1a3fbace287196078b219fe9b08d6cb6.ppt
- Количество слайдов: 26

Modern Methods of Ferritic Nitrocarburizing (FNC) H-13 section 28” dia 48” depth open basket Open bed at temperature Loaded basket, ready for carousel Removal from fluidized quench

Ferritic Nitrocarburizing (FNC) How we got here l 1900’s – Dr. Adolph Fry § Discovered that Nitrogen and Iron had affinity to one another. § Developed nitrogen iron equilibrium table § Nitralloy steels § Studied effect of adding other alloys Vanadium, Tungsten, Manganese, Molybdenum, Titanium

Ferritic Nitrocarburizing (FNC) Continued 2 l 1900’s Adolph Machlet – New Jersey § American Gas Company - Elizabeth § Applied for patents which were received June 24, 1913 § US saw no commercial benefit at the time

Ferritic Nitrocarburizing (FNC) Continued 3 l 1927 Pierre Aubert – Chicago § At SME convention presented research for practical applications in Europe § Included railway steel, machine tools, auto, aviation. § Benefits – hard surface, core not changed, high wear, unaffected by temper, corrosion resistance.
Ferritic Nitrocarburizing (FNC) Continued 4 l 1928 – Mc. Quaid and Ketchum § Timken – Detroit Axle Co. § Metallurgists – practical applications § Used work of Fry and Machlet as pivot point l 1929 – Robert Sergeson § Central Alloy Steel – Canton, OH § Varying Al content in nitro alloy steel with effect of nickel

Ferritic Nitrocarburizing (FNC) Others l V. O. Homberg & J. P. Walsted - MIT § Effect of varying temperature – white layer § Equipment preheat and decarburization effect l Dr. Carl F. Floe – Assoc MIT § Continued study of white (epsilon) layer § The Flow Process – methods to change compound layer l Eventually this research lead to “Ion Nitriding” – in effort to shorten cycle times, reduce distortion, and improve metallurgical properties.

Types of Nitriding l Fluidized Bed Nitriding and FNC l Nitempering l Controlled Nitrocarburizing l Soft Nitriding l Triniding l Nitroc Process l Vacuum Nitrocarburizing l Nitrotec Process l Austenite Nitrocarburizing

FNC – Thermochemical Diffusion l Process where N 2, C, and sometimes a very small degree of O 2 atoms are diffused into the surface of a ferrous substrate forming a compound layer and subsurface diffusion layer. l Done in relatively short period of time at sub critical steel temperatures l Wear properties, Corrosion (solder), and improved fatigue resistance.

Prominent Developments FNC l. Salt Bath Nitrocarburizing § Started about 55 years ago commercially. § 1959 – Germany patented Tuffride § 1970’s – EPA regs prohibiting cyanide base materials § Tufftride replaced with Melonite and French process called Sur-sulf § These two processes still in use today.

Prominent Developments FNC Continued l. Gas – Originally patented in 1961 by Joseph Lucas Industries Ltd. l 1965 – B. Presnosil Published results of study doing Gas. l. During following quarter of a century – developed Triniding (NH 3 and exothermic gas), Nitemper, Lindure and a two stage process (Deganit) from Germany
Fluidized (FNC) Bed Phenomenon l An 1879 patent discusses baking minerals under fluidized bed conditions l Bed of finely-divided heated particles, usually Aluminum oxide made to behave like a liquid by moving exothermic and reactive gases through the medium § Smooth or bubbly properties – determines fluidization quality. § Size and hetrogeneity (other offspring) of bubbles – influences rate of the solid mixing § Bed geometry, gas flow rate, type of gas distributor § Vessel features – baffles, screens, heat exchangers

Analogy of Fluidized Bed and Liquids l Plunging your hand into a fluidized bed (unheated of course) gives the sensation of placing your hand in a bucket of water. Light objects introduced in the bed will float if light enough. l Behaving as a liquid causes the entire introduced object (metal) to be in complete contact with the aluminum oxide separated by the reactive gas/gases that surround the media and cause diffusion and white layer creation. l The heat from the bed starts the diffusion
Fluidized (FNC) Heat Transfer Factors l Cleanliness of tool steel § Mild to aggressive alkaline bath at elevated temperature – bed & part contamination l Particle transfer diameter – influences heat § In practice 100 micro mm (. 3940 micro inches) l Bed material density § Optimum value 1280 -1600 kg/cu m or 80 -100 lb/cu. ft. l. Fluidized velocity of gas/gases

Optimizing heat transfer to bed l. Optimizing gas/gases flow rate § Between 2 to 3 times the minimum fluidization velocity l. Curve peaking § To high – particle entrapment – high gas consumption § To low - poor heat transfer – lack of uniformity § Bed screws do not provide consistency
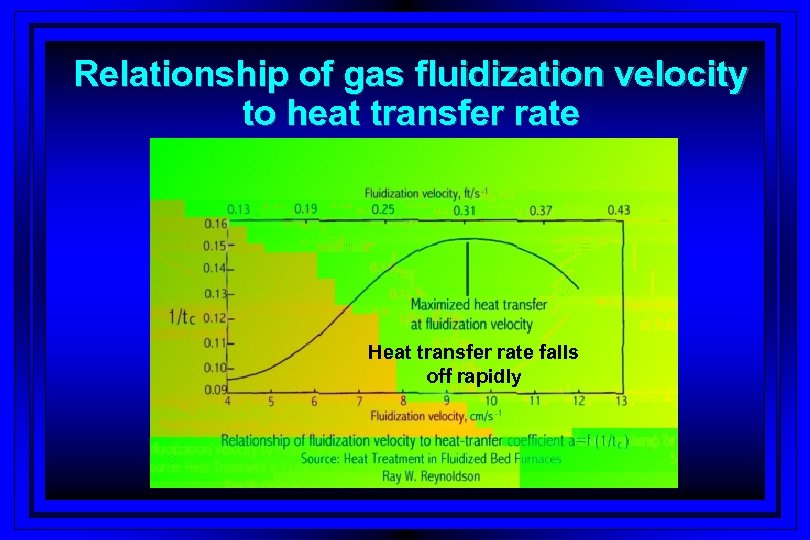
Relationship of gas fluidization velocity to heat transfer rate Heat transfer rate falls off rapidly
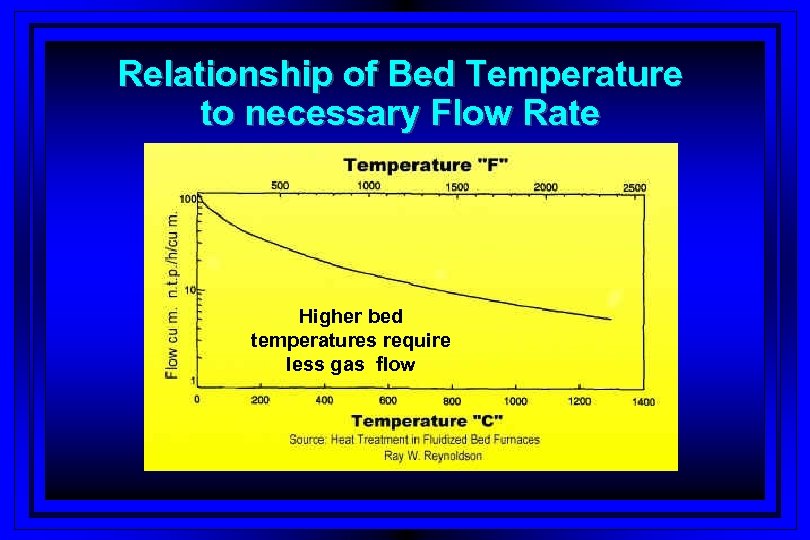
Relationship of Bed Temperature to necessary Flow Rate Higher bed temperatures require less gas flow
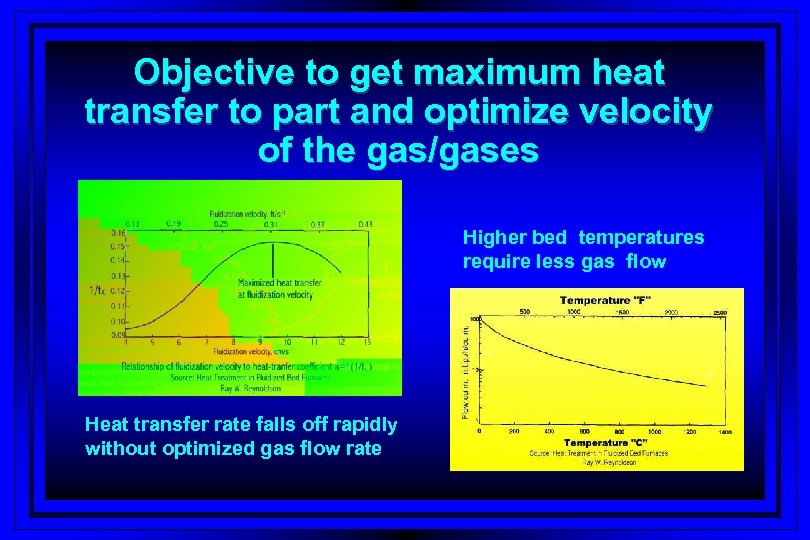
Objective to get maximum heat transfer to part and optimize velocity of the gas/gases Higher bed temperatures require less gas flow Heat transfer rate falls off rapidly without optimized gas flow rate

Now a better way to attain repeatability - flow and heat l. Computer control and automation l. Adjustable ceramic screens l. Eclipse valves with flow meters l. Sensor feedback to computer controls and automation system First Fully Automated System in the United States and Canada
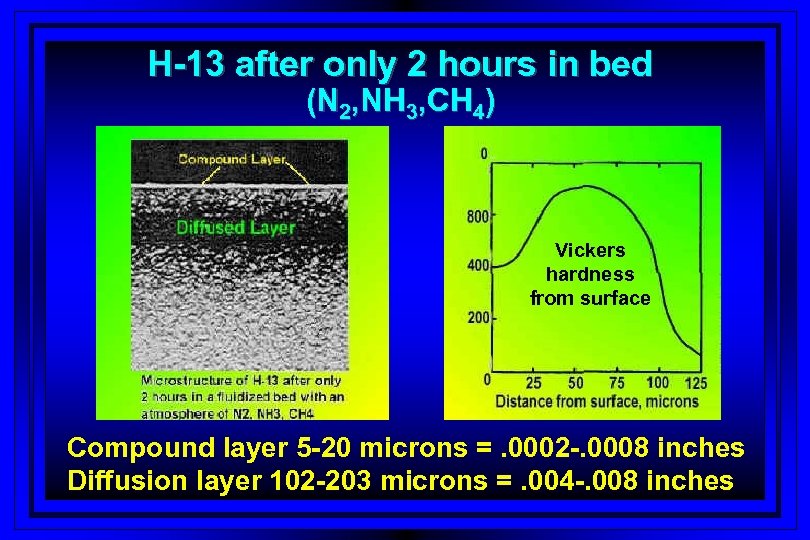
H-13 after only 2 hours in bed (N 2, NH 3, CH 4) Vickers hardness from surface Compound layer 5 -20 microns =. 0002 -. 0008 inches Diffusion layer 102 -203 microns =. 004 -. 008 inches

Future Uses of Fluidized Beds l Development of Hard PVD (below 700 o F) thermochemical surface treatments in Australia – grant by IR & D board l Coatings such as vanadium carbonitride, and chromium carbonitride at low temperature by diffusion-based treatments l Patents already applied for and equipment available to perform new range of low temperature surface treatments – Qab.
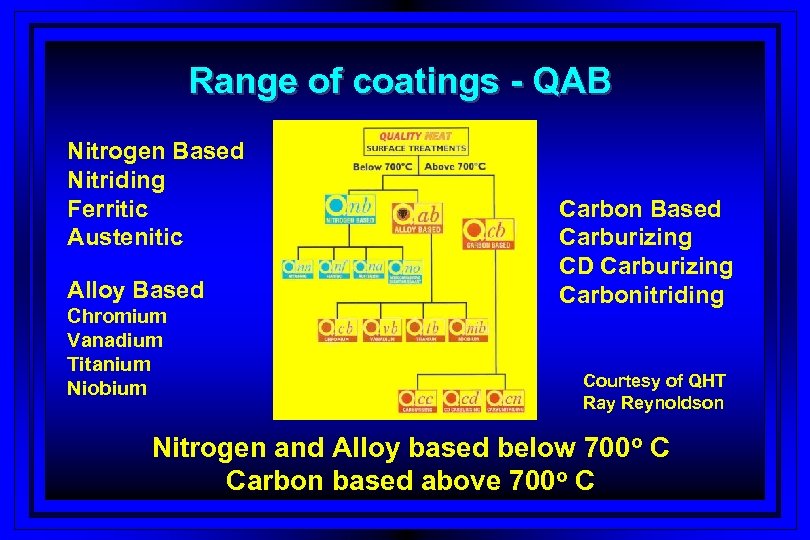
Range of coatings - QAB Nitrogen Based Nitriding Ferritic Austenitic Alloy Based Chromium Vanadium Titanium Niobium Carbon Based Carburizing CD Carburizing Carbonitriding Courtesy of QHT Ray Reynoldson Nitrogen and Alloy based below 700 o C Carbon based above 700 o C
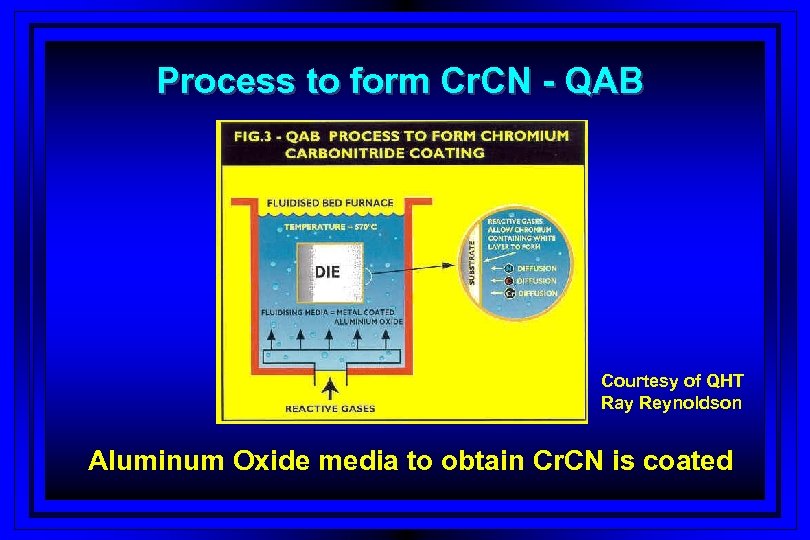
Process to form Cr. CN - QAB Courtesy of QHT Ray Reynoldson Aluminum Oxide media to obtain Cr. CN is coated
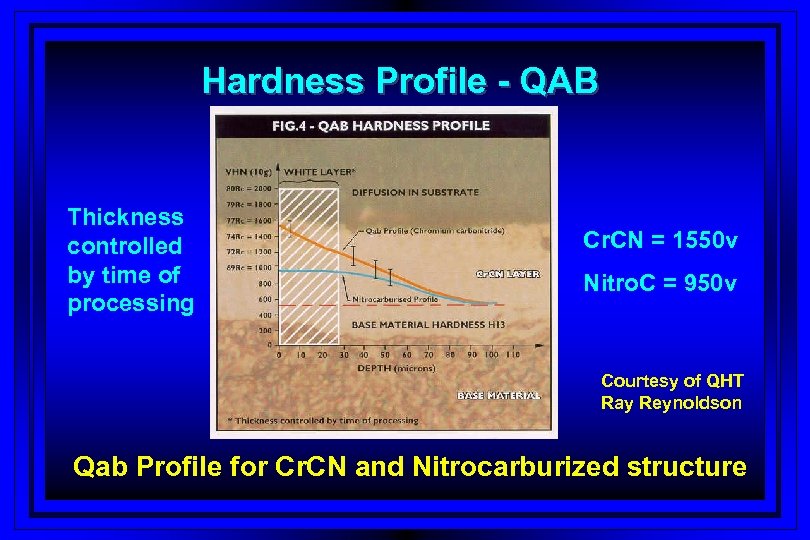
Hardness Profile - QAB Thickness controlled by time of processing Cr. CN = 1550 v Nitro. C = 950 v Courtesy of QHT Ray Reynoldson Qab Profile for Cr. CN and Nitrocarburized structure
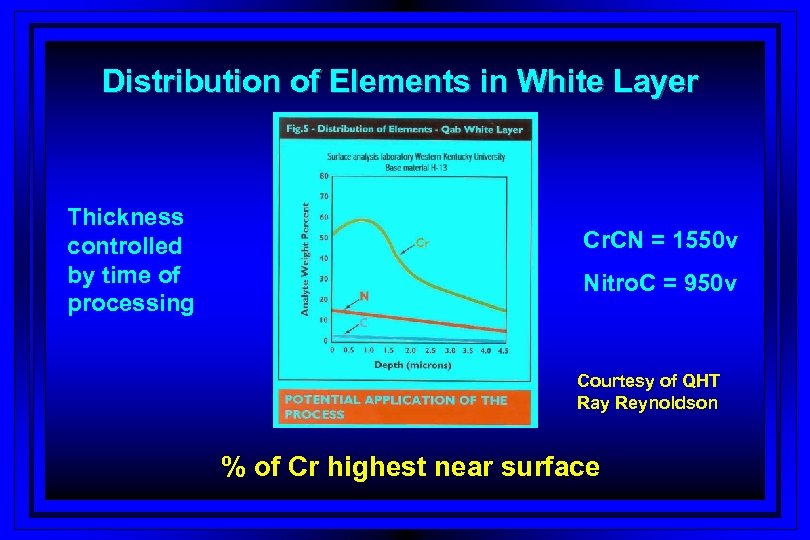
Distribution of Elements in White Layer Thickness controlled by time of processing Cr. CN = 1550 v Nitro. C = 950 v Courtesy of QHT Ray Reynoldson % of Cr highest near surface
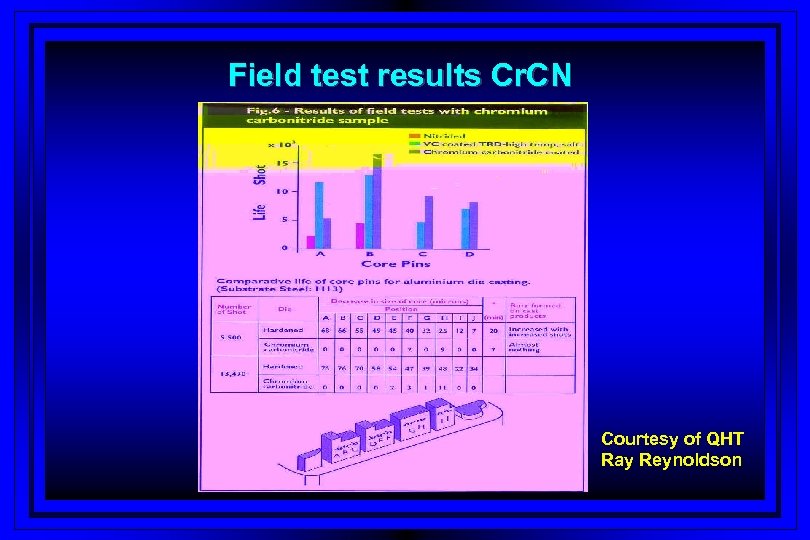
Field test results Cr. CN Courtesy of QHT Ray Reynoldson

TOOLING CIRCLE OF LIFE Want more information regarding our capabilities regarding fluidized bed treatments? Badger Metal Tech, Inc. 262 -252 -3956 Toll Free 800 -366 -1973 TOOLING CYCLE OF LIFE Visit our website and click on the flame logo at the top of our home page www. badgermetal. com
1a3fbace287196078b219fe9b08d6cb6.ppt