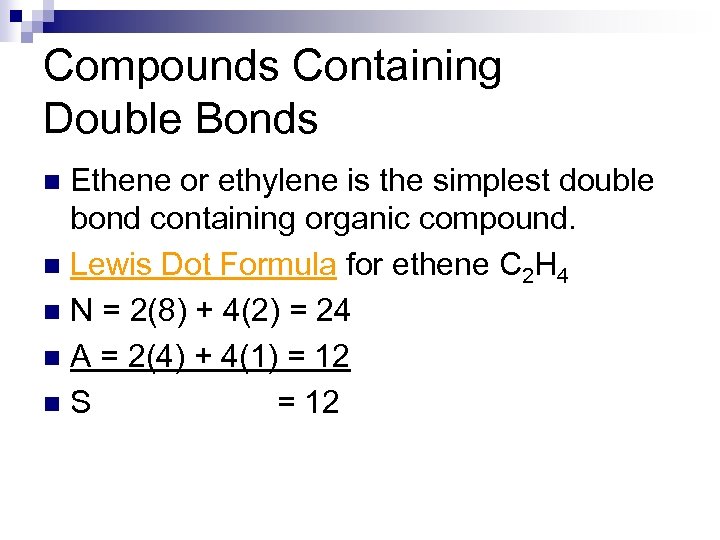
Compounds Containing Double Bonds Ethene or ethylene is the simplest double bond containing organic compound. n Lewis Dot Formula for ethene C 2 H 4 n N = 2(8) + 4(2) = 24 n A = 2(4) + 4(1) = 12 n. S = 12 n
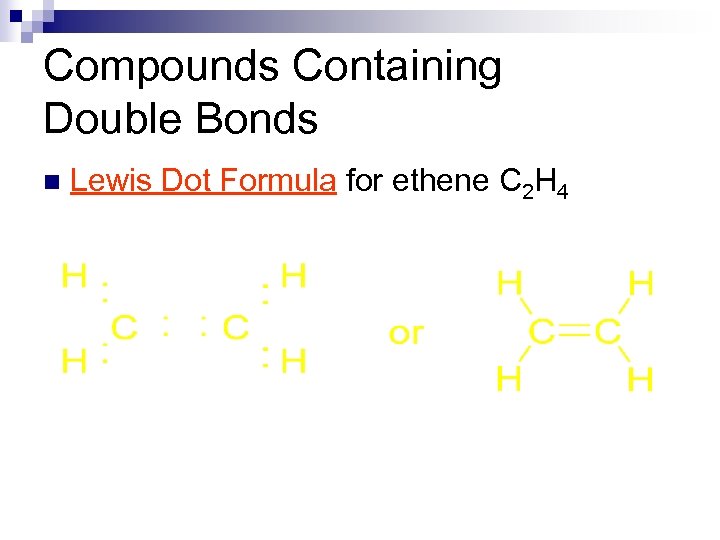
Compounds Containing Double Bonds n Lewis Dot Formula for ethene C 2 H 4
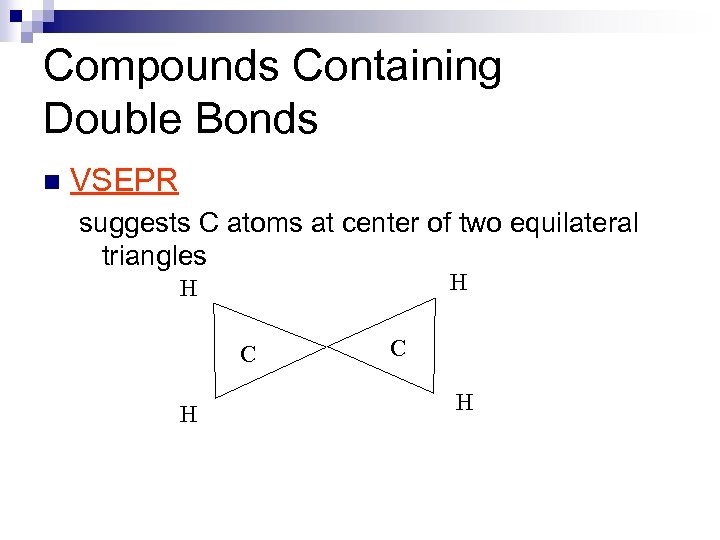
Compounds Containing Double Bonds n VSEPR suggests C atoms at center of two equilateral triangles H H C H
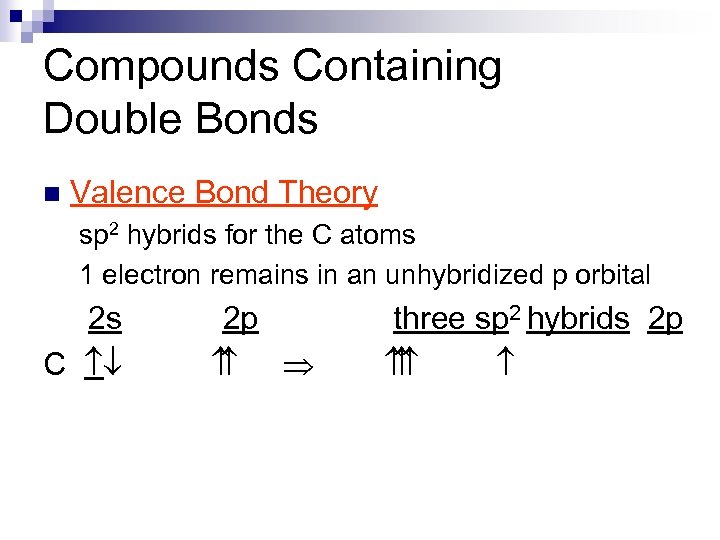
Compounds Containing Double Bonds n Valence Bond Theory sp 2 hybrids for the C atoms 1 electron remains in an unhybridized p orbital 2 s C ¯ 2 p Þ three sp 2 hybrids 2 p
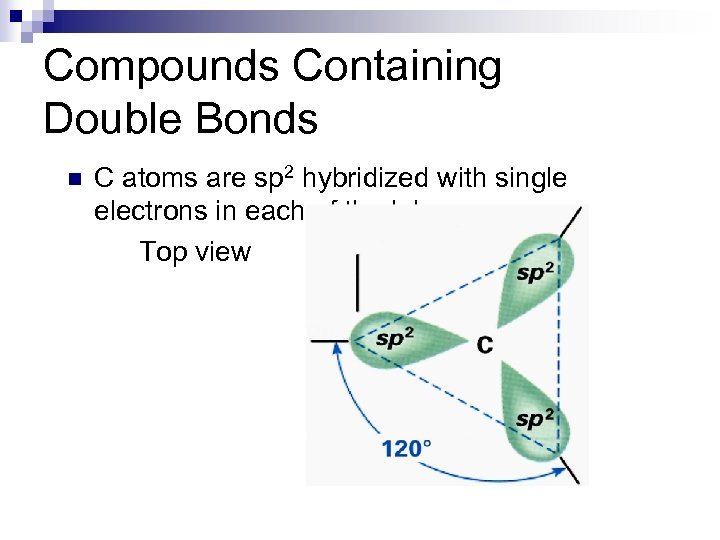
Compounds Containing Double Bonds n C atoms are sp 2 hybridized with single electrons in each of the lobes Top view
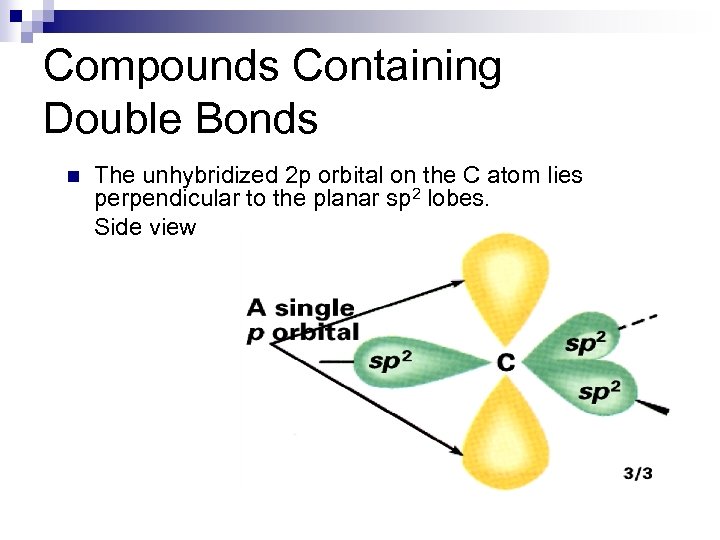
Compounds Containing Double Bonds n The unhybridized 2 p orbital on the C atom lies perpendicular to the planar sp 2 lobes. Side view
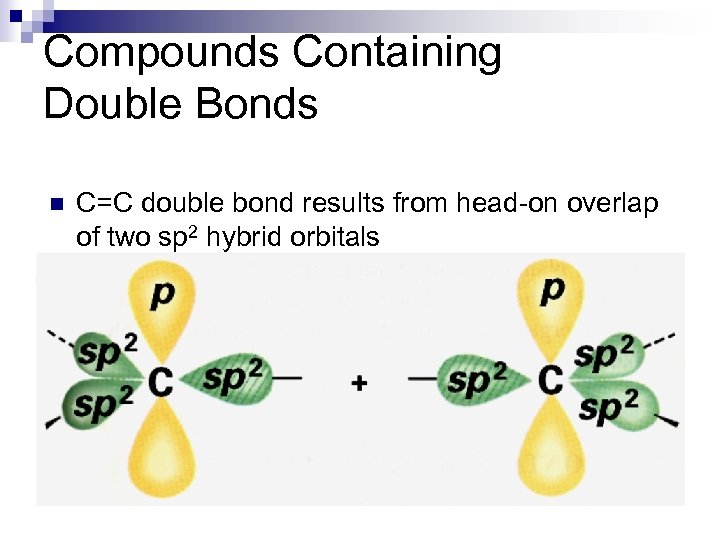
Compounds Containing Double Bonds n C=C double bond results from head-on overlap of two sp 2 hybrid orbitals
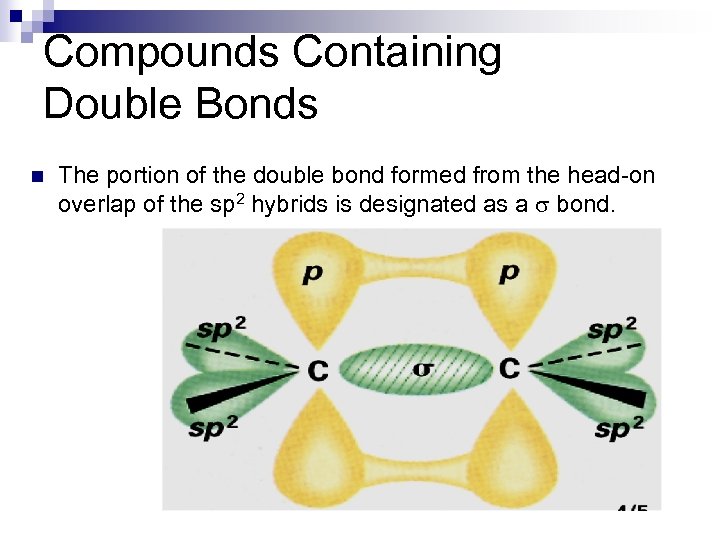
Compounds Containing Double Bonds n The portion of the double bond formed from the head-on overlap of the sp 2 hybrids is designated as a s bond.
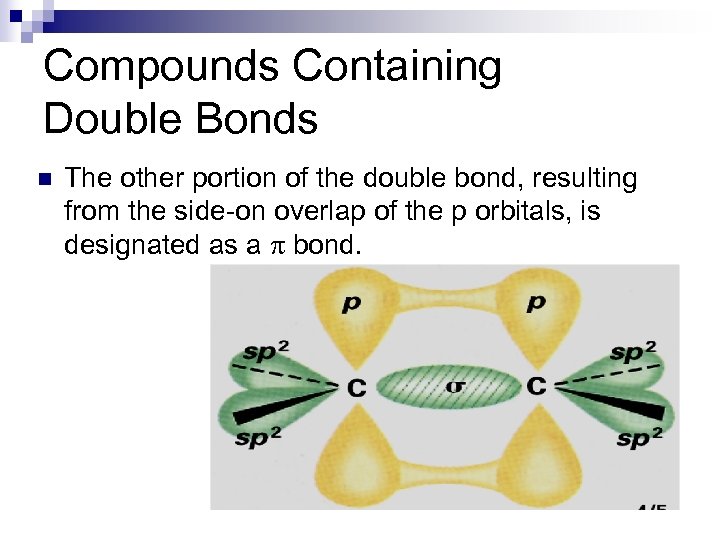
Compounds Containing Double Bonds n The other portion of the double bond, resulting from the side-on overlap of the p orbitals, is designated as a p bond.
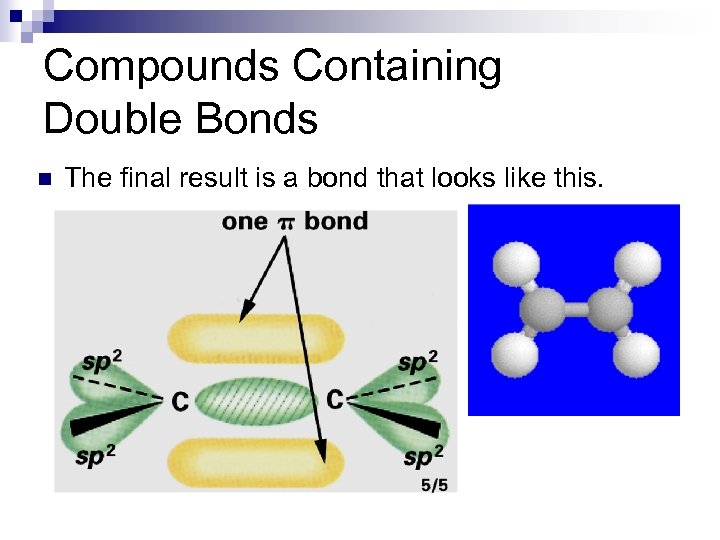
Compounds Containing Double Bonds n The final result is a bond that looks like this.
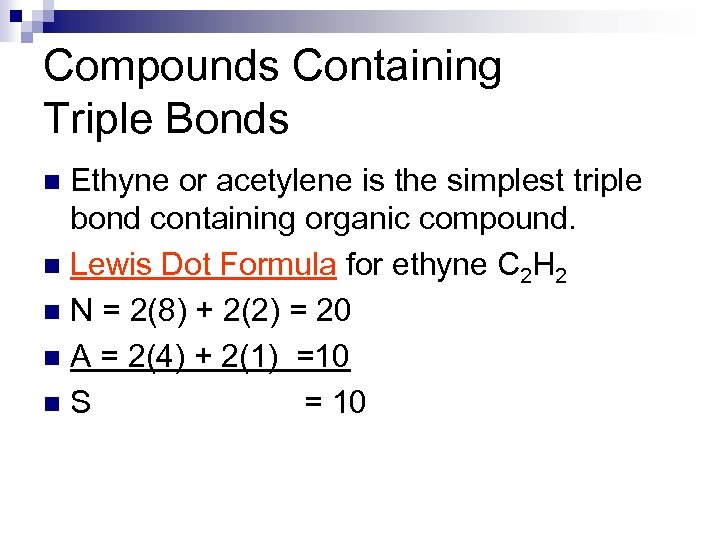
Compounds Containing Triple Bonds Ethyne or acetylene is the simplest triple bond containing organic compound. n Lewis Dot Formula for ethyne C 2 H 2 n N = 2(8) + 2(2) = 20 n A = 2(4) + 2(1) =10 n. S = 10 n
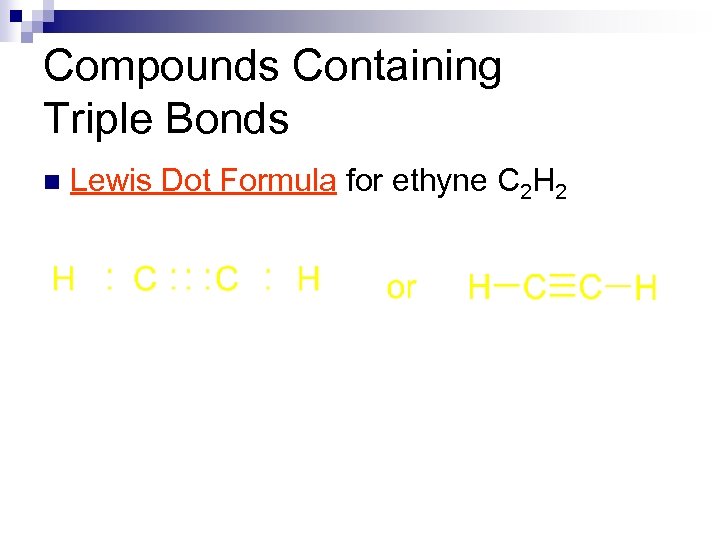
Compounds Containing Triple Bonds n Lewis Dot Formula for ethyne C 2 H 2

Compounds Containing Triple Bonds n VSEPR suggests C and H atoms are 180 o apart. H C C H

Compounds Containing Triple Bonds n Valence Bond Theory sp hybrids for the C atoms 2 electrons remain in unhybridized p orbitals
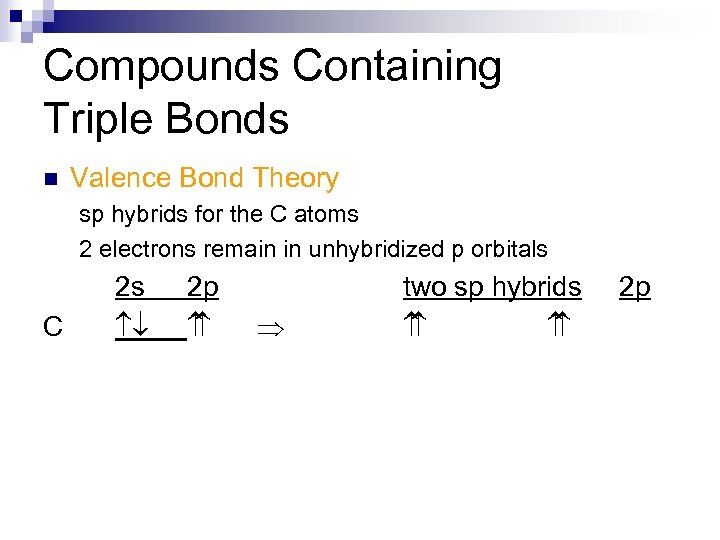
Compounds Containing Triple Bonds n Valence Bond Theory sp hybrids for the C atoms 2 electrons remain in unhybridized p orbitals C 2 s ¯ 2 p Þ two sp hybrids 2 p
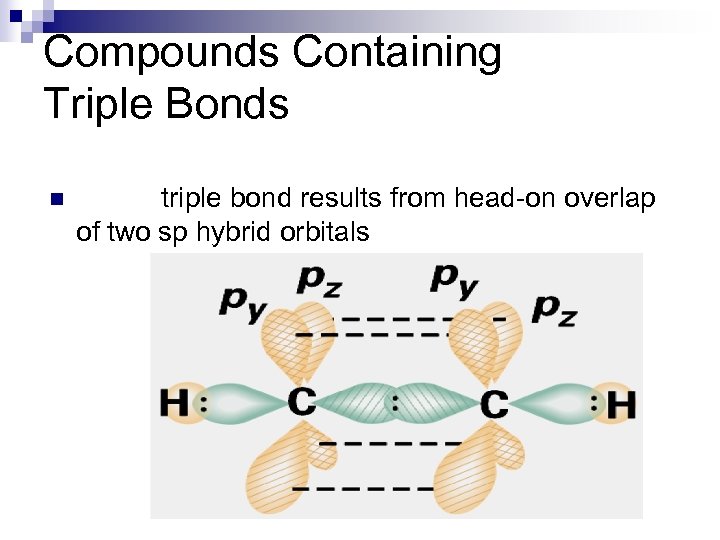
Compounds Containing Triple Bonds n triple bond results from head-on overlap of two sp hybrid orbitals
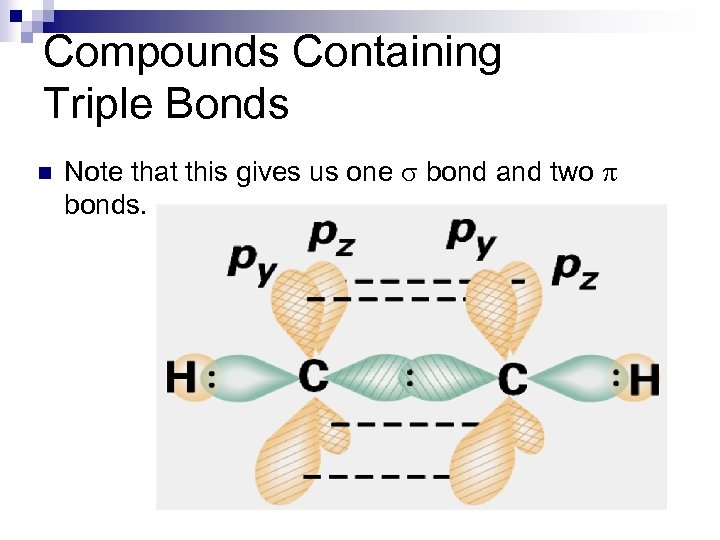
Compounds Containing Triple Bonds n Note that this gives us one s bond and two p bonds.
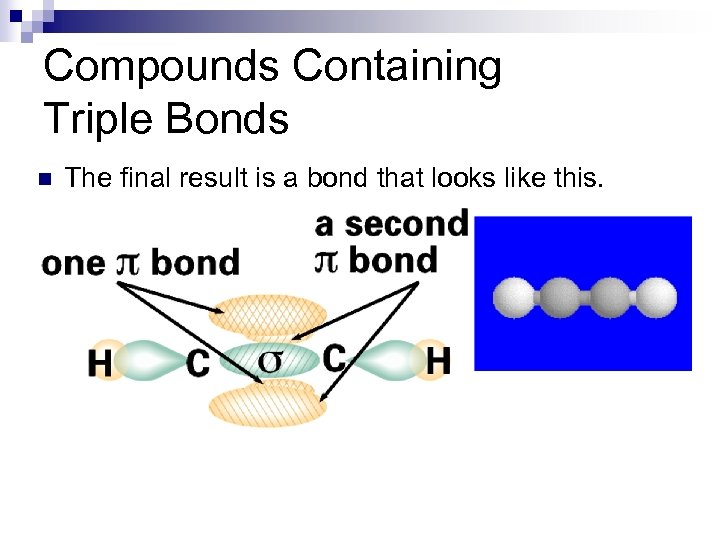
Compounds Containing Triple Bonds n The final result is a bond that looks like this.
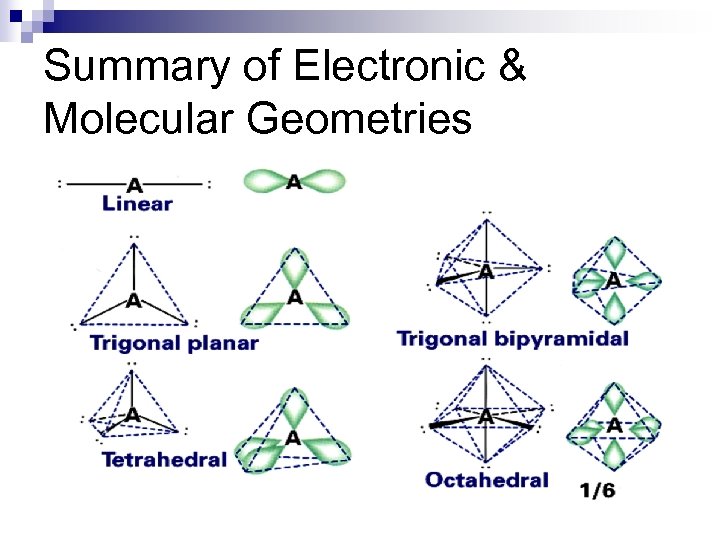
Summary of Electronic & Molecular Geometries
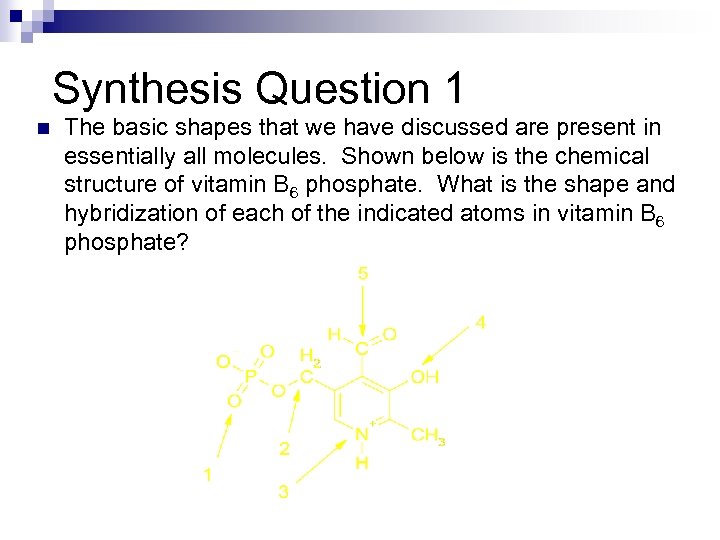
Synthesis Question 1 n The basic shapes that we have discussed are present in essentially all molecules. Shown below is the chemical structure of vitamin B 6 phosphate. What is the shape and hybridization of each of the indicated atoms in vitamin B 6 phosphate?
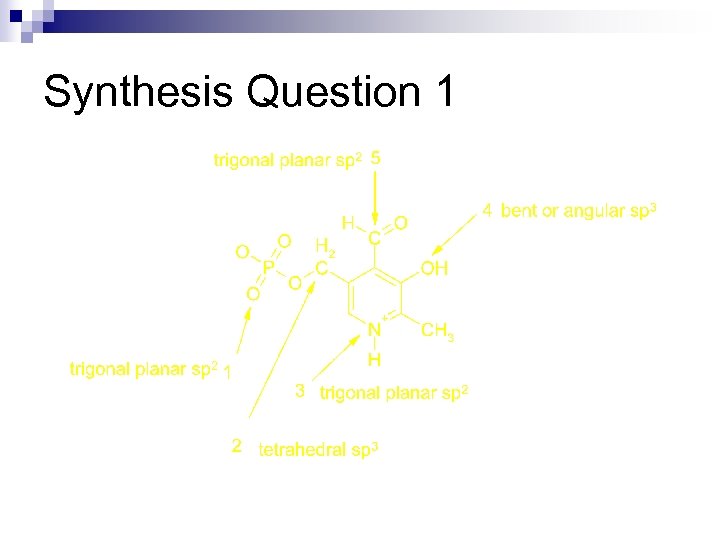
Synthesis Question 1
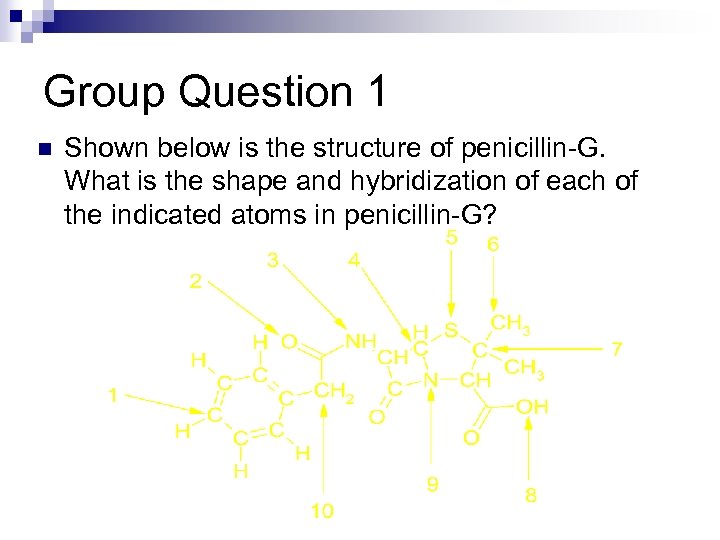
Group Question 1 n Shown below is the structure of penicillin-G. What is the shape and hybridization of each of the indicated atoms in penicillin-G?

Synthesis Question 2 n As we all know, in the wintertime we are more likely to get shocked when we walk across carpet and touch the door knob. Here is another wintertime experiment to perform. Turn on a water faucet until you have a continuous but small stream of water coming from the faucet. Brush your hair vigorously then hold the brush near the stream of water. You will notice that the stream bends towards the brush. Why does the water bend?

Synthesis Question 2 n Since water is a highly polar molecule, it is attracted by the electromagnetic field generated by the hair brush. This causes the stream to bend.

Group Question 2 n On a recent “infomercial” it was claimed that placing a small horseshoe magnet over the fuel intake line to your car’s carburetor would increase fuel mileage by 50%. The reason given for the mileage increase was that “the magnet aligned the molecules causing them to burn more efficiently. ” Will this work? Should you buy this product?
1bd03241bfd4bf0db6096b8da3a040f3.ppt