c507650ca4db521b4687869cbc21fa95.ppt
- Количество слайдов: 18
 Mole Madness How do chemists count molecules?
Mole Madness How do chemists count molecules?
 The th 13 Chick …. Mr. Zachmann presented to us the parable of the 13 th chicken. He asked us to consider a hen sitting on the 13 th nest. The hen observes the farmer approaching the hen house. He appears to be holding an empty egg carton and there is the smell of cooking bacon in the air. Zachmann asked us to put ourselves in the place of the 13 th hen. He then asked if we would fear for the safety of our future chick. After due consideration we told him that we did not fear for the safety of our chick. The “packaging size” for eggs is the dozen. Since the farmer always took the eggs in order of the nests the 13 th nest was safe.
The th 13 Chick …. Mr. Zachmann presented to us the parable of the 13 th chicken. He asked us to consider a hen sitting on the 13 th nest. The hen observes the farmer approaching the hen house. He appears to be holding an empty egg carton and there is the smell of cooking bacon in the air. Zachmann asked us to put ourselves in the place of the 13 th hen. He then asked if we would fear for the safety of our future chick. After due consideration we told him that we did not fear for the safety of our chick. The “packaging size” for eggs is the dozen. Since the farmer always took the eggs in order of the nests the 13 th nest was safe.
 Fitting the Package to the Task After a little discussion we agreed that a dozen was a reasonable packaging unit (container size) for eggs as eggs were a fairly big item and they do go bad over time. The next slide reminded us that a container size of 12 was not specific to only eggs.
Fitting the Package to the Task After a little discussion we agreed that a dozen was a reasonable packaging unit (container size) for eggs as eggs were a fairly big item and they do go bad over time. The next slide reminded us that a container size of 12 was not specific to only eggs.
 Dozen Paper Clips As it turns out large 2 inch paper clips come in containers that hold a dozen. Smaller paper clips come in containers of 50. When it comes to packaging, size does matter.
Dozen Paper Clips As it turns out large 2 inch paper clips come in containers that hold a dozen. Smaller paper clips come in containers of 50. When it comes to packaging, size does matter.
 The Baker’s Dozen Mr. Zachmann admitted that the baker’s dozen might confuse us as it actually contains 13 items. We then discussed the intentional error as a way to entice us to buy the larger unit as we would be getting more that we should…. .
The Baker’s Dozen Mr. Zachmann admitted that the baker’s dozen might confuse us as it actually contains 13 items. We then discussed the intentional error as a way to entice us to buy the larger unit as we would be getting more that we should…. .
 Sharpies The container size for sharpies happens to be 8 pens.
Sharpies The container size for sharpies happens to be 8 pens.
 The Shoebox When we buy shoes they come packaged in sets of two. Unless you are a centipede the packaging size seems appropriate for human anatomy.
The Shoebox When we buy shoes they come packaged in sets of two. Unless you are a centipede the packaging size seems appropriate for human anatomy.
 Main Point: Thought goes into container size The container size for the chemist is called the mole. Like a dozen the mole represents a specific number of things. Because things chemist work with are very small the number of items in a mole is very large. The number is actually named after a very special person, Amedeo Avogadro. The actual number is 6. 02 x 1023.
Main Point: Thought goes into container size The container size for the chemist is called the mole. Like a dozen the mole represents a specific number of things. Because things chemist work with are very small the number of items in a mole is very large. The number is actually named after a very special person, Amedeo Avogadro. The actual number is 6. 02 x 1023.
 Chemists do not count molecules directly (one at a time). If you lick you finger and touch it to the top of a sugar bowl and then place it on your tongue you will get about 1. 0 x 1023 molecules of sugar in your mouth. If you counted out those molecules one by one it would have taken you 3. 17 x 1015 years (see calculation on the next slide). Making a cake would be clearly out of the question unless we had a much more efficient and reliable method than direct counting. Fortunately, we do.
Chemists do not count molecules directly (one at a time). If you lick you finger and touch it to the top of a sugar bowl and then place it on your tongue you will get about 1. 0 x 1023 molecules of sugar in your mouth. If you counted out those molecules one by one it would have taken you 3. 17 x 1015 years (see calculation on the next slide). Making a cake would be clearly out of the question unless we had a much more efficient and reliable method than direct counting. Fortunately, we do.
 Time needed to count 1. 0 x 1023 molecules of sugar. (1. 0 x 1023 molecules of sugar) x [(1 s)/ (1 molecule)] x [(1 min)/(60 s)] x [(1 hr)/(60 min)] x [(1 Day)/(24 hrs)] x [(1 Year)/(365 Days)] = 3. 17 x 1015 years. The practical alternative is to count the population by delivering to a container the expected mass of the desired population. Before we use this method we need to prove that it works.
Time needed to count 1. 0 x 1023 molecules of sugar. (1. 0 x 1023 molecules of sugar) x [(1 s)/ (1 molecule)] x [(1 min)/(60 s)] x [(1 hr)/(60 min)] x [(1 Day)/(24 hrs)] x [(1 Year)/(365 Days)] = 3. 17 x 1015 years. The practical alternative is to count the population by delivering to a container the expected mass of the desired population. Before we use this method we need to prove that it works.
 Required Materials In this experiment we will change the number of plastic balls in a population and then find the mass of the population. In the pictures to the right you can see that we tarred the mass of the beaker to make reporting the mass of the population easier.
Required Materials In this experiment we will change the number of plastic balls in a population and then find the mass of the population. In the pictures to the right you can see that we tarred the mass of the beaker to make reporting the mass of the population easier.
 Mass of 3, 12, 15 and then 23 plastic balls.
Mass of 3, 12, 15 and then 23 plastic balls.
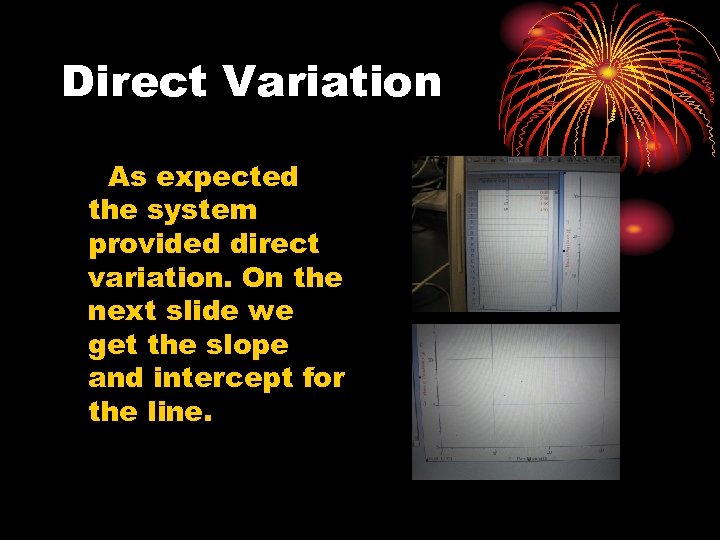 Direct Variation As expected the system provided direct variation. On the next slide we get the slope and intercept for the line.
Direct Variation As expected the system provided direct variation. On the next slide we get the slope and intercept for the line.
 Testing the System To test the technique of counting though massing we delivered an unknown number of plastic balls to the beaker. The mass of the plastic balls was 47. 22 grams. On the next page we use a logic chain to predict the number of balls present in the beaker.
Testing the System To test the technique of counting though massing we delivered an unknown number of plastic balls to the beaker. The mass of the plastic balls was 47. 22 grams. On the next page we use a logic chain to predict the number of balls present in the beaker.
 Verification of the Data • Mass of Population = 47. 72 g From the slope of the best fit line we know. 995 g = 1 Ball • Using the logic chain approach: 47. 72 g Balls X [(1 Ball) / (. 995 g Balls)] = 47. 457 Balls. Since we can not have a half a ball we round this value to the nearest whole number of 48 Balls. • When we actually counted the Balls in the unknown population 48 balls were present. • Conclusion: uniform things can be counted by massing.
Verification of the Data • Mass of Population = 47. 72 g From the slope of the best fit line we know. 995 g = 1 Ball • Using the logic chain approach: 47. 72 g Balls X [(1 Ball) / (. 995 g Balls)] = 47. 457 Balls. Since we can not have a half a ball we round this value to the nearest whole number of 48 Balls. • When we actually counted the Balls in the unknown population 48 balls were present. • Conclusion: uniform things can be counted by massing.
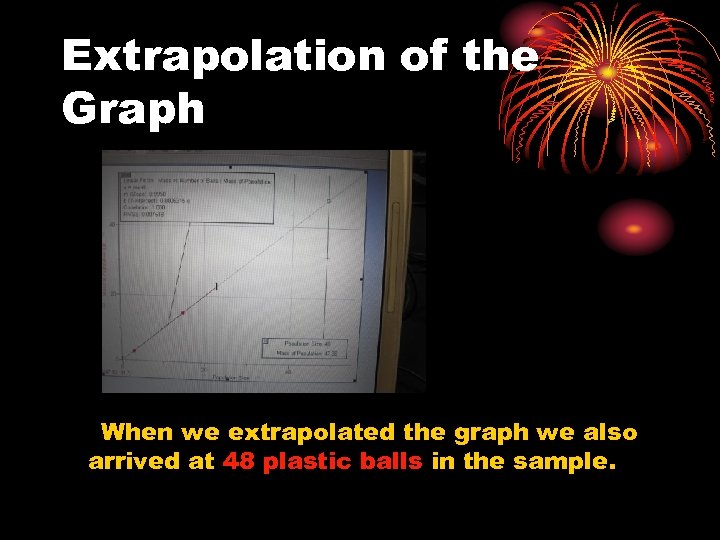 Extrapolation of the Graph When we extrapolated the graph we also arrived at 48 plastic balls in the sample.
Extrapolation of the Graph When we extrapolated the graph we also arrived at 48 plastic balls in the sample.
 The Great Truth Atomic Mass of one Number of atoms Mass of one Wt. (AMU) one AMU atom in g in one mole of (grams) Na 23 1. 66 x 10 -24 3. 82 x 10 -23 6. 02 x 1023 23 K 39 1. 66 x 10 -24 6. 47 x 10 -23 6. 02 x 1023 39 Ca 40 1. 66 x 10 -24 6. 64 x 10 -23 6. 02 x 1023 40 P 31 1. 66 x 10 -24 5. 15 x 10 -23 6. 02 x 1023 31 Ba 137 1. 66 x 10 -24 2. 27 x 10 -23 6. 02 x 1023 137 Element • In class we used this graphic to show you that the mass of one mole of any element is equal to the molecular mass in grams. This mass is also called the molar mass. • The Great Truth (as Mr. Zachmann calls it) says: The Molar Mass = 1 mole = 6. 02 x 1023 molecules
The Great Truth Atomic Mass of one Number of atoms Mass of one Wt. (AMU) one AMU atom in g in one mole of (grams) Na 23 1. 66 x 10 -24 3. 82 x 10 -23 6. 02 x 1023 23 K 39 1. 66 x 10 -24 6. 47 x 10 -23 6. 02 x 1023 39 Ca 40 1. 66 x 10 -24 6. 64 x 10 -23 6. 02 x 1023 40 P 31 1. 66 x 10 -24 5. 15 x 10 -23 6. 02 x 1023 31 Ba 137 1. 66 x 10 -24 2. 27 x 10 -23 6. 02 x 1023 137 Element • In class we used this graphic to show you that the mass of one mole of any element is equal to the molecular mass in grams. This mass is also called the molar mass. • The Great Truth (as Mr. Zachmann calls it) says: The Molar Mass = 1 mole = 6. 02 x 1023 molecules
 Triad of Truth We also call the Great Truth the “Triad of Truth. ” The triad is simply the molar mass = 1 mole = Avogadro’s number
Triad of Truth We also call the Great Truth the “Triad of Truth. ” The triad is simply the molar mass = 1 mole = Avogadro’s number


