88be9ce3ad49d543495a79655c4791e2.ppt
- Количество слайдов: 68

MMG /BIOC 352 Protein-DNA Interactions: Kinetics and Thermodynamics Example: the Bacteriophage l System Spring 2006 Scott W. Morrical with special thanks to Margaret A. Daugherty

Contact Information Scott W. Morrical Given B 407 656 -8260 Scott. Morrical@uvm. edu
Lecture outline: Introduction to the system Bacteriophage lambda Lysogeny vs. lysis The molecular switch PR, PRM, c. I repressor, cro Specific vs. Non-specific Interactions What makes a good DNA binding protein? Thermodynamic “Primer” DG = DH - TDS: importance Intrinsic Free Energy Cooperativity Techniques Quantitative DNAse Footprinting c. I repressor protein Structure Dimerization Data Cro protein Structure Dimerization & DNA Binding An example of induced fit Data Kinetic Aspects of c. I and cro binding Facilitated diffusion cro-DNA interactions Structure Analysis? cro-DNA vs. c. I-DNA interactions

Reference list for this topic: Ref 1: Ptashne, M. (1992) A Genetic Switch, 2 nd ed. , Cell Press & Blackwell Scientific Publications, Cambridge, MA. **This an excellent general review of bacteriophage l with simple descriptions of thermodynamics and regulation. Ref 2: Johnson, A. D. , Poteete, A. R. , Lauer, G. , Sauer, R. T. , Ackers, G. K. & Ptashne, M. (1981) l Repressor and cro - components of an efficient molecular switch. Nature 294: 217 -223. Review article of bacteriophage l, outdated, but ok for understanding the system in general. Ref 3: Chattophadhyay, R. & Ghosh, K. (2003) A comparative three-dimensional model of the carboxy-terminal domain of the lambda repressor and its use to build intact repressor tetramer models bound to adjacent operator sites. J. Struct. Biol. 141: 103 -114. Ref 4: Oda, M. & Nakamura, H. (2000) Thermodynamic and kinetic analyses for understanding sequence-specific DNA recognition. Genes to Cells 5: 319 -326. Just one of many reviews on thermo & kinetic aspects of DNA binding. Ref 5: Brenowitz, M. , Senear, D. F. , Shea, M. A. & Ackers, G. K. (1986) “Footprint” titrations yield valid thermodynamic isotherms. P. N. A. S. USA 83: 8462 -8466

Reference list - continued Ref 6: Koblan, K. S. & Ackers, G. K. (1992) Site-Specific Regulation of DNA Transcription at Bacteriophage l OR, Biochemistry 31: 57 -67. Ref 7: Darling, P. J. , Holt, J. M. & Ackers, G. K. (2000) Coupled Energetics of l cro Repressor Self-assembly and Site-specific DNA Operator Binding II: Cooperative Interactions of cro Dimers. J. Mol. Biol. 302: 625 -638. Ref 8: Albright, R. A. & Matthews, B. W. (1998) Crystal structure of l-cro bound to a Consensus Operator at 3. 0 Å Resolution, J. Mol. Biol. 280: 137 -151. Ref 9: Spolar, R. S. and Record, M. T. (1994) Coupling of Local Folding to Site-Specific Binding of Proteins to DNA. Science 263: 777 - 784 a classic “must know” paper! Ref 10: von Hippel (1994) Protein - DNA Recognition : New Perspectives and Underlying Themes. Science 263: 769 -770. (a review of Spolar & Record) Ref 11: Frankel, A. D. & Kim, P. S. (1991) Modular Structure of Transcription Factors: Implications for Gene Regulation. Cell 65: 717 -719 (quick reading - introduces notion of induced fit) Ref 12: Takeda, Y. , Ross. P. D. & Mudd, C. P. (1992) Thermodynamics of Cro-protein DNA interactions. Proc. Natl. Acad. Sci. USA 89: 8180 -8184.

Reference list - continued Ref 13: von Hippel, P. H. & Berg, O. G. (1989) Facilitated Target Location in Biological Systems. J. Biol. Chem. 264: 675 -678. Nice mini-review. Ref 14: Kim, J. G. , Takeda, Y. , Matthews, B. W. & Anderson, W. F. (1987) Kinetic Studies of Cro-Repressor Operator DNA Interaction, J. Mol. Biol. 196: 149 -158 Ref 15: Albright, R. A. and Matthews, B. W. (1998) How Cro and l-repressor distinguish between operators: The structural basis underlying a genetic switch. Proc. Natl. Acad. Sci. USA 95: 3431 -3436.
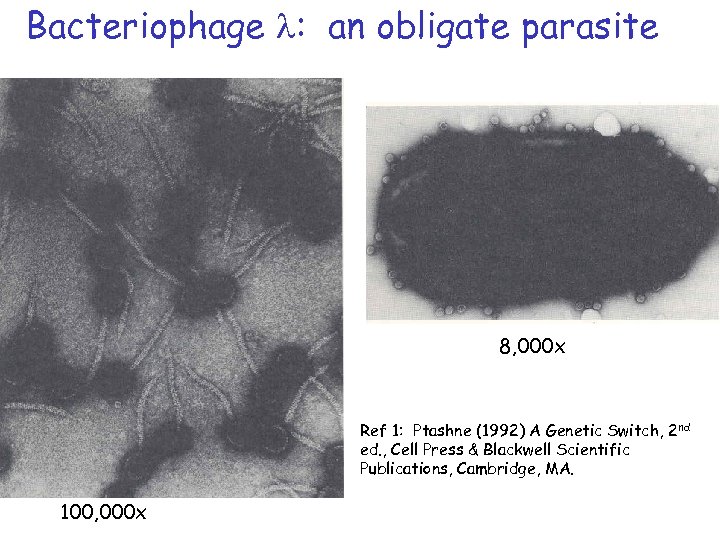
Bacteriophage l: an obligate parasite 8, 000 x Ref 1: Ptashne (1992) A Genetic Switch, 2 nd ed. , Cell Press & Blackwell Scientific Publications, Cambridge, MA. 100, 000 x
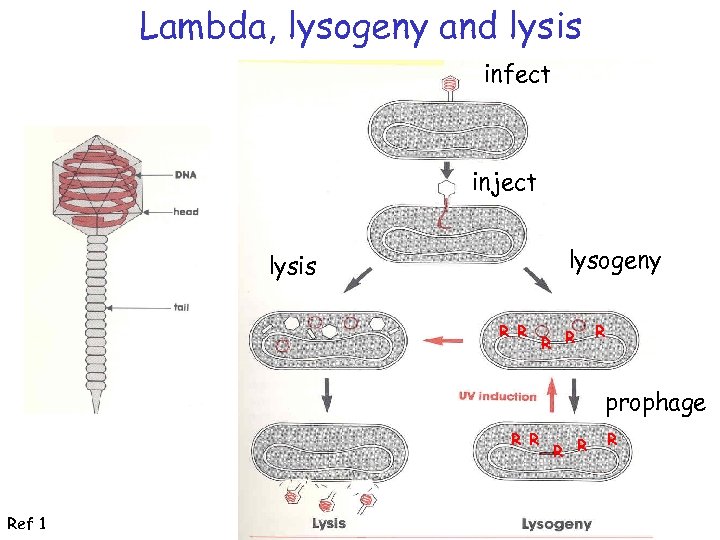
Lambda, lysogeny and lysis infect inject lysogeny lysis R R R prophage R R Ref 1 R R R
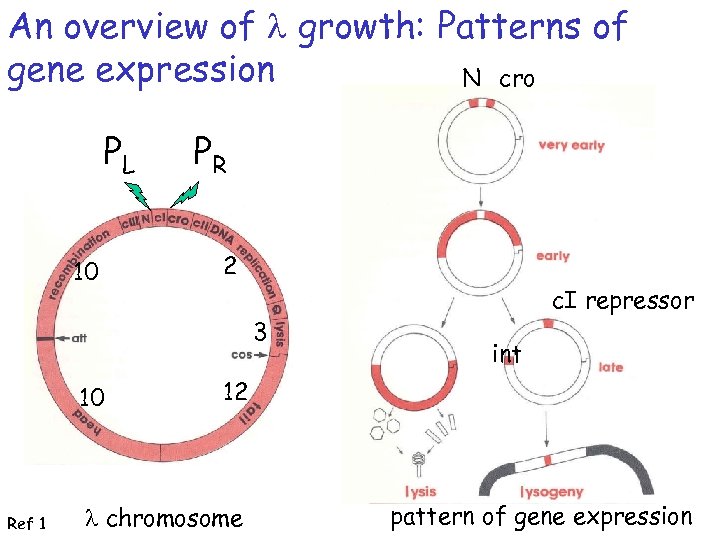
An overview of l growth: Patterns of gene expression N cro PL 10 PR 2 3 10 Ref 1 c. I repressor int 12 l chromosome pattern of gene expression
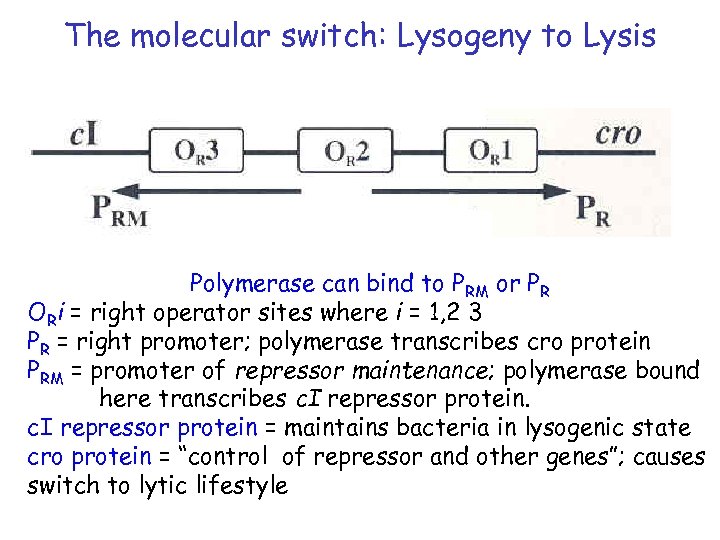
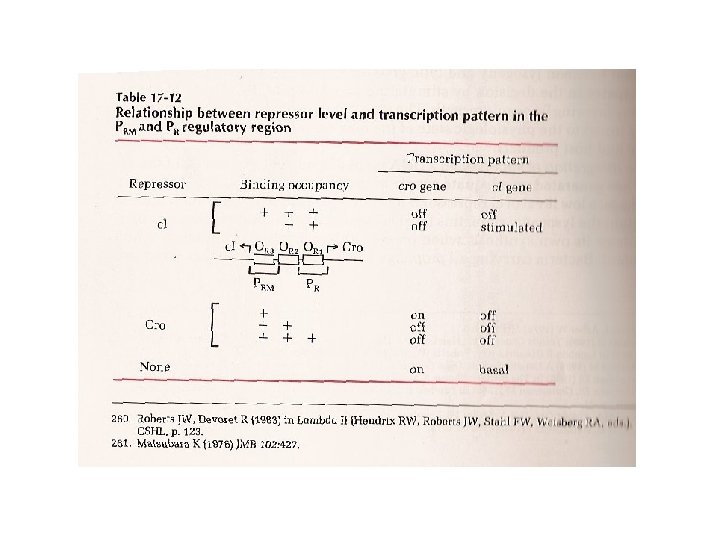
The molecular switch: Lysogeny to Lysis Polymerase can bind to PRM or PR ORi = right operator sites where i = 1, 2 3 PR = right promoter; polymerase transcribes cro protein PRM = promoter of repressor maintenance; polymerase bound here transcribes c. I repressor protein = maintains bacteria in lysogenic state cro protein = “control of repressor and other genes”; causes switch to lytic lifestyle
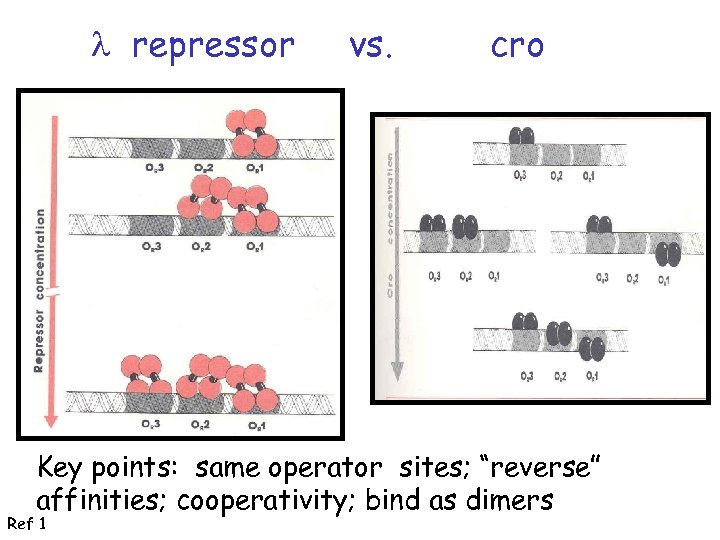
l repressor vs. cro Key points: same operator sites; “reverse” affinities; cooperativity; bind as dimers Ref 1
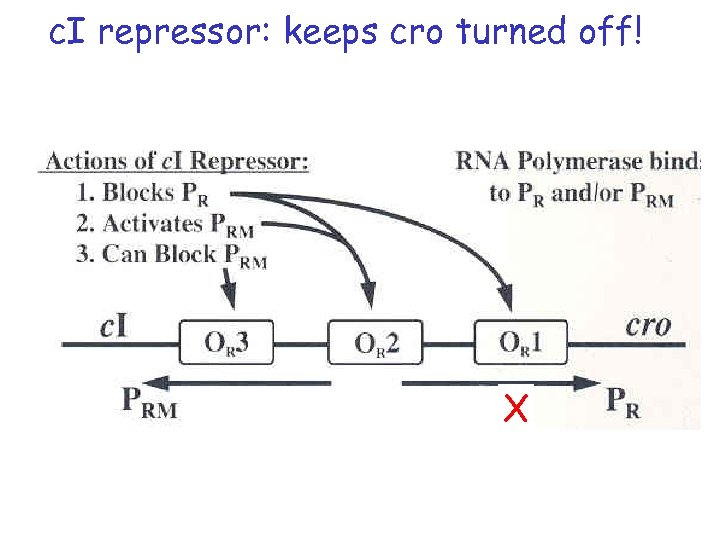
c. I repressor: keeps cro turned off! X
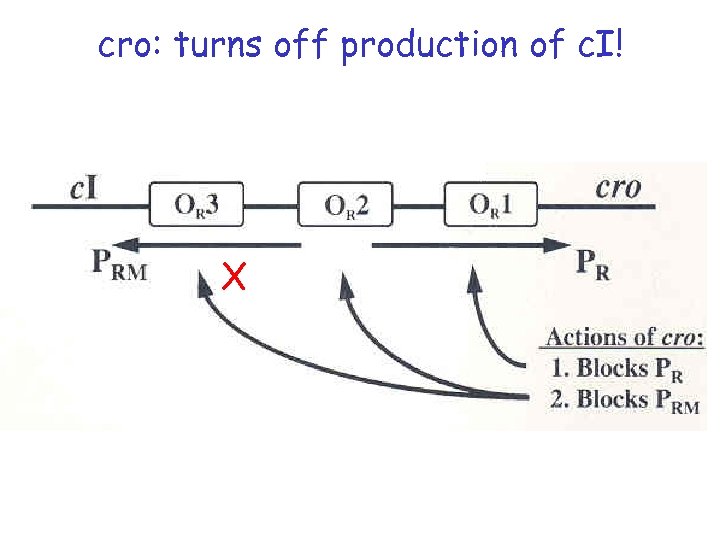
cro: turns off production of c. I! X
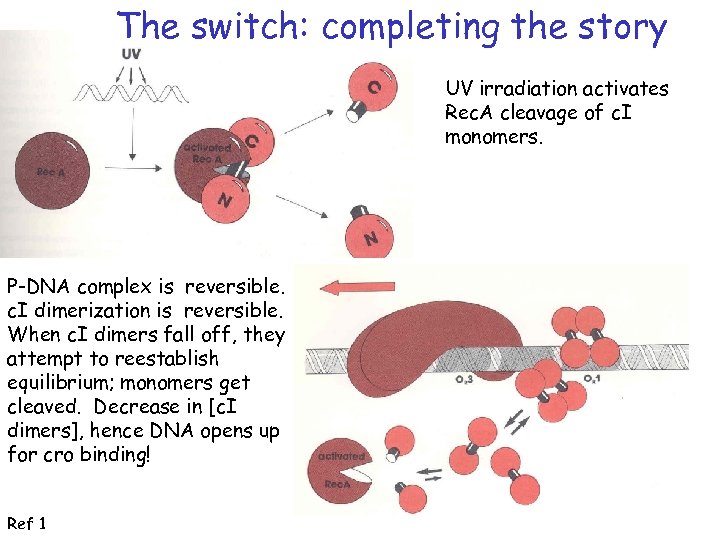
The switch: completing the story UV irradiation activates Rec. A cleavage of c. I monomers. P-DNA complex is reversible. c. I dimerization is reversible. When c. I dimers fall off, they attempt to reestablish equilibrium; monomers get cleaved. Decrease in [c. I dimers], hence DNA opens up for cro binding! Ref 1
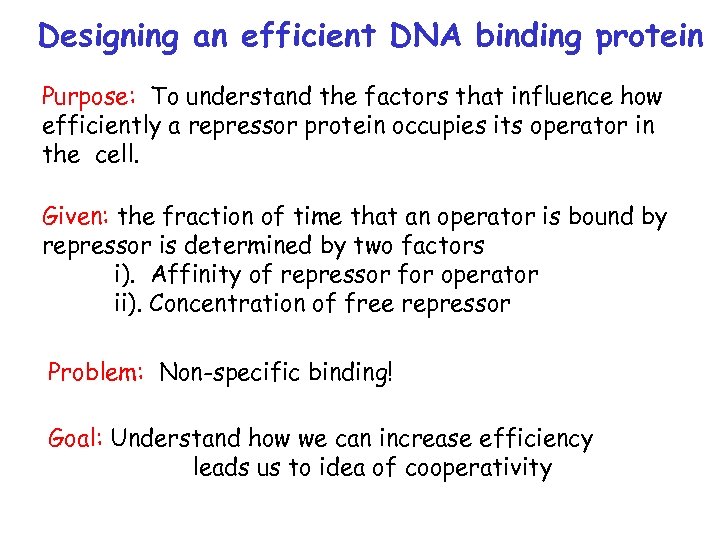
Designing an efficient DNA binding protein Purpose: To understand the factors that influence how efficiently a repressor protein occupies its operator in the cell. Given: the fraction of time that an operator is bound by repressor is determined by two factors i). Affinity of repressor for operator ii). Concentration of free repressor Problem: Non-specific binding! Goal: Understand how we can increase efficiency leads us to idea of cooperativity

Designing an efficient DNA binding protein Equations on board The rationale for the arguments are taken from Appendix One in reference 1

Designing an efficient DNA binding protein How can we increase specificity? 1). Increase protein concentration 2). Improve specificity directly *play with the KD/KOP ratio hold KD constant; improve KOP *increase number of contacts by increasing repressor twice the contacts, twice the energy! KOP = 10 -20 M; KD = 10 -8 M good idea, but…. Affinities become a problem, which give rise to kinetic problems!

Increased efficiency with cooperativity
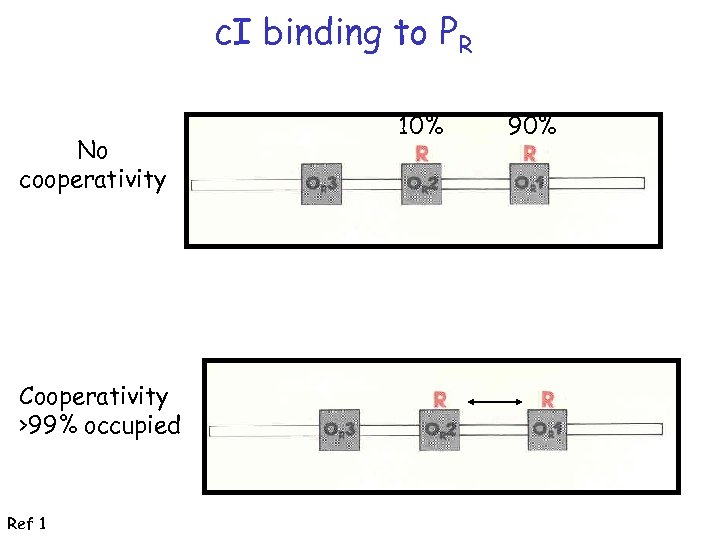
c. I binding to PR No cooperativity Cooperativity >99% occupied Ref 1 10% 90%
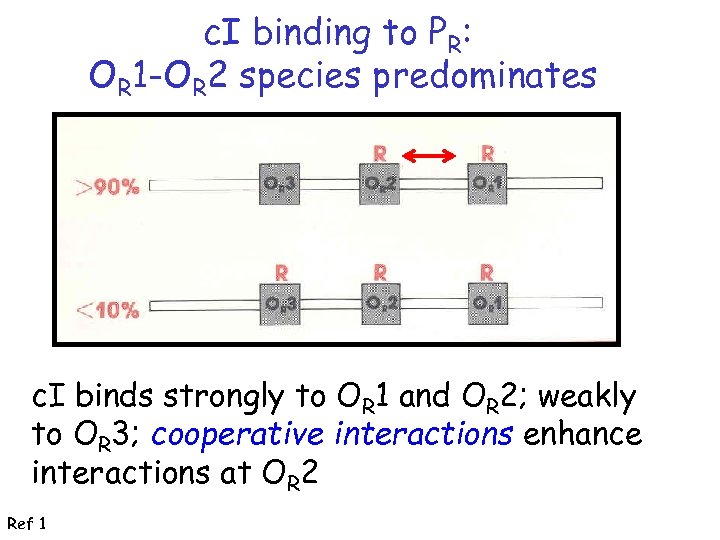
c. I binding to PR: OR 1 -OR 2 species predominates c. I binds strongly to OR 1 and OR 2; weakly to OR 3; cooperative interactions enhance interactions at OR 2 Ref 1
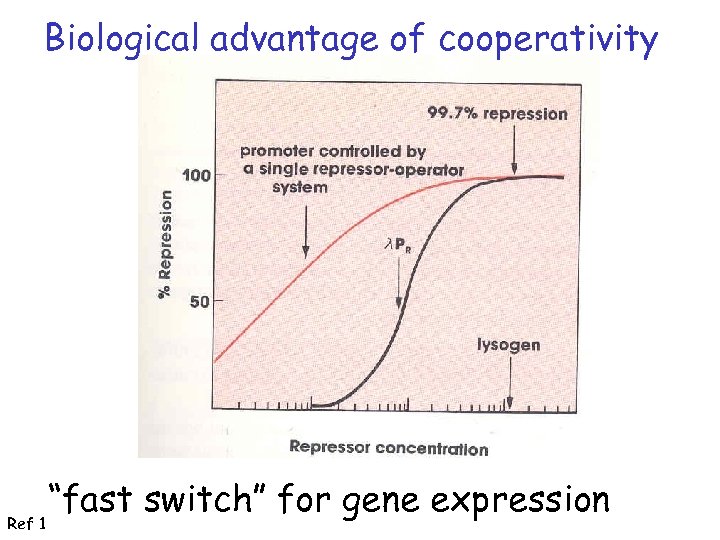
Biological advantage of cooperativity Ref 1 “fast switch” for gene expression
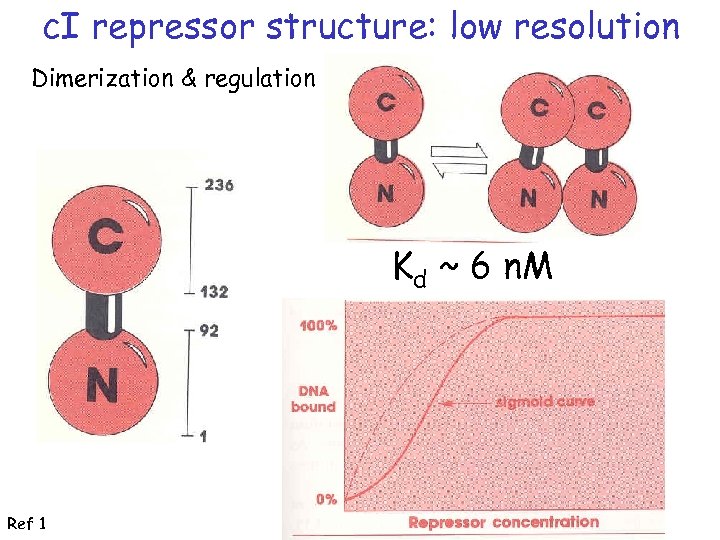
c. I repressor structure: low resolution Dimerization & regulation Kd ~ 6 n. M Ref 1
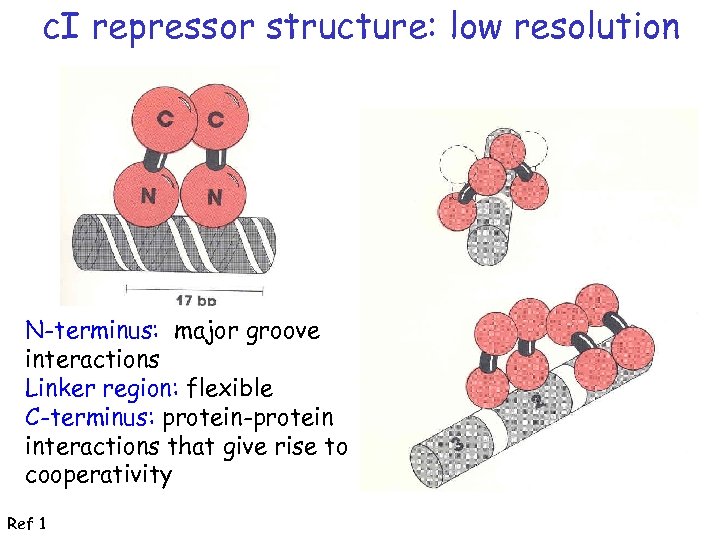
c. I repressor structure: low resolution N-terminus: major groove interactions Linker region: flexible C-terminus: protein-protein interactions that give rise to cooperativity Ref 1
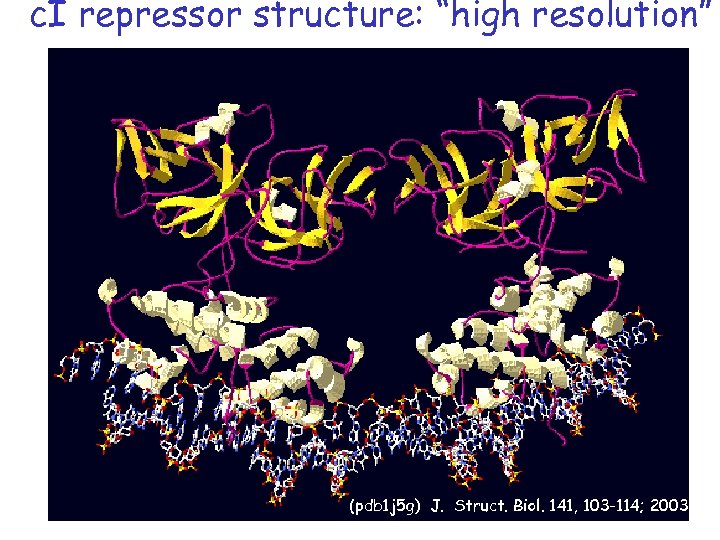
c. I repressor structure: “high resolution” (pdb 1 j 5 g) J. Struct. Biol. 141, 103 -114; 2003
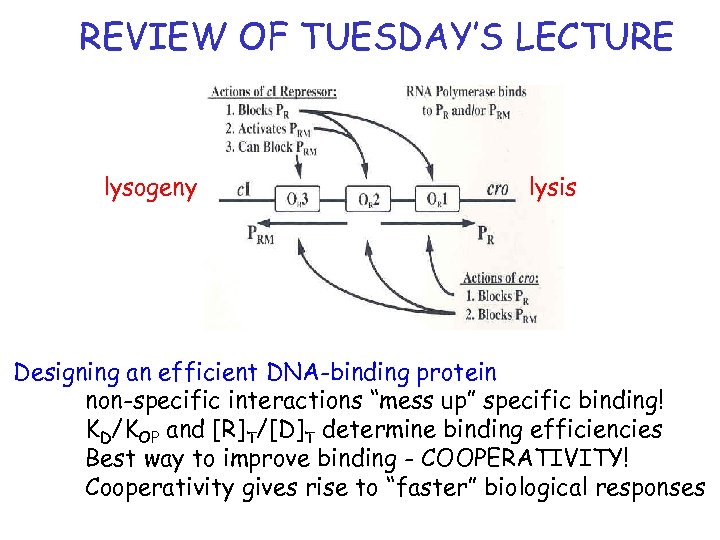
REVIEW OF TUESDAY’S LECTURE lysogeny lysis Designing an efficient DNA-binding protein non-specific interactions “mess up” specific binding! KD/KOP and [R]T/[D]T determine binding efficiencies Best way to improve binding - COOPERATIVITY! Cooperativity gives rise to “faster” biological responses
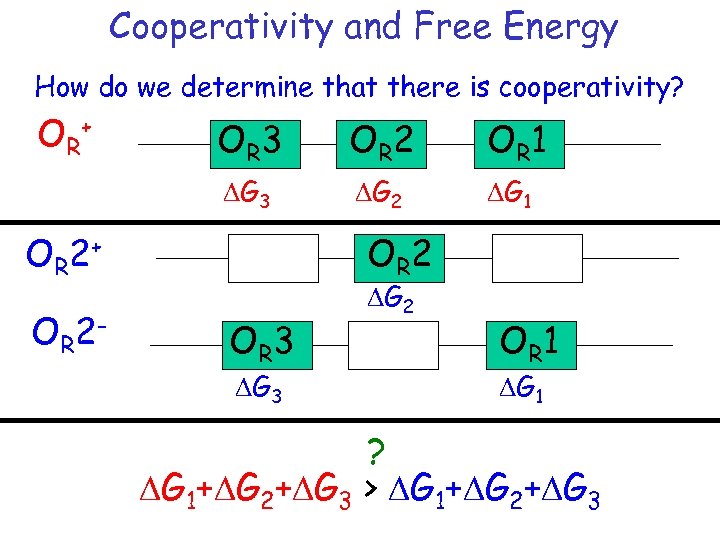
Cooperativity and Free Energy How do we determine that there is cooperativity? OR+ OR 3 OR 2 OR 1 DG 3 DG 2 DG 1 OR 2+ OR 2 - OR 2 OR 3 DG 2 OR 1 DG 1 ? DG 1+DG 2+DG 3 > DG 1+DG 2+DG 3

Thermodynamic Primer: Gibbs Free Energy P + DNA Keq P • DNA [P • DNA] Keq = [P] [DNA] o DG = -RTln Keq Remember: more negative, more favorable reaction!
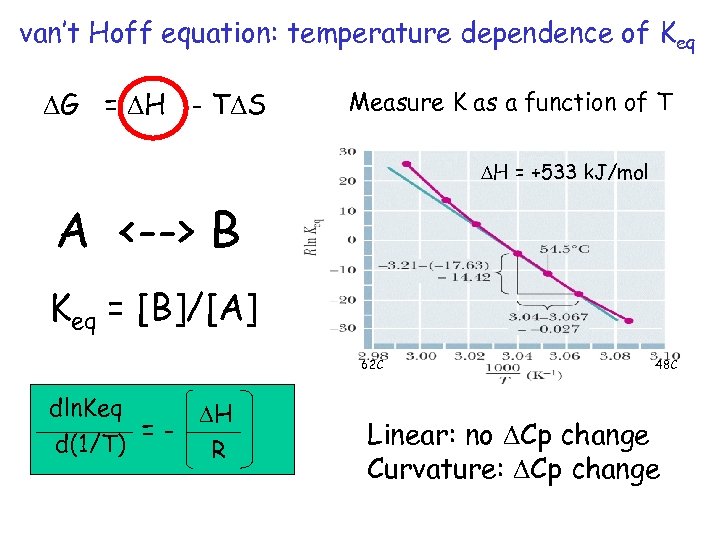
van’t Hoff equation: temperature dependence of Keq DG = DH - TDS Measure K as a function of T DH = +533 k. J/mol A <--> B Keq = [B]/[A] 62 C dln. Keq =d(1/T) DH R 48 C Linear: no DCp change Curvature: DCp change
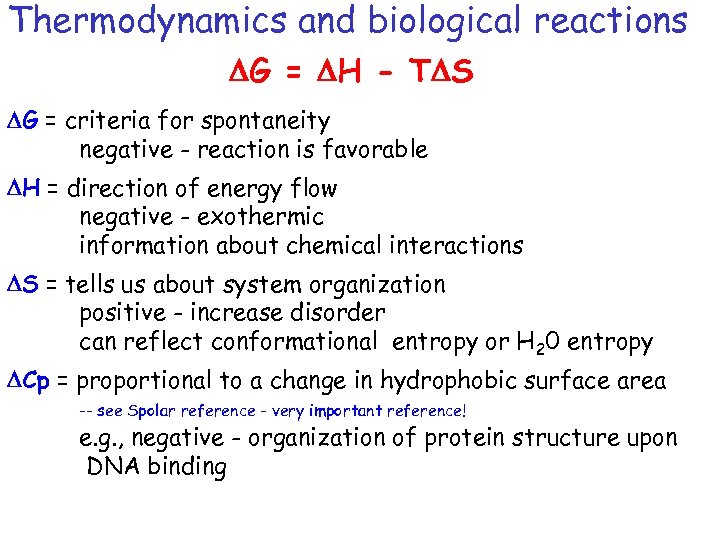
Thermodynamics and biological reactions DG = DH - TDS DG = criteria for spontaneity negative - reaction is favorable DH = direction of energy flow negative - exothermic information about chemical interactions DS = tells us about system organization positive - increase disorder can reflect conformational entropy or H 20 entropy DCp = proportional to a change in hydrophobic surface area -- see Spolar reference - very important reference! e. g. , negative - organization of protein structure upon DNA binding
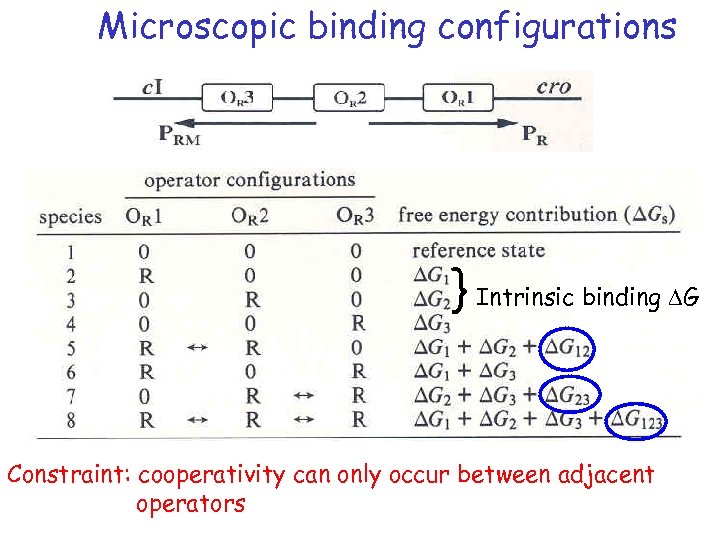
Microscopic binding configurations } Intrinsic binding DG Constraint: cooperativity can only occur between adjacent operators
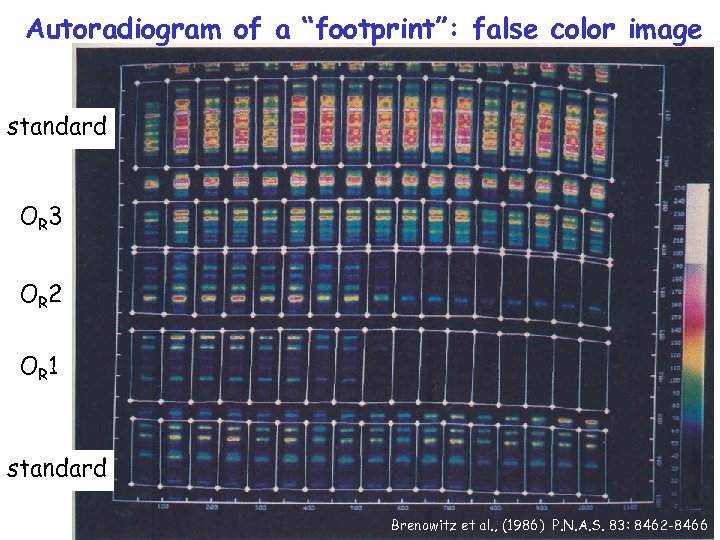
Autoradiogram of a “footprint”: false color image standard OR 3 OR 2 OR 1 standard Brenowitz et al. , (1986) P. N. A. S. 83: 8462 -8466
![Langmuir isotherm-- single site interactions: Y = K 1[X] / (1 + K 1[X]) Langmuir isotherm-- single site interactions: Y = K 1[X] / (1 + K 1[X])](https://present5.com/presentation/88be9ce3ad49d543495a79655c4791e2/image-33.jpg)
Langmuir isotherm-- single site interactions: Y = K 1[X] / (1 + K 1[X]) K 1 = 1/[X] at Y = 0. 5 For 2 -site cooperative interaction: Y 1 = (K 1[X] + K 1 K 2 K 12[X]2) / B Fractional saturation Individual site binding isotherms OR 3 Y 2 = (K 2[X] + K 1 K 2 K 12[X]2) / B where B = (1 + (K 1 + K 2)[X] + K 1 K 2 K 12[X]2) K 1 and K 2 are intrinsic binding constants for sites 1 & 2, and K 12 is the interaction (or cooperativity) constant. K 12 defines the extra free energy of binding 2 sites simultaneously compared to sum of individual free energies, i. e. DG 12 = DGtotal - (DG 1 + DG 2) where DG = RTln. K.

Individual site binding isotherms for c. I - OR interactions OR 3 OR 2 OR 1 1 OR 3 OR 2 2 3 OR 2 OR 1 1 3 Koblan, K. and Ackers, G. K. , (1992) Biochemistry 31: 57 -65.
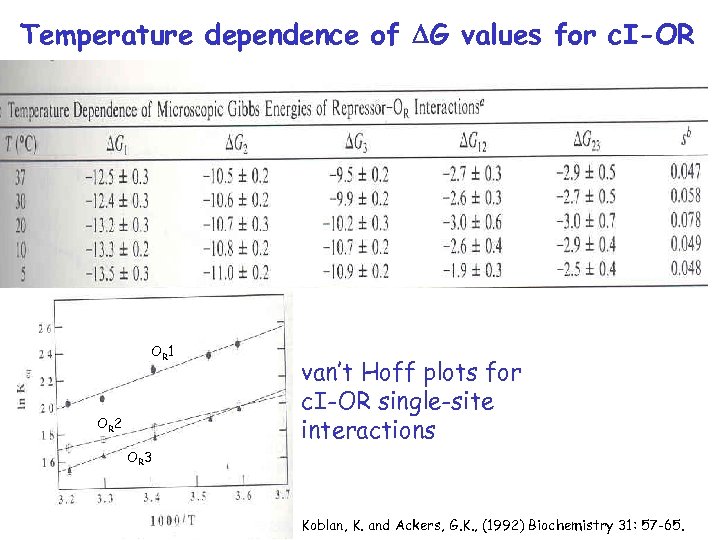
Temperature dependence of DG values for c. I-OR OR 1 OR 2 van’t Hoff plots for c. I-OR single-site interactions OR 3 Koblan, K. and Ackers, G. K. , (1992) Biochemistry 31: 57 -65.

c. I-OR Interactions are Enthalpically Driven Koblan, K. and Ackers, G. K. , (1992) Biochemistry 31: 57 -65.
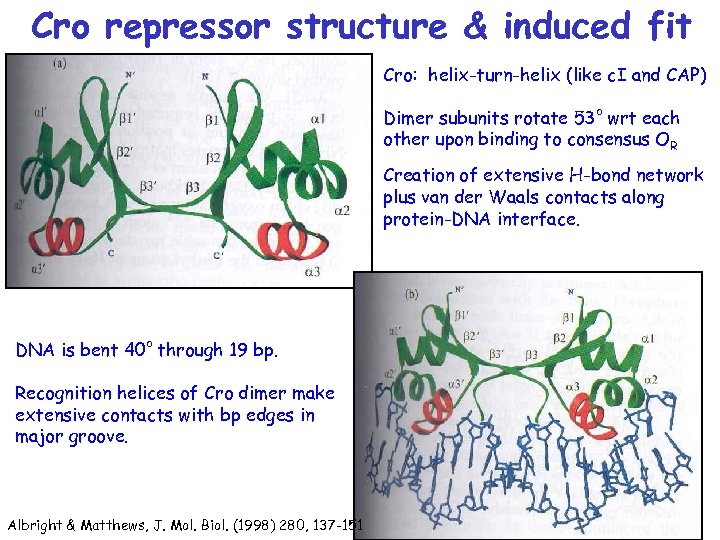
Cro repressor structure & induced fit Cro: helix-turn-helix (like c. I and CAP) Dimer subunits rotate 53 o wrt each other upon binding to consensus OR Creation of extensive H-bond network plus van der Waals contacts along protein-DNA interface. DNA is bent 40 o through 19 bp. Recognition helices of Cro dimer make extensive contacts with bp edges in major groove. Albright & Matthews, J. Mol. Biol. (1998) 280, 137 -151
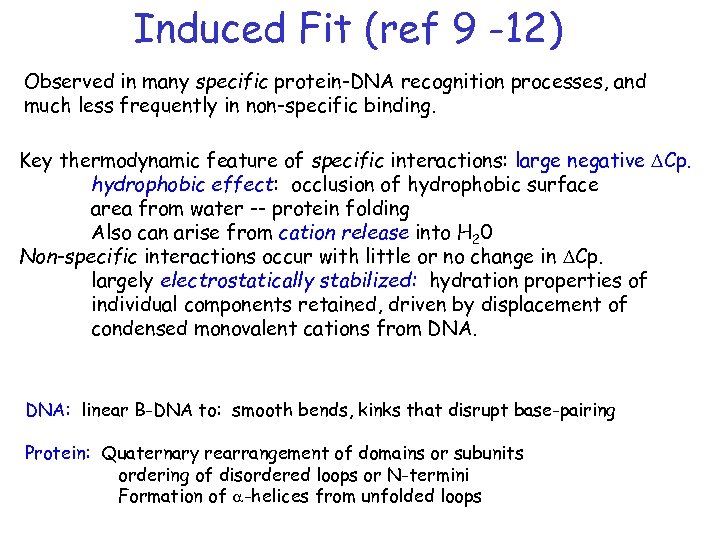
Induced Fit (ref 9 -12) Observed in many specific protein-DNA recognition processes, and much less frequently in non-specific binding. Key thermodynamic feature of specific interactions: large negative DCp. hydrophobic effect: occlusion of hydrophobic surface area from water -- protein folding Also can arise from cation release into H 20 Non-specific interactions occur with little or no change in DCp. largely electrostatically stabilized: hydration properties of individual components retained, driven by displacement of condensed monovalent cations from DNA: linear B-DNA to: smooth bends, kinks that disrupt base-pairing Protein: Quaternary rearrangement of domains or subunits ordering of disordered loops or N-termini Formation of a-helices from unfolded loops

Binding isotherms for Cro repressor OR+ 3 1 2 OR 1, OR 2 & OR 3 templates
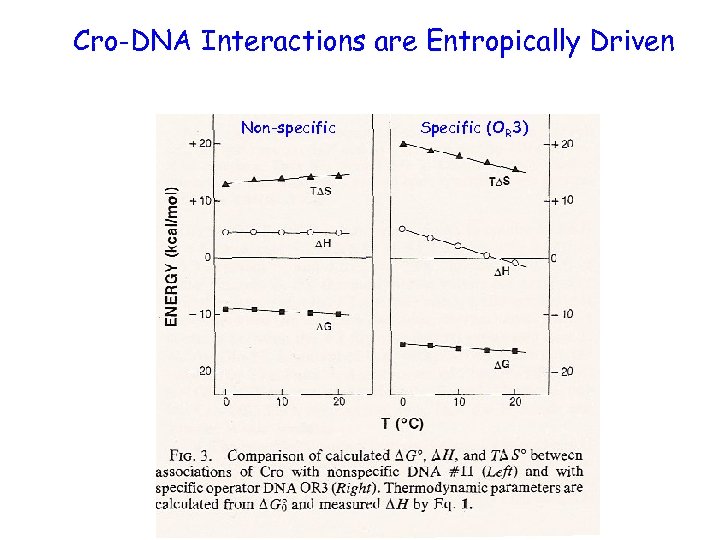
Cro-DNA Interactions are Entropically Driven Non-specific Specific (OR 3)
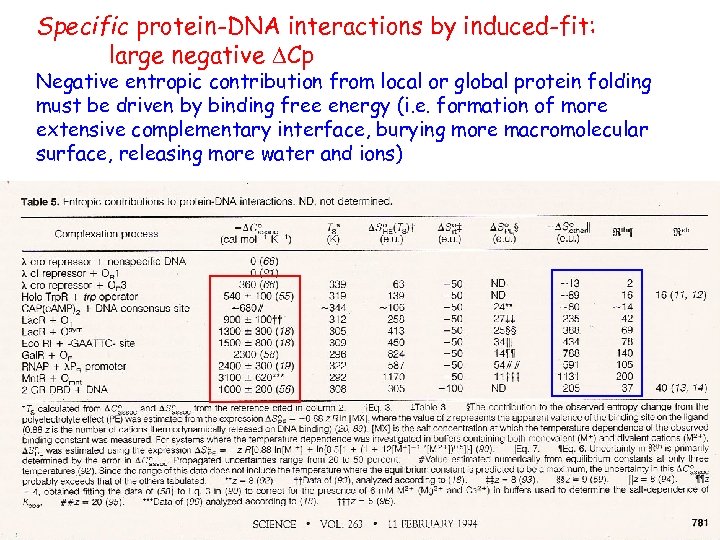
Specific protein-DNA interactions by induced-fit: large negative DCp Negative entropic contribution from local or global protein folding must be driven by binding free energy (i. e. formation of more extensive complementary interface, burying more macromolecular surface, releasing more water and ions)
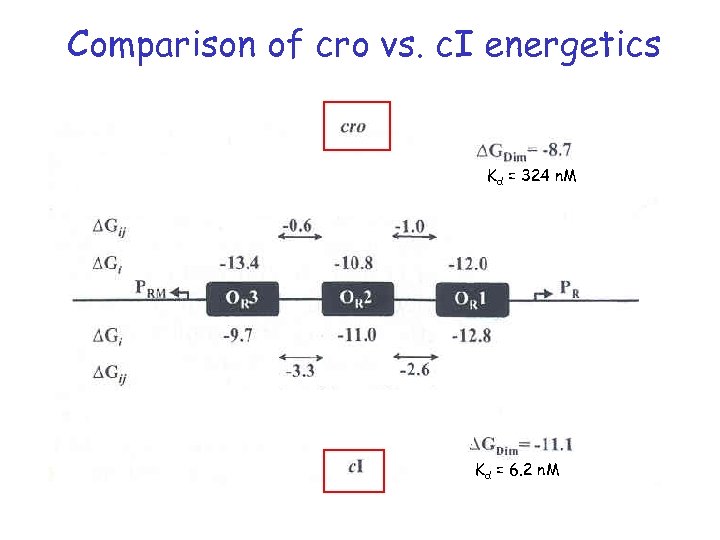
Comparison of cro vs. c. I energetics Kd = 324 n. M Kd = 6. 2 n. M

Kinetics of protein-DNA interactions For function, regulatory proteins must reach target DNA. Problem: regulatory proteins show affinity to non-specific DNA result: competition, potential slowing down of interactions Early results on lac repressor show rates were increased above simple diffusion rates: 100 - 1000 x faster!
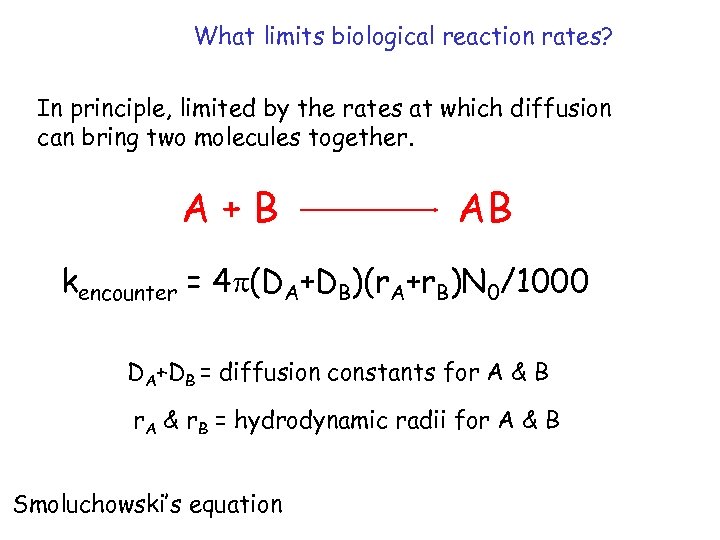
What limits biological reaction rates? In principle, limited by the rates at which diffusion can bring two molecules together. A+B AB kencounter = 4 p(DA+DB)(r. A+r. B)N 0/1000 DA+DB = diffusion constants for A & B r. A & r. B = hydrodynamic radii for A & B Smoluchowski’s equation

Kinetics of biological reactions are complex! surfaces of macromolecules not uniformly reactive electrostatic forces may be attractive or repulsive asymmetric molecules (rate of diffusion decreases) complex interaction distance kassoc = 4 pkaf(DA+DB)N 0/1000 k represents fraction of A & B that interact a is the interaction distance f reflects attractive/repulsive electrostatic forces Modified Smoluchowski equation lets us calculate kassoc for protein & DNA (lac-DNA) at 108 M-1 sec-1. In reality, kassoc= 5 x 1010 M-1 sec-1.

Forward rate constants for macromolecular association Biological molecules are not small inert spheres! Small diffusion constants General stickiness -- van der Waals Biological molecules are slow to drift apart! A classically defined “collision” can consist of many mini-collisions. Rotational rearrangements overcome steric factors Biological molecules are charged! Macromolecules set up electrostatic fields that guide substrates to functional sites; sometimes “shaped”
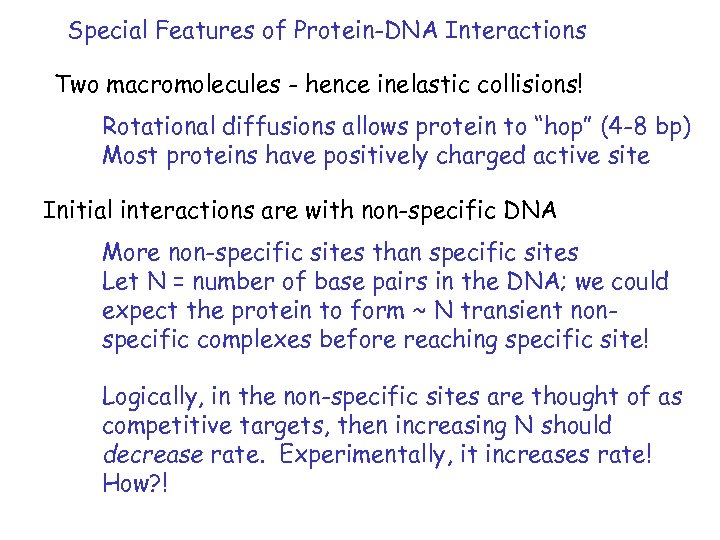
Special Features of Protein-DNA Interactions Two macromolecules - hence inelastic collisions! Rotational diffusions allows protein to “hop” (4 -8 bp) Most proteins have positively charged active site Initial interactions are with non-specific DNA More non-specific sites than specific sites Let N = number of base pairs in the DNA; we could expect the protein to form ~ N transient nonspecific complexes before reaching specific site! Logically, in the non-specific sites are thought of as competitive targets, then increasing N should decrease rate. Experimentally, it increases rate! How? !

Initial interactions are with non-specific DNA diffusion facilitated k 1 k 2 R+D+O <-> RO + D k-1 k-2
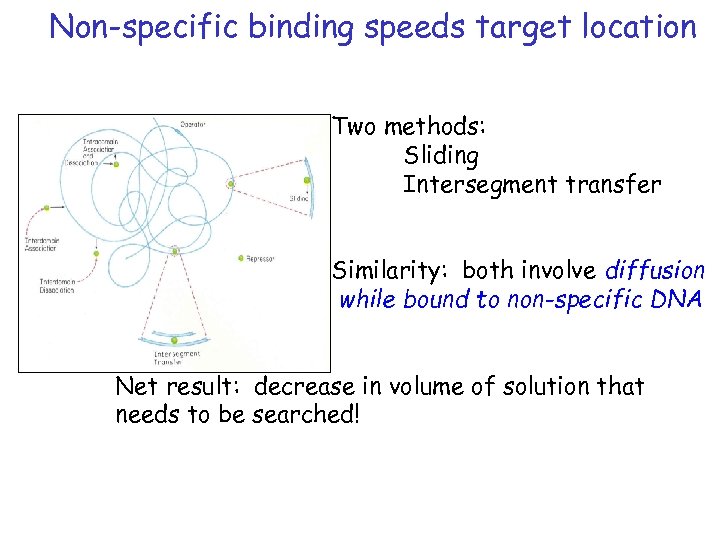
Non-specific binding speeds target location Two methods: Sliding Intersegment transfer Similarity: both involve diffusion while bound to non-specific DNA Net result: decrease in volume of solution that needs to be searched!
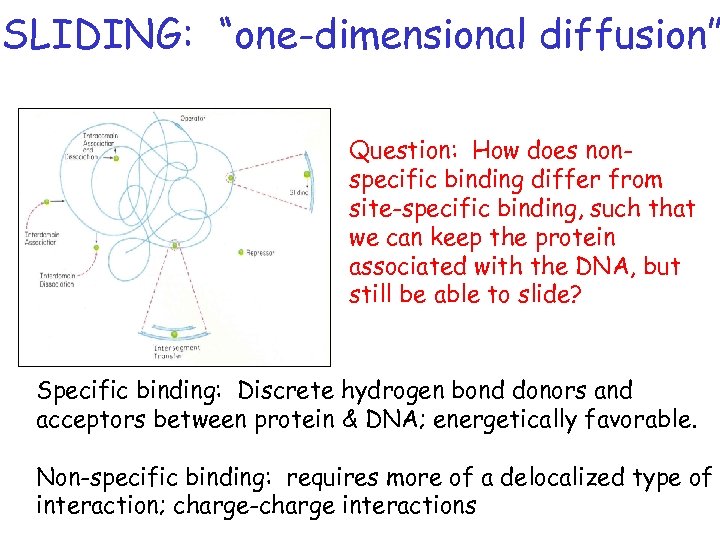
SLIDING: “one-dimensional diffusion” Question: How does nonspecific binding differ from site-specific binding, such that we can keep the protein associated with the DNA, but still be able to slide? Specific binding: Discrete hydrogen bond donors and acceptors between protein & DNA; energetically favorable. Non-specific binding: requires more of a delocalized type of interaction; charge-charge interactions
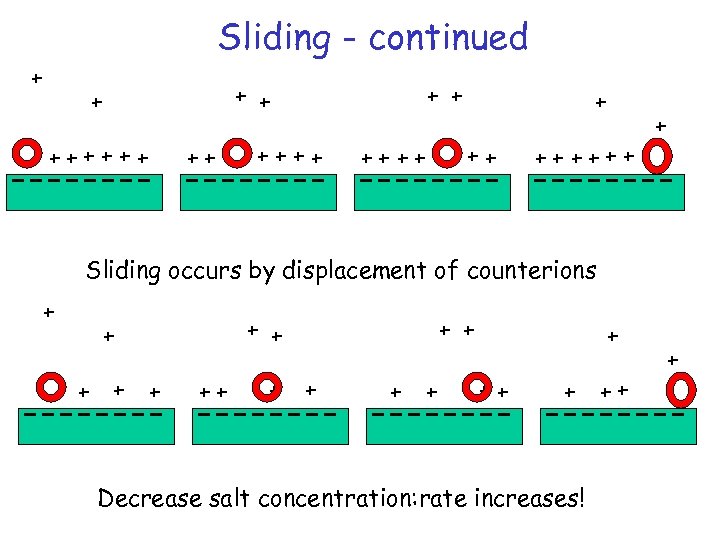
Sliding - continued + + ++++++ ++ + + ++++ ++ ++++ + Sliding occurs by displacement of counterions + + + ++ + Decrease salt concentration: rate increases! ++ +
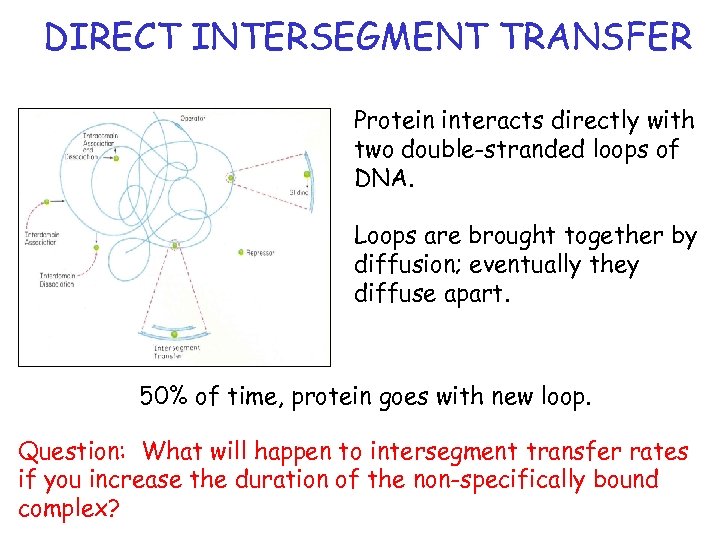
DIRECT INTERSEGMENT TRANSFER Protein interacts directly with two double-stranded loops of DNA. Loops are brought together by diffusion; eventually they diffuse apart. 50% of time, protein goes with new loop. Question: What will happen to intersegment transfer rates if you increase the duration of the non-specifically bound complex?

Diagnostics for sliding and other modes of facilitated diffusion 1). kassoc > kdiffusion small increases: electrostatic fields or docking large increases: sliding or intersegment transfer 2). Larger DNA fragments give rise to larger kassoc. 3). Decreased salt concentrations give rise to larger kassoc.
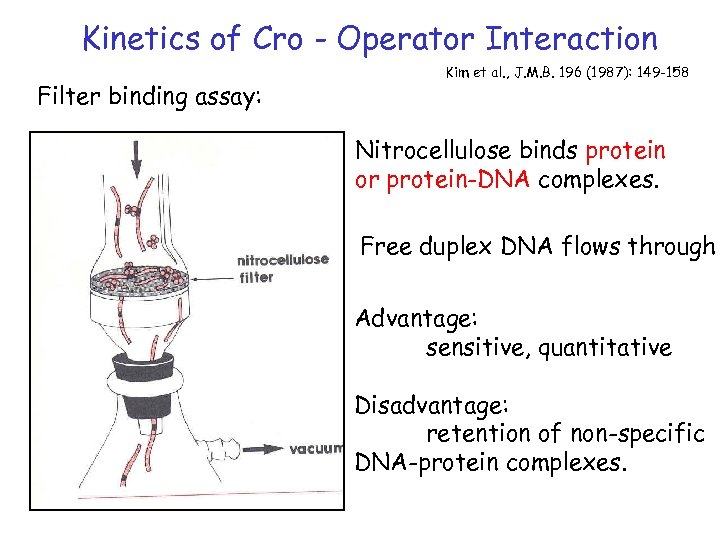
Kinetics of Cro - Operator Interaction Filter binding assay: Kim et al. , J. M. B. 196 (1987): 149 -158 Nitrocellulose binds protein or protein-DNA complexes. Free duplex DNA flows through Advantage: sensitive, quantitative Disadvantage: retention of non-specific DNA-protein complexes.
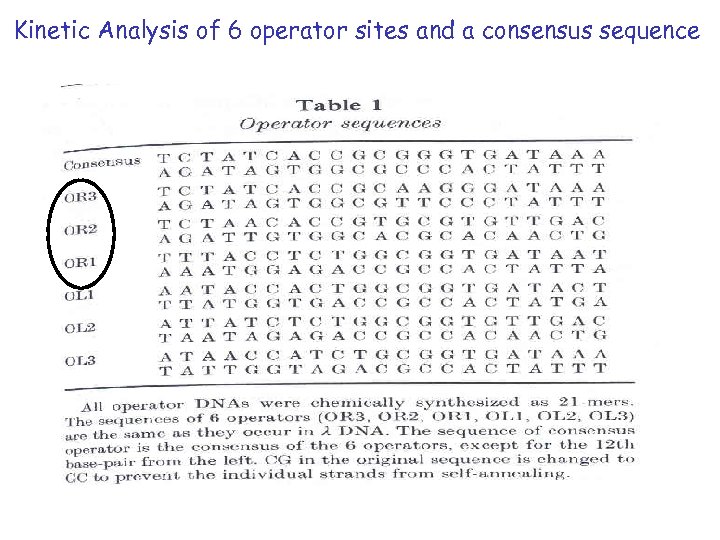
Kinetic Analysis of 6 operator sites and a consensus sequence
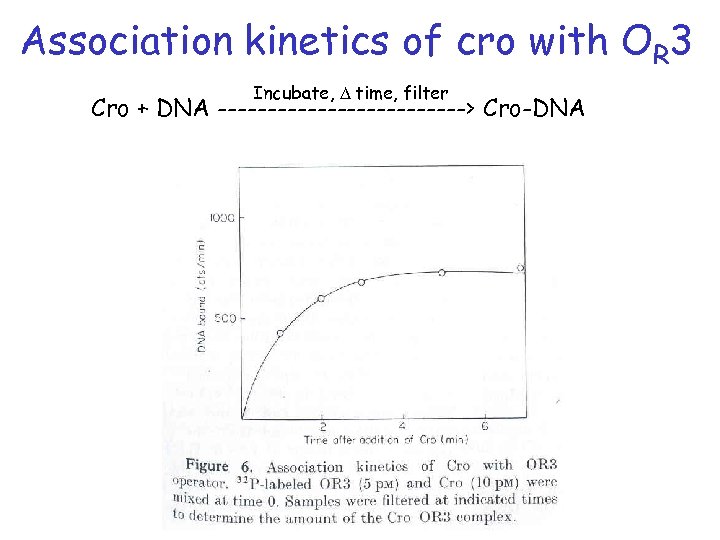
Association kinetics of cro with OR 3 Incubate, D time, filter Cro + DNA -------------> Cro-DNA
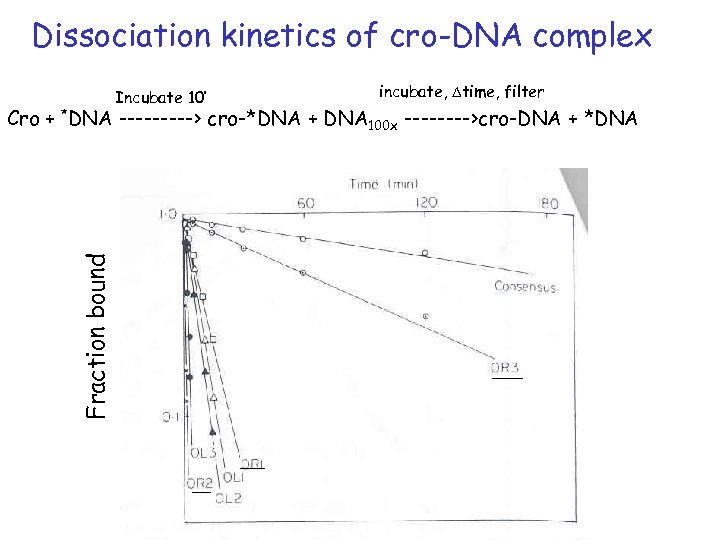
Dissociation kinetics of cro-DNA complex Incubate 10’ incubate, Dtime, filter Fraction bound Cro + *DNA -----> cro-*DNA + DNA 100 x ---->cro-DNA + *DNA
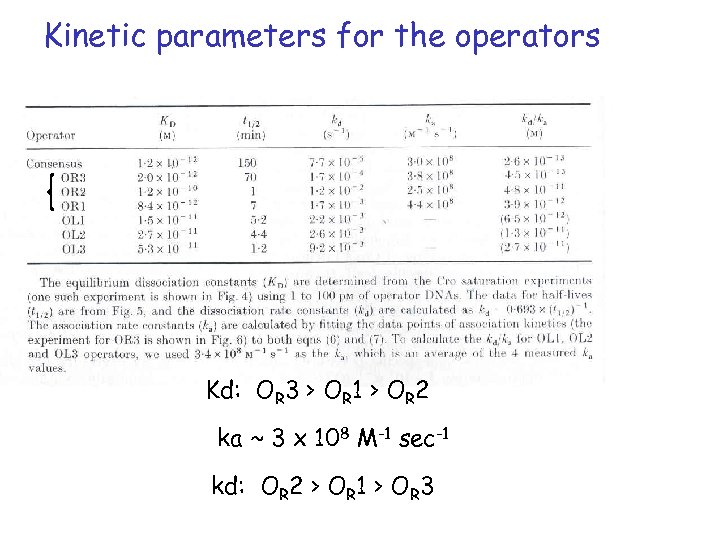
Kinetic parameters for the operators Kd: OR 3 > OR 1 > OR 2 ka ~ 3 x 108 M-1 sec-1 kd: OR 2 > OR 1 > OR 3
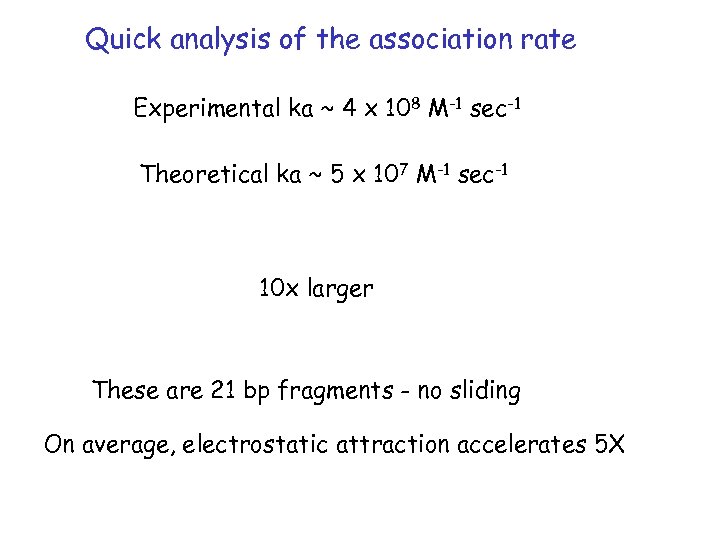
Quick analysis of the association rate Experimental ka ~ 4 x 108 M-1 sec-1 Theoretical ka ~ 5 x 107 M-1 sec-1 10 x larger These are 21 bp fragments - no sliding On average, electrostatic attraction accelerates 5 X

Kinetics of cro binding to variable-length OR fragments
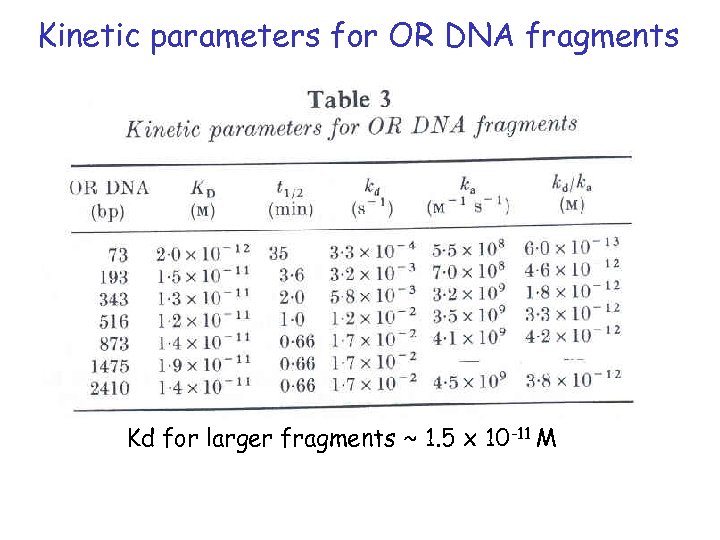
Kinetic parameters for OR DNA fragments Kd for larger fragments ~ 1. 5 x 10 -11 M

Summary of kinetic data for OR-DNA fragments KA KA decreases 7 fold kd increases 100 fold ka increases 10 fold kd ka DNA length (bp) 700 bp is break point

Theoretical test of the sliding mechanism k 1 k 2 R + D + O <-----> RO + D k-1 k-2 kd well described by sliding mechanism; ka described by a sliding mechanism enhanced by electrostatic effects
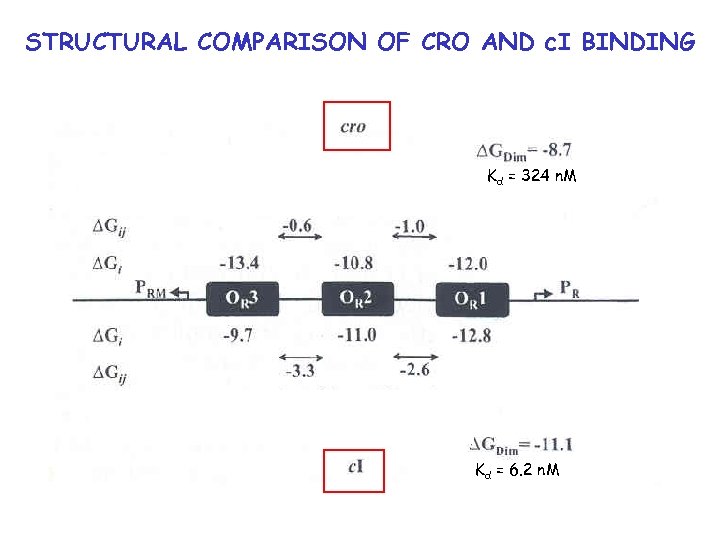
STRUCTURAL COMPARISON OF CRO AND c. I BINDING Kd = 324 n. M Kd = 6. 2 n. M

STRUCTURAL COMPARISON OF CRO AND c. I BINDING PNAS 95: 3431 -3436 (1998)
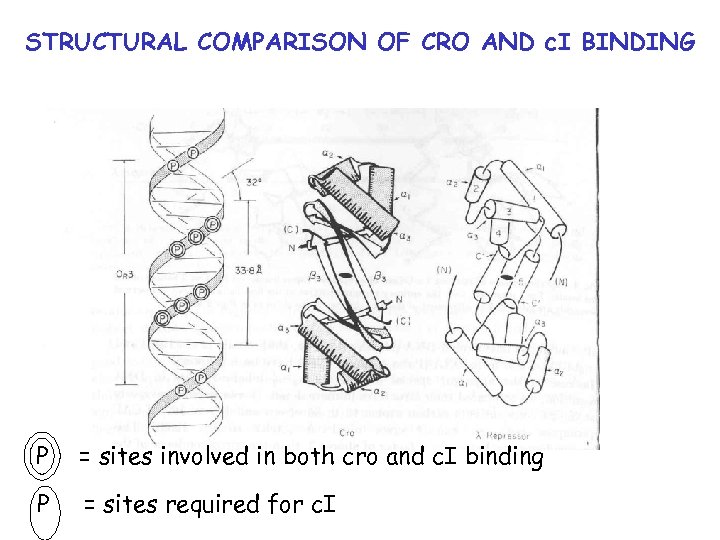
STRUCTURAL COMPARISON OF CRO AND c. I BINDING P = sites involved in both cro and c. I binding P = sites required for c. I
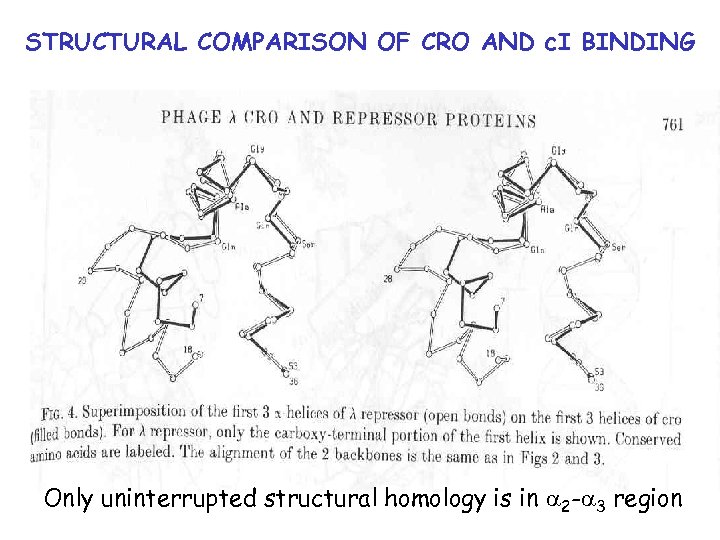
STRUCTURAL COMPARISON OF CRO AND c. I BINDING Only uninterrupted structural homology is in a 2 -a 3 region
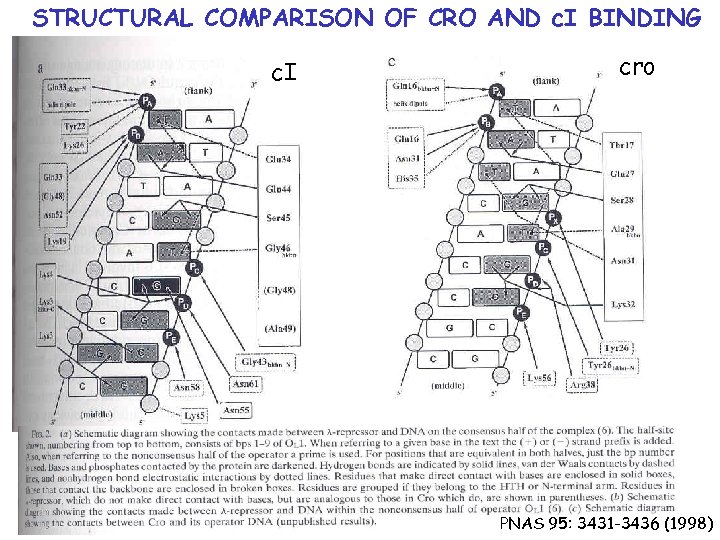
STRUCTURAL COMPARISON OF CRO AND c. I BINDING c. I cro PNAS 95: 3431 -3436 (1998)
88be9ce3ad49d543495a79655c4791e2.ppt