3d801d831711eed50ec3339b6d286e27.ppt
- Количество слайдов: 45

MLAB 2534: MICROBIOLOGY KERI BROPHY-MARTINEZ Haemophilus and Other Fastidious Gram-Negative Rods
HAEMOPHILUS AND OTHER FASTIDIOUS GRAM-NEGATIVE RODS The fastidious group of gram-negative bacilli include: Haemophilus HACEK( Haemophilus, Actinobacillus, Cardiobacteria, Eikenella & Kingella) Legionella Bordetella Pasteurella Brucella Francisella Bartonella

HAEMOPHILUS SPECIES Haemophilus = “blood loving” Require either heme (X factor) or NAD (V factor) Haemophilus is facultative and can grow anaerobically Organism is sensitive to drying and extremes in temperature Distinctive “mousy” or “bleach-like” odor

HAEMOPHILUS INFLUENZAE Misnamed – originally thought to cause the “flu” Now know that flu is caused by viruses In some cases of flu, H. influenzae is secondary infection

HAEMOPHILUS INFLUENZAE: VIRULENCE FACTORS Capsule Antiphagocytic Ig. A Protease Lipid A Cleaves Ig. A on mucosal surfaces Effects ciliated respiratory epithelium Pili Attachment
HAEMOPHILUS INFLUENZAE: CLINICAL INFECTIONS: TYPABLE STRAINS Acute epiglottitis or laryngotracheal infection in small children Cellulitis/arthritis Children under 6 years Contagious, vaccine has decreased incidence Pneumonia/septicemia cheek and upper extremities Meningitis Can cause airway obstruction needing immediate tracheostomy In children Conjunctivitis “pink eye” very contagious

HAEMOPHILUS INFLUENZAE: CLINICAL INFECTIONS: NONTYPABLE STRAINS Otitis media Children 6 months- 2 years Sinusitis Pneumonia, bronchitis In adults These sites are all in proximity to respiratory tract
HAEMOPHILUS SPECIES Haemophilus species require growth factors: X-factor ( hemin) Heat-stable substance Present in RBC and released with degradation of hemoglobin V-factor (NAD: nicotinamide adenine dinucleotide) Heat- labile Found in blood or secreted by certain organisms

HAEMOPHILUS SPECIES H. influenzae satellitism around and between the large, white, hemolytic staphylococci. This occurs when another organism produces V factor as a bi-product.
HAEMOPHILUS SPECIES Gram Stain Morphology Usually very small pleomorphic gram negative cb or rod May be able to observe a halo around the organism Gram stain can be enhanced by extending time for safranin to 2 minutes OR substitute carbolfuschin for safranin
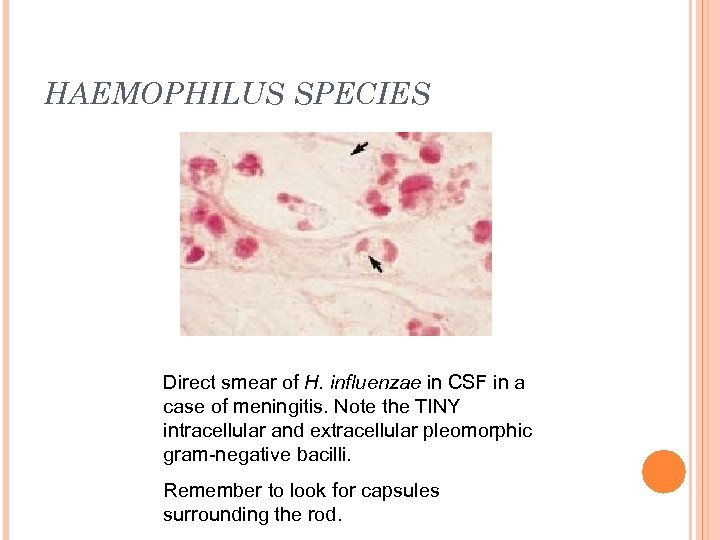
HAEMOPHILUS SPECIES Direct smear of H. influenzae in CSF in a case of meningitis. Note the TINY intracellular and extracellular pleomorphic gram-negative bacilli. Remember to look for capsules surrounding the rod.

HAEMOPHILUS SPECIES Colony Morphology No growth on BAP or MAC On CA: semi-opaque, gray-white, convex, mucoid.

HAEMOPHILUS SPECIES: IDENTIFICATION Gram stain Gram negative cocco-baccillus Catalase + Oxidase + X and V factor strips or disks Quad plates Rapid ID Panels NHI cards- automated

HAEMOPHILUS SPECIES: IDENTIFICATION This organism would be identified as H. influenzae because it is using both X and V factors.

HAEMOPHILUS SPECIES: IDENTIFICATION This organism would be identified as H. parainfluenzae because it is using V factor only.

HAEMOPHILUS SPECIES: IDENTIFICATION Quad plates Contain X and V factors & sheep blood agar

HAEMOPHILUS DUCREYI Causative agent of chancroid or soft chancre (STD), highly contagious Specimens should be collected from base of lesion, inoculated directly to enriched media and held for 5 days Gram stain appears as groups of coccbacilli that resemble a ‘school of fish” or “railroad tracks” Requires only X factor to grow
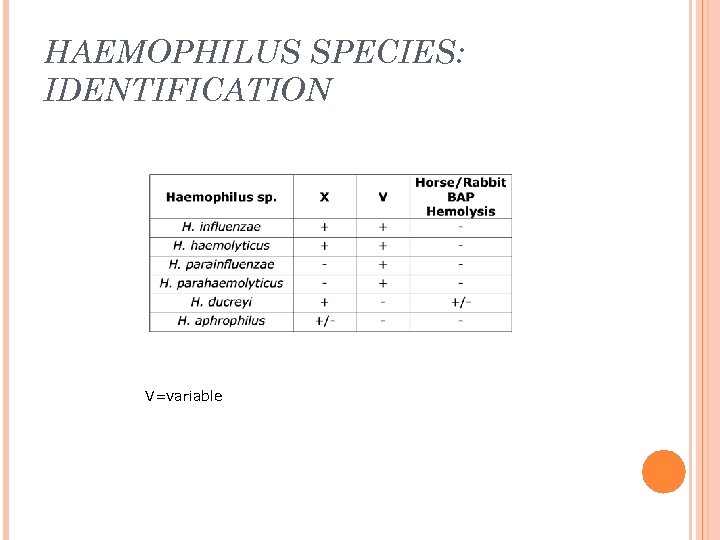
HAEMOPHILUS SPECIES: IDENTIFICATION V=variable
HAEMOPHILUS Antibiotic therapy Historically ampicillin was the drug of choice. However, resistance has developed due to production of beta-lactamase or altered penicillin binding proteins and cell wall permeability Susceptibility testing can be performed by disk diffusion, broth dilution or E-test Primary antibiotics include cefotaxime or ceftriaxone

TAKE 5!

HACEK GROUP HACEK is an acronym of the first initial of each genus that belong in the group: Haemophilus aphrophilus: o Actinobacillus actinomycetemcomitans Cardiobacterium hominis Eikenella corrodens Kingella species Habitat o NAME ALERT: Now called Aggregatibacter aphrophilus Not a true Haemophilus because does not need X nor V Commensals of oral cavity Clinical Significance Infective endocarditis Peridontal disease Dental caries Infections following dental procedures

HACEK GROUP: GENERAL CHARACTERISTICS Gram-negative bacilli Require an increased CO 2 (5%-10%) environment Slow/poor growers Usual flora of the oralpharyngeal cavity Opportunists in immunocompromised hosts

CAPNOCYTOPHAGA SP. Capnophilic Facultative anaerobe Part of the normal oralpharygeal flora Cause periodontal disease, sepsis
PASTEURELLA SPECIES General characteristics Colonizes mucous membranes of the upper respiratory tract and gastrointestinal tracts of mammals and birds Human infections occur from bites and scratches inflicted by animals, primarily felines Results in a localized, pus- producing infection Can cause life-threatening systemic disease Most common isolated species is Pasteurella multocida
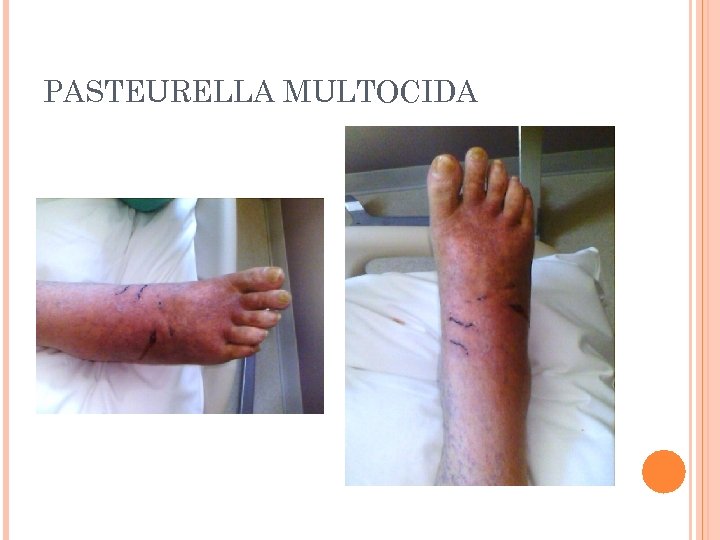
PASTEURELLA MULTOCIDA

PASTEURELLA MULTOCIDA Culture characteristics Growth on 5% blood or chocolate shows small, smooth, grayish, convex colonies Non-hemolytic “Musty” or earthy odor No growth on Mac. Conkey agar

PASTEURELLA MULTOCIDA Microscopic examination Very small gramnegative rods Bipolar staining with Giemsa or methylene blue “Safety-pin” appearance

PASTEURELLA MULTOCIDA: IDENTIFICATION Oxidase positive Indole positive Nonmotile Catalase positive Glucose fermenter

BRUCELLA SPECIES Causes infection in cattle (zoonosis) Acquired through aerosol, percutaneous and oral routes of exposure Brucellosis Primarily seen with animal handlers and those who handle animal products Also known as Malta or undulant fever Type 3 biohazard – can be transmitted through unbroken skin Category B Biological agent- easy to disseminate and cause moderate morbidity, but low mortality.

BRUCELLA SPECIES: IDENTIFICATION Colony Morphology Small, smooth, convex, nonhemolytic May require holding culture for 21 days Gram Stain Morphology Small gram-negative coccobaccilli Nonmotile Aerobic Oxidase positive Catalase positive Urease positive

FRANCISELLA TULARENSIS Highly infectious Type 3 biohazard – can be transmitted through unbroken skin, bite from an insect, direct contact with infected animals or inhalation of aerosols Category A Biological agent-it can be spread from person to person or disseminated, high mortality rates Infection in rabbits, sheep, squirrels and ticks Zoonotic infection in humans Tularemia

FRANCISELLA TULARENSIS: IDENTIFICATION Colony Morphology BAP = No growth MAC = No growth Choc = Small, smooth, gray gncb at 2 -5 days Requires special media (BCYE or MTM) Oxidase: negative Catalase: negative- weak positive Ferments glucose X and V negative NOTE: Usually identified by DFA or direct agglutination tests due to risk of lab acquired infection

LEGIONELLA SPECIES General characteristics Habitat Aquatic sources Cooling towers, condensers Ubiquitous gram-negative rods Acquired by humans primarily through inhalation of aerosols

LEGIONELLA SPECIES: CLINICAL INFECTIONS Legionnaire’s disease Disease with pneumonia and extrapulmonary involvement Malaise, rapid onset of dry cough and fever Illness is fatal in 15 -30% of cases not treated Pontiac fever Influenza-like Fever, headache, malaise Not fatal- short lived (2 -5 days)

LEGIONELLA SPECIES Specimen Handling & Processing BAL, bronchial washings, lung biopsy and pleural fluid are appropriate specimens Avoid aerosolization & transport ambient temperature Buffered Charcoal Yeast Extract (BCYE) most widely used Organism requires cysteine & iron salts for growth Incubate at 35 o C in 5 -10% CO 2 with increased humidity for 10 days Slow growth (2 -4 days)
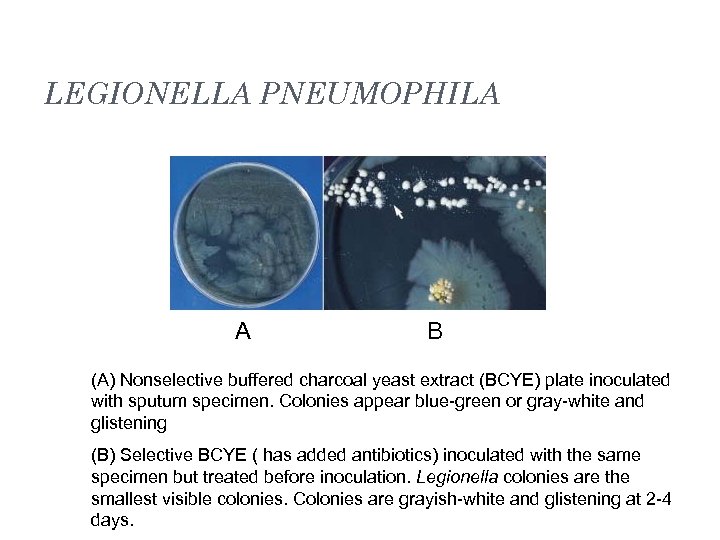
LEGIONELLA PNEUMOPHILA A B (A) Nonselective buffered charcoal yeast extract (BCYE) plate inoculated with sputum specimen. Colonies appear blue-green or gray-white and glistening (B) Selective BCYE ( has added antibiotics) inoculated with the same specimen but treated before inoculation. Legionella colonies are the smallest visible colonies. Colonies are grayish-white and glistening at 2 -4 days.

LEGIONELLA SPECIES: IDENTIFICATION Oxidase positive Catalase Positive Motile by polar flagella Short, thin GNR, may be faint staining

LEGIONELLA PNEUMOPHILA Misc. Identification methods Rapid Methods for Identification Urine Antigen test Direct Fluorescent Antibody test (DFA) DNA Detection Serological tests (IFA)

LEGIONELLA SPP. : TREATMENT Susceptibility testing not routinely performed Erythromycin alone or Rifampin used to treat

BORDETELLA SPP. B. pertussis and B. parapertussis Cause pertussis “Whooping cough” Highly communicable disease of children Strict human pathogen, spread by airborne droplets Lives in ciliated epithelium of URT Produces toxins and virulence factors Required vaccination (DTa. P)

BORDETELLA SPP: SPECIMEN COLLECTION, TRANSPORT AND PROCESSING Nasopharyngeal swab or aspirate is the specimen of choice. Swabs should be calcium alginate or dacron polyester Specimen should be plated at the bedside and a smear made OR placed in casamino acid for transport Regan-Lowe is recommended for transport

BORDETELLA SPP: IDENTIFICATION Requires Bordet-Gengou agar Cough plate Appears slightly beta hemolytic smooth, shiny, resembling a mercury droplet Regan-Lowe agar Domed and shiny with a white mother of pearl opalescence BAP & MAC: no growth Organism is a fastidious obligate aerobe Gram stain: small faint staining GN coccobacilli Can increase counterstain of safranin to 2 minutes for improved visibility Oxidase positive Nonmotile
BORDETELLA SPP. : MISC. IDENTIFICATION METHODS Serologic Identification Direct fluorescent antibody Slide agglutination tests Nucleic Acid Detection by PCR

BARTONELLA SPP. Facultative Intracellular gram negative cocco-bacillus Transmitted by direct contact or blood-sucking arthropods Infect RBCs and vascular endothelial cells in the host leading to circulatory system infections Clinical Infections Cat Scratch disease Others Carrion’s disease Trench fever

REFERENCES Engelkirk, P. G. , & Duben-Engelkirk, J. (2008). Laboratory Diagnosis of Infectious Diseases: Essentials of Diagnostic Microbiology. Baltimore, MD: Lippincott Williams & Willkins. Kiser, K. M. , Payne, W. C. , & Taff, T. (2011). Clinical Laboratory Microbiology: A Practical Approach. Upper Saddle River, NJ: Pearson Education, Inc. Mahon, C. R. , Lehman, D. C. , & Manuselis, G. (2011). Textbook of Diagnostic Microbiology (4 th ed. ). Maryland Heights, MO: Saunders.
3d801d831711eed50ec3339b6d286e27.ppt