2d668121dd4670bba262c3262aa49350.ppt
- Количество слайдов: 14

Molecular Dynamic Simulation of a-C Deposition by Energetic Carbon Atoms Seung-Hyeob Lee, Seung-Cheol Lee, Kwang-Ryeol Lee , Kyu. Hwan Lee, and June-Gunn Lee Future Technology Research Division, Korea Institute of Science and Technology, P. O. Box, 131 Cheongryang, Seoul, Korea E-mail : einshlee@kist. re. kr

Introduction q Tetrahedral amorphous carbon (ta-C) High ratio of sp 3 hybridization sites (>80%) Ø High hardness and wear resistance with optical transparency Ø Smooth surface Ø
Disadvantages of ta-C q Limitation of ta-C film High residual compressive stress → poor adhesion Ø Many attempts have been reported ü Substrate biasing , post-annealing, boron incorporation Ø Atomic level understanding of the amorphous carbon structure is required to control the residual stress.
High Energy Carbon Deposition by Classical MD Simulation
MD Simulation Condition q Interatomic Potential Ø Tersoff Potential ü q J. Tersoff, Phys. Rev. Lett. , 61 (1988) 2879. Diamond Substrate Number of atoms : 608 Ø Temperature : 300 K Ø Boundary condition : Y-Z axis Ø q Carbon Deposition Ø Ø Ø q Number of deposited atoms : 500 atoms Incident kinetic energy : 1 ~ 300 e. V Time step : 0. 155 ~ 0. 5 fs Interval between carbon arrival : 1 ps Full dynamics except fixed layer Structure Stabilization Ø Time for stabilization : 10 ps 32Å
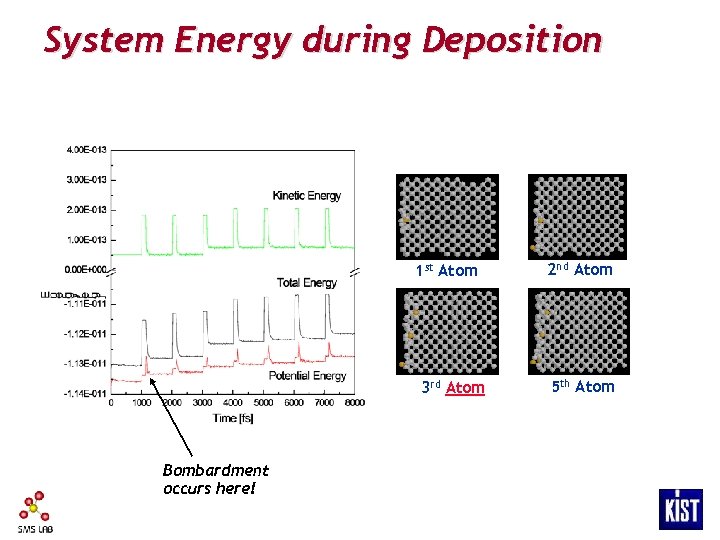
System Energy during Deposition 1 st Atom 3 rd Atom Bombardment occurs here! 2 nd Atom 5 th Atom
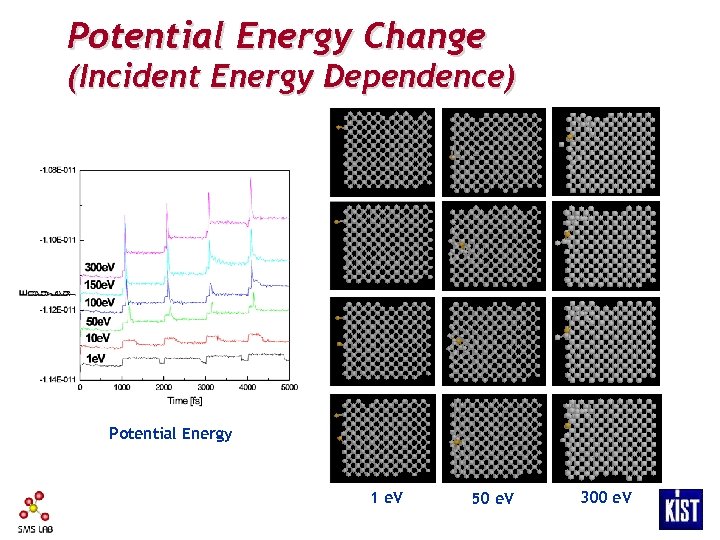
Potential Energy Change (Incident Energy Dependence) Potential Energy 1 e. V 50 e. V 300 e. V
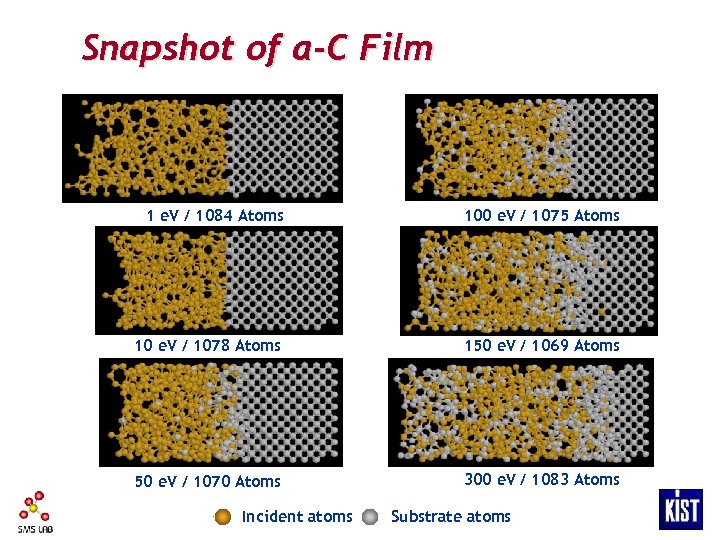
Snapshot of a-C Film 1 e. V / 1084 Atoms 100 e. V / 1075 Atoms 10 e. V / 1078 Atoms 150 e. V / 1069 Atoms 50 e. V / 1070 Atoms 300 e. V / 1083 Atoms Incident atoms Substrate atoms
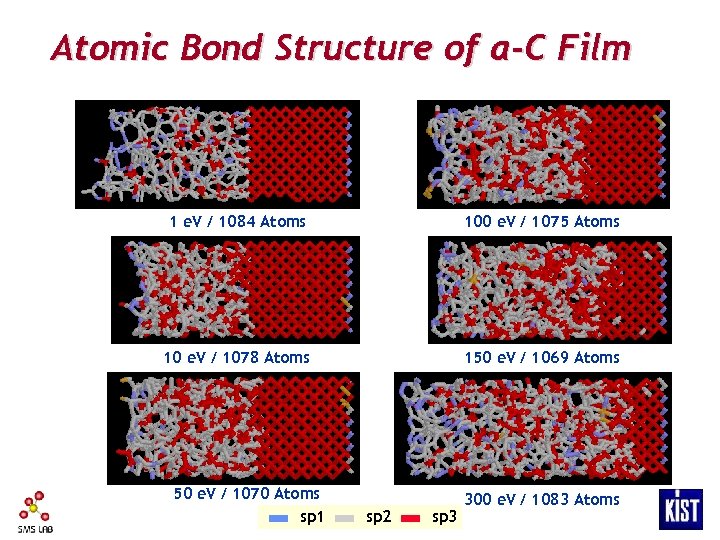
Atomic Bond Structure of a-C Film 1 e. V / 1084 Atoms 100 e. V / 1075 Atoms 10 e. V / 1078 Atoms 150 e. V / 1069 Atoms 50 e. V / 1070 Atoms sp 1 sp 2 sp 3 300 e. V / 1083 Atoms
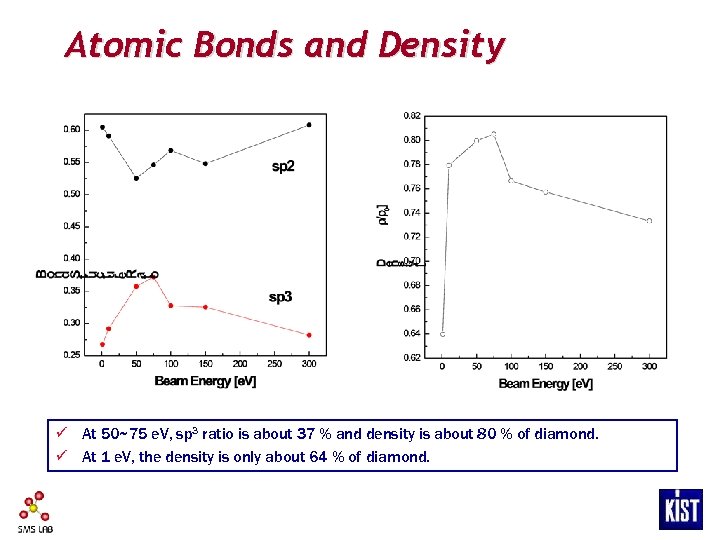
Atomic Bonds and Density ü At 50~75 e. V, sp 3 ratio is about 37 % and density is about 80 % of diamond. ü At 1 e. V, the density is only about 64 % of diamond.
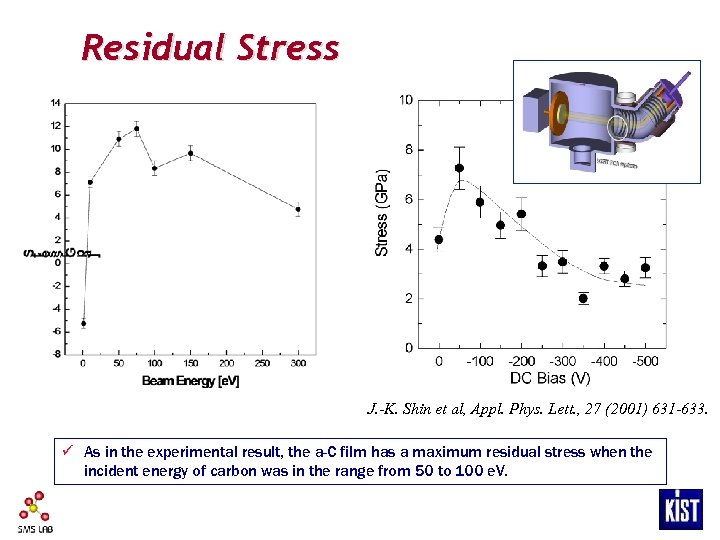
Residual Stress J. -K. Shin et al, Appl. Phys. Lett. , 27 (2001) 631 -633. ü As in the experimental result, the a-C film has a maximum residual stress when the incident energy of carbon was in the range from 50 to 100 e. V.
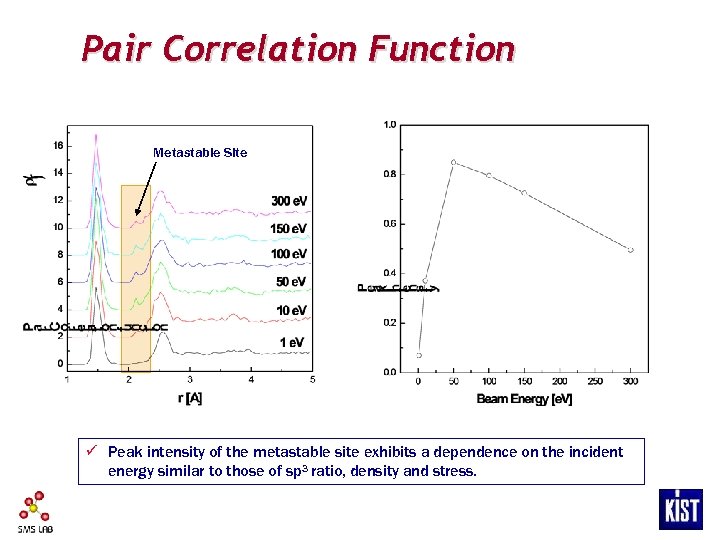
Pair Correlation Function Metastable Site ü Peak intensity of the metastable site exhibits a dependence on the incident energy similar to those of sp 3 ratio, density and stress.
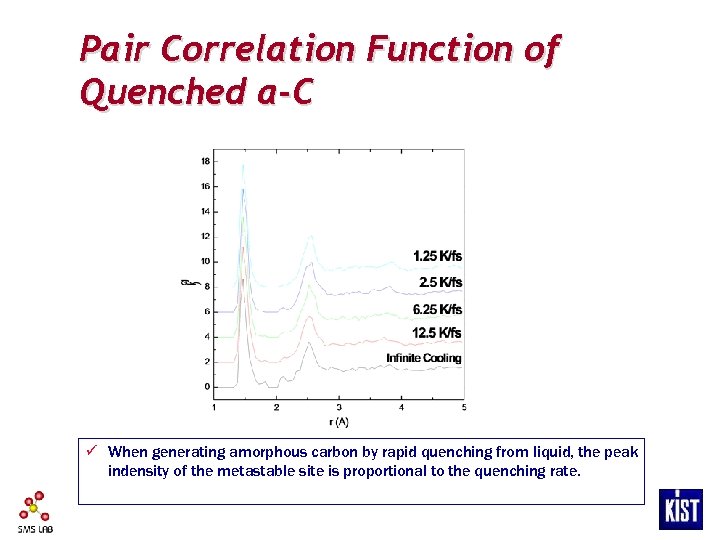
Pair Correlation Function of Quenched a-C ü When generating amorphous carbon by rapid quenching from liquid, the peak indensity of the metastable site is proportional to the quenching rate.
Conclusions q Atomic intermixing between the incident carbon and diamond occurred only when the incident energy was higher than 10 e. V. q The optimum incident energy for the highest sp 3 bond ratio and mass density was in the rage from 50 to 75 e. V, which is in good agreement with experimental observations. q At the optimum incident energy, significant amount of carbon atom was placed at a metastable site of distance 0. 22 nm. q From the metastable peak intensity, we could conclude that the atomic structure of the film was dependent on the quenching rate of thermal spike due to stopping the high energy incident atoms.
2d668121dd4670bba262c3262aa49350.ppt