ec1f249b2debc902051a2f2e4cd6e2f1.ppt
- Количество слайдов: 67

Module 3 Use, calibration and maintenance of laboratory equipment 1

Learning objectives At the end of this module you will be able to: Ø describe briefly the operating characteristics of biological safety cabinets, centrifuges, autoclaves, inspissators, p. H meters, incubators; Ø correctly use biological safety cabinets, centrifuges, autoclaves, inspissators, p. H meters, incubators; Ø describe the maintenance operations for biological safety cabinets, centrifuges, autoclaves, inspissators, p. H meters, incubators. 2

Content outline Principles, use and maintenance of: • • • biological safety cabinet (BSC) centrifuge autoclave inspissator (coagulator) p. H meter incubator 3

Biological safety cabinet (BSC) A ventilated contained area providing protection for the operator and the environment against infectious aerosols during the handling of hazardous microorganisms The most important equipment in all diagnostic mycobacteriology laboratories is the BSC. 4

HEPA filter HEPA = high-efficiency particulate air The HEPA filter traps and remove 99. 97% of airborne particles of diameter 0. 3 µm or more. 5

BSC class I and II • BSCs should be ducted or vented to the outside. • No recirculation into the room is allowed. • The BSC should be connected to a UPS (Universal Power Supply). BSC class I and II – main differences BSC class Minimal face velocity (m/s) Air flow (%) Exhaust system Re- Exhausted circulated I 0. 38 0 100 Hard duct IIA 1 0. 38 70 30 Thimble connection IIA 2 0. 51 70 30 Thimble connection IIB 1 0. 51 30 70 Hard duct IIB 2 0. 51 0 100 Hard duct 6
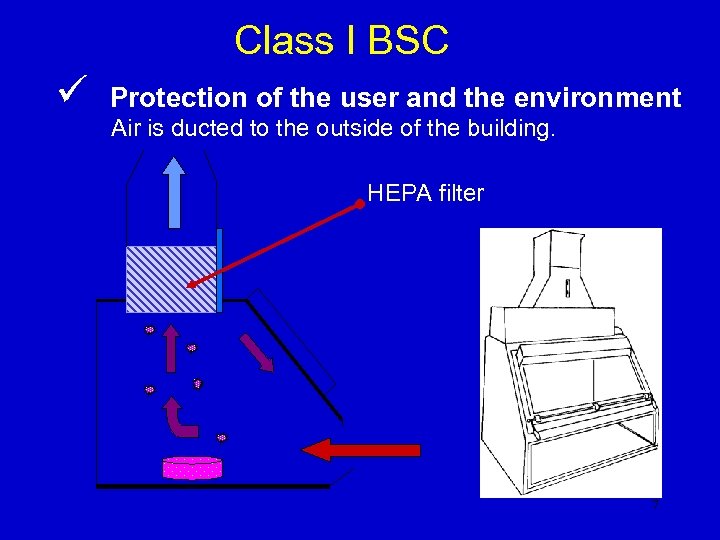
Class I BSC ü Protection of the user and the environment Air is ducted to the outside of the building. HEPA filter 7
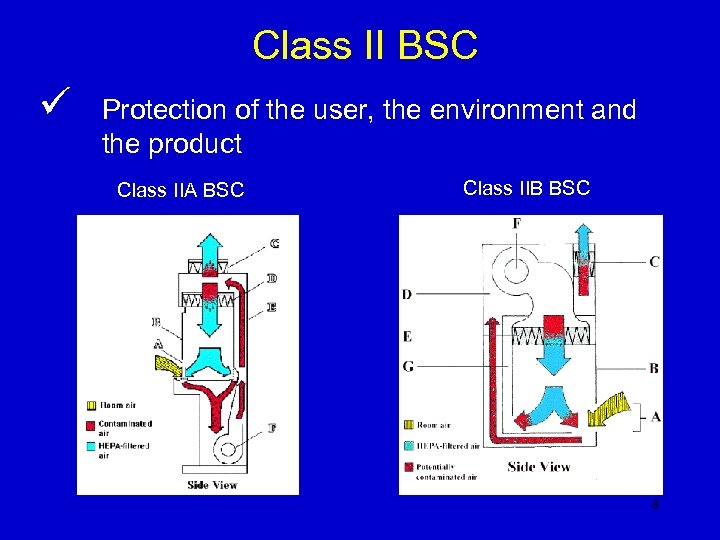
Class II BSC ü Protection of the user, the environment and the product Class IIA BSC Class IIB BSC 8
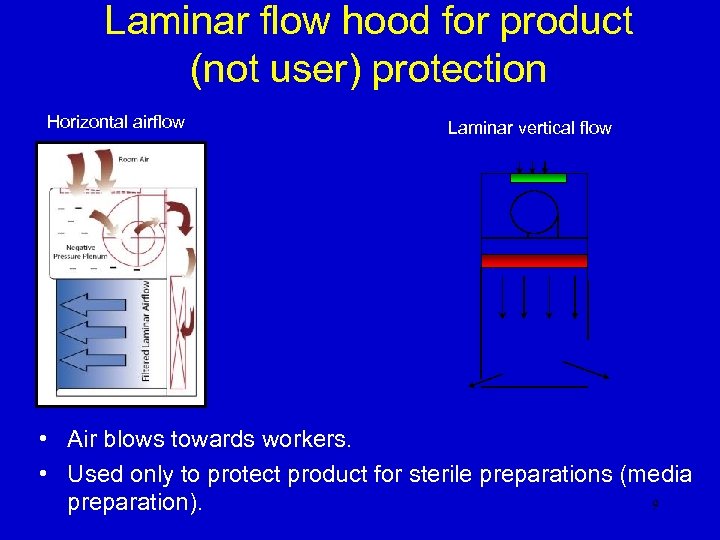
Laminar flow hood for product (not user) protection Horizontal airflow Laminar vertical flow • Air blows towards workers. • Used only to protect product for sterile preparations (media 9 preparation).
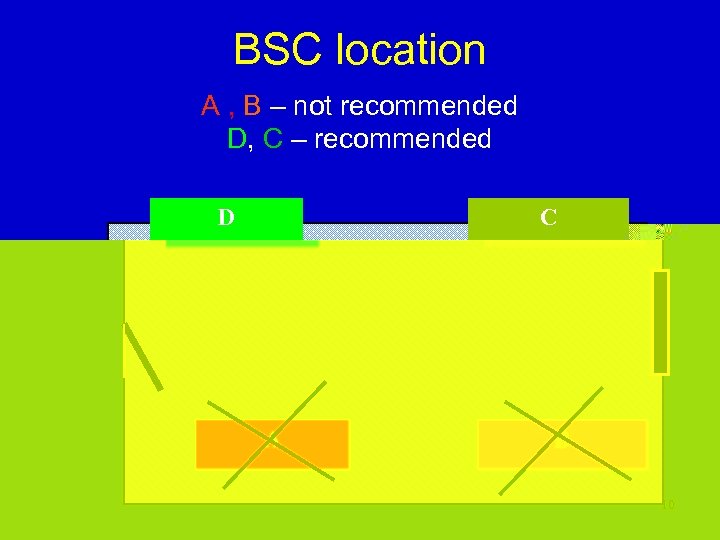
BSC location A , B – not recommended D, C – recommended D C A B 10

BSC – operations for correct use • Switch on the BSC airflow and the UV light (if present) 5– 15 minutes before use. • Check the airflow velocity on the display. • Complete the logbook, recording details of operation of the BSC. • Switch off the UV lamp. Never work with the UV lamp on. • Disinfect the work surface, interior walls and interior window surface with 70% ethanol or other suitable compounds. Do not use corrosive substances. 11

BSC – rules for correct use • Organize all items needed for work in the BSC to avoid frequent displacements. • Organize the work to minimize arm movements. • Do not overcrowd the working area – this disturbs the airflow. • Conduct all manipulations within the BSC as far towards the back of the unit as possible (at least 15 cm from the front grille). • In class II BSCs, never allow the front grille to be covered with anything. • Do not use large open flames in the BSC. • Do not accumulate waste in the BSC; remove it when activities are finished. 12

BSC – after use • Autoclave the waste. • Wipe down the inside of the BSC and work surface with 70% alcohol. • Switch on the UV light for 1 hour (if the lamp is in good working order). • Switch off the BSC fan. 13

Certification and maintenance Regular certification and maintenance, with replacement of filters, are needed and represent a major challenge in low-income countries. If maintenance is not carried out regularly, the filters may become clogged and tubercle bacilli may be blown into the face of the operator. A BSC that is not well maintained is more hazardous than protective. 14

BSC – evaluation of performance Performance of the BSC should be evaluated by the manufacturer or a qualified professional, with specific, well-calibrated equipment. 15

BSC – maintenance • Modern cabinets are equipped with airflow indicators and warning devices. • Maintenance, with replacement of filters, should be carried out by professionals when the airflow falls below the minimum recommended level. The BSC should not be used until this maintenance has been carried out. Ou to fo rde r 16

BSC – maintenance Airflow smoke pattern tests are performed to determine: – whether the airflow along the entire perimeter of the work access opening is inward; – whether airflow within the work area is downward with no dead spots or refluxing; – whether ambient air passes onto or over the work surface; – whethere is refluxing to the outside at the window wiper gasket and side seals. 17

BSC – maintenance A cabinet leak test is carried out: – to determine whether exterior surfaces of all plenums, welds, gaskets, and plenum penetrations or seals are free of leaks; – before initial installation or annually on fully installed cabinets 18

BSC – fumigation • • • Decontamination of the BSC with formaldehyde gas. Hydrogen peroxide. Fumigation should be performed only by professionals. System DECONTAKIT 19

BSC – fumigation Fumigation of the BSC should be carried out: – before release of the BSC for use after a major biohazardous spill; – before replacement of HEPA filters; – before repair work requiring access to the sealed plenum; – before service or replacement of the circulation fan or components; – before maintenance work in contaminated areas; – before performance tests requiring entry into contaminated areas; – before movement of the BSC to another laboratory or area of use; – before changing work programmes, e. g. to non-TB work; – before release of the BSC for resale or salvage. 20
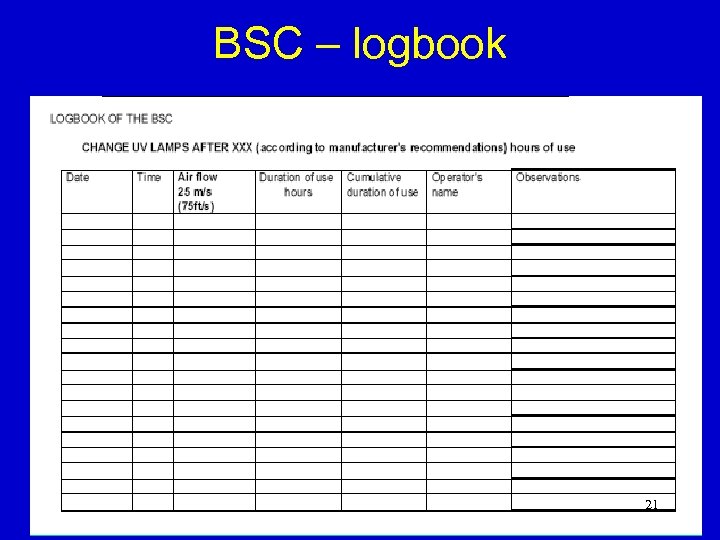
BSC – logbook 21
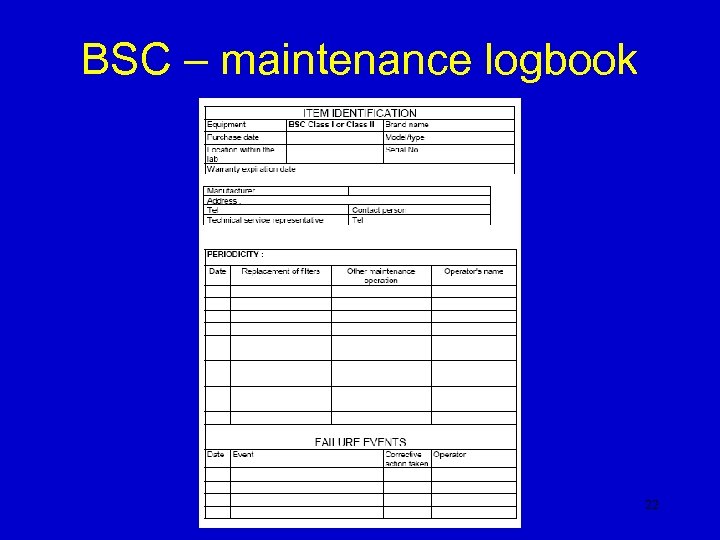
BSC – maintenance logbook 22
Centrifuge – selection of proper equipment for TB culture • The centrifuge should: • Have aerosol-free (O-ring sealed) swing buckets. • Have locking mechanism for protection to prevent opening before rotation has ceased. • Be able to generate 3000 g. • Be refrigerated. 23
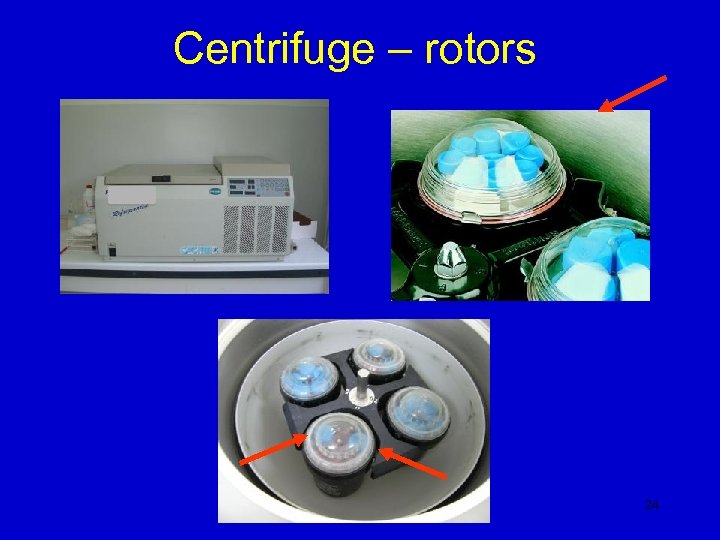
Centrifuge – rotors 24
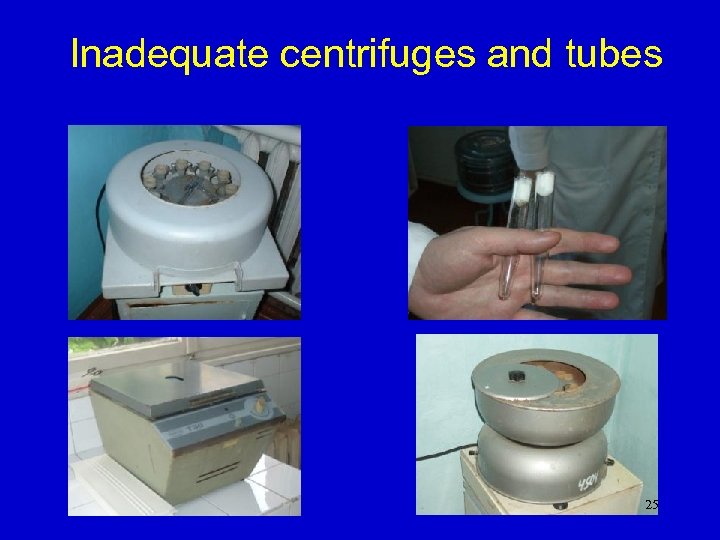
Inadequate centrifuges and tubes 25

Prevent accidents by regular maintenance 26

Centrifuge – use • Inspect the centrifuge chamber for liquid or signs of corrosion. • Check that there are shock-absorbing pads in the bottom of the centrifuge buckets. • Balance the sample tubes and insert them in the buckets in the appropriate position. –Never add water to a specimen to balance paired tubes –Use an empty tube filled with water as a “balance”. • Select the required time and speed. • Stop centrifuge immediately if any abnormal noise is noticed. • Never operate a centrifuge with the lid open. • Open the sealed buckets in the BSC. 27

Centrifuge tubes – adaptors • Centrifuge tubes must tolerate g-forces of at least 3000 g. • Centrifuge tubes must be used with appropriate rubber or plastic cushions matched to the tube and bucket-holder. • Use only adaptors recommended by the manufacturer of the centrifuge in use. 28
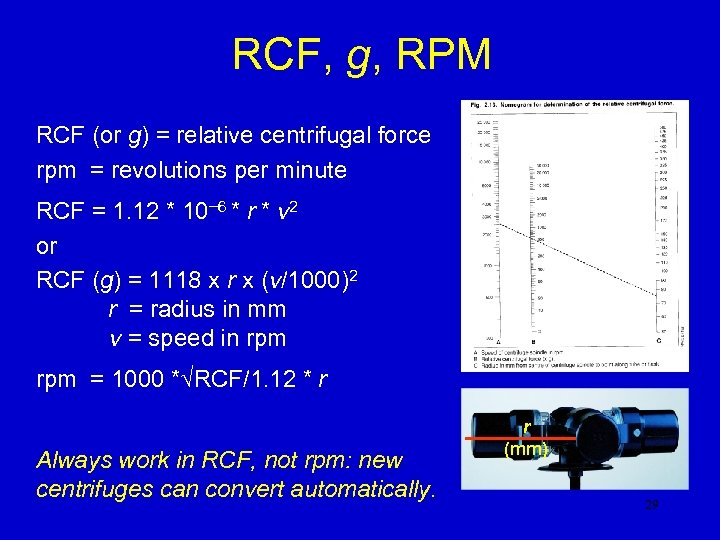
RCF, g, RPM RCF (or g) = relative centrifugal force rpm = revolutions per minute RCF = 1. 12 * 10− 6 * r * v 2 or RCF (g) = 1118 x r x (v/1000)2 r = radius in mm v = speed in rpm = 1000 *√RCF/1. 12 * r Always work in RCF, not rpm: new centrifuges can convert automatically. r (mm) 29

Centrifuge – installation • Ensure that the centrifuge is installed on a rigid, flat, level surface. • Because centrifuges produce vibration during operation, they must not be installed next to balances or other sensitive equipment. • Allow sufficient free space around the centrifuge for ventilation to prevent overheating. • Initial calibration should be performed only by a qualified service technician. 30

Centrifuge – daily maintenance • Wipe the inside bowl with disinfectant solution and rinse thoroughly. • If a refrigerated centrifuge is turned off at night, open the top to allow the bowl to dry. During the day when the unit is under refrigeration, keep the top closed to avoid condensation and ice build-up. 31

Centrifuge – monthly maintenance • Clean the centrifuge housing, rotor chamber, rotors and rotor accessories with a neutral cleaning agent. • Clean plastic and non-metal parts with a fresh solution of 5% sodium hypochlorite (bleach). 32

Centrifuge – annual maintenance Annual service should be carried out by a qualified service technician: centrifuge brushes, timer, speed and electrical leaks should all be checked. 33
Centrifuge – logbook 34
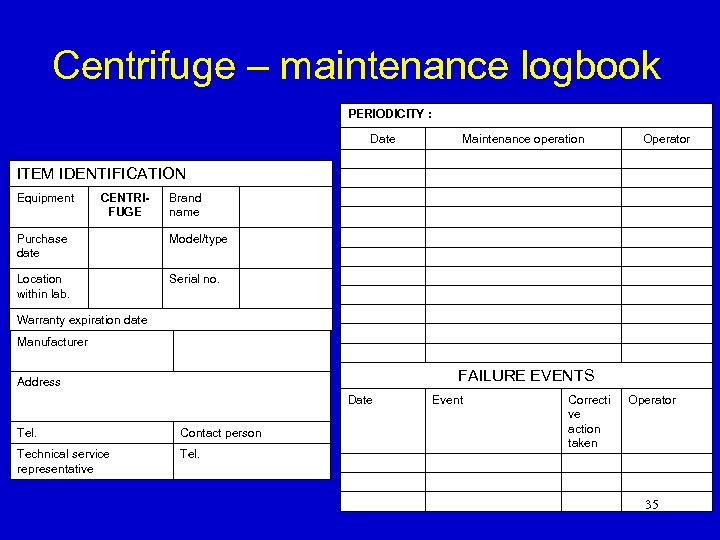
Centrifuge – maintenance logbook PERIODICITY : Date Maintenance operation Operator ITEM IDENTIFICATION Equipment CENTRIFUGE Brand name Purchase date Model/type Location within lab. Serial no. Warranty expiration date Manufacturer FAILURE EVENTS Address Date Tel. Contact person Technical service representative Tel. Event Correcti ve action taken Operator 35

Autoclave The autoclave using saturated steam under pressure is the most efficient means of: • sterilization in a diagnostic TB laboratory; • decontamination of biological material consisting of infectious waste. 36

Autoclave For optimum function: • All of the air in the chamber should be replaced by steam. • The temperature must be 121 ºC. • Materials to be sterilized must be packed loosely. 37

Tips for loading the autoclave • Only appropriate containers should be used. • Discarded cultures should be in solid-bottomed containers no more than 40 cm deep. • Leave a large air space around each container. • Never containers. • If autoclaving closed containers, add water to generate steam. Separate autoclaves should be used for sterilization of solutions or glassware (clean materials) and for decontamination of infectious materials. 38
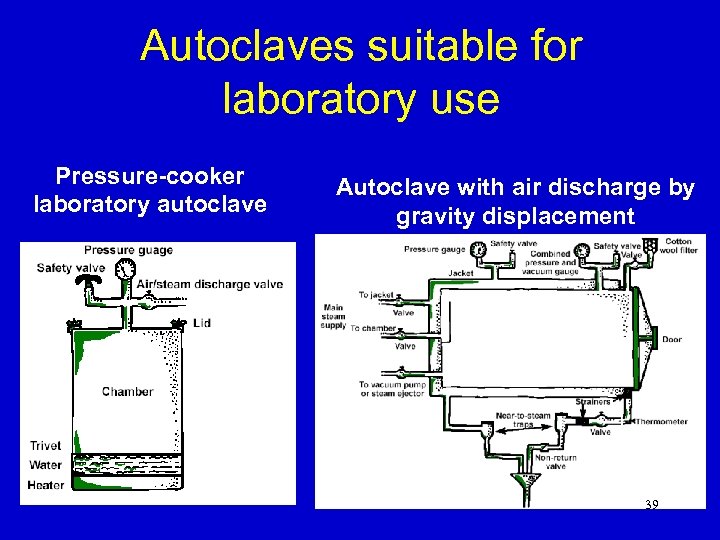
Autoclaves suitable for laboratory use Pressure-cooker laboratory autoclave Autoclave with air discharge by gravity displacement 39

Examples of autoclaves 40

Autoclave – use • Prepare material for autoclaving with thermosensitive paper. • Do not load material to be sterilized with material to be decontaminated. • Fill the bottom of the autoclave with distilled water. • Open the air outlet valve. • Turn on the heating. • Close the outlet valve. • Do not touch the drainage tap or the outlet or the safety valve while heating under pressure. • When the required time is up, turn off the heating completely. • When the temperature falls below 100 ºC, open the outlet valve slowly. 41

Autoclave – use • Never unscrew the lid clamps and open the lid until the hissing sound has stopped. • Leave the sterilized/decontaminated material to cool before removing it from the autoclave. • Check whether the autoclave tape has turned black and the covering paper has turned brown. If they have not, the material is not decontaminated. Check the autoclave for malfunction. • Biological indicators should be used after 40 hours of use. 42

Autoclave – suggested protocols • Solid material for sterilization: 121 ºC (appropriate pressure 115 k. Pa), 20 min. • Solid material for decontamination: 121 ºC (appropriate pressure 115 k. Pa), 30 min. • Liquid material for sterilization: 15 min at 121 ºC if “liquid cycle” is available. 43

Autoclave – maintenance • Check door gaskets for cracks and pitting. • Check for properation of door clamps and door locks. • Inspect valve discs and seats for signs of wear or cutting. • Check pressure-release safety valves and thermometers. 44
Autoclave – logbook 45

Autoclave – maintenance and incidents logbook 46

Inspissator • Essential when egg media are “home-made” (made in the laboratory). 47

Inspissator – use • Carefully control the amount of heating. • Ensure the required temperature has been reached before loading the tubes • Heat the tubes at constant temperature of 80– 85 ºC for 45 minutes. • Tubes or bottles loaded at an angle of 5– 10º ( 10° ). • Always check the quality of media after cooling. 48

Inspissator – maintenance • Check temperature at each use. • Clean after preparation of each batch of culture media. 49

Inspissator – logbook 50

Inspissator – maintenance and incidents logbook 51
p. H meter 52

p. H meter – use • Calibrate the p. H meter before use at three p. H values (p. H 4. 0, 7. 0 and 10. 0), using the calibration solutions provided by the manufacturer. • Immerse the electrode in the test solution. • Rinse the electrode after use. • Cover the p. H meter after use. 53
p. H meter – calibration • The p. H meter must be calibrated according to the manufacturer’s instructions. • Calibration must be performed once daily or, in case of infrequent use, at least on the day of p. H testing. It must be performed before the first measurement of the day. • Standardize with p. H 4. 0 and 7. 0 buffers before each test or series of tests. • Discard contaminated or cloudy standard buffers. • Calibration results are acceptable if the p. H values of the reference buffer solutions are within 0. 1 p. H units of the expected p. H. 54

p. H meter – Maintenance • Keep the instrument clean. Cover it after each use. • Rinse the electrode after use. • Make sure that the electrode is always filled with electrolyte according to the manufacturer’s instructions. • Do not touch the electrode membrane, which can be easily damaged. • A glass electrode should be kept immersed in a standard salt solution for long-term storage. • Calomel electrodes must be kept in a potassium chloride (KCl) buffer solution when not in use. • Date buffer solutions and discard when unsatisfactory. 55
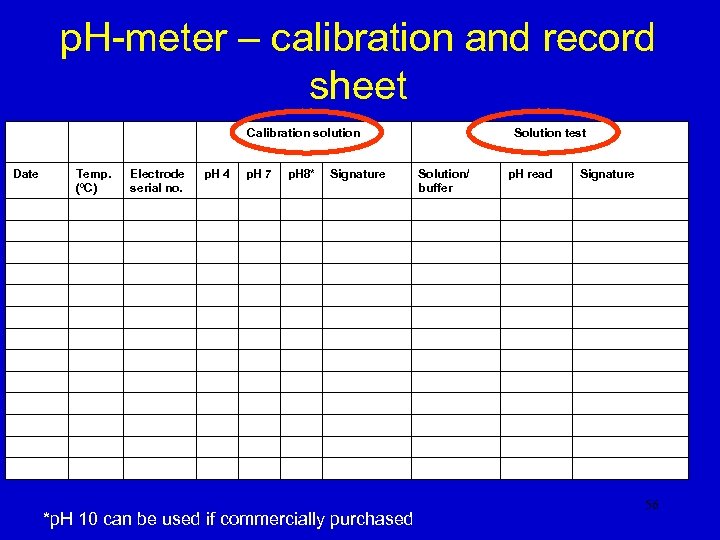
p. H-meter – calibration and record sheet Calibration solution Date Temp. (ºC) Electrode serial no. p. H 4 p. H 7 p. H 8* Signature *p. H 10 can be used if commercially purchased Solution test Solution/ buffer p. H read Signature 56
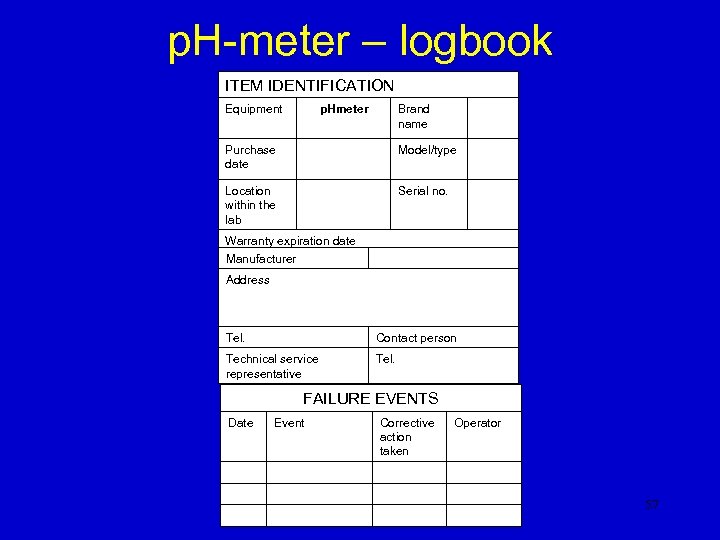
p. H-meter – logbook ITEM IDENTIFICATION Equipment p. Hmeter Brand name Purchase date Model/type Location within the lab Serial no. Warranty expiration date Manufacturer Address Tel. Contact person Technical service representative Tel. FAILURE EVENTS Date Event Corrective action taken Operator 57

Incubators ensure optimum growth conditions for culture of M. tuberculosis. Incubators are available in various sizes, from small (benchtop) models to large closets up to several hundred liters. Walk-in incubator rooms with circulating fans to obtain an homogeneous temperature also frequently used. 58

Incubator – use • Keep door(s) closed to prevent heat loss to the environment. • Ensure that rack positions are clearly marked. • Switch the incubator off when not in use. • Do not overload. 59

Incubator – maintenance • Record temperatures daily on appropriate forms. • Check that the actual temperature corresponds with thermostat setting. • Clean every 14 days with 70% ethanol. • Clean immediately after any infectious spills. 60

Incubator – calibration Calibrate: –before use; –after temperature changes have been detected and rectified; –following a power failure; –after cleaning of spillages. 61

Incubator – calibration • Ensure that the door is closed and that the incubator is switched on. • Set the required temperature using the temperature control and leave the incubator to run for 1 hour. • Place a thermometer in the centre of the incubator with the probe away from the heating element. • Take the temperature reading after 30 minutes. If the temperature is not 36 ± 1 ºC, adjust the control. • Repeat this process every 30 minutes until the required temperature is reached. • Continue to take readings until two consecutive readings (30 minutes apart) are 36 ± 1 ºC. 62
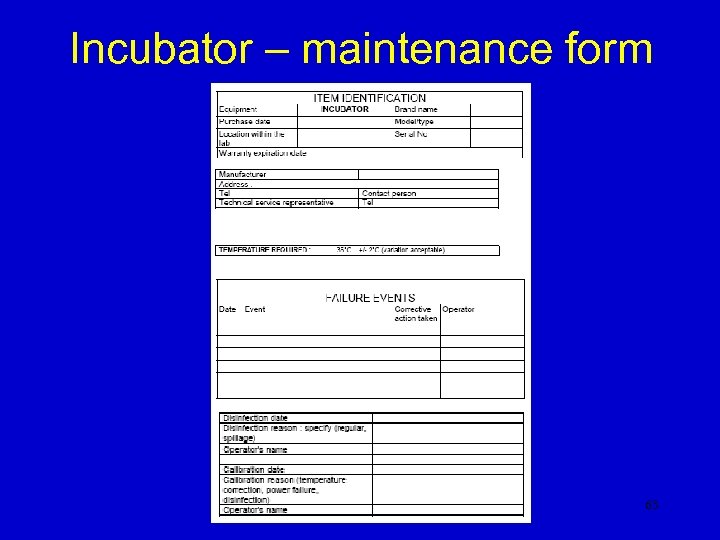
Incubator – maintenance form 63

Trimester : Month Year : Temp. Visa °C Month (Initials) 2 2 3 3 3 4 4 4 5 5 5 6 6 6 7 7 7 8 8 8 9 9 9 10 10 10 11 11 11 12 12 12 13 13 13 14 14 14 15 15 15 16 16 16 17 17 17 18 18 18 19 19 19 20 20 20 21 21 21 22 22 22 23 23 23 24 24 24 25 25 25 26 26 26 27 27 27 28 28 28 29 29 29 30 30 30 31 31 31 (Initials) 1 2 Incubator logbook 1 Visa °C 1 Temp. 64

True and false exercise 1. The biological safety cabinet is the most important equipment for TB culture laboratories. 2. Only class II BSCs can be used for TB culture. 3. Centrifuge speed should always be expressed in rpm. 4. Centrifugation may produce hazardous aerosols. 5. Checking the autoclave regularly is unnecessary if it is used only for decontamination. 6. Equipment maintenance is crucial for optimal performance. 7. Maintenance can always be performed in-house. 65

Module review: take-home messages 1. Equipment is expensive: all equipment must be treated with care and properly maintained. 2. Quality-control checks on equipment should be run regularly and results should be recorded on appropriate forms. 3. The biological safety cabinet is the most important item of equipment for TB culture under safe conditions. 4. Read carefully and always follow manufacturers’ instructions for routine work and maintenance. 5. Familiarize yourself with all working parts of all the instruments. 6. Call for help when troubleshooting any equipment malfunction. 66
Self-assessment • Describe the differences between the two types of BSC. • What precautions must you take when working in a BSC? • Describe the operations to be undertaken before starting a centrifuge. • Explain the principles of sterilization using an autoclave. 67
ec1f249b2debc902051a2f2e4cd6e2f1.ppt