440601029afbea85a6a236ed34990755.ppt
- Количество слайдов: 36
 Modeling short-range ordering (SRO) in solutions Arthur D. Pelton and Youn-Bae Kang Centre de Recherche en Calcul Thermochimique, Département de Génie Chimique, École Polytechnique P. O. Box 6079, Station "Downtown" Montréal, Québec H 3 C 3 A 7 Canada
Modeling short-range ordering (SRO) in solutions Arthur D. Pelton and Youn-Bae Kang Centre de Recherche en Calcul Thermochimique, Département de Génie Chimique, École Polytechnique P. O. Box 6079, Station "Downtown" Montréal, Québec H 3 C 3 A 7 Canada
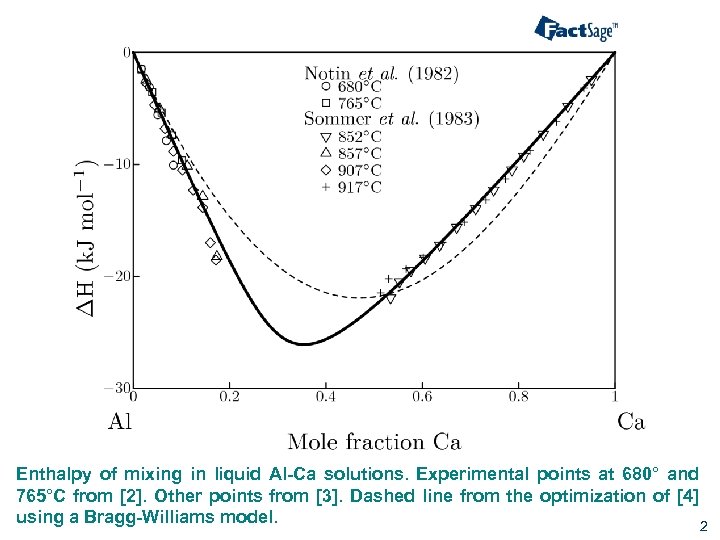 Enthalpy of mixing in liquid Al-Ca solutions. Experimental points at 680° and 765°C from [2]. Other points from [3]. Dashed line from the optimization of [4] using a Bragg-Williams model. 2
Enthalpy of mixing in liquid Al-Ca solutions. Experimental points at 680° and 765°C from [2]. Other points from [3]. Dashed line from the optimization of [4] using a Bragg-Williams model. 2
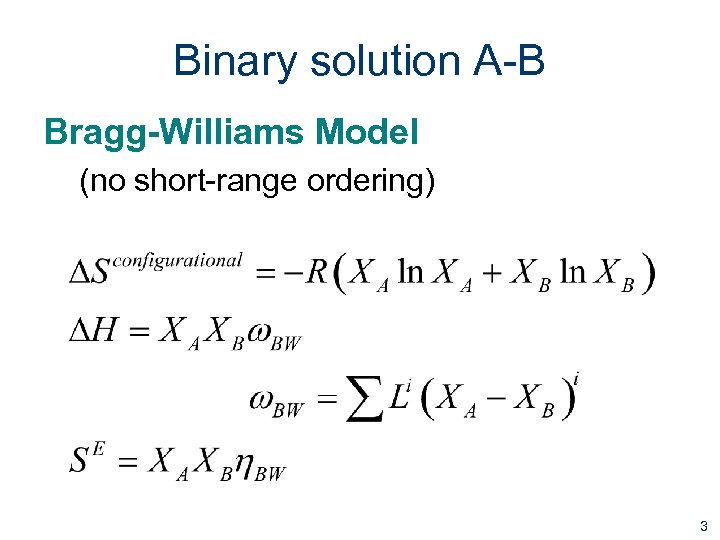 Binary solution A-B Bragg-Williams Model (no short-range ordering) 3
Binary solution A-B Bragg-Williams Model (no short-range ordering) 3
![Enthalpy of mixing in liquid Al-Sc solutions at 1600°C. Experimental points from [5]. Thick Enthalpy of mixing in liquid Al-Sc solutions at 1600°C. Experimental points from [5]. Thick](https://present5.com/presentation/440601029afbea85a6a236ed34990755/image-4.jpg) Enthalpy of mixing in liquid Al-Sc solutions at 1600°C. Experimental points from [5]. Thick line optimized [6] with the quasichemical model. Dashed line from the optimization of [7] using a BW model. 4
Enthalpy of mixing in liquid Al-Sc solutions at 1600°C. Experimental points from [5]. Thick line optimized [6] with the quasichemical model. Dashed line from the optimization of [7] using a BW model. 4
![Partial enthalpies of mixing in liquid Al-Sc solutions at 1600°C. Experimental points from [5]. Partial enthalpies of mixing in liquid Al-Sc solutions at 1600°C. Experimental points from [5].](https://present5.com/presentation/440601029afbea85a6a236ed34990755/image-5.jpg) Partial enthalpies of mixing in liquid Al-Sc solutions at 1600°C. Experimental points from [5]. Thick line optimized [6] with the quasichemical model. Dashed line from the optimization of [7] using a BW model. 5
Partial enthalpies of mixing in liquid Al-Sc solutions at 1600°C. Experimental points from [5]. Thick line optimized [6] with the quasichemical model. Dashed line from the optimization of [7] using a BW model. 5
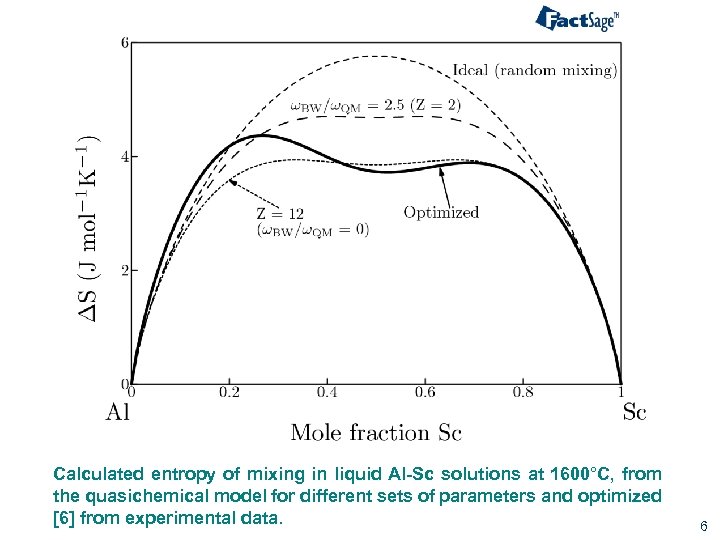 Calculated entropy of mixing in liquid Al-Sc solutions at 1600°C, from the quasichemical model for different sets of parameters and optimized [6] from experimental data. 6
Calculated entropy of mixing in liquid Al-Sc solutions at 1600°C, from the quasichemical model for different sets of parameters and optimized [6] from experimental data. 6
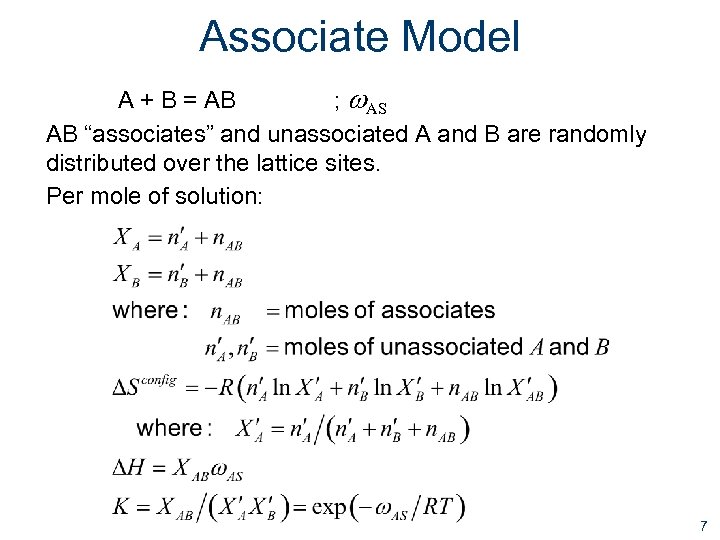 Associate Model A + B = AB ; w. AS AB “associates” and unassociated A and B are randomly distributed over the lattice sites. Per mole of solution: 7
Associate Model A + B = AB ; w. AS AB “associates” and unassociated A and B are randomly distributed over the lattice sites. Per mole of solution: 7
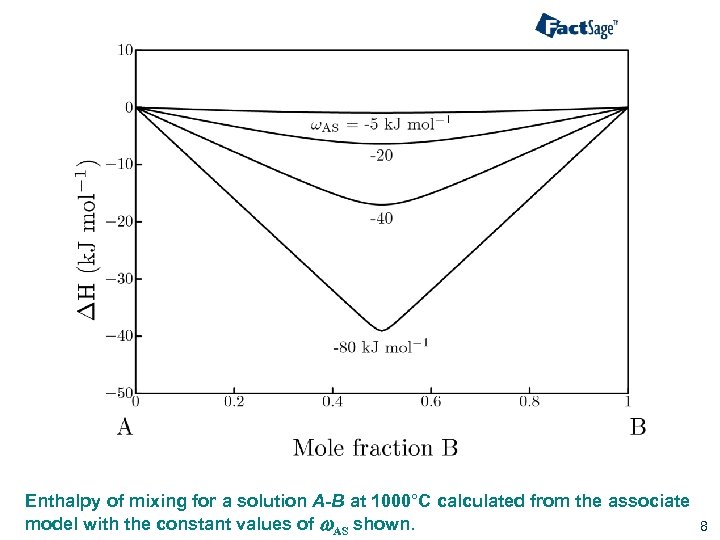 Enthalpy of mixing for a solution A-B at 1000°C calculated from the associate model with the constant values of w. AS shown. 8
Enthalpy of mixing for a solution A-B at 1000°C calculated from the associate model with the constant values of w. AS shown. 8
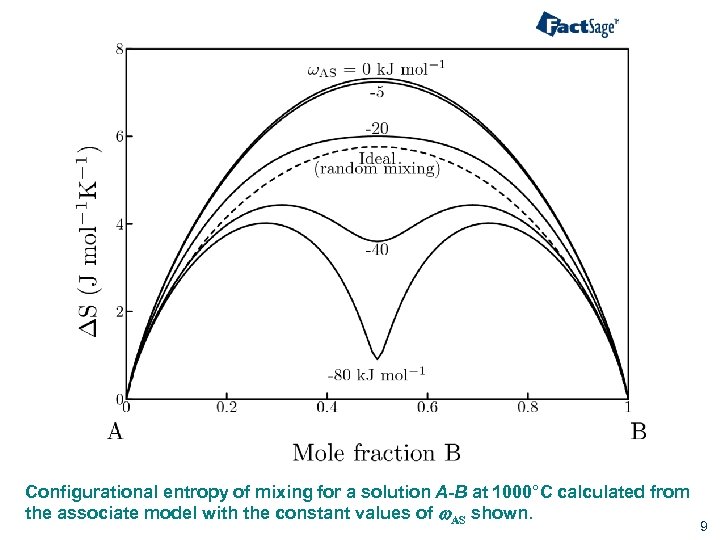 Configurational entropy of mixing for a solution A-B at 1000°C calculated from the associate model with the constant values of w. AS shown. 9
Configurational entropy of mixing for a solution A-B at 1000°C calculated from the associate model with the constant values of w. AS shown. 9
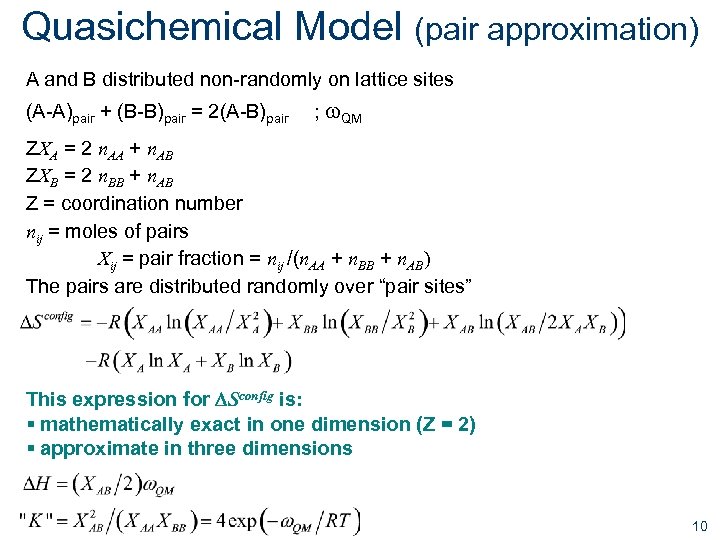 Quasichemical Model (pair approximation) A and B distributed non-randomly on lattice sites (A-A)pair + (B-B)pair = 2(A-B)pair ; w. QM ZXA = 2 n. AA + n. AB ZXB = 2 n. BB + n. AB Z = coordination number nij = moles of pairs Xij = pair fraction = nij /(n. AA + n. BB + n. AB) The pairs are distributed randomly over “pair sites” This expression for DSconfig is: § mathematically exact in one dimension (Z = 2) § approximate in three dimensions 10
Quasichemical Model (pair approximation) A and B distributed non-randomly on lattice sites (A-A)pair + (B-B)pair = 2(A-B)pair ; w. QM ZXA = 2 n. AA + n. AB ZXB = 2 n. BB + n. AB Z = coordination number nij = moles of pairs Xij = pair fraction = nij /(n. AA + n. BB + n. AB) The pairs are distributed randomly over “pair sites” This expression for DSconfig is: § mathematically exact in one dimension (Z = 2) § approximate in three dimensions 10
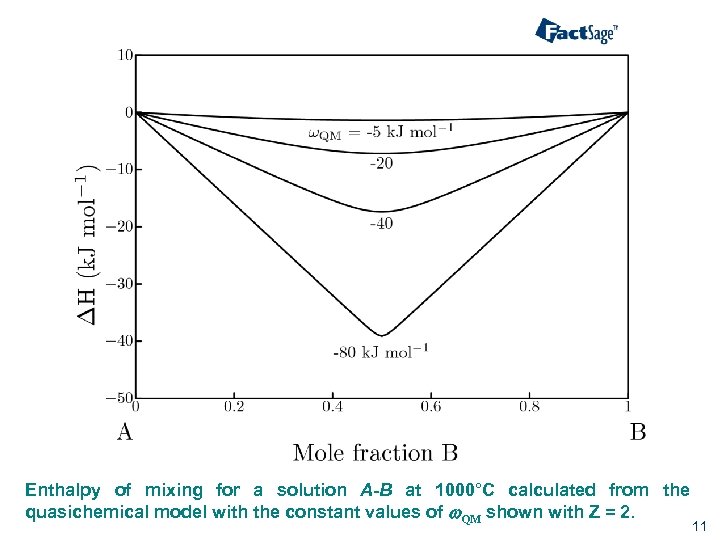 Enthalpy of mixing for a solution A-B at 1000°C calculated from the quasichemical model with the constant values of w. QM shown with Z = 2. 11
Enthalpy of mixing for a solution A-B at 1000°C calculated from the quasichemical model with the constant values of w. QM shown with Z = 2. 11
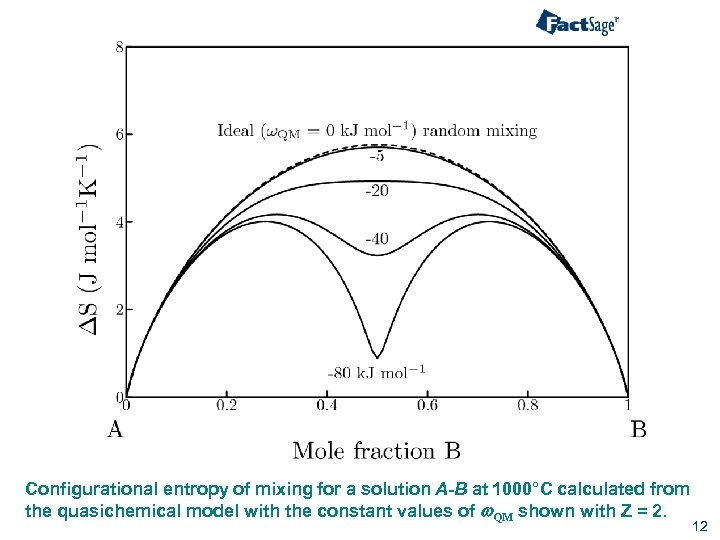 Configurational entropy of mixing for a solution A-B at 1000°C calculated from the quasichemical model with the constant values of w. QM shown with Z = 2. 12
Configurational entropy of mixing for a solution A-B at 1000°C calculated from the quasichemical model with the constant values of w. QM shown with Z = 2. 12
 The quasichemical model with Z = 2 tends to give DH and DSconfig functions with minima which are too sharp. (The associate model also has this problem. ) Combining the quasichemical and Bragg-Williams models Term for nearestneighbor interactions Term for remaining lattice interactions DSconfig as for quasichemical model 13
The quasichemical model with Z = 2 tends to give DH and DSconfig functions with minima which are too sharp. (The associate model also has this problem. ) Combining the quasichemical and Bragg-Williams models Term for nearestneighbor interactions Term for remaining lattice interactions DSconfig as for quasichemical model 13
![Enthalpy of mixing in liquid Al-Sc solutions at 1600°C. Experimental points from [5]. Curves Enthalpy of mixing in liquid Al-Sc solutions at 1600°C. Experimental points from [5]. Curves](https://present5.com/presentation/440601029afbea85a6a236ed34990755/image-14.jpg) Enthalpy of mixing in liquid Al-Sc solutions at 1600°C. Experimental points from [5]. Curves calculated from the quasichemical model for various ratios (w. BW/w. QM) with Z = 2, and for various values of with Z = 0. 14
Enthalpy of mixing in liquid Al-Sc solutions at 1600°C. Experimental points from [5]. Curves calculated from the quasichemical model for various ratios (w. BW/w. QM) with Z = 2, and for various values of with Z = 0. 14
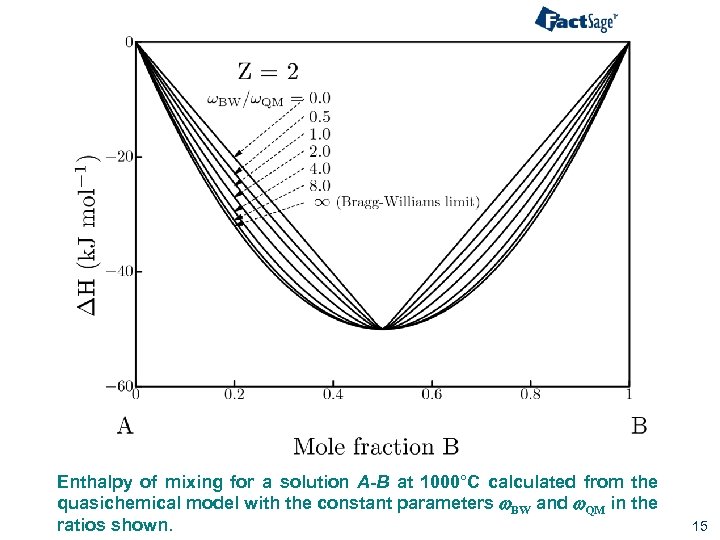 Enthalpy of mixing for a solution A-B at 1000°C calculated from the quasichemical model with the constant parameters w. BW and w. QM in the ratios shown. 15
Enthalpy of mixing for a solution A-B at 1000°C calculated from the quasichemical model with the constant parameters w. BW and w. QM in the ratios shown. 15
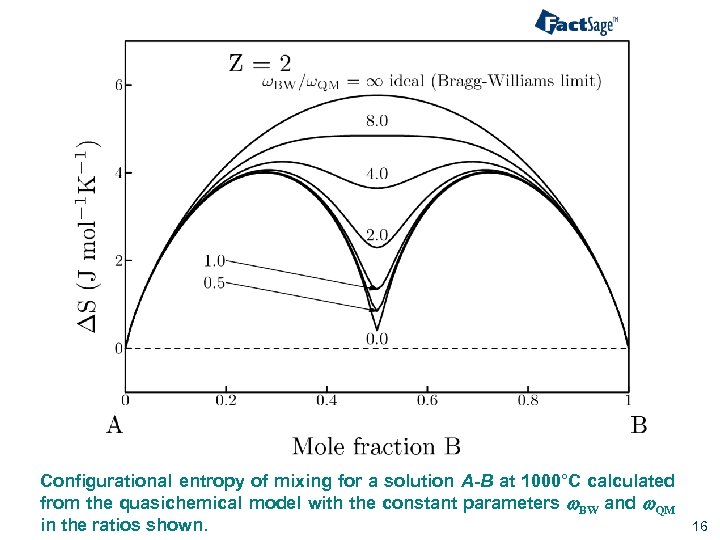 Configurational entropy of mixing for a solution A-B at 1000°C calculated from the quasichemical model with the constant parameters w. BW and w. QM in the ratios shown. 16
Configurational entropy of mixing for a solution A-B at 1000°C calculated from the quasichemical model with the constant parameters w. BW and w. QM in the ratios shown. 16
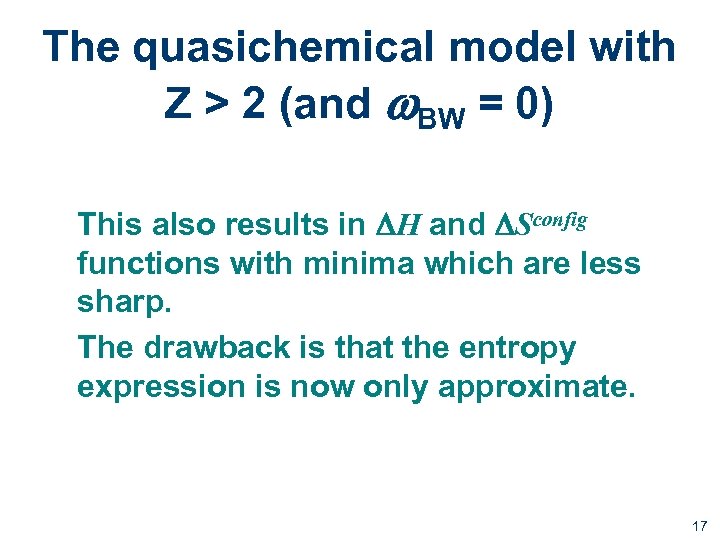 The quasichemical model with Z > 2 (and w. BW = 0) This also results in DH and DSconfig functions with minima which are less sharp. The drawback is that the entropy expression is now only approximate. 17
The quasichemical model with Z > 2 (and w. BW = 0) This also results in DH and DSconfig functions with minima which are less sharp. The drawback is that the entropy expression is now only approximate. 17
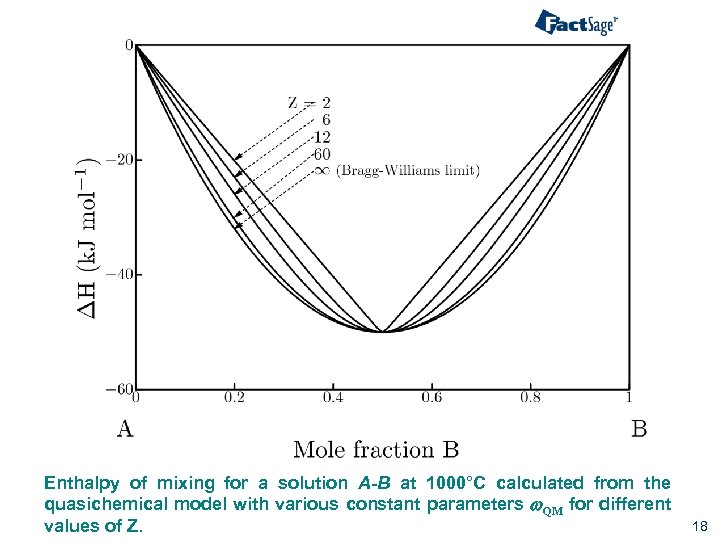 Enthalpy of mixing for a solution A-B at 1000°C calculated from the quasichemical model with various constant parameters w. QM for different values of Z. 18
Enthalpy of mixing for a solution A-B at 1000°C calculated from the quasichemical model with various constant parameters w. QM for different values of Z. 18
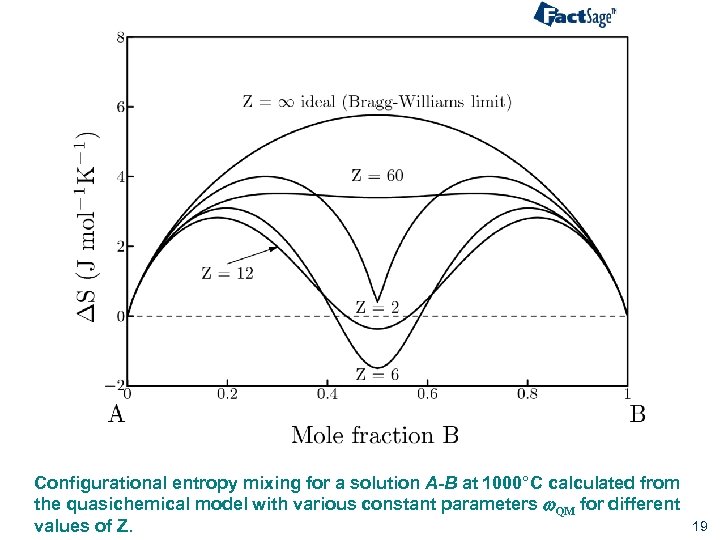 Configurational entropy mixing for a solution A-B at 1000°C calculated from the quasichemical model with various constant parameters w. QM for different values of Z. 19
Configurational entropy mixing for a solution A-B at 1000°C calculated from the quasichemical model with various constant parameters w. QM for different values of Z. 19
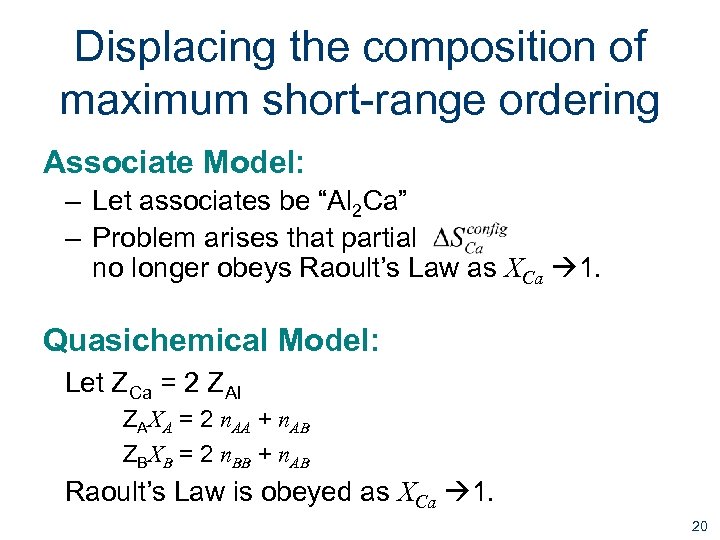 Displacing the composition of maximum short-range ordering Associate Model: – Let associates be “Al 2 Ca” – Problem arises that partial no longer obeys Raoult’s Law as XCa 1. Quasichemical Model: Let ZCa = 2 ZAl ZAXA = 2 n. AA + n. AB ZBXB = 2 n. BB + n. AB Raoult’s Law is obeyed as XCa 1. 20
Displacing the composition of maximum short-range ordering Associate Model: – Let associates be “Al 2 Ca” – Problem arises that partial no longer obeys Raoult’s Law as XCa 1. Quasichemical Model: Let ZCa = 2 ZAl ZAXA = 2 n. AA + n. AB ZBXB = 2 n. BB + n. AB Raoult’s Law is obeyed as XCa 1. 20
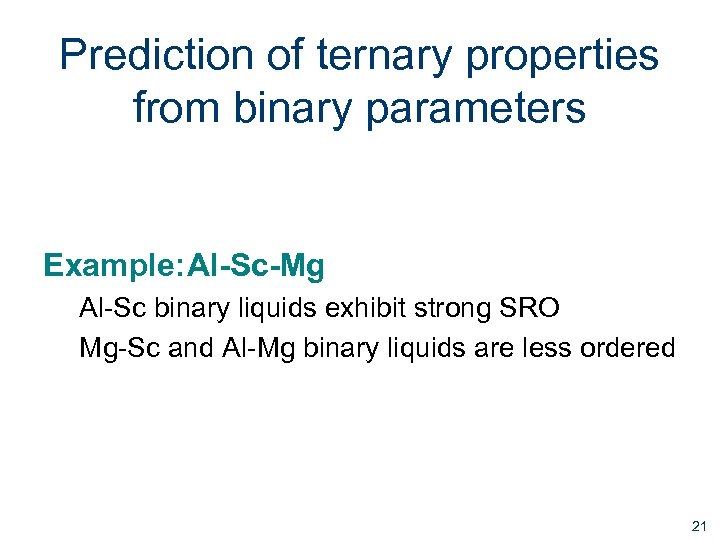 Prediction of ternary properties from binary parameters Example: Al-Sc-Mg Al-Sc binary liquids exhibit strong SRO Mg-Sc and Al-Mg binary liquids are less ordered 21
Prediction of ternary properties from binary parameters Example: Al-Sc-Mg Al-Sc binary liquids exhibit strong SRO Mg-Sc and Al-Mg binary liquids are less ordered 21
![Optimized polythermal liquidus projection of Al-Sc-Mg system [18]. 22 Optimized polythermal liquidus projection of Al-Sc-Mg system [18]. 22](https://present5.com/presentation/440601029afbea85a6a236ed34990755/image-22.jpg) Optimized polythermal liquidus projection of Al-Sc-Mg system [18]. 22
Optimized polythermal liquidus projection of Al-Sc-Mg system [18]. 22
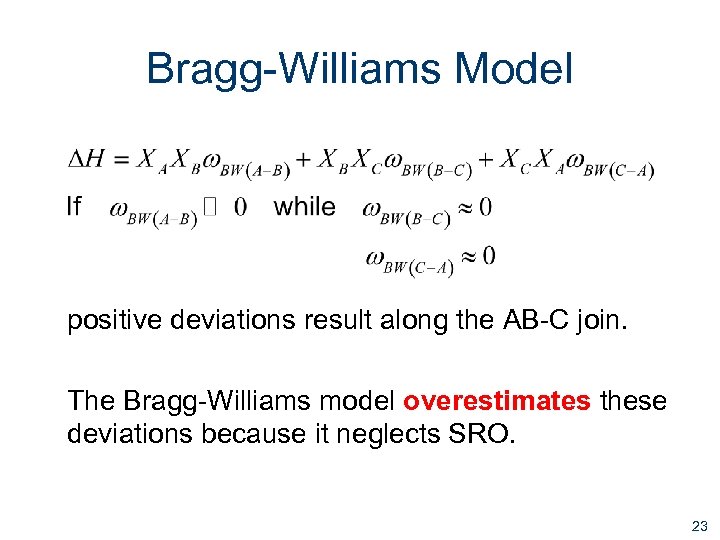 Bragg-Williams Model positive deviations result along the AB-C join. The Bragg-Williams model overestimates these deviations because it neglects SRO. 23
Bragg-Williams Model positive deviations result along the AB-C join. The Bragg-Williams model overestimates these deviations because it neglects SRO. 23
![Al 2 Sc-Mg join in the Al-Mg-Sc phase diagram. Experimental liquidus points [19] compared Al 2 Sc-Mg join in the Al-Mg-Sc phase diagram. Experimental liquidus points [19] compared](https://present5.com/presentation/440601029afbea85a6a236ed34990755/image-24.jpg) Al 2 Sc-Mg join in the Al-Mg-Sc phase diagram. Experimental liquidus points [19] compared to calculations from optimized binary parameters with various models [18]. 24
Al 2 Sc-Mg join in the Al-Mg-Sc phase diagram. Experimental liquidus points [19] compared to calculations from optimized binary parameters with various models [18]. 24
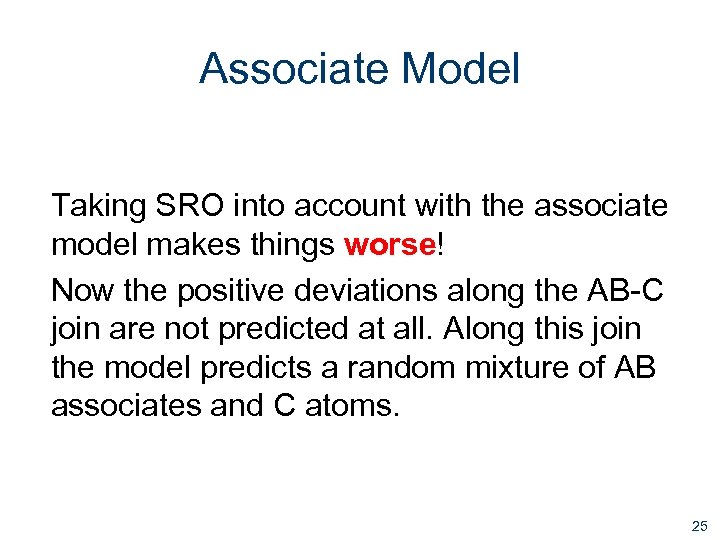 Associate Model Taking SRO into account with the associate model makes things worse! Now the positive deviations along the AB-C join are not predicted at all. Along this join the model predicts a random mixture of AB associates and C atoms. 25
Associate Model Taking SRO into account with the associate model makes things worse! Now the positive deviations along the AB-C join are not predicted at all. Along this join the model predicts a random mixture of AB associates and C atoms. 25
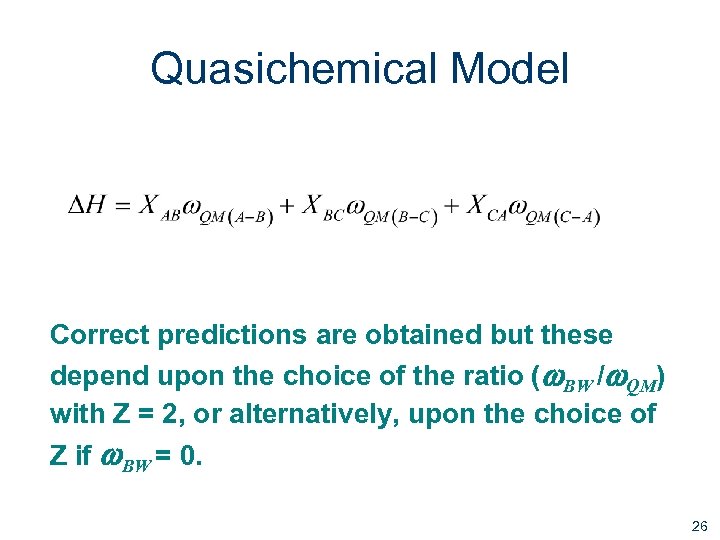 Quasichemical Model Correct predictions are obtained but these depend upon the choice of the ratio (w. BW /w. QM) with Z = 2, or alternatively, upon the choice of Z if w. BW = 0. 26
Quasichemical Model Correct predictions are obtained but these depend upon the choice of the ratio (w. BW /w. QM) with Z = 2, or alternatively, upon the choice of Z if w. BW = 0. 26
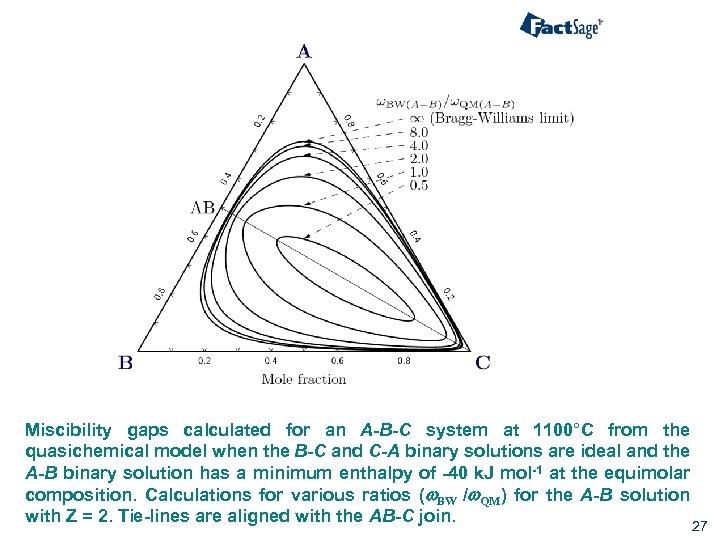 Miscibility gaps calculated for an A-B-C system at 1100°C from the quasichemical model when the B-C and C-A binary solutions are ideal and the A-B binary solution has a minimum enthalpy of -40 k. J mol-1 at the equimolar composition. Calculations for various ratios (w. BW /w. QM) for the A-B solution with Z = 2. Tie-lines are aligned with the AB-C join. 27
Miscibility gaps calculated for an A-B-C system at 1100°C from the quasichemical model when the B-C and C-A binary solutions are ideal and the A-B binary solution has a minimum enthalpy of -40 k. J mol-1 at the equimolar composition. Calculations for various ratios (w. BW /w. QM) for the A-B solution with Z = 2. Tie-lines are aligned with the AB-C join. 27
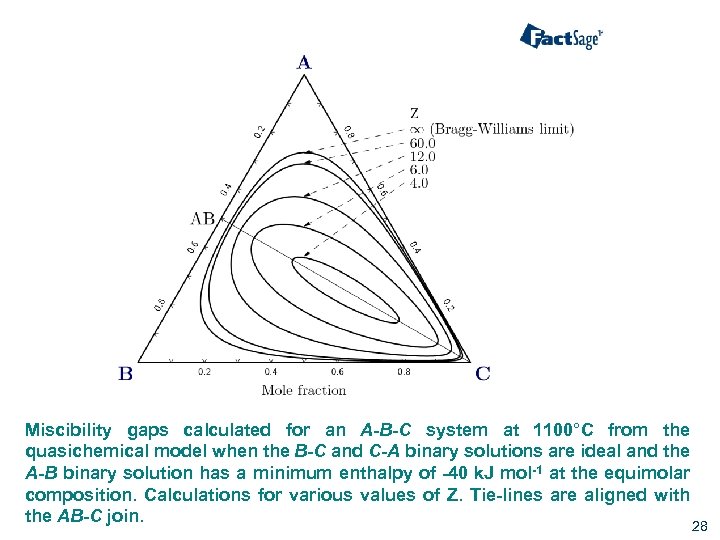 Miscibility gaps calculated for an A-B-C system at 1100°C from the quasichemical model when the B-C and C-A binary solutions are ideal and the A-B binary solution has a minimum enthalpy of -40 k. J mol-1 at the equimolar composition. Calculations for various values of Z. Tie-lines are aligned with the AB-C join. 28
Miscibility gaps calculated for an A-B-C system at 1100°C from the quasichemical model when the B-C and C-A binary solutions are ideal and the A-B binary solution has a minimum enthalpy of -40 k. J mol-1 at the equimolar composition. Calculations for various values of Z. Tie-lines are aligned with the AB-C join. 28
 Binary Systems Short-range ordering with positive deviations from ideality (clustering) Bragg-Williams model with w. BW > 0 gives miscibility gaps which often are too rounded. (Experimental gaps have flatter tops. ) 29
Binary Systems Short-range ordering with positive deviations from ideality (clustering) Bragg-Williams model with w. BW > 0 gives miscibility gaps which often are too rounded. (Experimental gaps have flatter tops. ) 29
![Ga-Pb phase diagram showing miscibility gap. Experimental points from [14]. Curves calculated from the Ga-Pb phase diagram showing miscibility gap. Experimental points from [14]. Curves calculated from the](https://present5.com/presentation/440601029afbea85a6a236ed34990755/image-30.jpg) Ga-Pb phase diagram showing miscibility gap. Experimental points from [14]. Curves calculated from the quasichemical model and the BW model for various sets of parameters as shown (k. J mol-1). 30
Ga-Pb phase diagram showing miscibility gap. Experimental points from [14]. Curves calculated from the quasichemical model and the BW model for various sets of parameters as shown (k. J mol-1). 30
 Quasichemical Model With Z = 2 and w. QM > 0, positive deviations are predicted, but immiscibility never results. 31
Quasichemical Model With Z = 2 and w. QM > 0, positive deviations are predicted, but immiscibility never results. 31
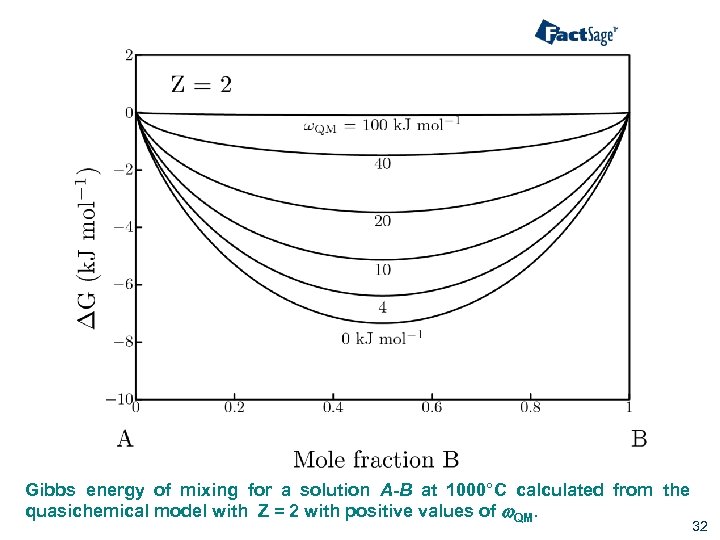 Gibbs energy of mixing for a solution A-B at 1000°C calculated from the quasichemical model with Z = 2 with positive values of w. QM. 32
Gibbs energy of mixing for a solution A-B at 1000°C calculated from the quasichemical model with Z = 2 with positive values of w. QM. 32
 With proper choice of a ratio (w. BW / w. QM) with Z = 2, or alternatively, with the proper choice of Z (with w. BW = 0), flattened miscibility gaps can be reproduced which are in good agreement with measurements. 33
With proper choice of a ratio (w. BW / w. QM) with Z = 2, or alternatively, with the proper choice of Z (with w. BW = 0), flattened miscibility gaps can be reproduced which are in good agreement with measurements. 33
![Ga-Pb phase diagram showing miscibility gap. Experimental points from [14]. Curves calculated from the Ga-Pb phase diagram showing miscibility gap. Experimental points from [14]. Curves calculated from the](https://present5.com/presentation/440601029afbea85a6a236ed34990755/image-34.jpg) Ga-Pb phase diagram showing miscibility gap. Experimental points from [14]. Curves calculated from the quasichemical model and the BW model for various sets of parameters as shown (k. J mol-1). 34
Ga-Pb phase diagram showing miscibility gap. Experimental points from [14]. Curves calculated from the quasichemical model and the BW model for various sets of parameters as shown (k. J mol-1). 34
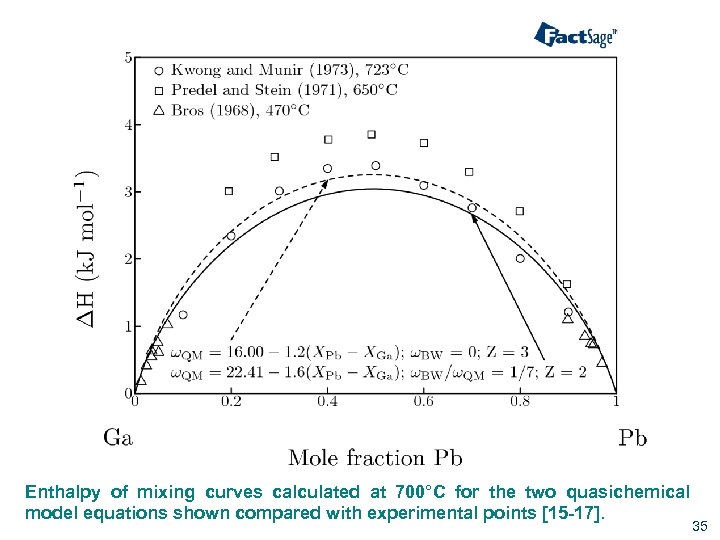 Enthalpy of mixing curves calculated at 700°C for the two quasichemical model equations shown compared with experimental points [15 -17]. 35
Enthalpy of mixing curves calculated at 700°C for the two quasichemical model equations shown compared with experimental points [15 -17]. 35
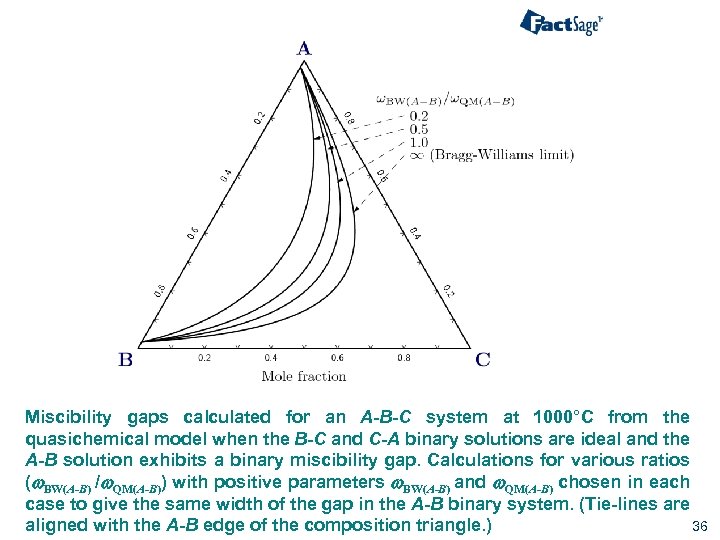 Miscibility gaps calculated for an A-B-C system at 1000°C from the quasichemical model when the B-C and C-A binary solutions are ideal and the A-B solution exhibits a binary miscibility gap. Calculations for various ratios (w. BW(A-B) /w. QM(A-B)) with positive parameters w. BW(A-B) and w. QM(A-B) chosen in each case to give the same width of the gap in the A-B binary system. (Tie-lines are 36 aligned with the A-B edge of the composition triangle. )
Miscibility gaps calculated for an A-B-C system at 1000°C from the quasichemical model when the B-C and C-A binary solutions are ideal and the A-B solution exhibits a binary miscibility gap. Calculations for various ratios (w. BW(A-B) /w. QM(A-B)) with positive parameters w. BW(A-B) and w. QM(A-B) chosen in each case to give the same width of the gap in the A-B binary system. (Tie-lines are 36 aligned with the A-B edge of the composition triangle. )


