fcd5a7a00bc325e39b10055ee89e7d50.ppt
- Количество слайдов: 41

MLAB 2401: Clinical Chemistry Quality Control, Quality Assessment and Statistics 1

Quality Assurance/Assessment (QA) ¨ An all inclusive / comprehensive system monitoring the accuracy of test results where all steps before, during and after the testing process are considered. Includes pre-analytic, analytic and post analytic factors ¨ Essentials include commitment to quality, facilities, resources, competent staff, and reliable procedures, methods and instrumentation ¨ Provides a structure for achieving lab and hospital quality goals 2

Quality Control (QC) ¨ QC systems monitor the analytical process; detect and minimize errors during the analysis and prevent reporting of erroneous test results. ¨ It uses statistical analysis of test system data ¨ Requires following published rules n Westgard Rules 3
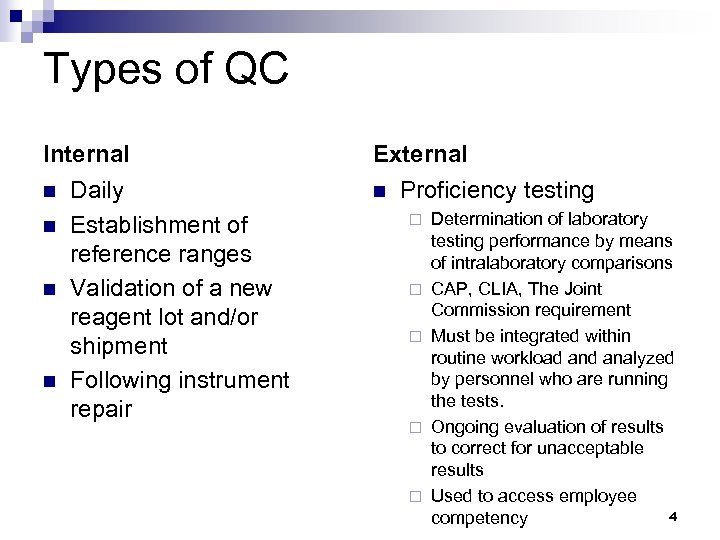
Types of QC Internal n n Daily Establishment of reference ranges Validation of a new reagent lot and/or shipment Following instrument repair External n Proficiency testing ¨ ¨ ¨ Determination of laboratory testing performance by means of intralaboratory comparisons CAP, CLIA, The Joint Commission requirement Must be integrated within routine workload analyzed by personnel who are running the tests. Ongoing evaluation of results to correct for unacceptable results Used to access employee 4 competency
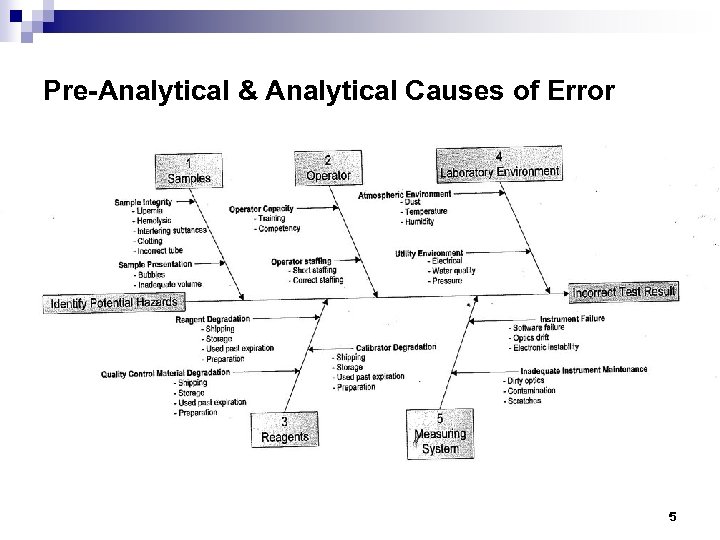
Pre-Analytical & Analytical Causes of Error 5
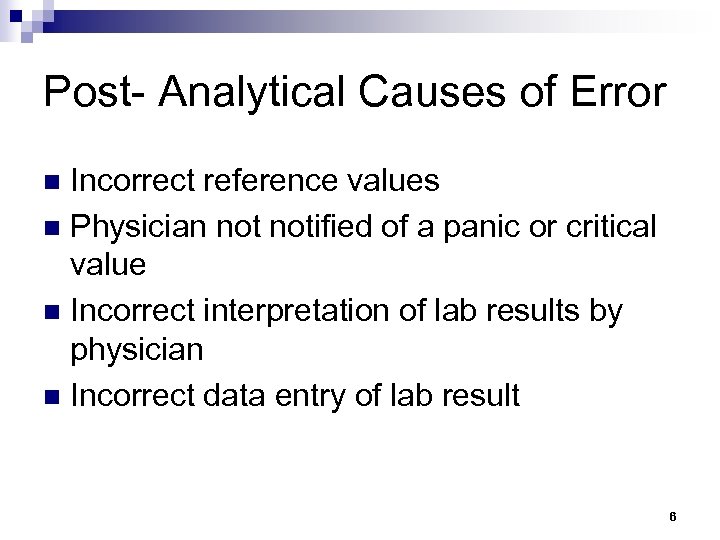
Post- Analytical Causes of Error Incorrect reference values n Physician notified of a panic or critical value n Incorrect interpretation of lab results by physician n Incorrect data entry of lab result n 6
Introduction to Statistical Analysis n When evaluating laboratory results, how do we determine what is normal or acceptable? In other words: What is “normal” or “OK”? n When does a laboratory test result become “weird” or “abnormal” ? When do we become uncomfortable with a result? n At some point we have to draw a “line in the sand” … on this side of the line you’re normal … on the other side of the line you’re abnormal. Where and how do we “draw the line” ? n Answer: Statistics are used to determine the lines of ‘normal’ and ‘acceptable’. 7
n Statistical Concepts ¨ Statistics is a (science of )branch of mathematics that collects, analyzes, summarizes and presents information about “observations. ” ¨ In the clinical lab, these “observations” are usually numerical test results ¨ A statistical analysis of lab test data can help us to define n Reference ranges for patient’s (normal and abnormal) n Acceptable ranges for control specimens ( “in” and “out” of control) 8
Measures of Central Tendency n n n Mean (x ) - the mathematical average of a group of numbers, determined by adding a group of numbers (events) and dividing the result by the number of events Median - determined as the ‘middle’ of a group of numbers that have been arranged in sequential order. That is to say, there an equal number of numbers on either side of the ‘middle’ number. In an odd # of observations, it is the middle observation. In an even # of observations, average the two middle values. Mode - the number that appears most frequently in a group of numbers. There can be more than mode, or none at all. 9
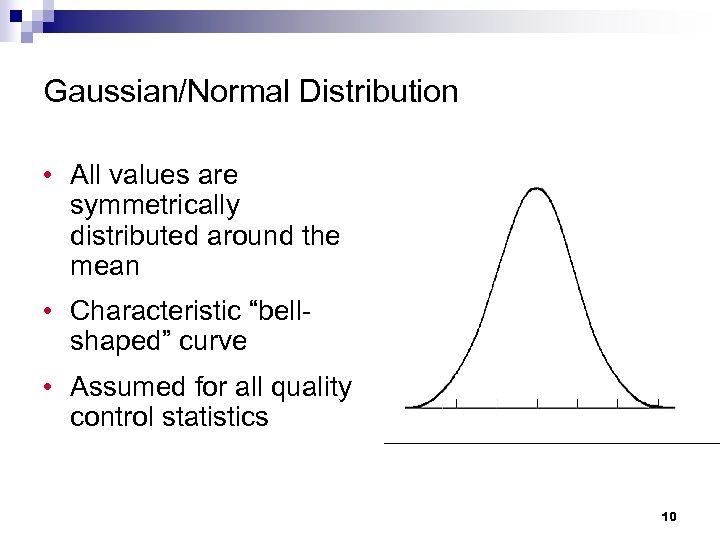
Gaussian/Normal Distribution • All values are symmetrically distributed around the mean • Characteristic “bellshaped” curve • Assumed for all quality control statistics 10
11
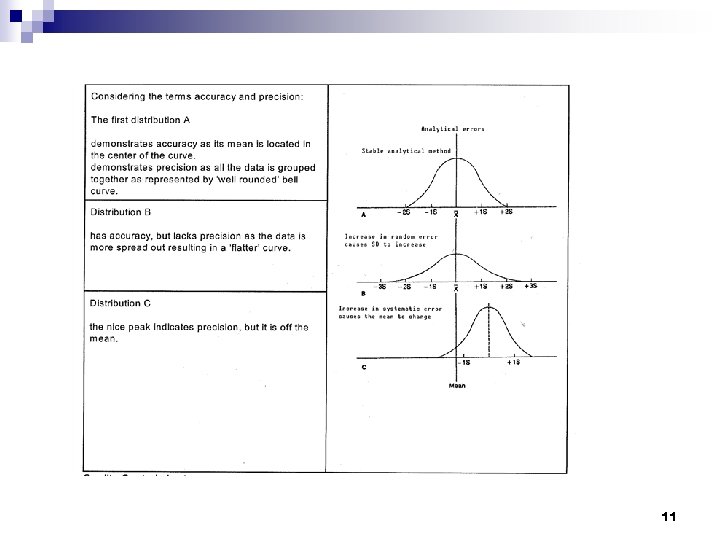
Accuracy and Precision n The degree of fluctuation in the measurements is indicative of the precision of the assay. ¨ Precision-refers to the ability to get the same (but not necessarily ‘true’) result time after time. n The closeness of measurements to the true value is indicative of the accuracy of the assay. ¨ Accuracy - An accurate result is one that is the ‘true’ result. 12

Precise and Accurate
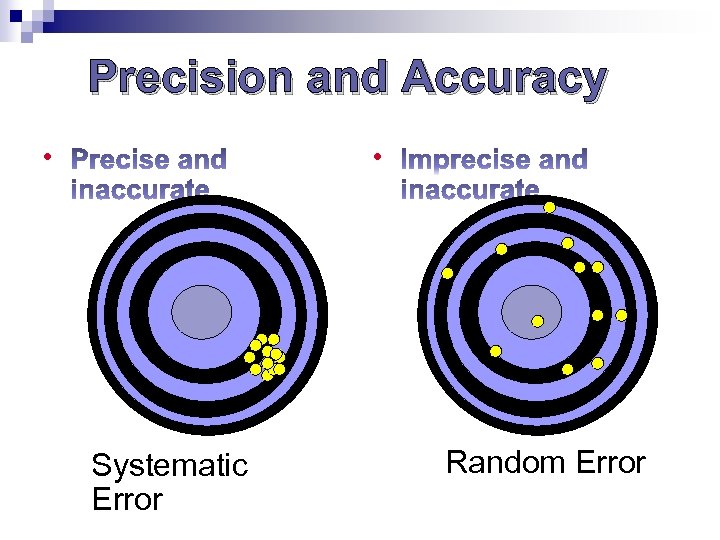
Precision and Accuracy • • Systematic Error Random Error
Systematic error Systematic change in the test system resulting in a displacement of the mean from the original value n Systematic error of an analytic system is predictable and causes shifts or trends on control charts that are consistently low or high n 15
Causes of Systematic Error n n n n n Change in reagent or calibrator lot numbers Wrong calibrator values Improperly prepared reagents Deterioration of reagents or calibrators Inappropriate storage of reagents or calibrators Variation in sample or reagent volumes due to pipettor misalignments Variation in temperature or reaction chambers Deterioration of photometric light source Variation in procedure between technologists 16

Random Error n Imprecision of the test system causing a scatter or spread of control values around the mean 17

Causes of Random Error n n n Air bubbles in reagent Improperly mixed reagents Reagent lines, sampling, or reagent syringes Improperly fitting pipette tips Clogged or imprecise pipetter Fluctuations in power supply 18
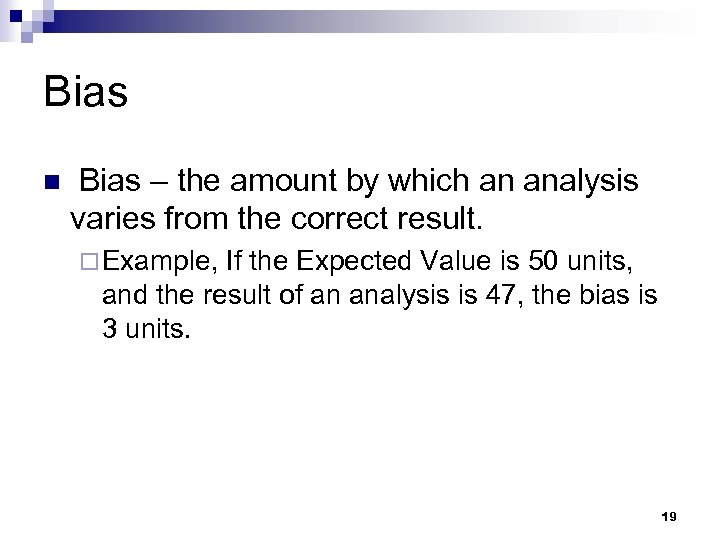
Bias n Bias – the amount by which an analysis varies from the correct result. ¨ Example, If the Expected Value is 50 units, and the result of an analysis is 47, the bias is 3 units. 19
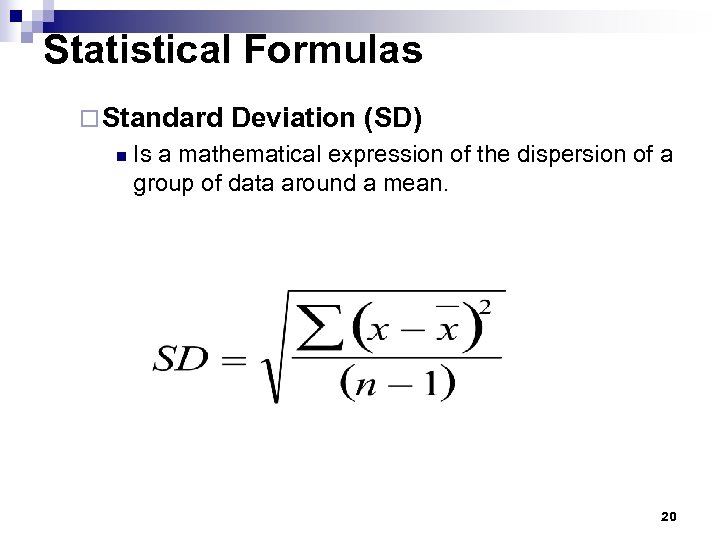
Statistical Formulas ¨ Standard n Deviation (SD) Is a mathematical expression of the dispersion of a group of data around a mean. 20
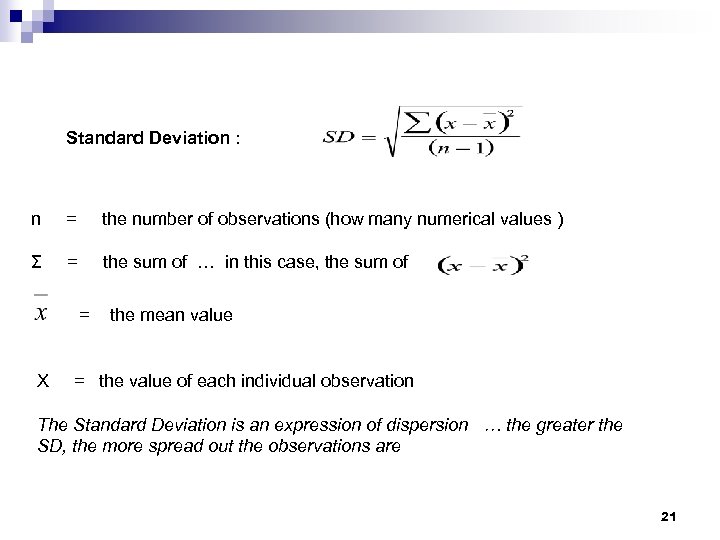
Standard Deviation : n = the number of observations (how many numerical values ) Σ = the sum of … in this case, the sum of = the mean value X = the value of each individual observation The Standard Deviation is an expression of dispersion … the greater the SD, the more spread out the observations are 21
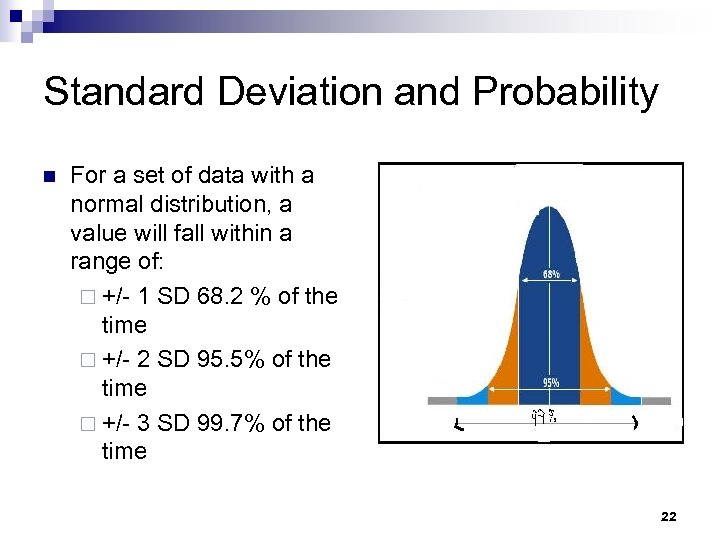
Standard Deviation and Probability n For a set of data with a normal distribution, a value will fall within a range of: ¨ +/- 1 SD 68. 2 % of the time ¨ +/- 2 SD 95. 5% of the time ¨ +/- 3 SD 99. 7% of the time 22
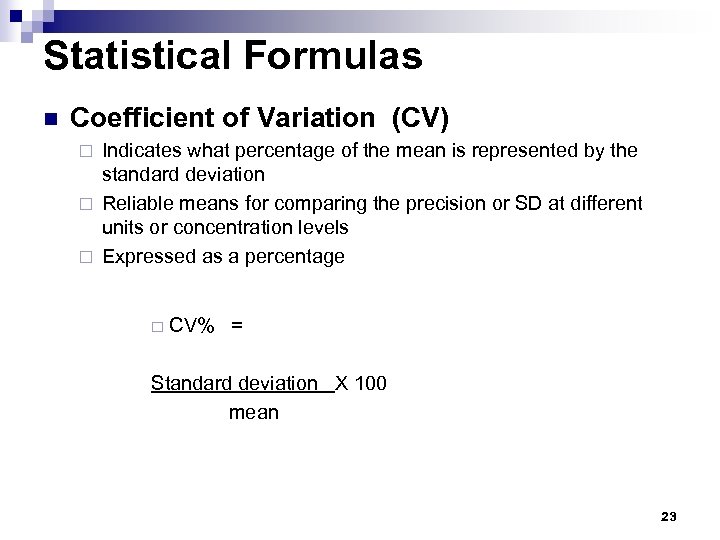
Statistical Formulas n Coefficient of Variation (CV) Indicates what percentage of the mean is represented by the standard deviation ¨ Reliable means for comparing the precision or SD at different units or concentration levels ¨ Expressed as a percentage ¨ ¨ CV% = Standard deviation X 100 mean 23
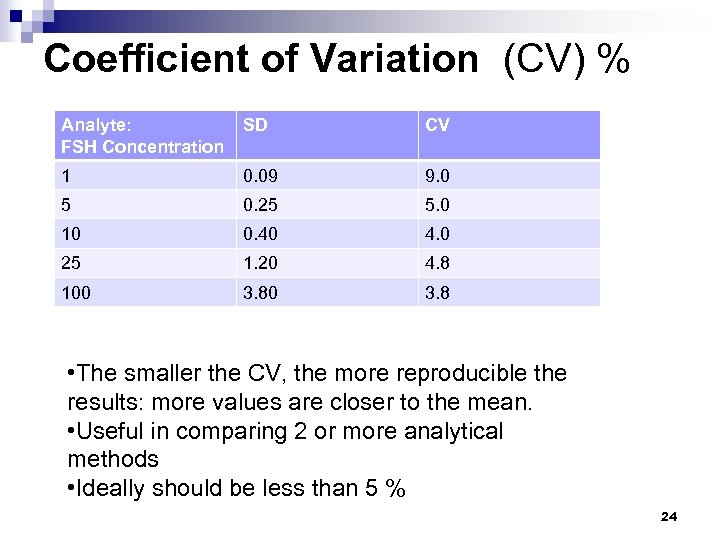
Coefficient of Variation (CV) % Analyte: FSH Concentration SD CV 1 0. 09 9. 0 5 0. 25 5. 0 10 0. 40 4. 0 25 1. 20 4. 8 100 3. 8 • The smaller the CV, the more reproducible the results: more values are closer to the mean. • Useful in comparing 2 or more analytical methods • Ideally should be less than 5 % 24
Establishment of a QC System n Two or three levels of control material used ¨ A control is a material or preparation used to monitor the stability of the test system within predetermined limits ¨ Measure of precision and reproducibility ¨ Purpose: verify the analytic measurement range of instrument for a specific analyte 25
Establishment of a QC System n Control material matrix should resemble actual specimens tested ¨ Lyophilized/liquid ¨ Assayed n Mean calculated by the manufacturer n Must verify in the laboratory ¨ Unassayed n Less expensive n Must perform data analysis in house 26
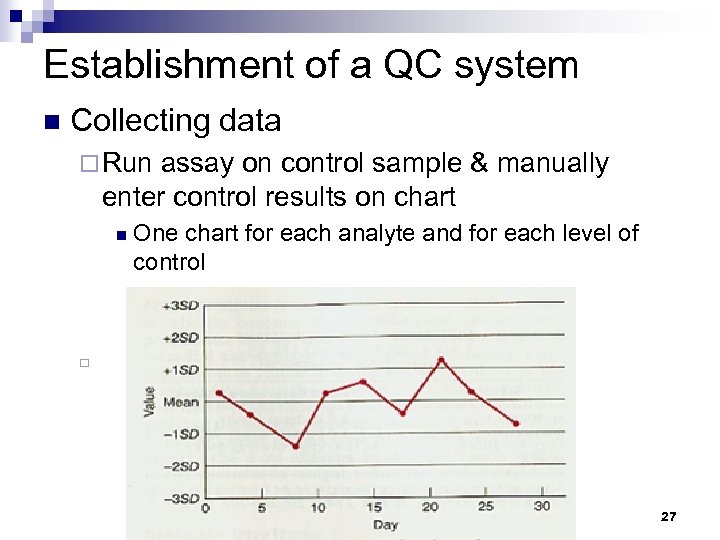
Establishment of a QC system n Collecting data ¨ Run assay on control sample & manually enter control results on chart n One chart for each analyte and for each level of control ¨ 27

Establishment of a QC system n Collecting data ¨ Many modern chemistry analyzers have computer program that maintains the QC log. n i. e Dade Dimension 28
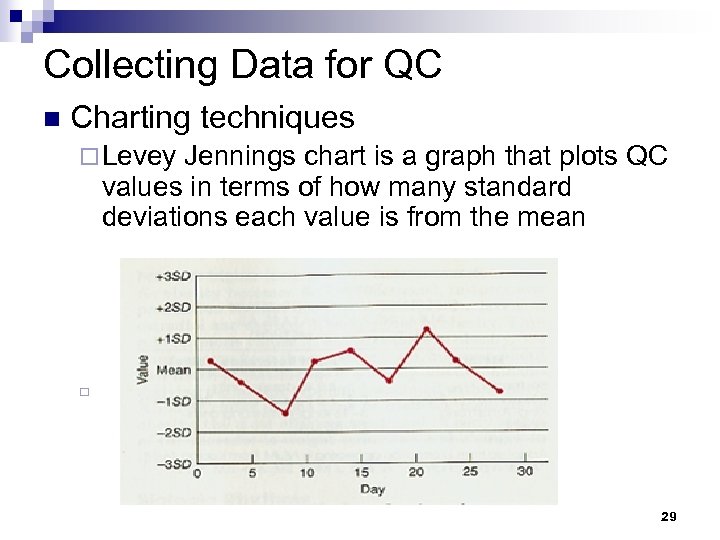
Collecting Data for QC n Charting techniques ¨ Levey Jennings chart is a graph that plots QC values in terms of how many standard deviations each value is from the mean ¨ 29
Use of Standard Deviation n Once you have determined the standard deviation, must use the information to evaluate current/ future analysis. n Most labs make use of ± 2 SD or 95% confidence limit. To put this into a workable form, you must establish the range of the ± 2 SDs 30
So, how do we determine the range of acceptable results ? n Scenario ¨ Mean of group of control values = 104 mg/d. L ¨ Standard Deviation = ± 5 mg/d. L ¨ Determine the Range of ± 2 SD; (which will allow you to evaluate acceptability of performance of the control on subsequent days. ) n Is a control value of 100 mg/d. L acceptable? 31

Shifts and Trends n Shift ¨ QC data results are distributed on one side of the mean for 6 -7 consecutive days § Trend ¨ Consistent increase or decrease of QC data points over a period of 6 -7 days 32

But what if your control specimen is “out of control? ” n “Out of control” means that there is too much dispersion in your result compared with the rest of the results n This suggests that something is wrong with the process that generated that observation n Patient test results cannot be reported to physicians when there is something wrong with the testing process that is generating inaccurate reports n Remember … No information is better than wrong information 33
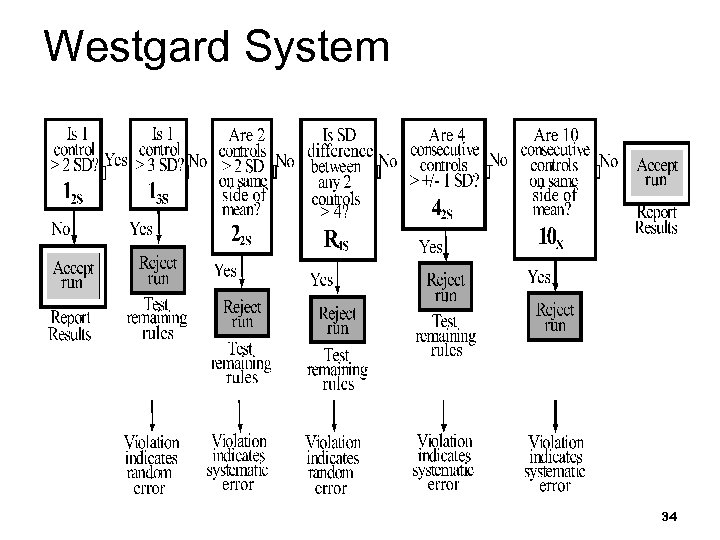
Westgard System 34

But what if your control specimen is “out of control? ” n Corrective methods Things that can go Wrong Corrective Action Instrument malfunction Identify malfunction and fix Reagents: preparation, contamination, volume New reagents Tech error Identify error and repeat test Control specimen is old or prepared improperly Use new control 35
QC terms n n AMR= Analytical Measurement Range ¨ Range of analyte values that a method can directly measure on the specimen without any dilution, concentration or other pretreatment CRR= Clinical Reportable Range ¨ Range of analyte values that a method can report as a quantitative result, allowing for specimen dilution, concentration, or other pretreatment used to expand the direct AMR. 36
System Flags n Delta check ¨ Comparison of individual patient results throughout the day or week with computer detection of changes from earlier individual patient results ¨ Helpful to identify pre-analytical errors Test Change Time Frame, hours Sodium, adult 7% 24 Creatinine 50% 72 Hemoglobin 3. 0 g/dl 48 Other Transfusion/ Bleeding? 37
Establishment of Reference Ranges n Reference ranges – the ‘normals’ ¨ The normal or expected value for patients. ¨ Are defined as being within +2 Standard Deviations from the mean ¨ A large sampling of clinical normal representatives. n Each lab must establish its own reference ranges based on local population 38
Establishment of Reference Ranges Factors affecting reference ranges: ¨ ¨ ¨ Age Sex Diet Medications Physical activity Pregnancy Personal habits (smoking, alcohol) Geographic location (altitude) Body weight Laboratory instrumentation (methodologies) Laboratory reagents 39

Test results n Critical values and read back of results ¨ Values that indicate a life-threatening situation for the patient ¨ Require notification of the value to nurse or physician ¨ Nurse or physician must “read back” the results to the technician Test Results Decreased Significancelow results Results Increased Significance- high results Glucose, adult < 50 mg/d. L Brain damage >500 mg/d. L Diabetic coma Sodium <120 m. EQ/L Paralysis, arrhythmias >160 m. EQ/L Dehydration, heart failure 40
References n n n Astles, J. R. , Stang, H. , & Alspach, T. (2013, September). CLIA requirements for proficiency testing: the basics for laboratory professionals. MLO, 45(9), 8 -15. Bishop, M. , Fody, E. , & Schoeff, l. (2010). Clinical Chemistry: Techniques, principles, Correlations. Baltimore: Wolters Kluwer Lippincott Williams & Wilkins. Sunheimer, R. , & Graves, L. (2010). Clinical Laboratory Chemistry. Upper Saddle River: Pearson. 41
fcd5a7a00bc325e39b10055ee89e7d50.ppt