e12c2f4a11ebecd90acd4e81ccefcdfb.ppt
- Количество слайдов: 108
Molecular Biology II Molecular cloning and mouse knockout principles 1

1 - Molecular cloning 2
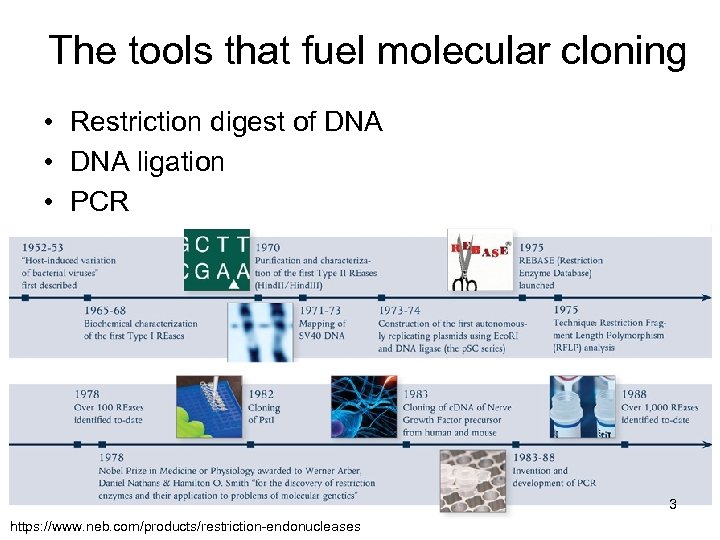
The tools that fuel molecular cloning • Restriction digest of DNA • DNA ligation • PCR 3 https: //www. neb. com/products/restriction-endonucleases

The Nobel Prize in Physiology or Medicine 1978 jointly to Werner Arber (Switzerland), Daniel Nathans (USA) and Hamilton O. Smith (USA) for the discovery of restriction enzymes and their application to problems of molecular genetics. 4
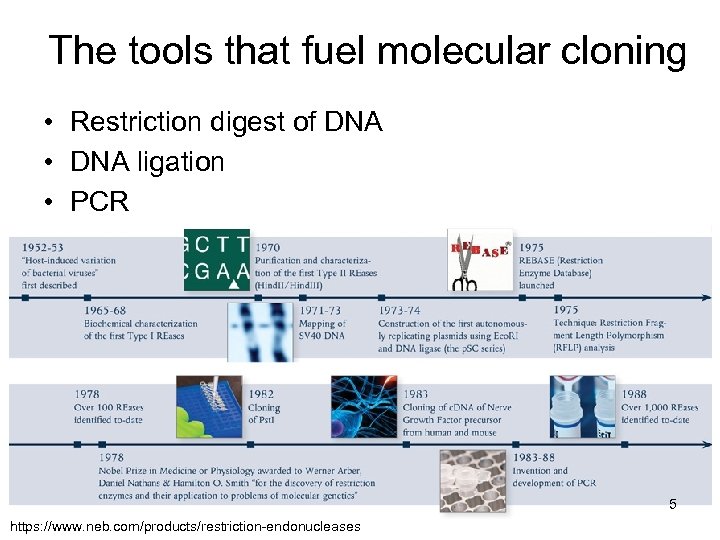
The tools that fuel molecular cloning • Restriction digest of DNA • DNA ligation • PCR 5 https: //www. neb. com/products/restriction-endonucleases

The Nobel Prize in Chemistry 1993 Michael Smith (Canada), for his fundamental contributions to the establishment of oligonucleotide-based, site-directed mutagenesis and its development for protein studies Kary B. Mullis (USA), for his invention of the polymerase chain reaction (PCR) method 6
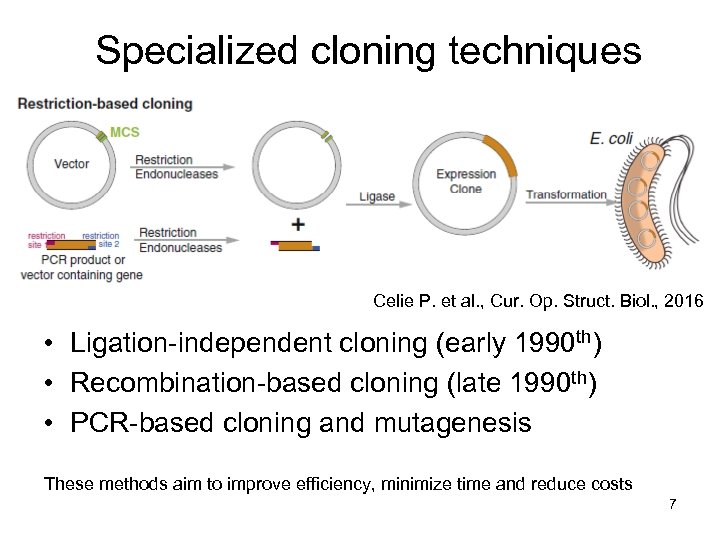
Specialized cloning techniques Celie P. et al. , Cur. Op. Struct. Biol. , 2016 • Ligation-independent cloning (early 1990 th) • Recombination-based cloning (late 1990 th) • PCR-based cloning and mutagenesis These methods aim to improve efficiency, minimize time and reduce costs 7

Restriction Endonucleases 8
Restriction Endonucleases • Found in Bacteria or Archaea • Cleave DNA depending on the presence of specific recognition sites (vary from short palindromyc sequences like GAATTC to long asymmetrical sequences like TGAN 8 TGCT) • Do not cleave DNA of the producing organism, which is protected by methylation (there a few exceptions) • Part of a simple bacterial “immune system” 9 protecting from foreign DNA (bacteriophages)
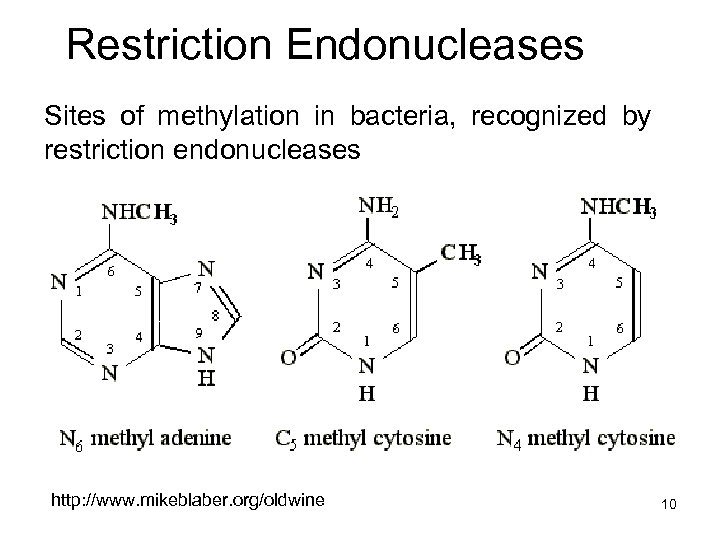
Restriction Endonucleases Sites of methylation in bacteria, recognized by restriction endonucleases http: //www. mikeblaber. org/oldwine 10
Naming of Restriction Endonucleases • The first three letters refer to the organism from which the restriction enzyme was originally isolated • The fourth letter (if present) refers to the strain • The Roman numerals serve as indices if the same organism contains several different restriction enzymes. Bam. H I: Bacillus amyloliquefaciens, strain H, enzyme I Hind III: Haemophilus influenzae strain d, enzyme III 11
Restriction Endonucleases • Type I: cuts the DNA at a random site far (>1000 bp) from the long asymmetrical recognition sequence (Eco. KI: AACN 6 GTGC). Consists of 3 different subunits (for DNA modification, cleavage and sequence recognition). Requires ATP. • Type II: cuts the DNA specificly within or near the recognition sequence (4 -8 bp). Usially homodimers (only DNA cleavage and sequence recognition; no methyltransferase activity). Does not require ATP. 12
Restriction Endonucleases • Type III: cuts the DNA about 20 -25 base pairs from the 5 -7 bp recognition sequence (Eco. PI: AGACC (25/27)). Consists of 2 different subunits (for DNA recognition/modification and cleavage). Requires ATP. • Type IV: cuts only modified (e. g. methylated) DNA at a random site. Their recognition sequences have usually not been well defined (exception Eco. KMcr. A: Ym. CGR). Consists of 2 different subunits (for DNA recognition and cleavage). Requires GTP. 13
Restriction Endonucleases More than 4, 100 restriction endonucleases are known, recognizing more than 300 distinct sequences (see REBASE® at rebase. neb. com) REBASE® lists (September 2015) only 112 Type I, 22 Type III and 19 Type IV restriction endonucleases (sequence analysis predicts approximately 29% Type I, 45% Type II, 8% Type III and 18% Type IV) Thus, in common usage, the term “restriction enzyme” is usually identified with the restriction endonucleases of type II 14
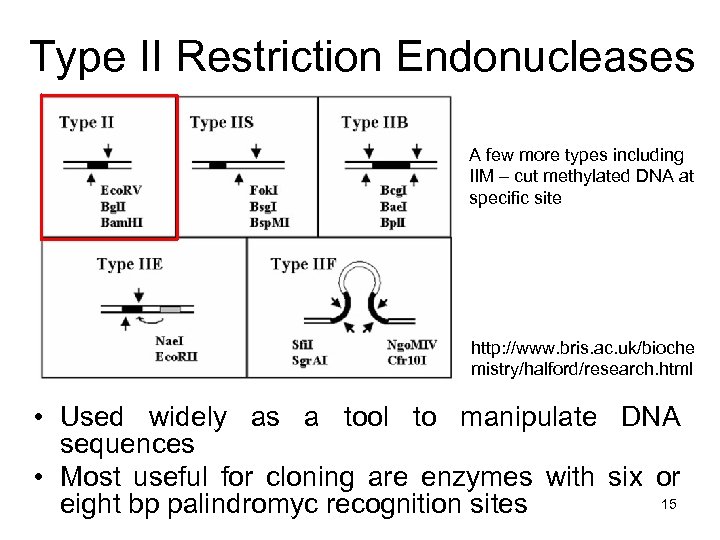
Type II Restriction Endonucleases A few more types including IIM – cut methylated DNA at specific site http: //www. bris. ac. uk/bioche mistry/halford/research. html • Used widely as a tool to manipulate DNA sequences • Most useful for cloning are enzymes with six or 15 eight bp palindromyc recognition sites
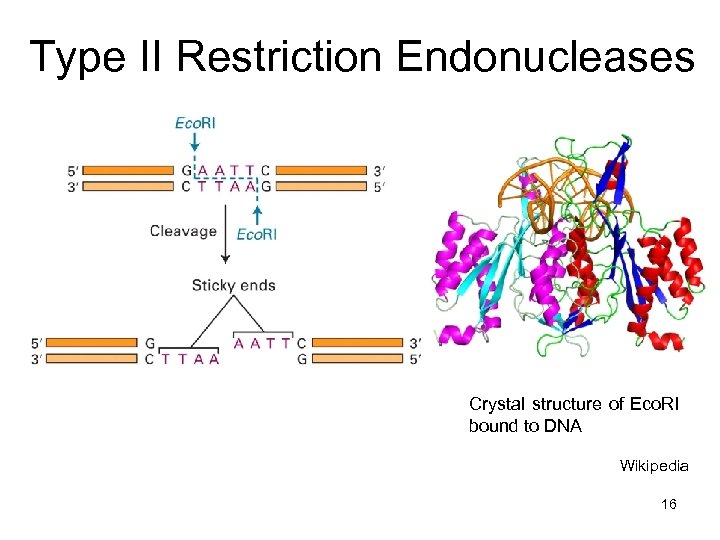
Type II Restriction Endonucleases Crystal structure of Eco. RI bound to DNA Wikipedia 16
Frequency of restriction sites • If the base composition is assumed to be completely random, the restriction site frequency can be calculated as 4 x, with X being the length of the recognition sequence For a four-base cutter: 44 = 256 one cutting site every 256 bp “ “ six “ : 46 = 4096 one cutting site every 4096 bp However, base compositions are not completely random and strongly depend on the organism, and hence so does the frequency of restriction sites. 17
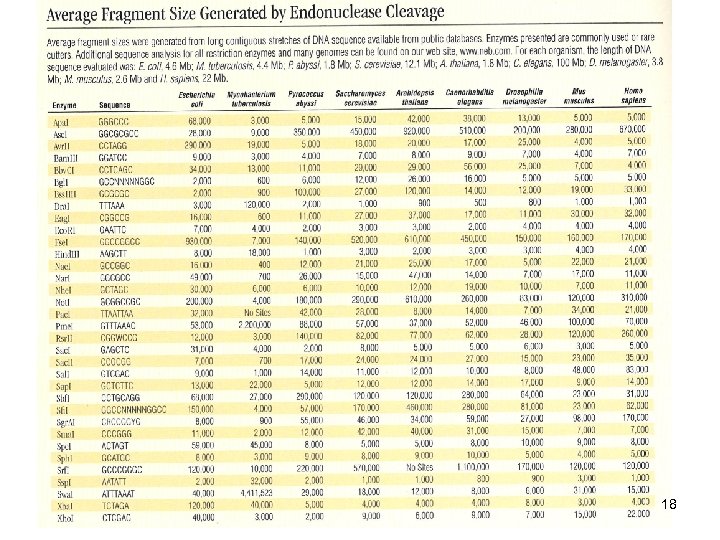
18

Restriction digests • Preparative digest (to obtain DNA fragments for cloning; 100 -500 ng DNA) – ≥ 20 units of enzyme(s), max 10% of total reaction volume (20 -50 ul) • (really, you may use more than 20 units considering the enzyme quality, especially when cutting the vector to which you ligate your DNA) – >1 hour incubation • Analytical digest (to verify construct) – 5 units of enzyme, reaction volume as little as 10 ul – 1 hour incubation 19

General practice for restriction digests • Aliquot your buffers – Sharing is fun, but it is not a good idea to have ten people dip into the same solutions for their digests, as this increases the likelihood of contaminations • Prepare digests in a clean working environment – Work on a clean bench – You may want to consider wearing gloves 20

21

Know your enzyme! • “Standard” conditions work “usually”, but depending on the enzyme used, you will have to pay attention to specific features • A wide variety of information is available for you from the manufacturer’s websites – but how do you read/interpret these properly? – what information is important for you? 22
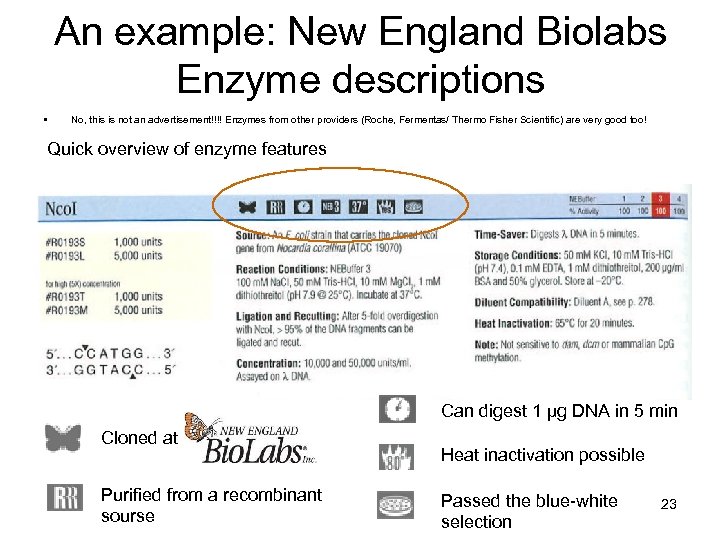
An example: New England Biolabs Enzyme descriptions • No, this is not an advertisement!!!! Enzymes from other providers (Roche, Fermentas/ Thermo Fisher Scientific) are very good too! Quick overview of enzyme features Can digest 1 µg DNA in 5 min Cloned at NEB, Inc. Purified from a recombinant sourse Heat inactivation possible Passed the blue-white selection 23
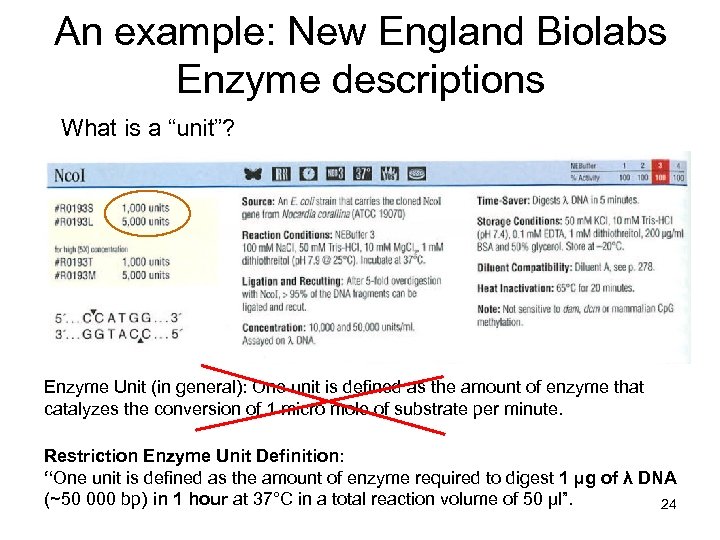
An example: New England Biolabs Enzyme descriptions What is a “unit”? Enzyme Unit (in general): One unit is defined as the amount of enzyme that catalyzes the conversion of 1 micro mole of substrate per minute. Restriction Enzyme Unit Definition: “One unit is defined as the amount of enzyme required to digest 1 µg of λ DNA (~50 000 bp) in 1 hour at 37°C in a total reaction volume of 50 µl”. 24

• The unit definition refers to cleavage of linear (phage λ) DNA • But: Plasmid DNA is usually supercoiled! * *More about this later! 25
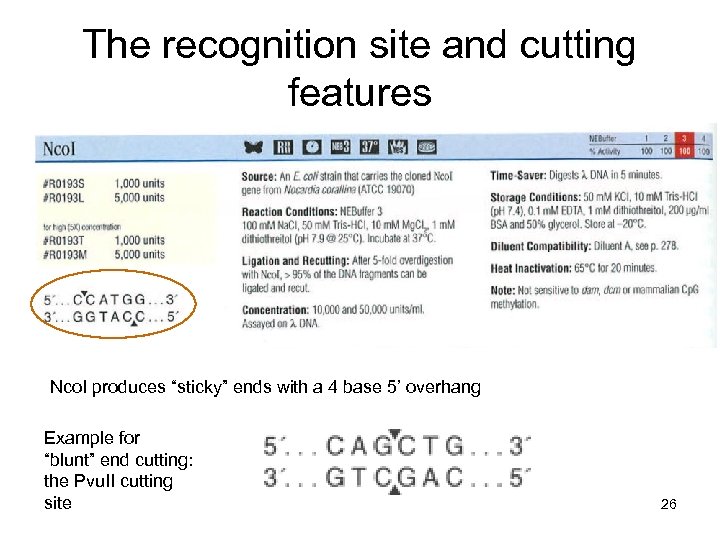
The recognition site and cutting features Nco. I produces “sticky” ends with a 4 base 5’ overhang Example for “blunt” end cutting: the Pvu. II cutting site 26
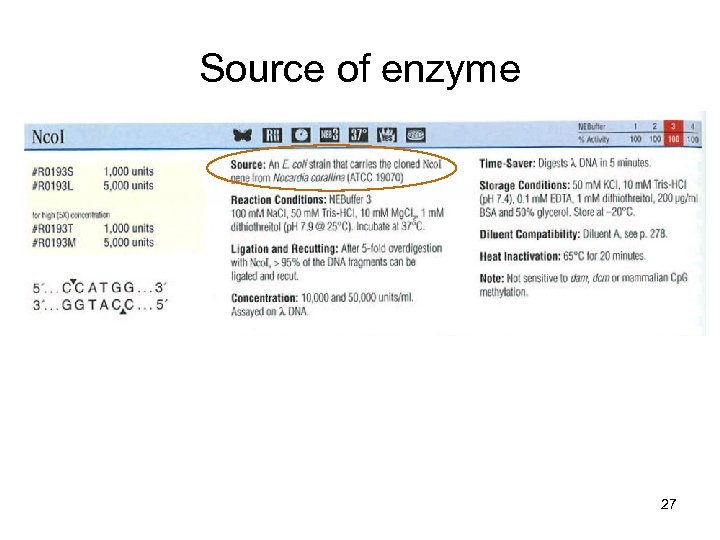
Source of enzyme 27
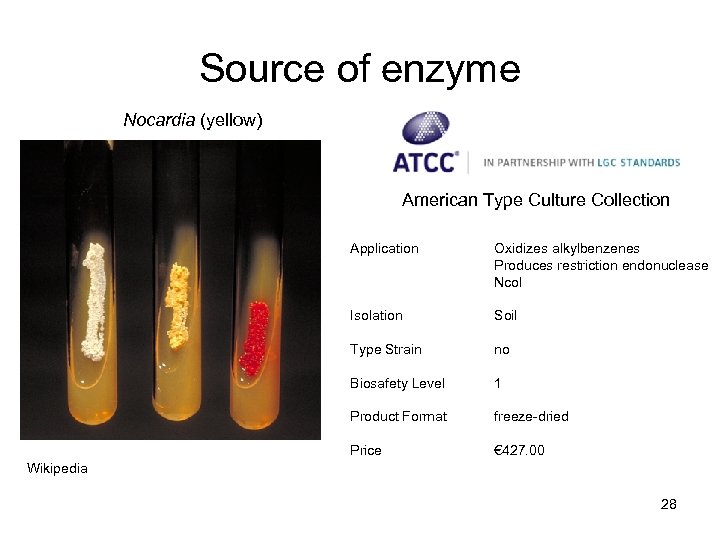
Source of enzyme Nocardia (yellow) American Type Culture Collection Application Oxidizes alkylbenzenes Produces restriction endonuclease Nco. I Isolation Soil Type Strain no Biosafety Level 1 Product Format freeze-dried Price € 427. 00 Wikipedia 28
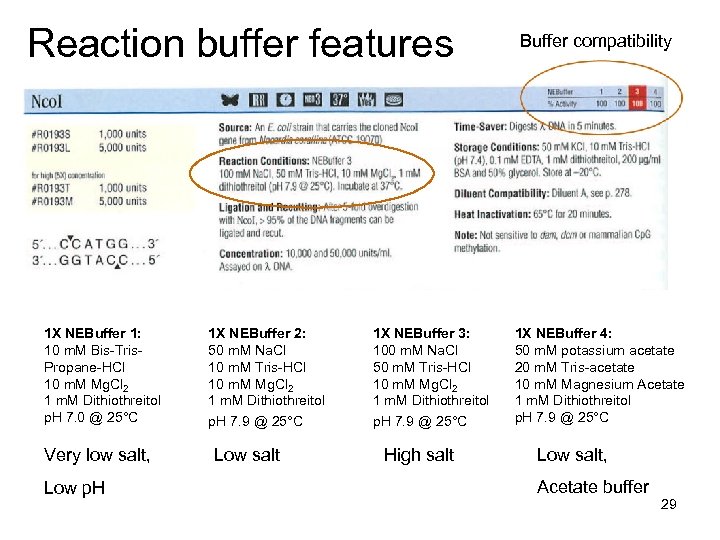
Reaction buffer features 1 X NEBuffer 1: 10 m. M Bis-Tris. Propane-HCl 10 m. M Mg. Cl 2 1 m. M Dithiothreitol p. H 7. 0 @ 25°C Very low salt, Low p. H 1 X NEBuffer 2: 50 m. M Na. Cl 10 m. M Tris-HCl 10 m. M Mg. Cl 2 1 m. M Dithiothreitol p. H 7. 9 @ 25°C Low salt 1 X NEBuffer 3: 100 m. M Na. Cl 50 m. M Tris-HCl 10 m. M Mg. Cl 2 1 m. M Dithiothreitol p. H 7. 9 @ 25°C High salt Buffer compatibility 1 X NEBuffer 4: 50 m. M potassium acetate 20 m. M Tris-acetate 10 m. M Magnesium Acetate 1 m. M Dithiothreitol p. H 7. 9 @ 25°C Low salt, Acetate buffer 29
• Some enzymes require special buffers • Sometimes additives are required BSA = bovine serum albumin SAM = S-adenosylmethionine (sourse of methyl groups) • Using the wrong buffer or leaving out required additives can result in: – Decreased or no activity (p. H too low or too high; salt too high; omission of BSA or SAM) – Star activity (loss of cutting specificity) • Digests with two enzymes sometimes may require compromises in buffer usage 30
What is STAR activity? • Some enzymes lose specificity if they are used in the wrong buffer conditions. Instead of recognizing a six base-pair sequence, the enzymes are able to cleave four basepair sites • Example: Eco RI restriction site is GAATTC • Eco. RI STAR sites are AATT Eco. RI becomes a four-base cutter! 31
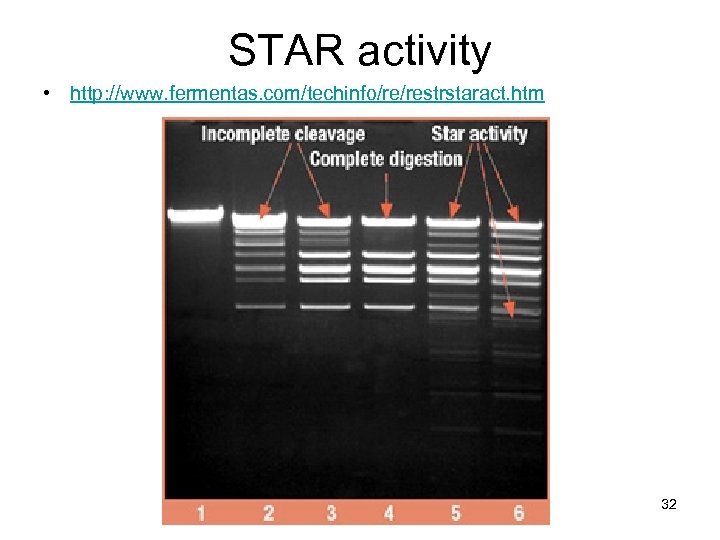
STAR activity • http: //www. fermentas. com/techinfo/re/restrstaract. htm 32

What conditions allow for STAR activity (and how is it prevented)? • Low salt ( use salt concentrations >100 m. M) • Low p. H ( use p. H of about 8. 0) • Glycerol concentration higher than 5% ( the fraction of restriction enzyme preparation should not exceed 10% of the total digest volume) • High enzyme concentrations ( don’t use excessive amounts) 33
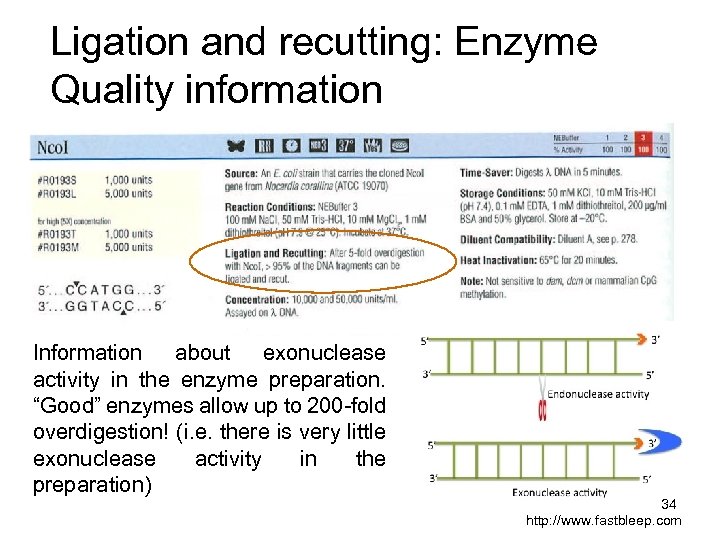
Ligation and recutting: Enzyme Quality information Information about exonuclease activity in the enzyme preparation. “Good” enzymes allow up to 200 -fold overdigestion! (i. e. there is very little exonuclease activity in the preparation) 34 http: //www. fastbleep. com
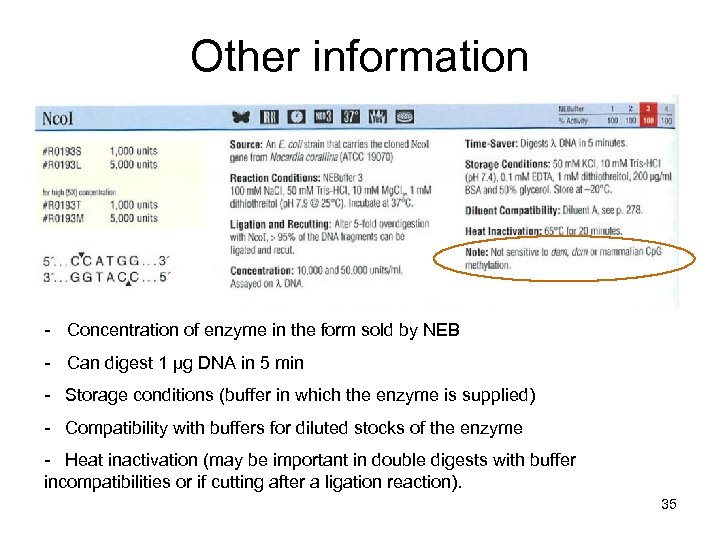
Other information - Concentration of enzyme in the form sold by NEB - Can digest 1 µg DNA in 5 min - Storage conditions (buffer in which the enzyme is supplied) - Compatibility with buffers for diluted stocks of the enzyme - Heat inactivation (may be important in double digests with buffer incompatibilities or if cutting after a ligation reaction). 35
About dam, dcm and Cp. G methylation • REBASE lists more then 2300 methyltransferases from various bacterial species • dam and dcm methylation is carried out by most E. coli strains and these DNA modifications can interfere with restriction enzyme function – Dam methylase modifies the adenine in the sequence GATC – Dcm methylase “ internal cytosine in the sequences CCAGG and CCTGG • Example of dam sensitive enzyme: Bcl I TGATCA • Dcm sensitive enzyme: Eco. R II CC(A/T)GG • Only DNA isolated from dam+ or dcm+ bacterial strains will be modified; PCR products do not carry the modification • dam or dcm methylation can be reversed by passing vectors through dam- or dcm - E. coli strains http: //www. flickriver. com/photos/agathman/tags/dna/ 36
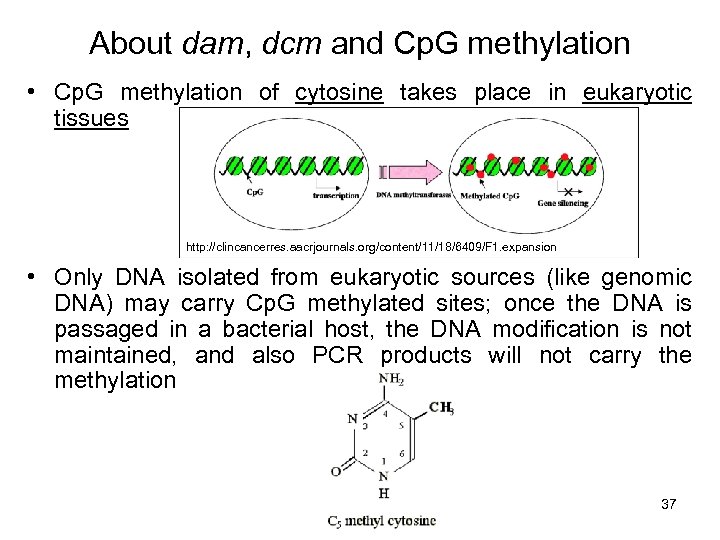
About dam, dcm and Cp. G methylation • Cp. G methylation of cytosine takes place in eukaryotic tissues http: //clincancerres. aacrjournals. org/content/11/18/6409/F 1. expansion • Only DNA isolated from eukaryotic sources (like genomic DNA) may carry Cp. G methylated sites; once the DNA is passaged in a bacterial host, the DNA modification is not maintained, and also PCR products will not carry the methylation 37
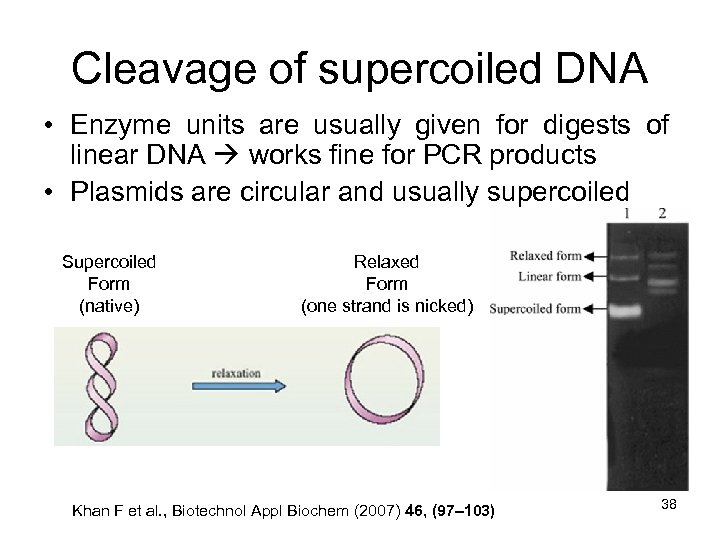
Cleavage of supercoiled DNA • Enzyme units are usually given for digests of linear DNA works fine for PCR products • Plasmids are circular and usually supercoiled Supercoiled Form (native) Relaxed Form (one strand is nicked) Khan F et al. , Biotechnol Appl Biochem (2007) 46, (97– 103) 38
Cleavage of supercoiled DNA • Some enzymes cleave supercoiled DNA at much lower rates • For some enzymes, reduction in activity can be as high as 10 -fold! • Problem if only one enzyme is used, or if both enzymes in a double digest are inefficient in cleaving supercoiled DNA (e. g. Sal. I and Nhe. I) • add more enzyme 39

Cleavage close to ends • Enzymes may need a bit of extra DNA to “sit” on in addition to their recognition sequence • This becomes important when cutting at the end of DNA sequences, e. g. PCR products or sequences in close proximity in a multiple cloning site (MCS) • add the appropriate amount of extra bases to your PCR primer if introducing restriction sites to the ends of DNA sequences (four or more? ) • avoid choosing restriction sites that are adjacent in an MCS – If you absolutely have no other choice, try using in the mixture of 2 restriction endonucleases first the enzyme that needs extra adjacent bases and then an enzyme that tolerates proximity to ends 40
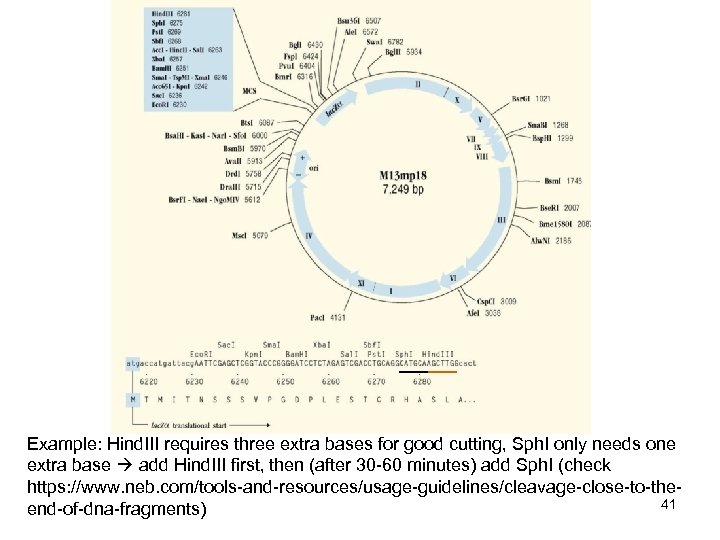
Example: Hind. III requires three extra bases for good cutting, Sph. I only needs one extra base add Hind. III first, then (after 30 -60 minutes) add Sph. I (check https: //www. neb. com/tools-and-resources/usage-guidelines/cleavage-close-to-the 41 end-of-dna-fragments)
Keep in mind! There also: • Isoschizomers – Enzymes that have the same recognition sequence • Neoschizomers – Enzymes that have the same recognition sequence, but cleave in a different position Aat. II (recognition sequence: GACGT↓C) and Zra. I (recognition sequence: GAC↓GTC) are neoschizomers of one another, while Hpa. II (recognition sequence: C↓CGG) and Msp. I (recognition sequence: C↓CGG) are 42 isoschizomers.
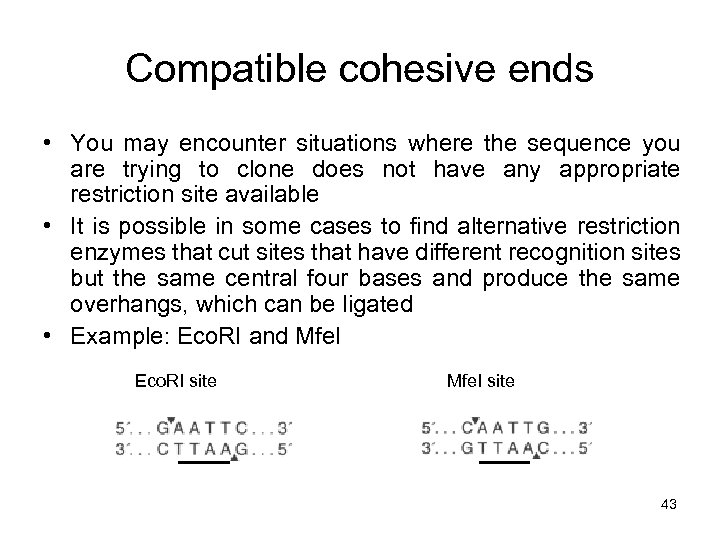
Compatible cohesive ends • You may encounter situations where the sequence you are trying to clone does not have any appropriate restriction site available • It is possible in some cases to find alternative restriction enzymes that cut sites that have different recognition sites but the same central four bases and produce the same overhangs, which can be ligated • Example: Eco. RI and Mfe. I Eco. RI site Mfe. I site 43
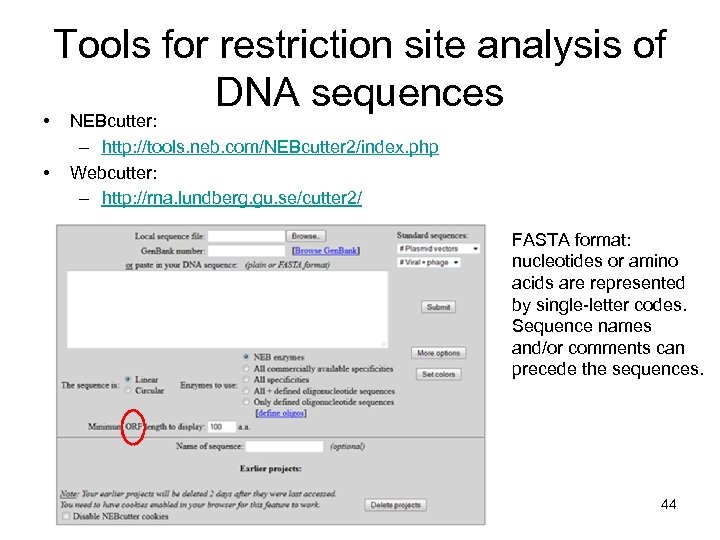
• • Tools for restriction site analysis of DNA sequences NEBcutter: – http: //tools. neb. com/NEBcutter 2/index. php Webcutter: – http: //rna. lundberg. gu. se/cutter 2/ FASTA format: nucleotides or amino acids are represented by single-letter codes. Sequence names and/or comments can precede the sequences. 44
How to Make a Circular DNA Map https: //www. addgene. org/ analyze-sequence/ aaaaaaaaaaaaaaaaaaa aaaatttttttttttttttttttttttttttttttccatggggggggggggggggggg gcccccccccccccccccccc 45

Ligation 46
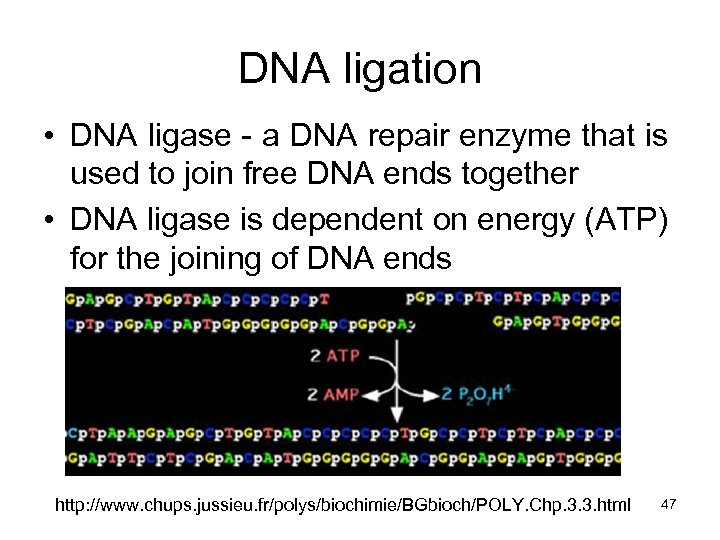
DNA ligation • DNA ligase - a DNA repair enzyme that is used to join free DNA ends together • DNA ligase is dependent on energy (ATP) for the joining of DNA ends http: //www. chups. jussieu. fr/polys/biochimie/BGbioch/POLY. Chp. 3. 3. html 47
DNA ligation Usually for molecular cloning DNA ligase from bacteriophage T 4 is used (most versatile) • T 4 DNA ligase does not appear to have any role in nucleic acid metabolism in bacteriophage T 4 infected E. coli, but instead appears to be required for the attachment of the bacteriophage’s tail fibers to its base plate during bacteriophage assembly • T 4 DNA ligase is available from several different suppliers (Fermentas/ Thermo Fisher Scientific, 48 Roche, NEB, Epicentre…)
DNA ligation: Important principles • Both blunt ends and “sticky” ends can be ligated with T 4 DNA ligase • Incubation temperature is a compromise between maximal enzymatic activity (the higher, the better – up to 37 o. C) and stabilization of interactions of the DNA ends (the lower, the better – down to 4 o. C) 49

Fast ligation kits • Formerly, ligation was carried out overnight at 4 o. C or for 4 -6 hours at 15 o. C • Better enzyme preparations allow for faster ligation (room temp; 20 -30 min for sticky ends, ~2 hrs for blunt ends) • Nowadays, almost all manufacturers offer Fast ligation kits (5 -15 min ligation time), e. g. – Epicentre Fast Link http: //www. epibio. com/item. asp? ID=296 – NEB Quick ligation kit https: //www. neb. com/products/m 2200 -quick-ligation-kit – Thermo. Fisher Rapid ligation kit https: //www. thermofisher. com/order/catalog/product/K 1422 – All these kits probably use addition of polyethylene glycol (PEG) to improve ligation 50
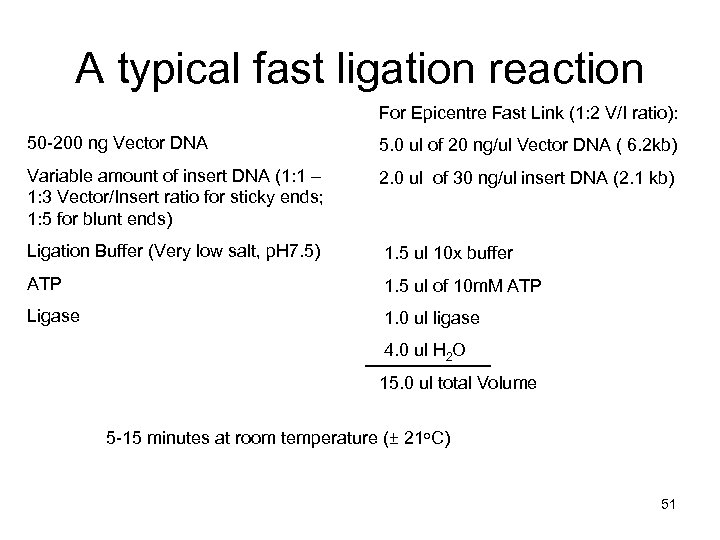
A typical fast ligation reaction For Epicentre Fast Link (1: 2 V/I ratio): 50 -200 ng Vector DNA 5. 0 ul of 20 ng/ul Vector DNA ( 6. 2 kb) Variable amount of insert DNA (1: 1 – 1: 3 Vector/Insert ratio for sticky ends; 1: 5 for blunt ends) 2. 0 ul of 30 ng/ul insert DNA (2. 1 kb) Ligation Buffer (Very low salt, p. H 7. 5) 1. 5 ul 10 x buffer ATP 1. 5 ul of 10 m. M ATP Ligase 1. 0 ul ligase 4. 0 ul H 2 O 15. 0 ul total Volume 5 -15 minutes at room temperature (± 21 o. C) 51
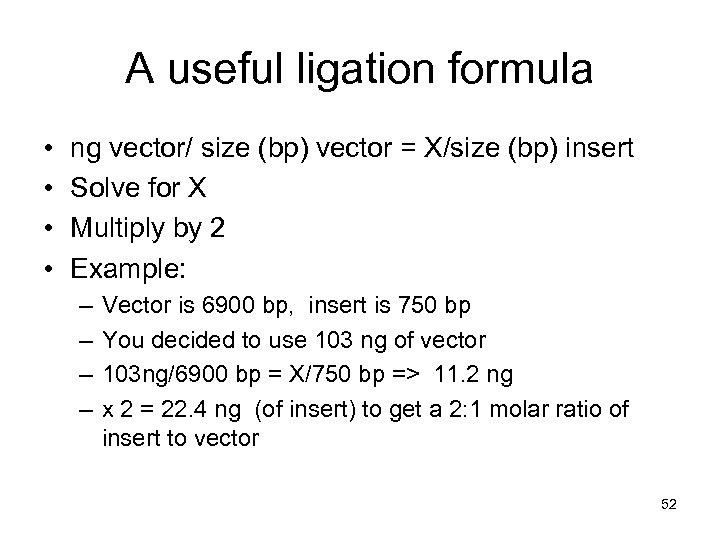
A useful ligation formula • • ng vector/ size (bp) vector = X/size (bp) insert Solve for X Multiply by 2 Example: – – Vector is 6900 bp, insert is 750 bp You decided to use 103 ng of vector 103 ng/6900 bp = X/750 bp => 11. 2 ng x 2 = 22. 4 ng (of insert) to get a 2: 1 molar ratio of insert to vector 52
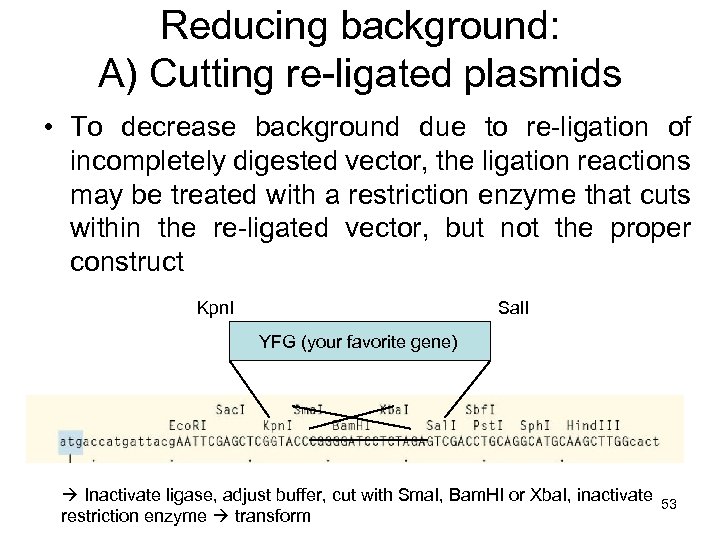
Reducing background: A) Cutting re-ligated plasmids • To decrease background due to re-ligation of incompletely digested vector, the ligation reactions may be treated with a restriction enzyme that cuts within the re-ligated vector, but not the proper construct Kpn. I Sal. I YFG (your favorite gene) Inactivate ligase, adjust buffer, cut with Sma. I, Bam. HI or Xba. I, inactivate 53 restriction enzyme transform
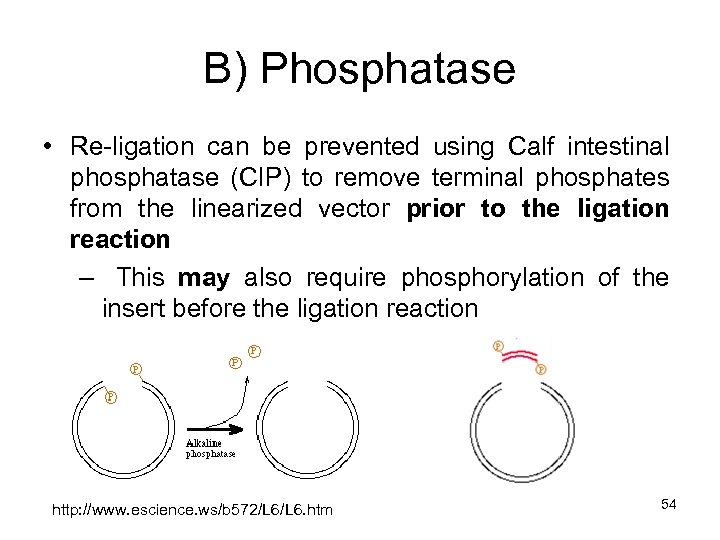
B) Phosphatase • Re-ligation can be prevented using Calf intestinal phosphatase (CIP) to remove terminal phosphates from the linearized vector prior to the ligation reaction – This may also require phosphorylation of the insert before the ligation reaction http: //www. escience. ws/b 572/L 6. htm 54
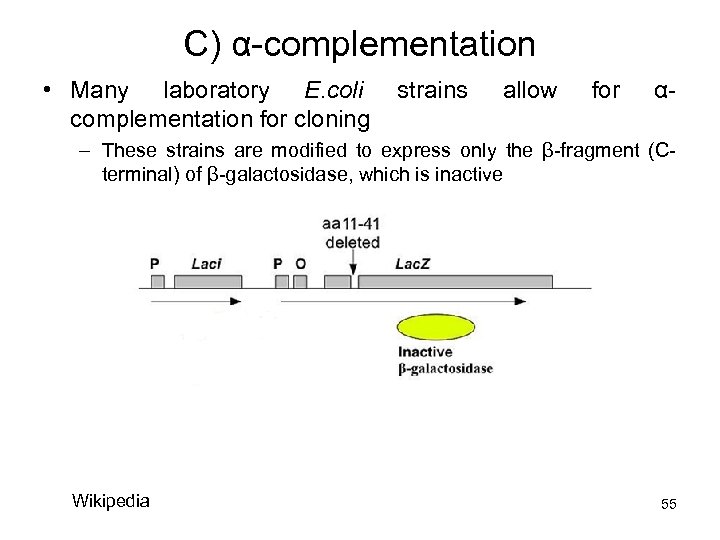
C) α-complementation • Many laboratory E. coli complementation for cloning strains allow for α- – These strains are modified to express only the β-fragment (Cterminal) of β-galactosidase, which is inactive Wikipedia 55
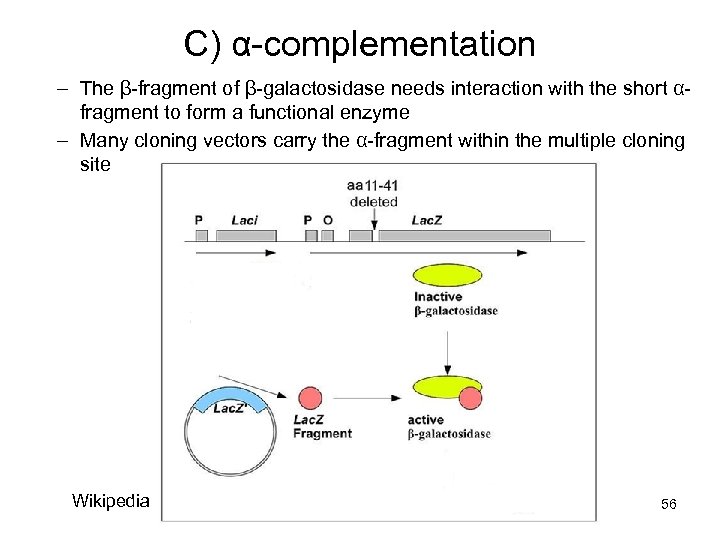
C) α-complementation – The β-fragment of β-galactosidase needs interaction with the short αfragment to form a functional enzyme – Many cloning vectors carry the α-fragment within the multiple cloning site Wikipedia 56
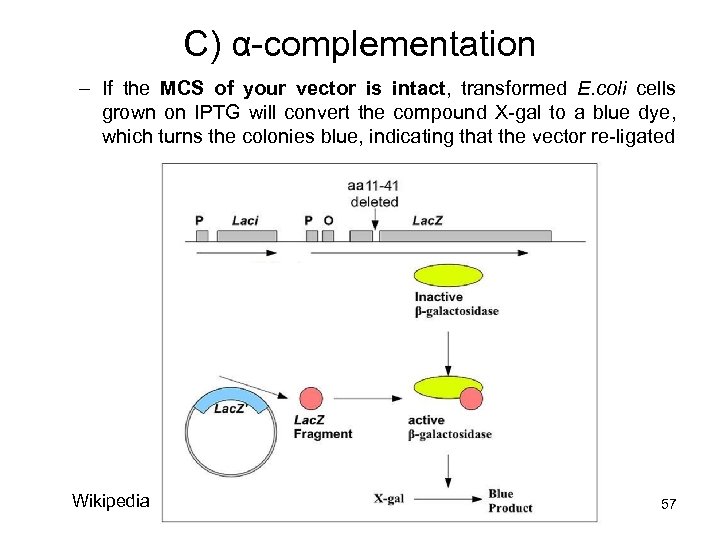
C) α-complementation – If the MCS of your vector is intact, transformed E. coli cells grown on IPTG will convert the compound X-gal to a blue dye, which turns the colonies blue, indicating that the vector re-ligated Wikipedia 57
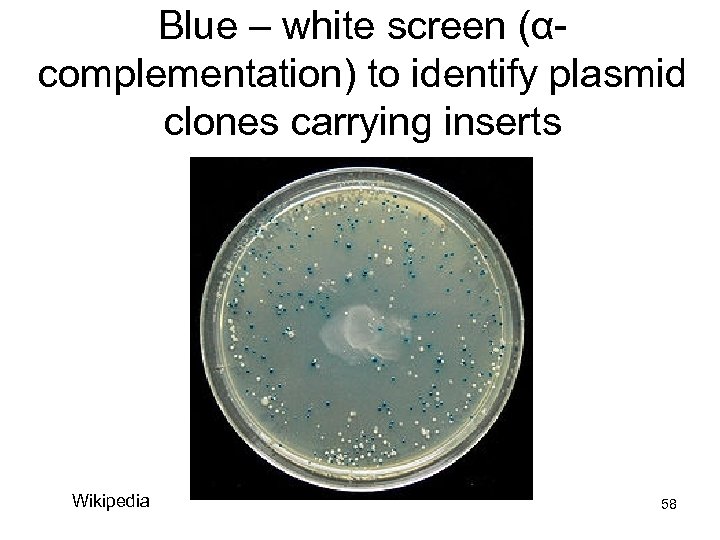
Blue – white screen (αcomplementation) to identify plasmid clones carrying inserts Wikipedia 58
General recommendations • Keep a clean working environment • Aliquot buffer, ATP • Do not let ATP warm up to room temperature • Heat inactivation of ligase after reaction (70 o. C for 15 -20 min) is recommended to reach optimal transformation frequencies 59

If you’re only interested in cloning a PCR product (and frame, etc. are not important) • Cut vector with a blunt end cutter (e. g. Sma. I or Pvu. II) • Add purified PCR product at the proper concentration • Ligate with blunt end method • Transform E. coli 60
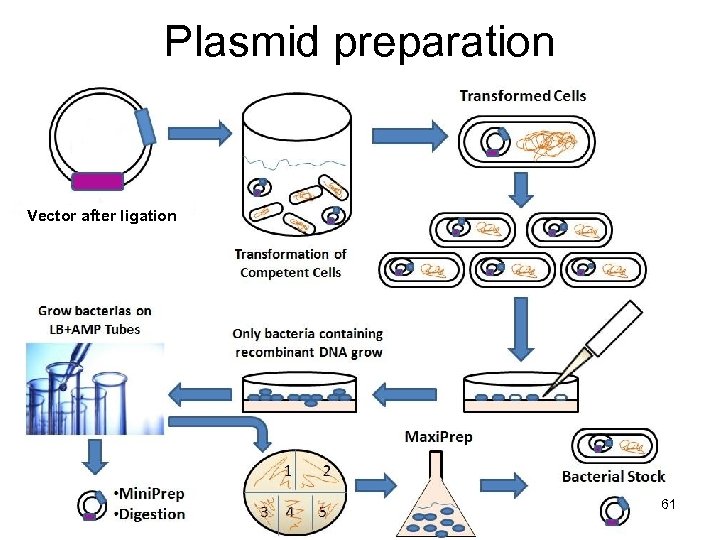
Plasmid preparation Vector after ligation 61
Analysis of plasmid constructs • Isolate plasmid DNA (miniprep) • Perform analytical digests (10 ul digest volume may be enough) – First, digest with the enzyme(s) used to insert your DNA into the Vector – Second, digest candidates with a second (set of) enzyme(s) – For one of these digests, include uncut DNA as a control • If any of the DNAs used in the construct were obtained by PCR: Sequence 62
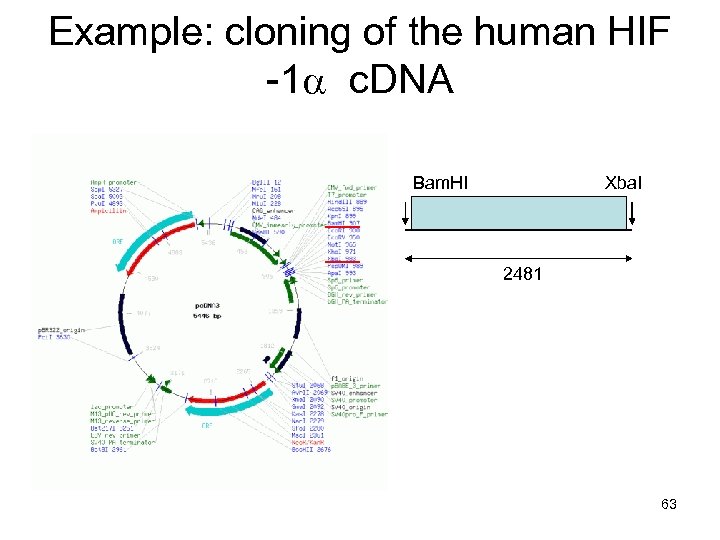
Example: cloning of the human HIF -1 a c. DNA Bam. HI Xba. I 2481 63
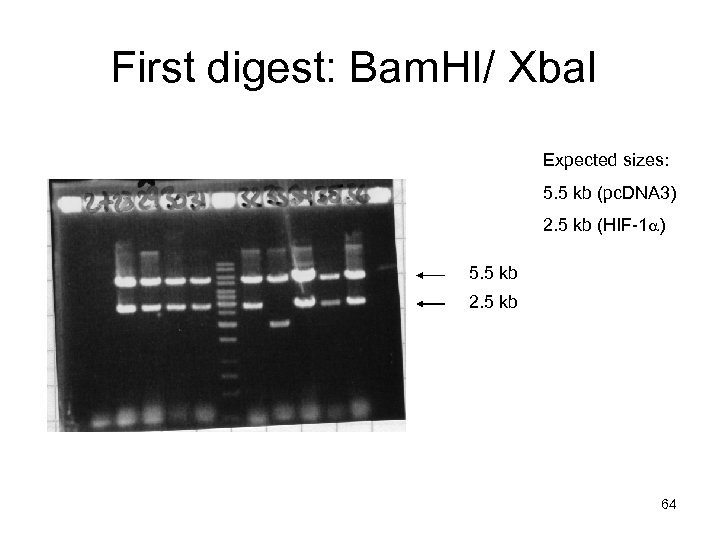
First digest: Bam. HI/ Xba. I Expected sizes: 5. 5 kb (pc. DNA 3) 2. 5 kb (HIF-1 a) 5. 5 kb 2. 5 kb 64
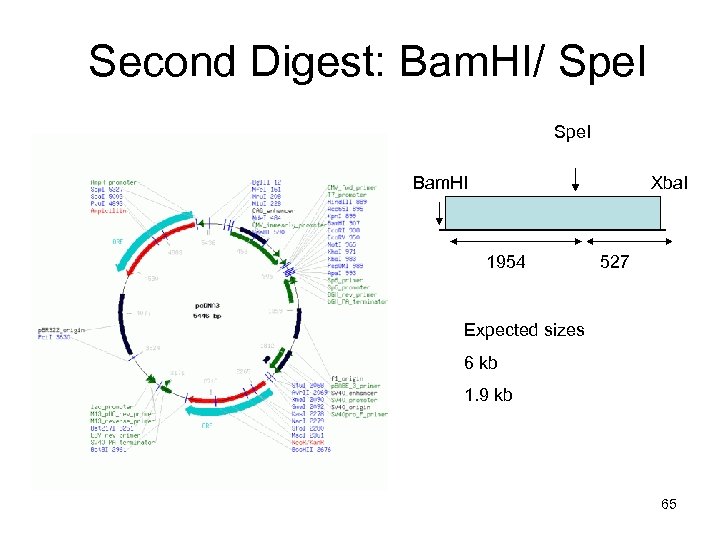
Second Digest: Bam. HI/ Spe. I Bam. HI Xba. I 1954 527 Expected sizes 6 kb 1. 9 kb 65
Remember dam methylation • Example: Xba. I site is TCTAGA • dam site is Gm. ATC (m= methylated base A) • If you generate a Xba. I site by PCR, but don’t pay attention to the following bases, you might end up with TCTAGATC • The A in your restriction site will be methylated (TCTAGm. ATC) once the DNA has passed through a dam+ E. coli strain, and Xba. I will not cut anymore • You also have to verify restriction maps that indicate sites for enzymes that are sensitive to dcm (or Cp. G) methylation. • Be sure you check both ends!! (Gm. ATCTAGA is also methylated) 66
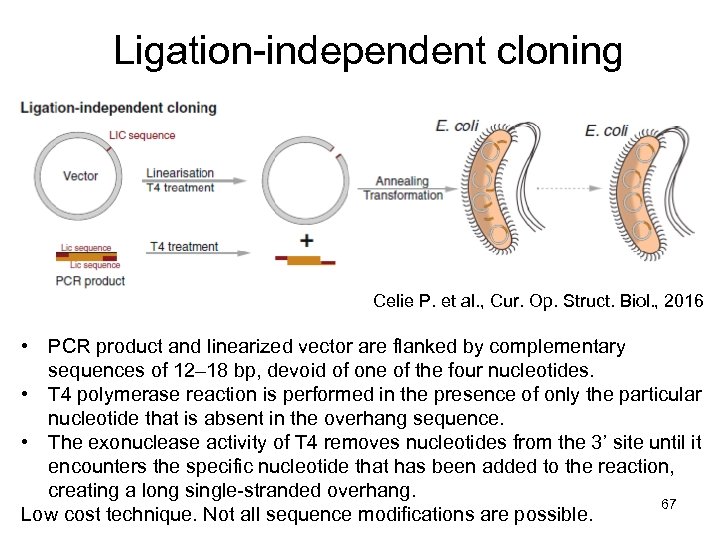
Ligation-independent cloning Celie P. et al. , Cur. Op. Struct. Biol. , 2016 • PCR product and linearized vector are flanked by complementary sequences of 12– 18 bp, devoid of one of the four nucleotides. • T 4 polymerase reaction is performed in the presence of only the particular nucleotide that is absent in the overhang sequence. • The exonuclease activity of T 4 removes nucleotides from the 3’ site until it encounters the specific nucleotide that has been added to the reaction, creating a long single-stranded overhang. 67 Low cost technique. Not all sequence modifications are possible.
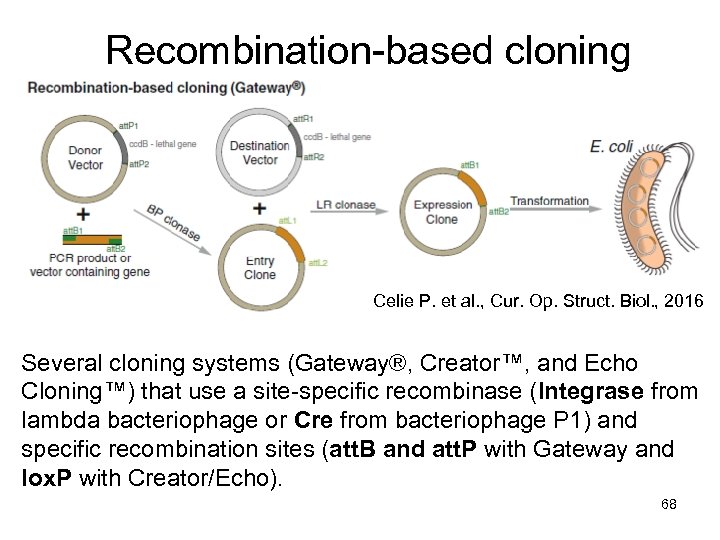
Recombination-based cloning Celie P. et al. , Cur. Op. Struct. Biol. , 2016 Several cloning systems (Gateway®, Creator™, and Echo Cloning™) that use a site-specific recombinase (Integrase from lambda bacteriophage or Cre from bacteriophage P 1) and specific recombination sites (att. B and att. P with Gateway and lox. P with Creator/Echo). 68
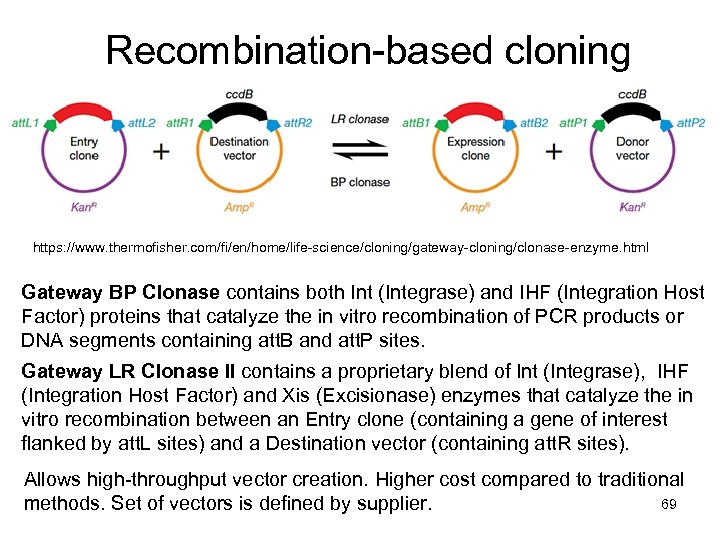
Recombination-based cloning https: //www. thermofisher. com/fi/en/home/life-science/cloning/gateway-cloning/clonase-enzyme. html Gateway BP Clonase contains both Int (Integrase) and IHF (Integration Host Factor) proteins that catalyze the in vitro recombination of PCR products or DNA segments containing att. B and att. P sites. Gateway LR Clonase II contains a proprietary blend of Int (Integrase), IHF (Integration Host Factor) and Xis (Excisionase) enzymes that catalyze the in vitro recombination between an Entry clone (containing a gene of interest flanked by att. L sites) and a Destination vector (containing att. R sites). Allows high-throughput vector creation. Higher cost compared to traditional 69 methods. Set of vectors is defined by supplier.

PCR 70
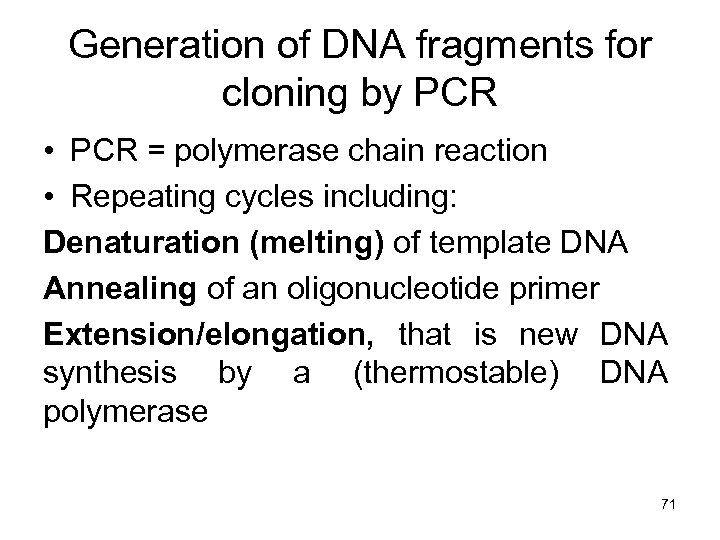
Generation of DNA fragments for cloning by PCR • PCR = polymerase chain reaction • Repeating cycles including: Denaturation (melting) of template DNA Annealing of an oligonucleotide primer Extension/elongation, that is new DNA synthesis by a (thermostable) DNA polymerase 71
![Principle of PCR Exponential amplification of template DNA (25 cycles [DNA at beginning] x Principle of PCR Exponential amplification of template DNA (25 cycles [DNA at beginning] x](https://present5.com/presentation/e12c2f4a11ebecd90acd4e81ccefcdfb/image-72.jpg)
Principle of PCR Exponential amplification of template DNA (25 cycles [DNA at beginning] x 225 33 x 106 fold 72 amplification)
Applications of PCR 1. Medical - Clinical diagnostic tests for some genetic disorders - Clinical diagnostic tests for various infectious agents - Blood screening to quantify the ammount of virus 2. Research - Molecular cloning - Genetic engineering - Mapping of the human genome 3. Forensic - Molecular archaeology and ancient DNA - Criminal investigations, parental testing etc. 73
Requirements for PCR • Thermocycler • Thermostable DNA polymerase • Template DNA (VERY little, 0. 1 -1 ng plasmid, down to 1 pg) • Target-specific oligonucleotide primers • Proper buffer conditions • d. NTPs 74

Thermocycler http: //www. molecularstation. com/molecular-biology-images/509 -pcr-pictures/71 -thermocycler-old-pcr- 75 machine. html? size=big
Thermostable DNA polymerases An automated thermocycler-based DNA amplification became possible only after Taq polymerase, that remains active even after DNA denaturation at 95 °C, was used. Purified from thermophilic bacterium, Thermus aquaticus, which naturally lives in hot (50 to 80 °C) environments such as hot springs of Yellowstone national park. Wikipedia 76
Thermostable DNA polymerases • Available from a variety of sources – Taq (NEB and others), Dynazyme (Finnzymes/ Thermo Fisher Scientific): cheap, no proofreading activity; good for analytical PCR and short DNA fragments – Platinum Taq Hi Fi (Invitrogen), Dynazyme EXT (Finnzymes): proofreading activity, higher fidelity and longer products – Pfu (varying sources): better proofreading activity, low processivity; good for high fidelity cloning of shorter DNA fragments – Pfu turbo (Stratagene): proofreading like Pfu, but better processivity; also good for longer PCR products (>4 kb, up to over 10 kb) – Phusion (Finnzymes and NEB), Pfu Ultra (Stratagene), KAPA Hi. Fi (Kapa Biosystems): Very high fidelity, good for long products 77 – Many others….
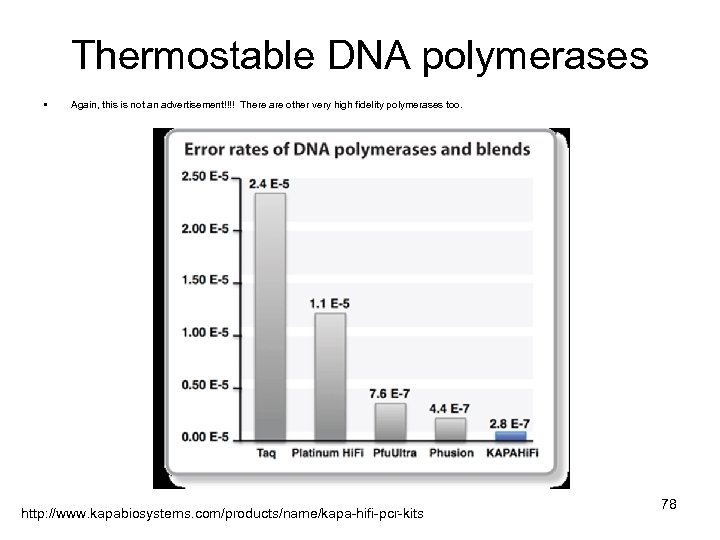
Thermostable DNA polymerases • Again, this is not an advertisement!!!! There are other very high fidelity polymerases too. http: //www. kapabiosystems. com/products/name/kapa-hifi-pcr-kits 78
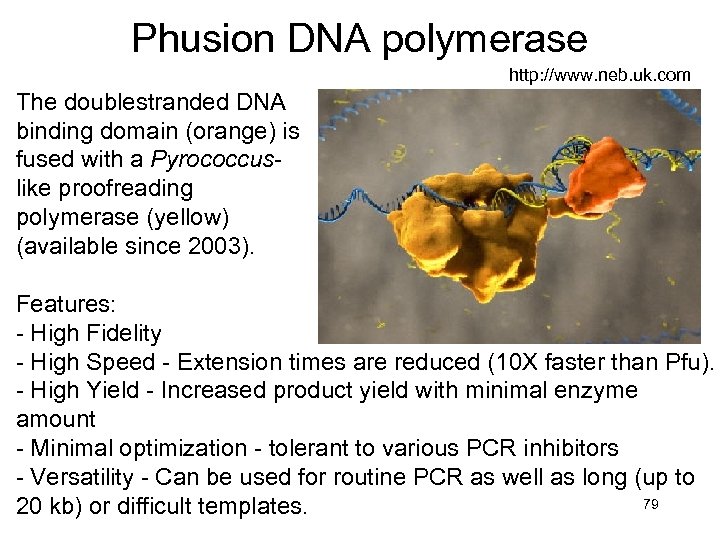
Phusion DNA polymerase http: //www. neb. uk. com The doublestranded DNA binding domain (orange) is fused with a Pyrococcuslike proofreading polymerase (yellow) (available since 2003). Features: - High Fidelity - High Speed - Extension times are reduced (10 X faster than Pfu). - High Yield - Increased product yield with minimal enzyme amount - Minimal optimization - tolerant to various PCR inhibitors - Versatility - Can be used for routine PCR as well as long (up to 79 20 kb) or difficult templates.
Concentrations of PCR components • Vary depending on manufacturer and enzymes – Check instructions carefully!! • Typically: – 10 -250 ng of template DNA – Buffer with 1. 5 m. M Mg. Cl 2 (final concentration) – 200 u. M of each d. NTP – 0. 3 -1. 0 u. M of each primer – Enzyme according to manufacturer Amplification of difficult sequences (GC-rich etc. ) may be improved by additives such as DMSO (usually 3%), formamide or glycerol 80
PCR conditions 1) Initialization. 95 °C (or 98 °C if extremely thermostable polymerase is used) for 1 min. 2) Denaturation. 95 °C for 50 seconds. Double-stranded DNA template is melted into two single-stranded DNA molecules. 3) Annealing. 50– 65 °C for 50 seconds. The primers anneal to the single-stranded DNA template. 4) Extension/elongation. Temperature depends on 25 -35 polymerase used (68 °C for Taq, 72 °C for Phusion). A new cycles DNA strand complementary to the DNA template is synthesized. The extension time depends both on the DNA polymerase used and on the length of the DNA fragment to be amplified (about thousand bases per minute for Taq; per 15 -30 seconds for Phusion). 5) Final elongation. 72 °C for 5– 15 min. 6) Final hold. 4°C for an indefinite time for short-term storage of the reaction products. 81
PCR conditions • Manufacturer’s instructions – NEB Phusion: https: //www. neb. com/protocols/1/01/01/pcr-protocol-m 0530 – Finnzymes/Thermo Fisher Phusion: https: //www. thermofisher. com/fi/en/home/brands/thermo-scientific/molecularbiology/thermo-scientific-molecular-biologyproducts/phusion. html#/legacy=www. finnzymes. com – Pfu Ultra http: //www. genomics. agilent. com/literature. jsp? crumb. Action=push&tab. Id=AG-PR -1149&content. Type=User+Manual – Taq polymerase https: //www. neb. com/protocols/1/01/01/taq-dna-polymerase-with-standard-taq buffer-m 0273 82
Things to pay attention to • Avoid the use of buffers containing EDTA – Because of the low concentration of Mg. Cl 2, PCR reactions are sensitive even to small amounts of EDTA • It is common practice to assemble PCR reactions on ice – There is a danger of getting unspecific PCR products due to low enzymatic activity of polymerase at room temperature – If you encounter problems with unspecific products, try a Hot Start (adding the enzyme just before the first elongation cycle) or use Hot Start polymerase preparations (contain specific inhibitor that inhibits polymerase activity at temperatures below 45°C, but releases the enzyme during normal cycling conditions) 83
Is It Really Necessary to Assemble PCR Reactions on Ice? The purity of Taq polymerase in the early 1990 s was not very high: enzyme preparations contained bacterial DNA contaminations. Therefore, there was the possibility of primers binding the bacterial DNA template at room temperature. Current polymerase purification techniques are much improved. None of the PCR components degrade at room temperature by itself. However, PCR primers may degrade due to the 3’ to 5’ exonuclease activity of Pfu DNA Polymerase. If you keep your PCR reaction mixture at room temperature, it is always safer to have the original concentrated components on ice. 84 Add the polymerase at the very last step!
Things to pay attention to • Prepare PCR in a clean working environment 85
PCR sensitivity Sensitivity is both a blessing and a curse for people who use it to analyze DNA. • PCR is very vulnerable to contamination (like DNA samples from investigator’s own body) • The ability to amplify everything in the sample means that a PCR can be used to find DNA which may only be present in trace amounts in a sample (perhaps as low as 1 molecule). 86

PCR sensitivity Neanderthal genomic DNA was sequenced using material obtained from fossilized bones that are tens of thousands of years old (despite contaminations with microbal and modern human’s DNA). 87
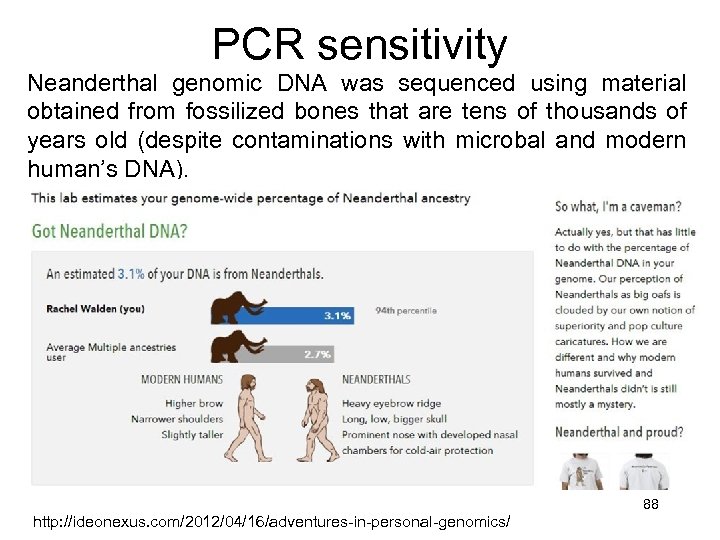
PCR sensitivity Neanderthal genomic DNA was sequenced using material obtained from fossilized bones that are tens of thousands of years old (despite contaminations with microbal and modern human’s DNA). http: //ideonexus. com/2012/04/16/adventures-in-personal-genomics/ 88
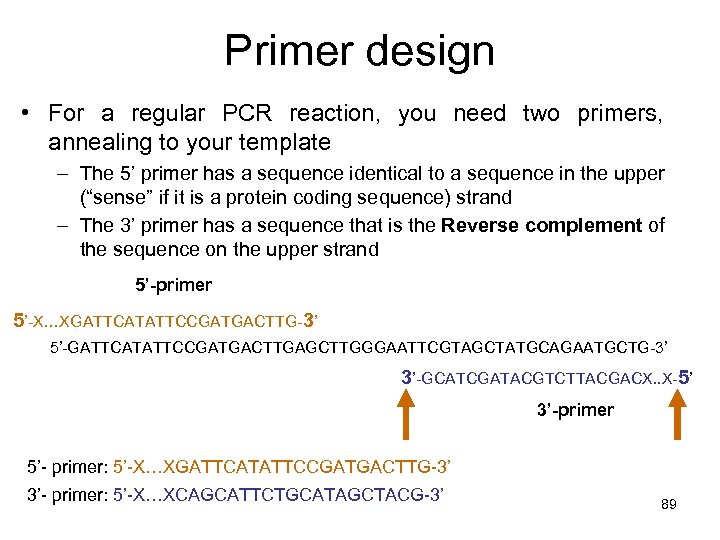
Primer design • For a regular PCR reaction, you need two primers, annealing to your template – The 5’ primer has a sequence identical to a sequence in the upper (“sense” if it is a protein coding sequence) strand – The 3’ primer has a sequence that is the Reverse complement of the sequence on the upper strand 5’-primer 5’-X…XGATTCATATTCCGATGACTTG-3’ 5’-GATTCATATTCCGATGACTTGAGCTTGGGAATTCGTAGCTATGCAGAATGCTG-3’ 3’-GCATCGATACGTCTTACGACX. . X-5’ 3’-primer 5’- primer: 5’-X…XGATTCATATTCCGATGACTTG-3’ 3’- primer: 5’-X…XCAGCATTCTGCATAGCTACG-3’ 89
Primer design • Length (depending on template source) 18 -25 bp homologous to gene/c. DNA intended for cloning • The last (3’-most) base should be a G or a C • Addition of required restriction site + extra bases 5’ to restriction site (3 -6? ) • • Kozak sequence CCACC before ATG ? (initiation of the translation). What stop codon do you need? • If you have a choice – 50 -60 % GC content – Avoid long runs (>3) of any one nucleotide – Avoid primer dimers and strong secondary structure 90
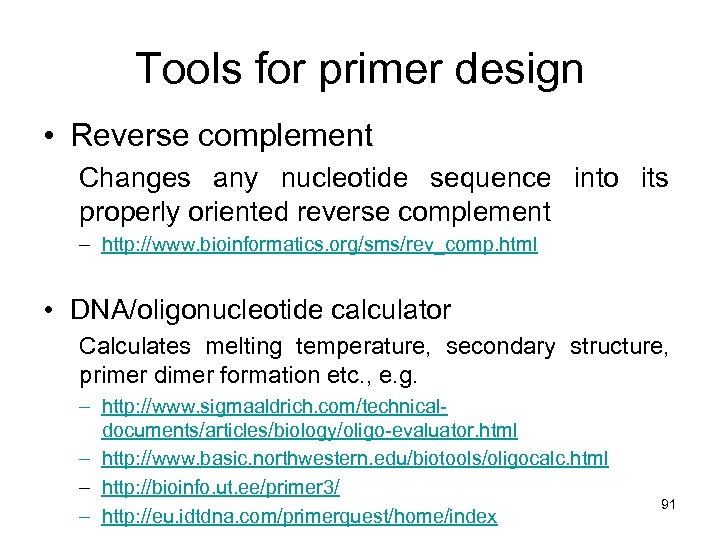
Tools for primer design • Reverse complement Changes any nucleotide sequence into its properly oriented reverse complement – http: //www. bioinformatics. org/sms/rev_comp. html • DNA/oligonucleotide calculator Calculates melting temperature, secondary structure, primer dimer formation etc. , e. g. – http: //www. sigmaaldrich. com/technicaldocuments/articles/biology/oligo-evaluator. html – http: //www. basic. northwestern. edu/biotools/oligocalc. html – http: //bioinfo. ut. ee/primer 3/ – http: //eu. idtdna. com/primerquest/home/index 91
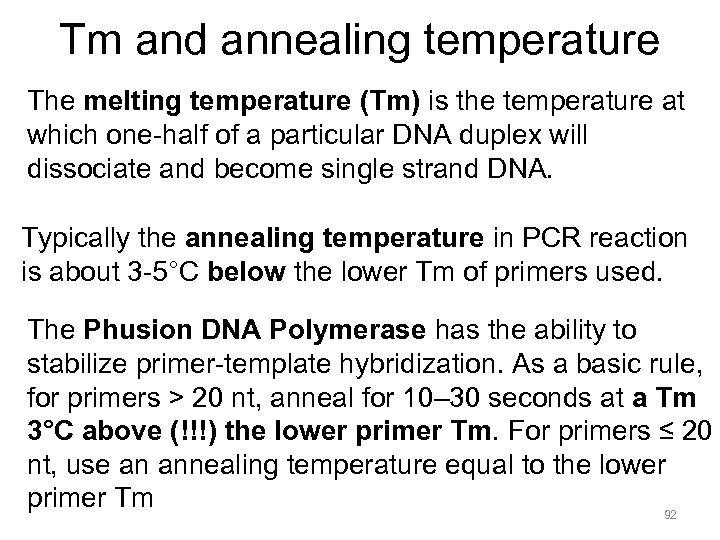
Tm and annealing temperature The melting temperature (Tm) is the temperature at which one-half of a particular DNA duplex will dissociate and become single strand DNA. Typically the annealing temperature in PCR reaction is about 3 -5°C below the lower Tm of primers used. The Phusion DNA Polymerase has the ability to stabilize primer-template hybridization. As a basic rule, for primers > 20 nt, anneal for 10– 30 seconds at a Tm 3°C above (!!!) the lower primer Tm. For primers ≤ 20 nt, use an annealing temperature equal to the lower primer Tm 92
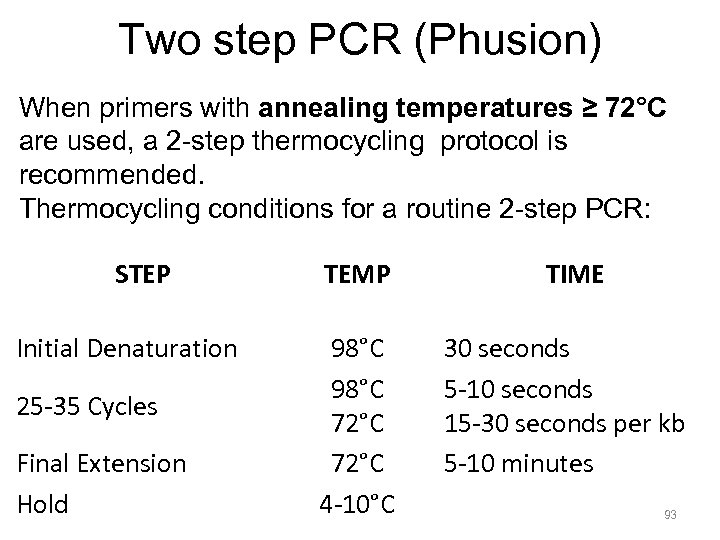
Two step PCR (Phusion) When primers with annealing temperatures ≥ 72°C are used, a 2 -step thermocycling protocol is recommended. Thermocycling conditions for a routine 2 -step PCR: STEP Initial Denaturation 25 -35 Cycles Final Extension Hold TEMP TIME 98°C 72°C 4 -10°C 30 seconds 5 -10 seconds 15 -30 seconds per kb 5 -10 minutes 93
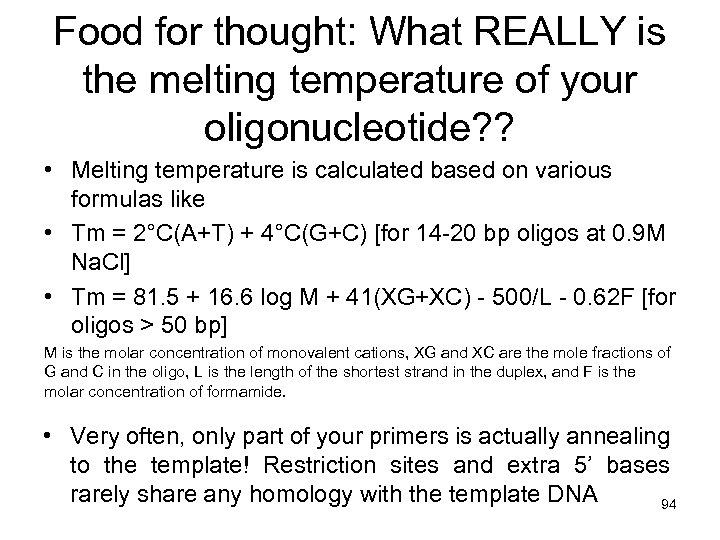
Food for thought: What REALLY is the melting temperature of your oligonucleotide? ? • Melting temperature is calculated based on various formulas like • Tm = 2°C(A+T) + 4°C(G+C) [for 14 -20 bp oligos at 0. 9 M Na. Cl] • Tm = 81. 5 + 16. 6 log M + 41(XG+XC) - 500/L - 0. 62 F [for oligos > 50 bp] M is the molar concentration of monovalent cations, XG and XC are the mole fractions of G and C in the oligo, L is the length of the shortest strand in the duplex, and F is the molar concentration of formamide. • Very often, only part of your primers is actually annealing to the template! Restriction sites and extra 5’ bases rarely share any homology with the template DNA 94

An example: Xho. I site 5’-TTATCTCGAGGATTCATATTCCGATGACTTG-3’ 5’…. . CTAGGCCTTAGATTCATATTCCGATGACTTGAGCTTGGGAATTCGTAGCTATGCAGAATGCTG…. -3’ In rare cases, you may not get any product at all, or get unspecific product… 95

Is there a solution? • Most PCRs appear to work well under “Standard” conditions (annealing temperature of 54 -56 o. C) • If you have problems getting any product, you may – Try a PCR reaction with an annealing temperature adjusted to the “homologous” part of your primers (you can also run the first ten or so cycles at this annealing temperature and then increase it) – Determine the optimal annealing temperature using a gradient PCR machine (reaction will be performed at different annealing temperatures, with all other values being the same; the annealing gradient should extend up to the extension temperature). 96
Processing and verification of PCR product • If the product was obtained by PCR from genomic DNA: – Run out a small aliquot (e. g. 3 -5 ul) on gel to determine if product is present and has right size – If O. K. purify the rest of the product directly from PCR mix • If product was obtained from a plasmid template – Run out entire reaction on agarose gel, check size and purify from gel • Ultimate verification: always sequence PCR products! 97
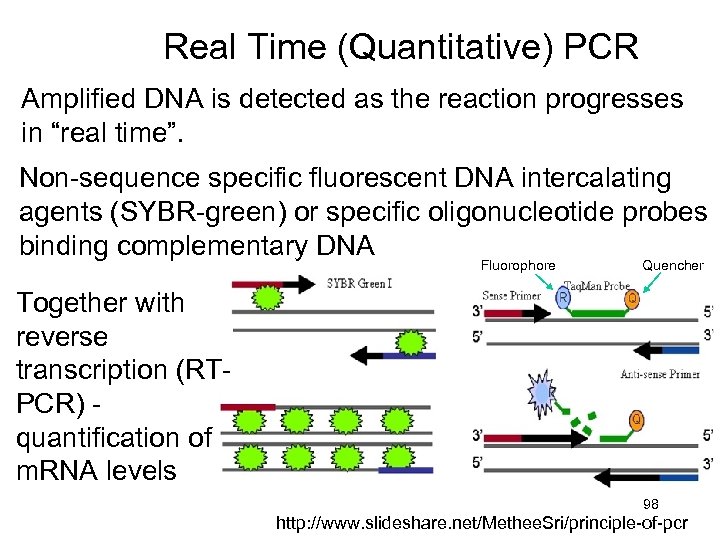
Real Time (Quantitative) PCR Amplified DNA is detected as the reaction progresses in “real time”. Non-sequence specific fluorescent DNA intercalating agents (SYBR-green) or specific oligonucleotide probes binding complementary DNA Fluorophore Quencher Together with reverse transcription (RTPCR) quantification of m. RNA levels 98 http: //www. slideshare. net/Methee. Sri/principle-of-pcr
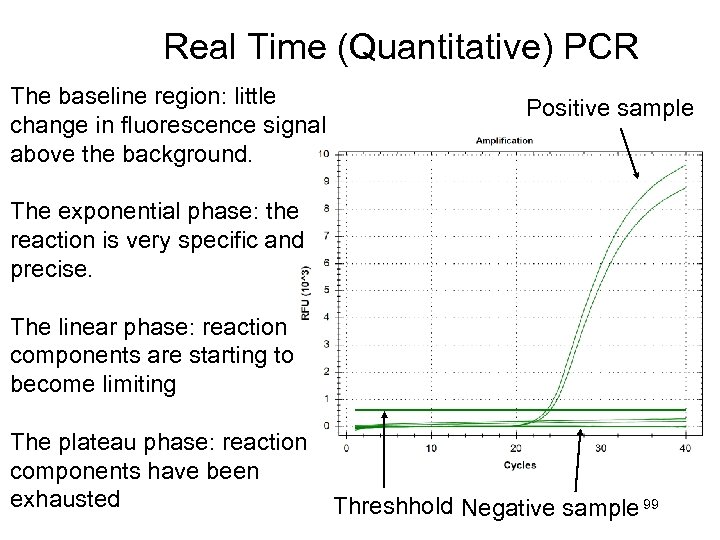
Real Time (Quantitative) PCR The baseline region: little change in fluorescence signal above the background. Positive sample The exponential phase: the reaction is very specific and precise. The linear phase: reaction components are starting to become limiting The plateau phase: reaction components have been exhausted Threshhold Negative sample 99
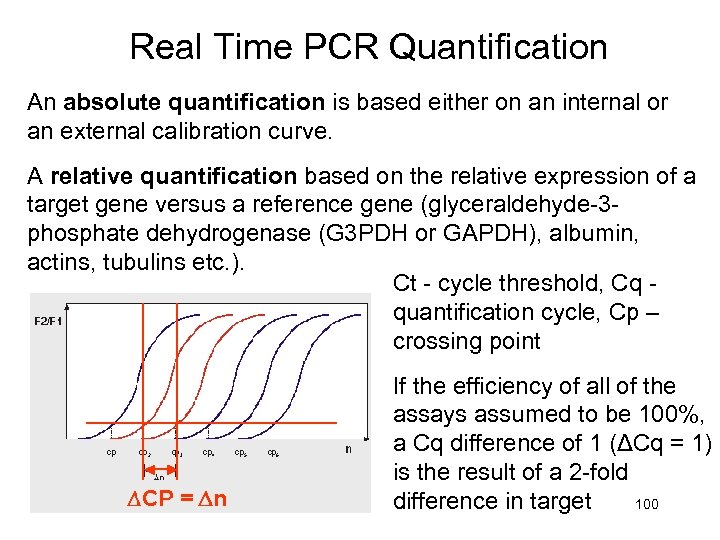
Real Time PCR Quantification An absolute quantification is based either on an internal or an external calibration curve. A relative quantification based on the relative expression of a target gene versus a reference gene (glyceraldehyde-3 phosphate dehydrogenase (G 3 PDH or GAPDH), albumin, actins, tubulins etc. ). Ct - cycle threshold, Cq - quantification cycle, Cp – crossing point If the efficiency of all of the assays assumed to be 100%, a Cq difference of 1 (ΔCq = 1) is the result of a 2 -fold difference in target 100
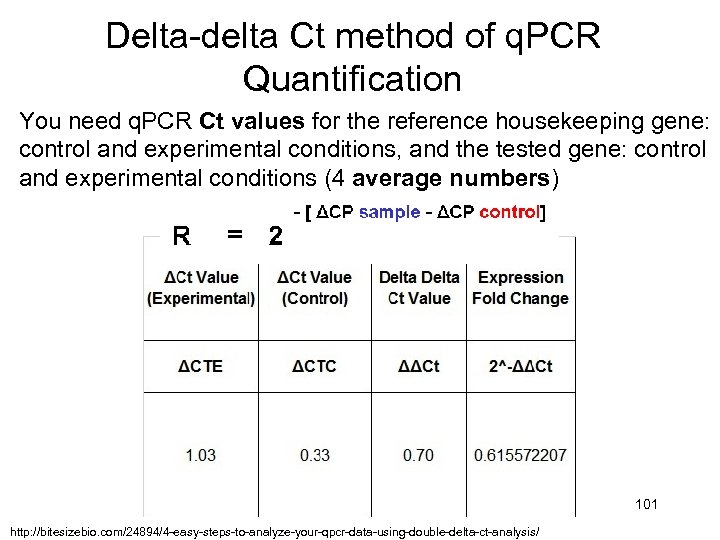
Delta-delta Ct method of q. PCR Quantification You need q. PCR Ct values for the reference housekeeping gene: control and experimental conditions, and the tested gene: control and experimental conditions (4 average numbers) 101 http: //bitesizebio. com/24894/4 -easy-steps-to-analyze-your-qpcr-data-using-double-delta-ct-analysis/
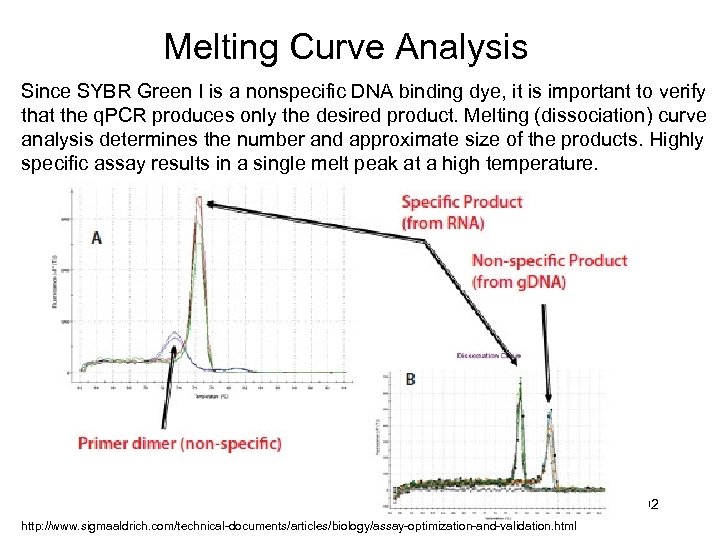
Melting Curve Analysis Since SYBR Green I is a nonspecific DNA binding dye, it is important to verify that the q. PCR produces only the desired product. Melting (dissociation) curve analysis determines the number and approximate size of the products. Highly specific assay results in a single melt peak at a high temperature. 102 http: //www. sigmaaldrich. com/technical-documents/articles/biology/assay-optimization-and-validation. html
Primer design for q. PCR • Length (depending on template source) 18 -23 bp (optimal 20) homologous to c. DNA • The last (3’-most) base should be a G or a C • Tm 57 -63 (optimal 60) • Product size 100 -300 (better till 200) • Intron-exon span • If you have a choice – 50 -60 % GC content – Avoid long runs (>3) of any one nucleotide – Avoid primer dimers and strong secondary structure 103

Information to be included on your assignment solutions • Restriction Maps of vector (commercially available plasmid) and insert (c. DNA prepared from total tissue RNA) • PCR and Mutagenesis: – – Oligonucleotide sequences PCR reaction mix Cycling conditions Processing? (Analytical gel + direct purification or preparative agarose gel + how can you be sure that the mutagenesis works? ) • Preparative restriction digests: – Digest mixes indicate enzymes and units used, indicate DNA concentrations • Ligation: – Ligation mix • Analytical restriction digest – Digest mixes – Expected restriction patterns for the first and second digest (you may also want to draw a circular map) • Point Mutagenesis (Stratagene) 104
Materials/assumed conditions for assignment (only recommendations) • Enzymes: – PCR: NEB Phusion protocol – Restriction digests: NEB enzymes https: //www. neb. com/tools-andresources/interactive-tools/double-digest-finder – Ligation: Epicentre Fast-Link™ DNA Ligation Kit – Site-directed mutagenesis: Stratagene protocol 105
Useful Databases for assignments • Pub. Med (Nucleotide) – http: //www. ncbi. nlm. nih. gov/nucleotide • Stratagene (Site-directed mutagenesis) – http: //www. tufts. edu/~mcourt 01/Documents/Stratagene%20 Quik change%20 mutagenesis. pdf (NOTE: only page number five is needed for the primer design - not the entire manual) • Ensembl Genome Browser – http: //www. ensembl. org/index. html • Expert Protein Analysis System – http: //au. expasy. org/ • Human Protein Reference Database (Human Proteinpedia) – http: //www. hprd. org/ 106

Vector databases • https: //www. addgene. org/vector-database/ • https: //www. neb. com/tools-and-resources/interactivetools/dna-sequences-and-maps-tool • https: //www. snapgene. com/resources/plasmid_files/your _time_is_valuable/ Textbook Molecular Cloning: A Laboratory Manual, 3 Vol. (3 rd edition 2000) Joseph Sambrook, David W. Russell 107

Deadline and submission • Assignments have to be sent in by the end of September • Please send to: Anatoliy. Samoylenko@oulu. fi 108
e12c2f4a11ebecd90acd4e81ccefcdfb.ppt