69632bd3948cd056a3c1b6f4a5f3d162.ppt
- Количество слайдов: 22
Modeling Escherichia coli signal peptidase complex with bound substrate: Determinants in mature peptide influencing signal peptide cleavage Khar Heng Choo & Joo Chuan Tong (I 2 R) Shoba Ranganathan Professor and Chair – Bioinformatics Adjunct Professor Biotechnology Research Institute Dept. of Biochemistry Macquarie University National University of Singapore Sydney, Australia Singapore (shoba. ranganathan@mq. edu. au) (shoba@bic. nus. edu. sg)

Protein targeting Discovered by Günter Blobel in the 1970 s and was awarded Nobel Prize in Physiology or Medicine in 1999 for his discovery that “Proteins have intrinsic signals that govern their transport and localization in the cell”
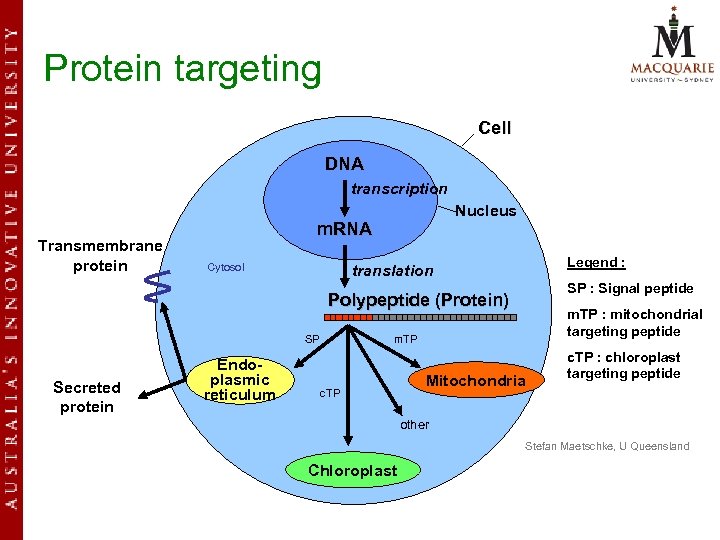
Protein targeting Cell DNA transcription Transmembrane protein Nucleus m. RNA Cytosol Legend : translation SP : Signal peptide Polypeptide (Protein) SP m. TP Endoplasmic Secreted protein plasmic reticulum m. TP : mitochondrial targeting peptide c. TP Mitochondria c. TP : chloroplast targeting peptide other Stefan Maetschke, U Queensland Chloroplast
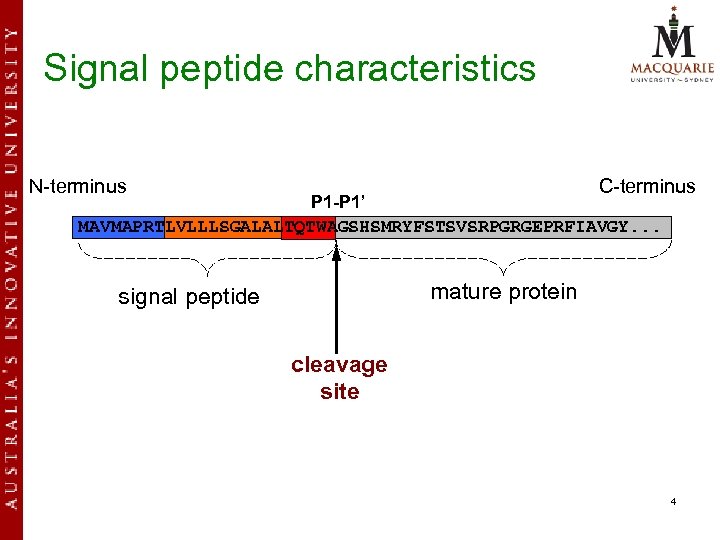
Signal peptide characteristics N-terminus C-terminus P 1 -P 1’ MAVMAPRTLVLLLSGALALTQTWAGSHSMRYFSTSVSRPGRGEPRFIAVGY. . . mature protein signal peptide cleavage site 4
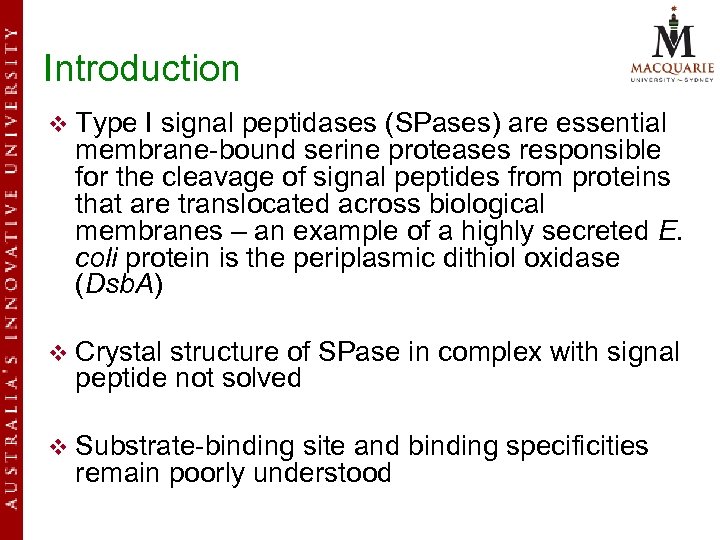
Introduction v Type I signal peptidases (SPases) are essential membrane-bound serine proteases responsible for the cleavage of signal peptides from proteins that are translocated across biological membranes – an example of a highly secreted E. coli protein is the periplasmic dithiol oxidase (Dsb. A) v Crystal structure of SPase in complex with signal peptide not solved v Substrate-binding site and binding specificities remain poorly understood
Our aims v To develop a structure-based model for E. coli periplasmic dithiol oxidase (Dsb. A) 13 -25 (P 7 P 6’: H 2 N-LAFSASAΔAQYEDG-COOH) in complex with its endogenous type I SPase v To understand how the peptide modeled in this study could be used to understand the observed E. coli repertoire of secreted signals, compiled by our manually curated signal peptide database.
Data : Sequences & Structures v 107 experimentally validated E. coli type I SPase peptide substrates extracted from the manually curated signal peptide database (SPdb) at http: //proline. bic. nus. edu. sg/spdb/ (Choo, Tan and Ranganathan, BMC Bioinformatics 2005, 6: 249) v Redundancy reduction at 80% using CD-HIT (Li and Godzik, Bioinformatics 2006, 22: 1658 -1659) v E. coli type I SPase-bound β-lactam (1 B 12) and lipopeptide (1 T 7 D) inhibitors were retrieved from the Protein Data Bank (PDB)
What do we know? v We have the structures of two inhibitor peptides at the active site of SPase I: these peptides mimic the transition state structure of the bound signal peptide to the signal peptidase. v These inhibitors also occupy positions that are complementary, with little overlap. v Determinants in the sequence of the mature protein affect signal peptide processing and secretion.
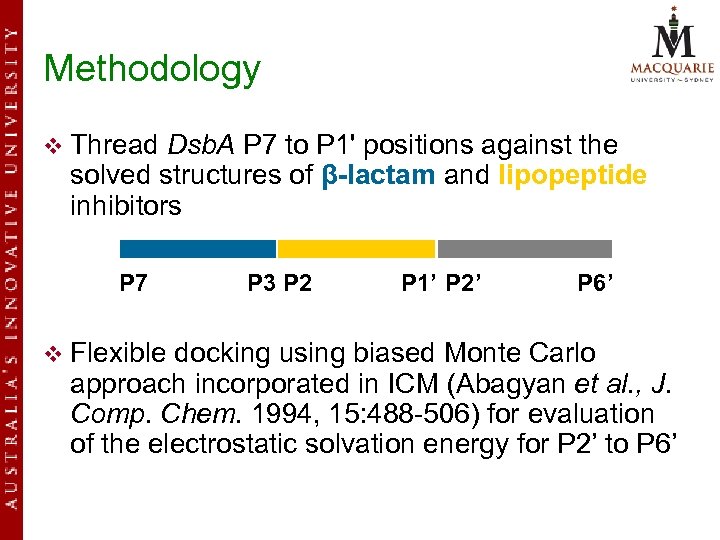
Methodology v Thread Dsb. A P 7 to P 1' positions against the solved structures of β-lactam and lipopeptide inhibitors P 7 v Flexible P 3 P 2 P 1’ P 2’ P 6’ docking using biased Monte Carlo approach incorporated in ICM (Abagyan et al. , J. Comp. Chem. 1994, 15: 488 -506) for evaluation of the electrostatic solvation energy for P 2’ to P 6’
Methodology (cont. ) v Structures relaxed using ICM software package conjugate gradient minimization. v In each iteration, new conformations for P 2’ to P 6’ were selected based on the Metropolis criterion with a temperature of 5000 K. The simulation was terminated after 20, 000 energy evaluations. v Intermolecular hydrogen bonds was calculated using HBPLUS (Mc. Donald and Thornton, J. Mol. Biol. 1994, 238: 777 -793).
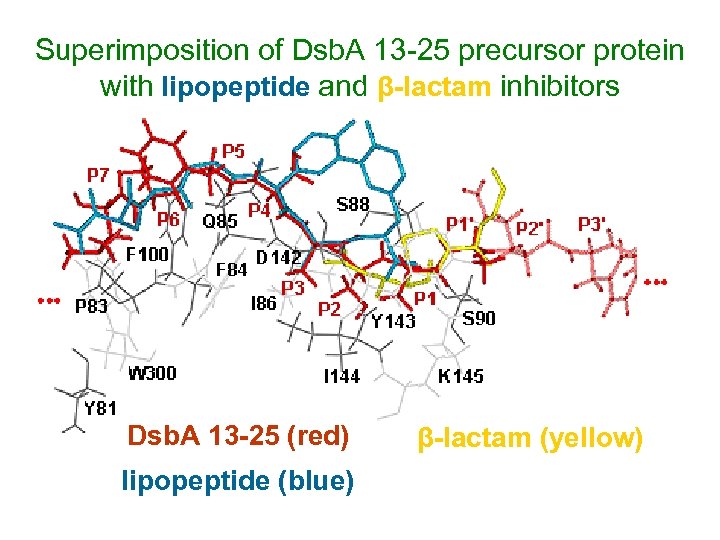
Superimposition of Dsb. A 13 -25 precursor protein with lipopeptide and β-lactam inhibitors Dsb. A 13 -25 (red) lipopeptide (blue) β-lactam (yellow)
Results v 13 enzyme subsites S 7 to S 6’ were identified within the SPase substrate binding site that might be critical to catalysis v Narrow clefts at S 3, S 2, S 1 and S 1’ play direct roles in the high specificity of the signal peptide residues v Larger clefts at S 3’ and S 4’ may be responsible for the specificity of the mature moieties.
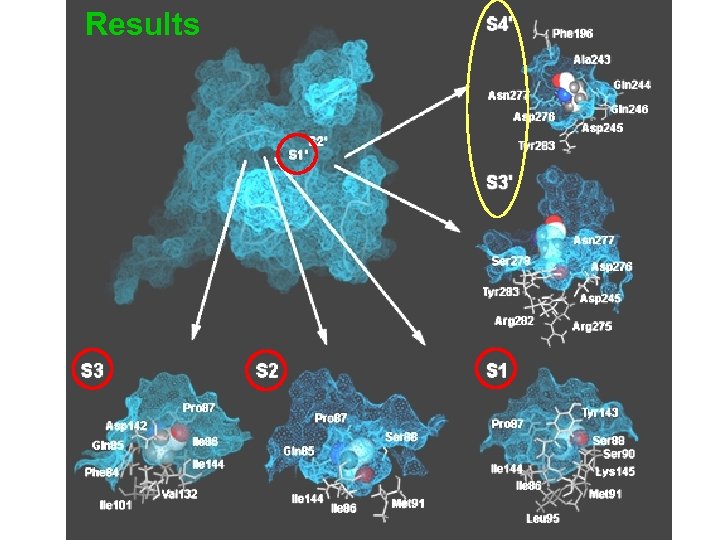
Results
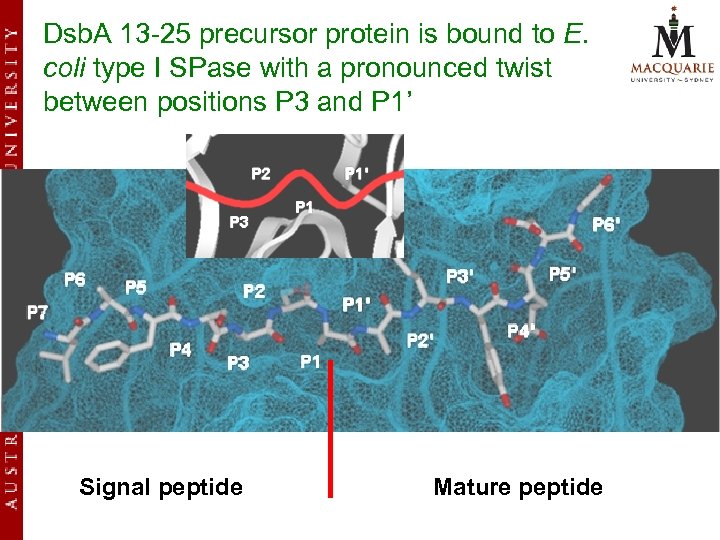
Dsb. A 13 -25 precursor protein is bound to E. coli type I SPase with a pronounced twist between positions P 3 and P 1’ Signal peptide Mature peptide
Results (cont. ) v Our model suggests that the enzymesubstrate contact points extend all the way from P 7 to P 6’ of the Dsb. A precursor protein. v Other models described earlier only focused on the P 3 -P 1’ segment and did not analyze in full the different substrate binding pockets on either side of the scissile bond.

Results (cont. ) v The S 2 subsite has the deepest cavity Ø can accommodate residues with large side chains Ø appears to play an important role in substrate specificity of E. coli type I SPase Ø formerly proposed as the S 1 by Paetzel et al. , largely overlaps with the S 1 subsite due to a pronounced twist in the P 3 to P 1’ binding conformation
Results (cont. ) v In contrast to the analysis by Paetzel et al. (2002), our model reveals that the Ser 18 (P 2) side chain is not solvent exposed but is completely buried. v Disparity between our model and Paetzel et al. may be attributable to the selection of different template structures where the structures of the covalently bound peptide inhibitor complex and the analogous enzyme Lex. A were used to guide the P 1 and P 3 to P 6 positions.
Results (cont. ) v The conformation of P 3' and P 4' allow their corresponding side-chains to extend into a large cavity (S 3'/S 4' subsites) Ø medium or large residues are preferred at these two positions for favorable interactions Ø medium or large residues both at P 3’ (81%) and at P 4’ (90%) positions
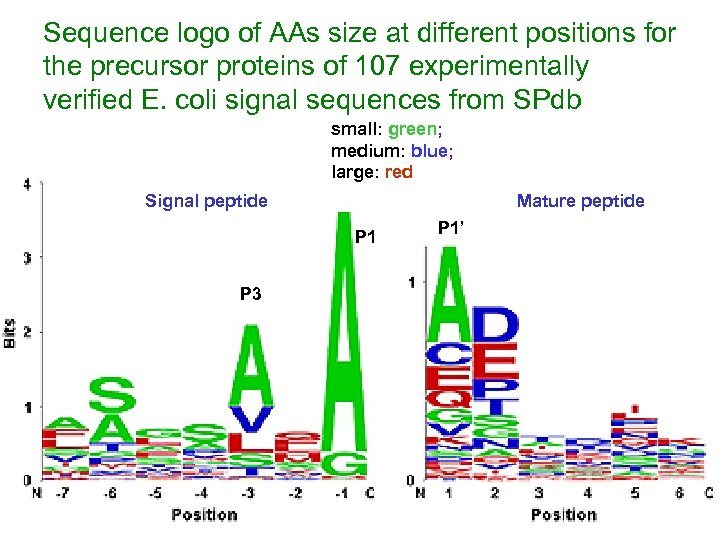
Sequence logo of AAs size at different positions for the precursor proteins of 107 experimentally verified E. coli signal sequences from SPdb small: green; medium: blue; large: red Signal peptide Mature peptide P 1 P 3 P 1’

Results (cont. ) v Most residues are tolerated at the +1 (P 1’) position of the mature moiety, with the exception of the large hydrophobes, Ile, Met and Trp and Pro, and Arg v Pro is avoided as the rigid positioning of its backbone hinders docking interactions with SPase at positions P 2’ to P 6’
Conclusions v This is the first report on the modeling of a precursor protein into the entire SPase binding site v Our model provides insights into the binding conformation of signal peptides and the substratebinding site of E. coli SPase I v SPdb data suggests that signal and mature moieties should be considered in the development of predictive tools

Acknowledgements v Khar Heng Choo v Joo Chuan Tong v Dept. of Biochemistry, Yong Loo Lin School of Medicine, National University of Singapore v In. Co. B organizers
69632bd3948cd056a3c1b6f4a5f3d162.ppt