4c4f0e1f226a98a9ba14d05355170598.ppt
- Количество слайдов: 15

Modified Directly Observed Therapy for First Virologic Failure: ACTG A 5234 PI Dr A Chisada Presenter: Dr W Samaneka MBCh. B, MSc UZ-UCSF ARD 17 April 2015

Background • Sustained and consistent adherence is a key factor for durable ART success • 2 nd line therapy is typically more complex and expensive than 1 st line • Conventional DOT is logistically challenging • Enhanced partner support may benefit patients with prior treatment failure.
Main Objective To test whether a partner-based modified DOT (m. DOT) intervention would result in higher rates of virologic suppression compared with standard of care after first-line treatment failure

Methods • • • Study duration: Apr 2009 – Sep 2011 Confirmed virologic failure on 1 st line Identifiable m. DOT partner (family, friend, etc. ) 1: 1 allocation to m. DOT and standard of care 2 nd line: lopinavir/r (400 mg/100 mg) BID + TDF/FTC (300 mg/200 mg) q. D Brazil, Botswana, Haiti, Peru, SA, Uganda, Zambia, Zimbabwe
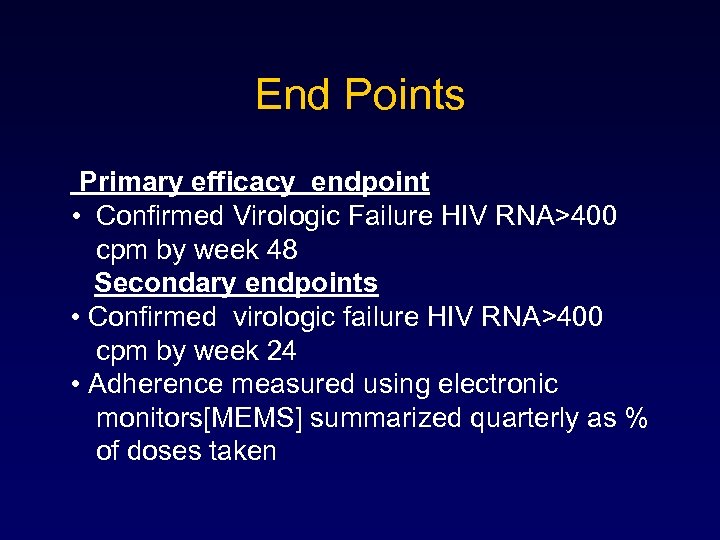
End Points Primary efficacy endpoint • Confirmed Virologic Failure HIV RNA>400 cpm by week 48 Secondary endpoints • Confirmed virologic failure HIV RNA>400 cpm by week 24 • Adherence measured using electronic monitors[MEMS] summarized quarterly as % of doses taken
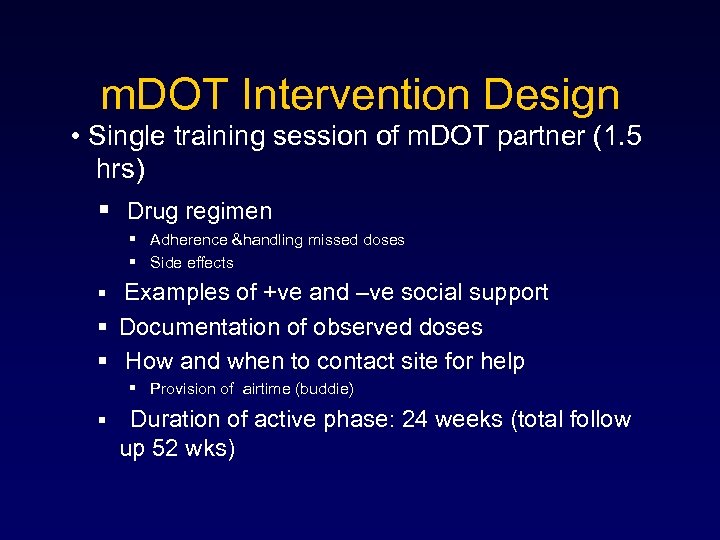
m. DOT Intervention Design • Single training session of m. DOT partner (1. 5 hrs) Drug regimen Adherence &handling missed doses Side effects Examples of +ve and –ve social support Documentation of observed doses How and when to contact site for help Provision of airtime (buddie) Duration of active phase: 24 weeks (total follow up 52 wks)
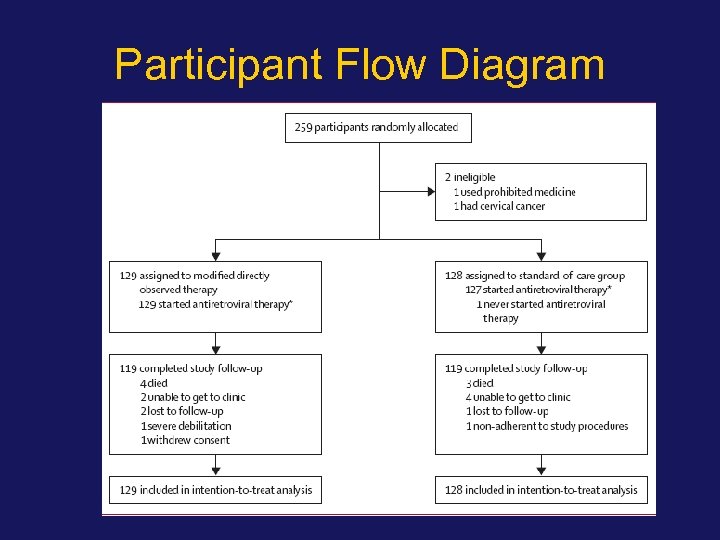
Participant Flow Diagram
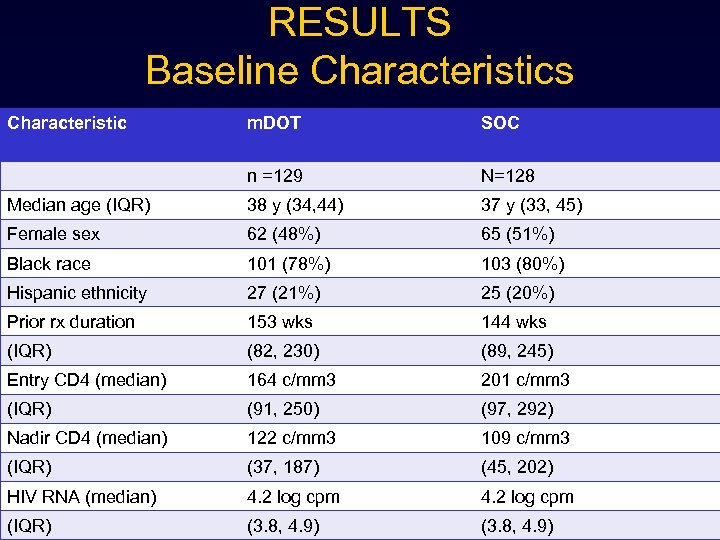
RESULTS Baseline Characteristics Characteristic m. DOT SOC n =129 N=128 Median age (IQR) 38 y (34, 44) 37 y (33, 45) Female sex 62 (48%) 65 (51%) Black race 101 (78%) 103 (80%) Hispanic ethnicity 27 (21%) 25 (20%) Prior rx duration 153 wks 144 wks (IQR) (82, 230) (89, 245) Entry CD 4 (median) 164 c/mm 3 201 c/mm 3 (IQR) (91, 250) (97, 292) Nadir CD 4 (median) 122 c/mm 3 109 c/mm 3 (IQR) (37, 187) (45, 202) HIV RNA (median) 4. 2 log cpm (IQR) (3. 8, 4. 9)
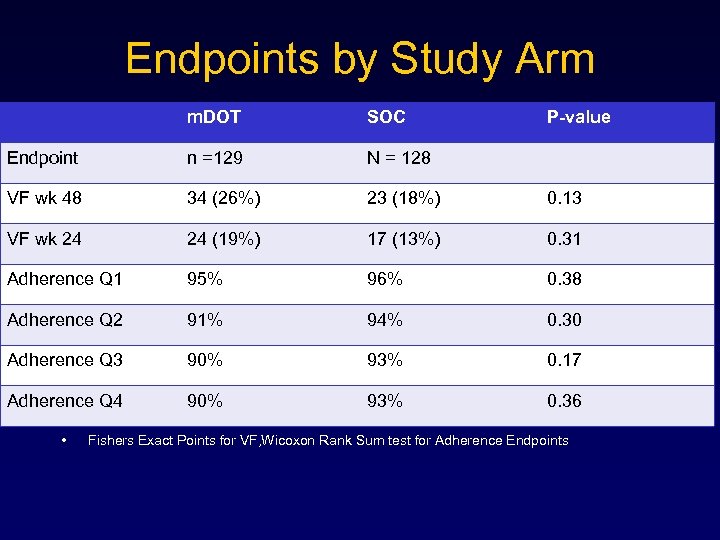
Endpoints by Study Arm m. DOT SOC Endpoint n =129 N = 128 VF wk 48 34 (26%) 23 (18%) 0. 13 VF wk 24 24 (19%) 17 (13%) 0. 31 Adherence Q 1 95% 96% 0. 38 Adherence Q 2 91% 94% 0. 30 Adherence Q 3 90% 93% 0. 17 Adherence Q 4 90% 93% 0. 36 • P-value Fishers Exact Points for VF, Wicoxon Rank Sum test for Adherence Endpoints
Virologic suppression ≤ 400 • At all time points after week 12 undetectable VL was higher in SOC, however not significant • Week 48, 75% (CI 67 -83) in m. DOT and 82% in SOC (CI 74 -89) had VL ≤ 400, (p=0. 37) • No sig diff between groups at week 24; p=0. 18
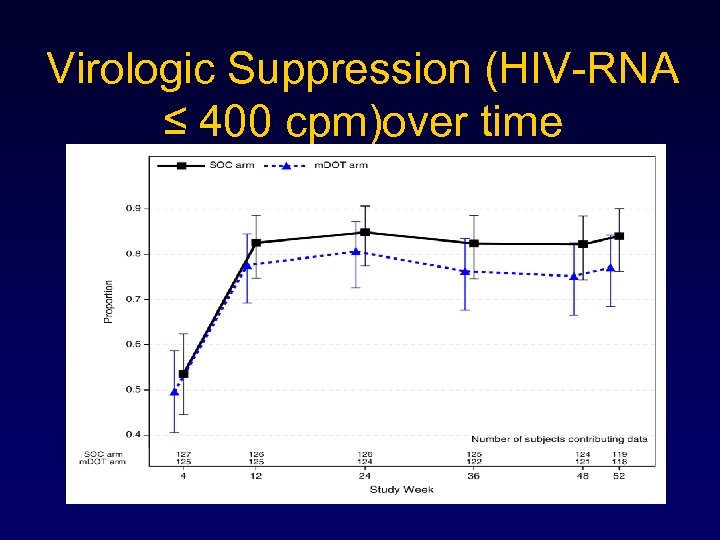
Virologic Suppression (HIV-RNA ≤ 400 cpm)over time

Conclusion • m. DOT had no significant impact on virologic failure Ø High rate of suppression in both arms • m. DOT had no significant impact on adherence Ø High rate of adherence in both arms • Other interventions still need to be tested in this setting

• Although m. DOT intervention not successful findings are encouraging > high rates of virologic success on second line therapy in RLS

Acknowledgements • Sponsors – NIAID - Abbott Laboratories - Gilead Sciences • Study participants + partners • CAB • CRS Leader- Prof JG Hakim • ACTG site staff • UZ-UCSF

THANK YOU
4c4f0e1f226a98a9ba14d05355170598.ppt