инкапсуляция 01.04.2016 - копия.pptx
- Количество слайдов: 28
MINISTRY OF EDUCATION AND SCIENCE OF THE REPUBLIC OF KAZAKHSTAN SOUTH KAZAKHSTAN STATE PEDAGOGICAL INSTITUTE GRADUATE SCHOOL OF NATURAL SCIENCES AND MATHEMATICS DEPARTMENT OF CHEMISTRY «THE USE OF POLYMERS IN THE ENCAPSULATION PROCESS» Performed Satybaldy U. N. Scientific adviser c. ch. s. , associate professor Madibekova G. M. Consultant c. p. s. , senior lecturer Karakulova A. M Shymkent - 2016 y.
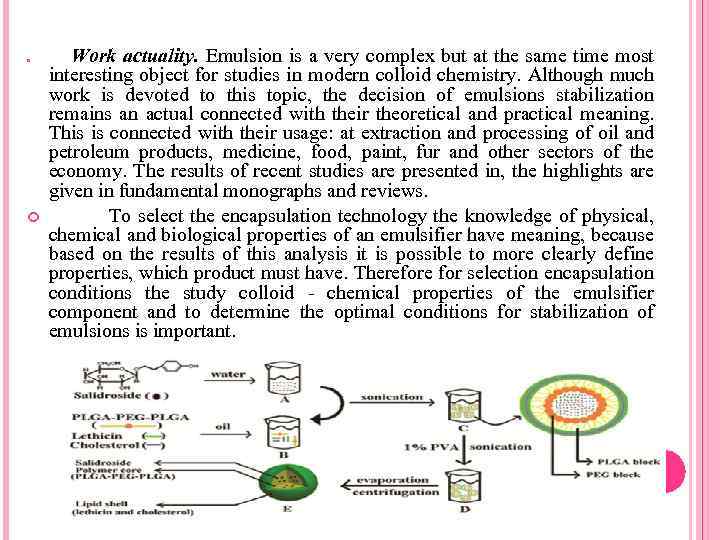
Work actuality. Emulsion is a very complex but at the same time most interesting object for studies in modern colloid chemistry. Although much work is devoted to this topic, the decision of emulsions stabilization remains an actual connected with their theoretical and practical meaning. This is connected with their usage: at extraction and processing of oil and petroleum products, medicine, food, paint, fur and other sectors of the economy. The results of recent studies are presented in, the highlights are given in fundamental monographs and reviews. To select the encapsulation technology the knowledge of physical, chemical and biological properties of an emulsifier have meaning, because based on the results of this analysis it is possible to more clearly define properties, which product must have. Therefore for selection encapsulation conditions the study colloid - chemical properties of the emulsifier component and to determine the optimal conditions for stabilization of emulsions is important.
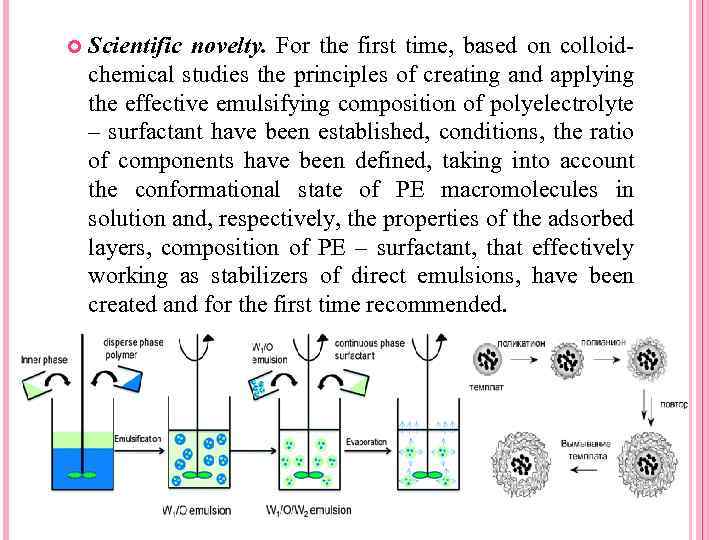
Scientific novelty. For the first time, based on colloidchemical studies the principles of creating and applying the effective emulsifying composition of polyelectrolyte – surfactant have been established, conditions, the ratio of components have been defined, taking into account the conformational state of PE macromolecules in solution and, respectively, the properties of the adsorbed layers, composition of PE – surfactant, that effectively working as stabilizers of direct emulsions, have been created and for the first time recommended.
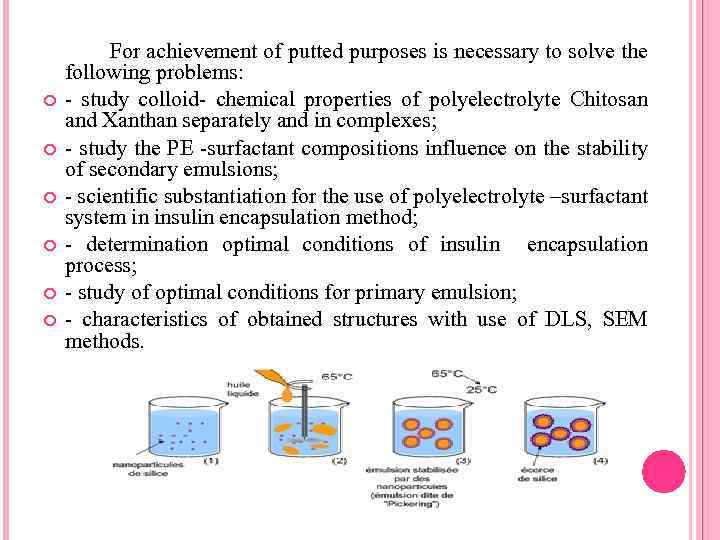
For achievement of putted purposes is necessary to solve the following problems: - study colloid- chemical properties of polyelectrolyte Chitosan and Xanthan separately and in complexes; - study the PE -surfactant compositions influence on the stability of secondary emulsions; - scientific substantiation for the use of polyelectrolyte –surfactant system in insulin encapsulation method; - determination optimal conditions of insulin encapsulation process; - study of optimal conditions for primary emulsion; - characteristics of obtained structures with use of DLS, SEM methods.
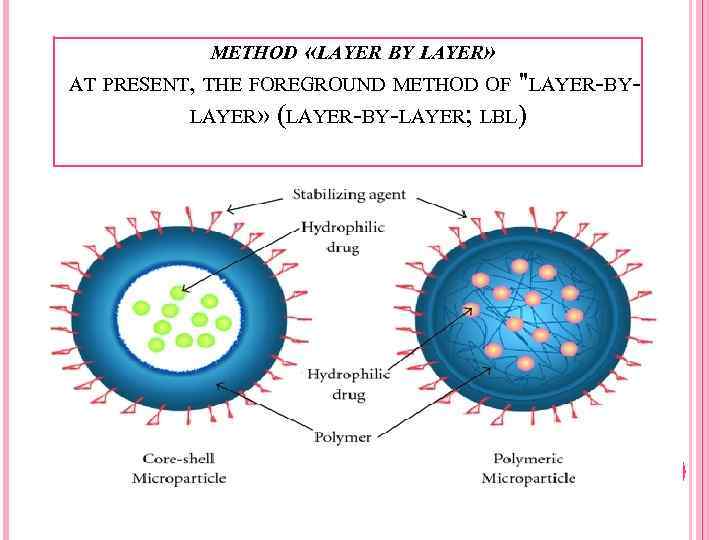
METHOD «LAYER BY LAYER» AT PRESENT, THE FOREGROUND METHOD OF "LAYER-BYLAYER» (LAYER-BY-LAYER; LBL)
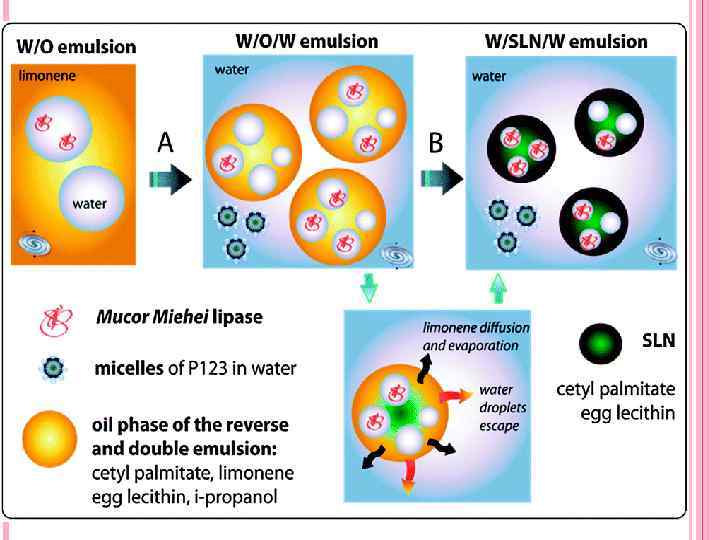

2 EXPERIMENTAL PART 2. 1 Objects of study
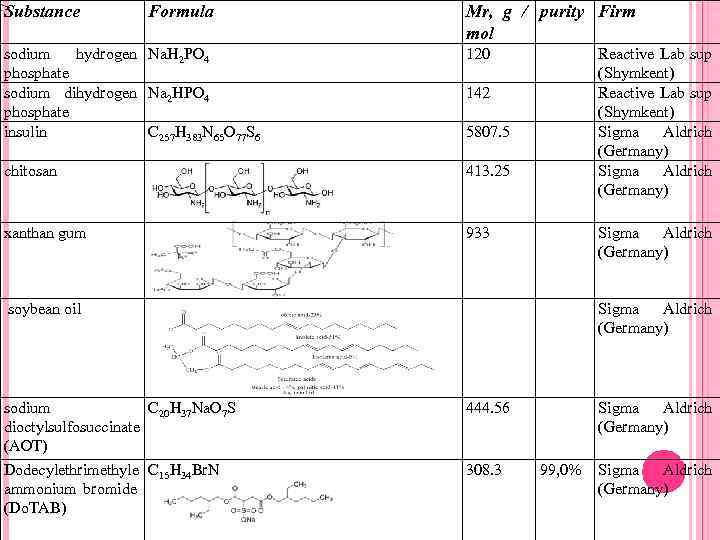
Substance Formula Mr, g / purity Firm mol sodium hydrogen Na. H 2 PO 4 phosphate sodium dihydrogen Na 2 HPO 4 phosphate insulin C 257 H 383 N 65 O 77 S 6 120 chitosan 413. 25 xanthan gum Reactive Lab sup (Shymkent) Sigma Aldrich (Germany) 933 142 5807. 5 Sigma Aldrich (Germany) soybean oil sodium C 20 H 37 Na. O 7 S dioctylsulfosuccinate (АОТ) Dodecylethrimethyle C 15 H 34 Br. N ammonium bromide (Do. TAB) Sigma Aldrich (Germany) 444. 56 308. 3 Sigma Aldrich (Germany) 99, 0% Sigma Aldrich (Germany)
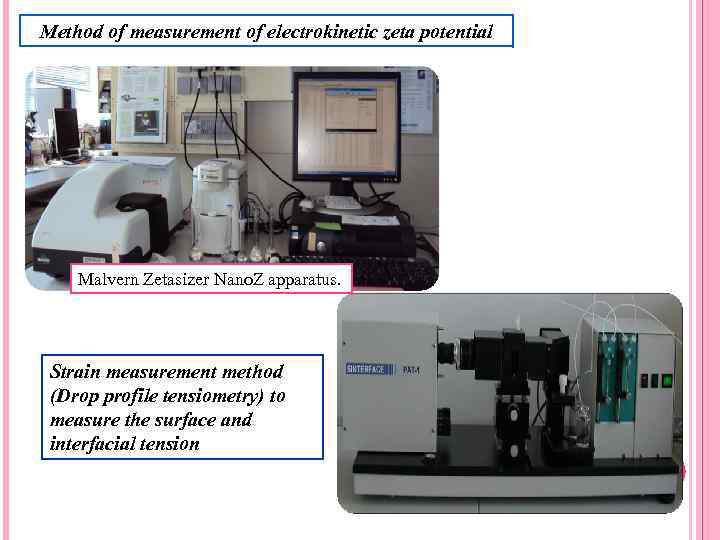
Method of measurement of electrokinetic zeta potential Malvern Zetasizer Nano. Z apparatus. Strain measurement method (Drop profile tensiometry) to measure the surface and interfacial tension
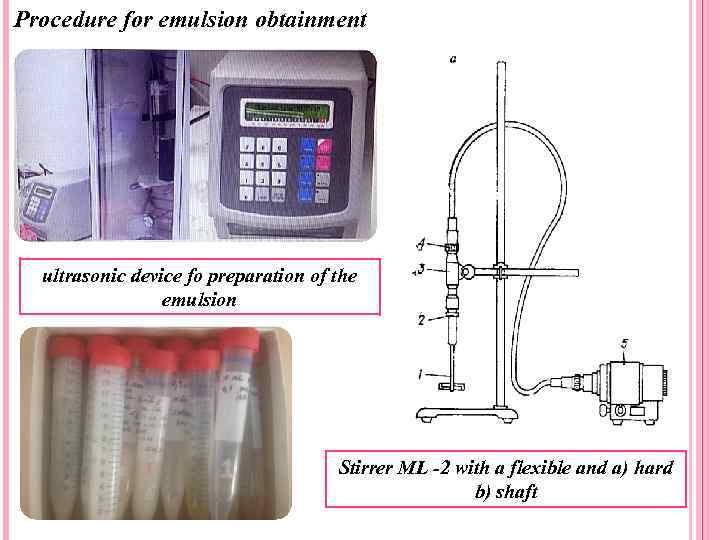
Procedure for emulsion obtainment ultrasonic device fo preparation of the emulsion Stirrer ML -2 with a flexible and a) hard b) shaft

Laboratory centrifuges Medical OPN – 8 Drying oven SHSU-М Microscope Mikromed 1 4 х/0, 1 160/0, 17; 10 х/0, 25 160/0, 17

Тип микроскопа Тип насадки Увеличение, крат Окуляры биологические, световые/оптические монокулярные 40– 1000 10 х/18 дополнительно: 16 х/15 Объективы 4 х0, 1; 10 х0, 25; 40 х0, 65; 100 х1, 25 МИ (ахроматические) дополнительно: 60 х0, 85 Предметный столик, мм 110 x 126 Конденсор центрируемый конденсор Аббе N. A. 1, 25 Подсветка галогенная Назначение школьные/учебные, лабораторные/медицинские Расположение подсветки нижняя Метод исследования светлое поле
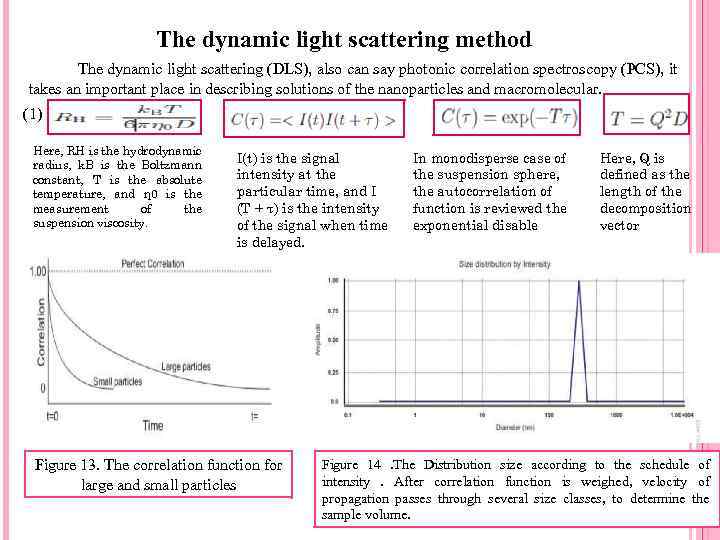
The dynamic light scattering method The dynamic light scattering (DLS), also can say photonic correlation spectroscopy (PCS), it takes an important place in describing solutions of the nanoparticles and macromolecular. (1) Here, RH is the hydrodynamic radius, k. B is the Boltzmann constant, T is the absolute temperature, and η 0 is the measurement of the suspension viscosity. I(t) is the signal intensity at the particular time, and I (T + τ) is the intensity of the signal when time is delayed. Figure 13. The correlation function for large and small particles In monodisperse case of the suspension sphere, the autocorrelation of function is reviewed the exponential disable Here, Q is defined as the length of the decomposition vector Figure 14. The Distribution size according to the schedule of intensity. After correlation function is weighed, velocity of propagation passes through several size classes, to determine the sample volume.
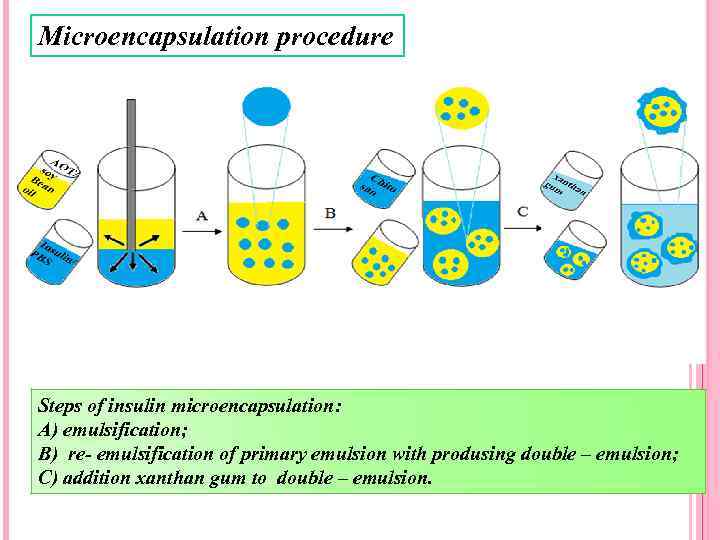
Microencapsulation procedure Steps of insulin microencapsulation: A) emulsification; B) re- emulsification of primary emulsion with produsing double – emulsion; C) addition xanthan gum to double – emulsion.

Morphology of double emulsion Morphology observation of the NPs micrographs showed similar NP sizes as those shown by Zeta sizer Malvern and Microscope Mikromed 1. INS - Ch/Xan NPs appeared irregular, and bigger than INS - loaded primary emulsion droplets, which displayed a more spherical shape and a more compact, small-sized structure. This is a double emulsion formation and also result of Ch-Xan Gum competition around the inner phase. The results testified that Ch-xan Gum complexe was suitable to stabilize primary emulsion and protect coalescence of inner droplets and external water phase, resulting in high encapsulation efficiency and stability. 3101 d. nm Double emulsion size distribution. Stabilized by 1, 12∙ 10 -3 M AOT in soybean oil at IP: OP 0, 1 : 10, 5 min. 40 -20, PE (primary emulsion): EP (external phase)=3 ml: 25 ml was 3101 d. nm.
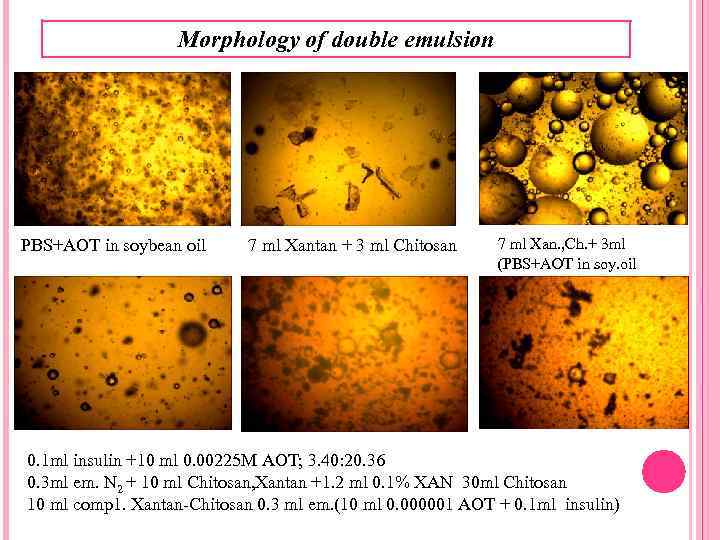
Morphology of double emulsion PBS+AOT in soybean oil 7 ml Xantan + 3 ml Chitosan 7 ml Xan. , Ch. + 3 ml (PBS+AOT in soy. oil 0. 1 ml insulin +10 ml 0. 00225 M AOT; 3. 40: 20. 36 0. 3 ml em. N 2 + 10 ml Chitosan, Xantan +1. 2 ml 0. 1% XAN 30 ml Chitosan 10 ml comp 1. Xantan-Chitosan 0. 3 ml em. (10 ml 0. 000001 AOT + 0. 1 ml insulin)
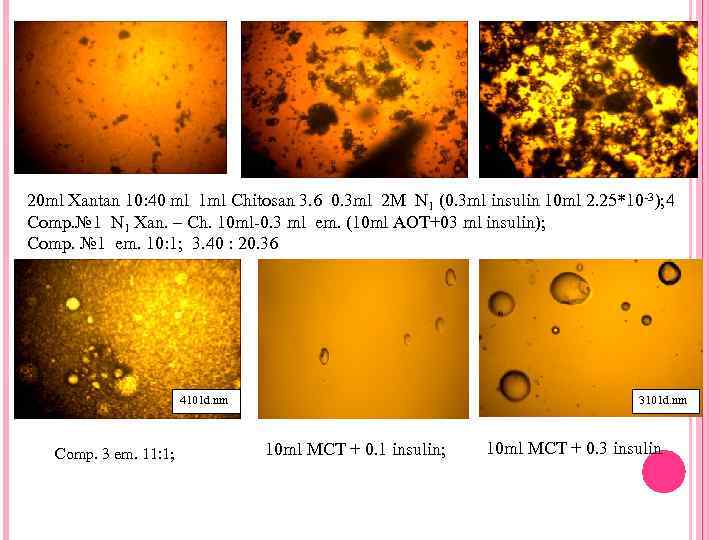
20 ml Xantan 10: 40 ml 1 ml Chitosan 3. 6 0. 3 ml 2 M N 1 (0. 3 ml insulin 10 ml 2. 25*10 -3); 4 Comp. № 1 N 1 Xan. – Ch. 10 ml-0. 3 ml em. (10 ml AOT+03 ml insulin); Comp. № 1 em. 10: 1; 3. 40 : 20. 36 4101 d. nm Comp. 3 em. 11: 1; 3101 d. nm 10 ml MCT + 0. 1 insulin; 10 ml MCT + 0. 3 insulin
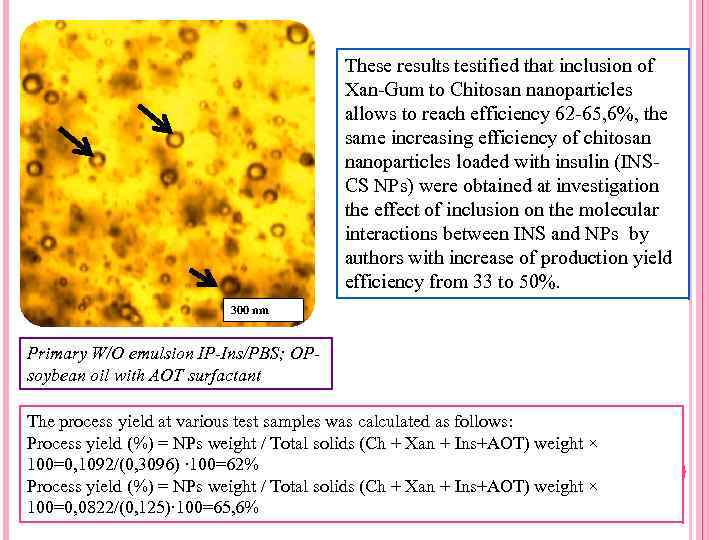
These results testified that inclusion of Xan-Gum to Chitosan nanoparticles allows to reach efficiency 62 -65, 6%, the same increasing efficiency of chitosan nanoparticles loaded with insulin (INSCS NPs) were obtained at investigation the effect of inclusion on the molecular interactions between INS and NPs by authors with increase of production yield efficiency from 33 to 50%. 300 nm Primary W/O emulsion IP-Ins/PBS; OPsoybean oil with AOT surfactant The process yield at various test samples was calculated as follows: Process yield (%) = NPs weight / Total solids (Ch + Xan + Ins+AOT) weight × 100=0, 1092/(0, 3096) ∙ 100=62% Process yield (%) = NPs weight / Total solids (Ch + Xan + Ins+AOT) weight × 100=0, 0822/(0, 125)∙ 100=65, 6%
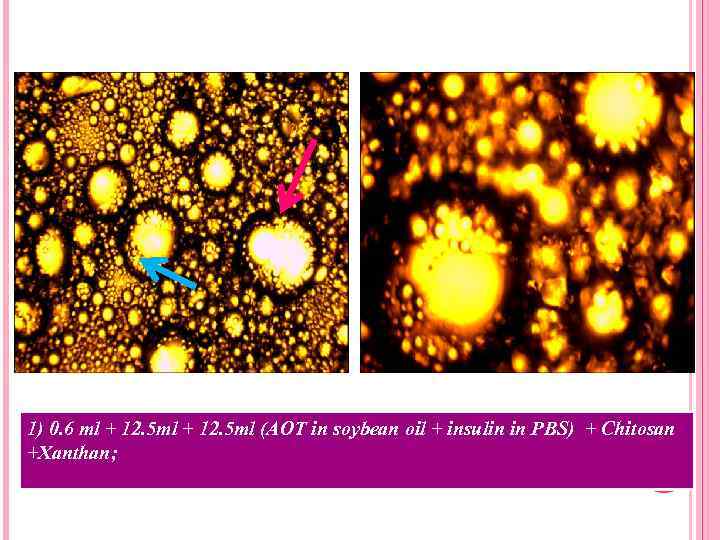
1) 0. 6 ml + 12. 5 ml (AOT in soybean oil + insulin in PBS) + Chitosan +Xanthan;
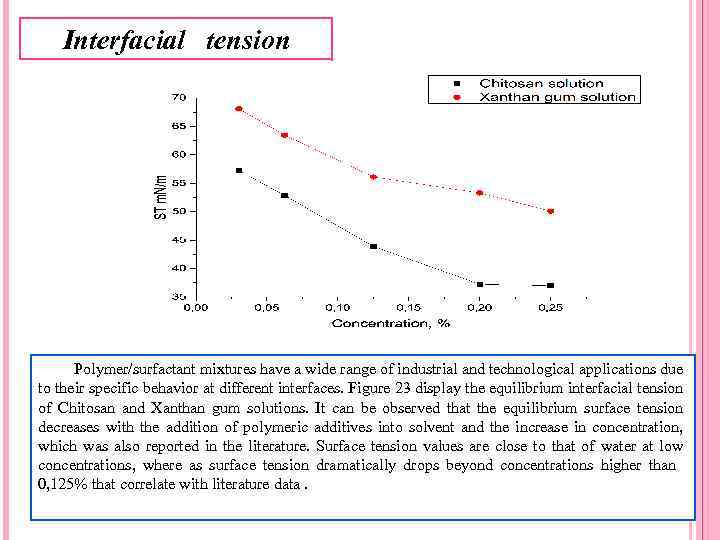
Interfacial tension Polymer/surfactant mixtures have a wide range of industrial and technological applications due to their specific behavior at different interfaces. Figure 23 display the equilibrium interfacial tension of Chitosan and Xanthan gum solutions. It can be observed that the equilibrium surface tension decreases with the addition of polymeric additives into solvent and the increase in concentration, which was also reported in the literature. Surface tension values are close to that of water at low concentrations, where as surface tension dramatically drops beyond concentrations higher than 0, 125% that correlate with literature data.
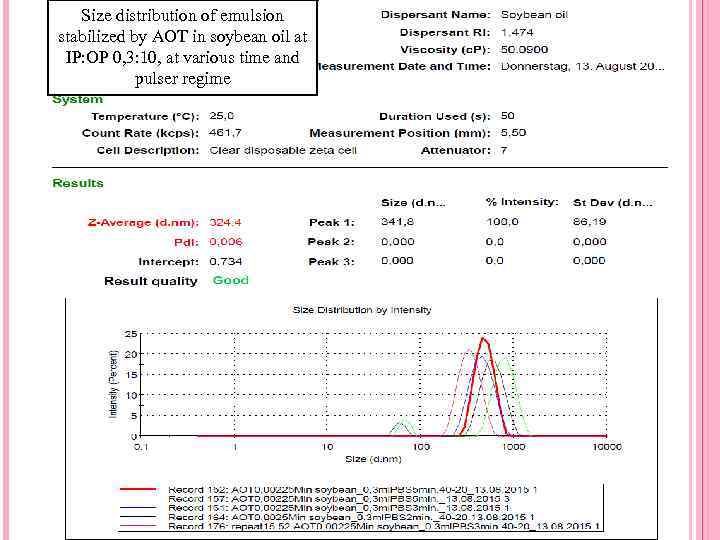
Size distribution of emulsion stabilized by AOT in soybean oil at IP: OP 0, 3: 10, at various time and pulser regime
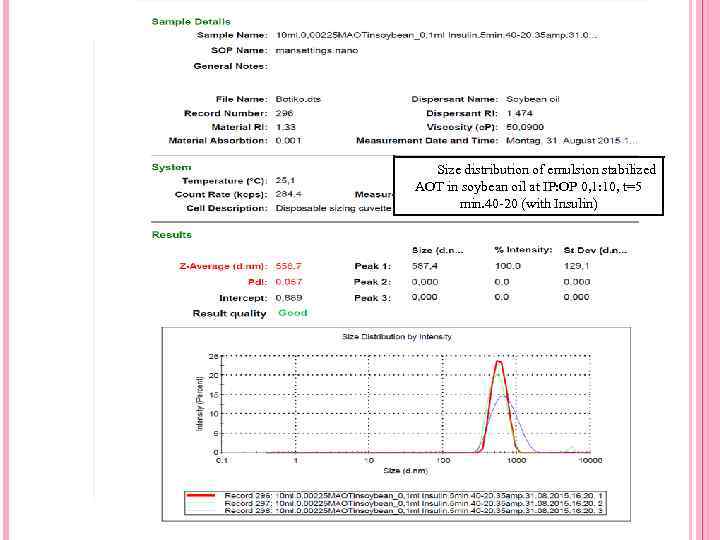
Size distribution of emulsion stabilized AOT in soybean oil at IP: OP 0, 1: 10, t=5 min. 40 -20 (with Insulin)
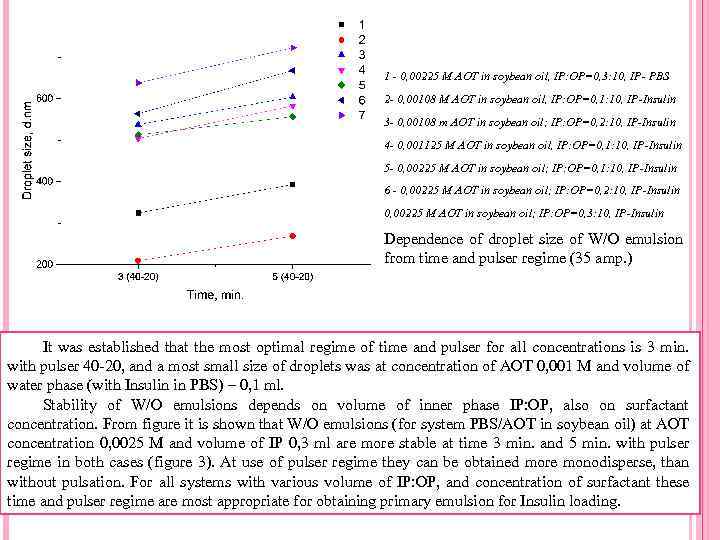
1 - 0, 00225 M AOT in soybean oil, IP: OP=0, 3: 10, IP- PBS 2 - 0, 00108 M AOT in soybean oil, IP: OP=0, 1: 10, IP-Insulin 3 - 0, 00108 m AOT in soybean oil; IP: OP=0, 2: 10, IP-Insulin 4 - 0, 001125 M AOT in soybean oil, IP: OP=0, 1: 10, IP-Insulin 5 - 0, 00225 M AOT in soybean oil; IP: OP=0, 1: 10, IP-Insulin 6 - 0, 00225 M AOT in soybean oil; IP: OP=0, 2: 10, IP-Insulin 0, 00225 M AOT in soybean oil; IP: OP=0, 3: 10, IP-Insulin Dependence of droplet size of W/O emulsion from time and pulser regime (35 amp. ) It was established that the most optimal regime of time and pulser for all concentrations is 3 min. with pulser 40 -20, and a most small size of droplets was at concentration of AOT 0, 001 M and volume of water phase (with Insulin in PBS) – 0, 1 ml. Stability of W/O emulsions depends on volume of inner phase IP: OP, also on surfactant concentration. From figure it is shown that W/O emulsions (for system PBS/AOT in soybean oil) at AOT concentration 0, 0025 M and volume of IP 0, 3 ml are more stable at time 3 min. and 5 min. with pulser regime in both cases (figure 3). At use of pulser regime they can be obtained more monodisperse, than without pulsation. For all systems with various volume of IP: OP, and concentration of surfactant these time and pulser regime are most appropriate for obtaining primary emulsion for Insulin loading.
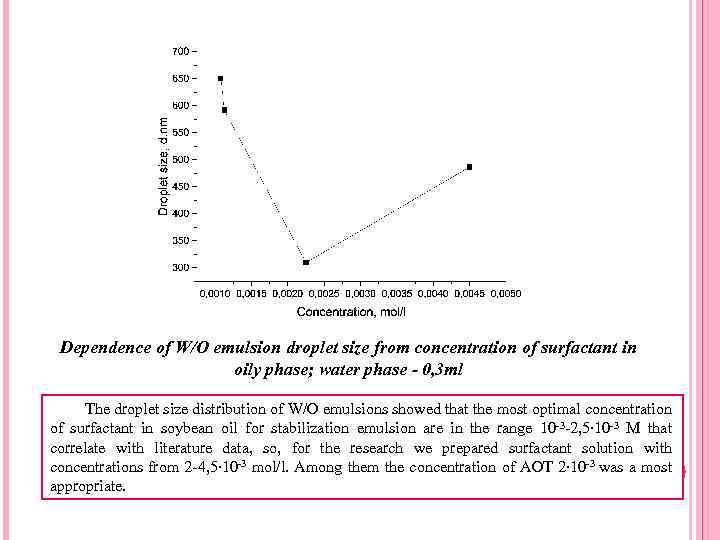
Dependence of W/O emulsion droplet size from concentration of surfactant in oily phase; water phase - 0, 3 ml The droplet size distribution of W/O emulsions showed that the most optimal concentration of surfactant in soybean oil for stabilization emulsion are in the range 10 -3 -2, 5∙ 10 -3 M that correlate with literature data, so, for the research we prepared surfactant solution with concentrations from 2 -4, 5∙ 10 -3 mol/l. Among them the concentration of AOT 2∙ 10 -3 was a most appropriate.
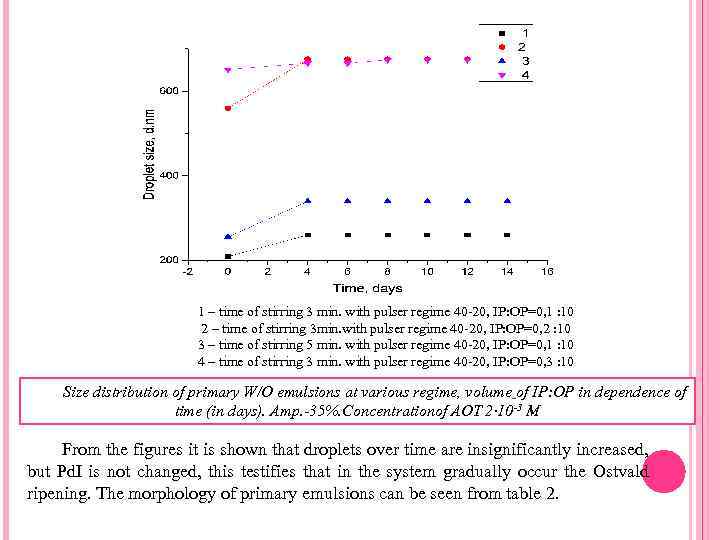
1 – time of stirring 3 min. with pulser regime 40 -20, IP: OP=0, 1 : 10 2 – time of stirring 3 min. with pulser regime 40 -20, IP: OP=0, 2 : 10 3 – time of stirring 5 min. with pulser regime 40 -20, IP: OP=0, 1 : 10 4 – time of stirring 3 min. with pulser regime 40 -20, IP: OP=0, 3 : 10 Size distribution of primary W/O emulsions at various regime, volume of IP: OP in dependence of time (in days). Amp. -35%. Concentrationof AOT 2∙ 10 -3 M From the figures it is shown that droplets over time are insignificantly increased, but Pd. I is not changed, this testifies that in the system gradually occur the Ostvald ripening. The morphology of primary emulsions can be seen from table 2.
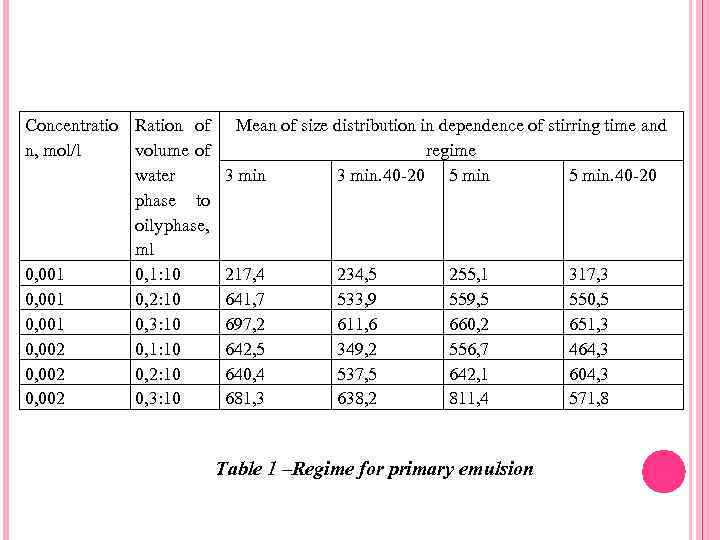
Concentratio Ration of n, mol/l volume of water phase to oily phase, ml 0, 001 0, 1: 10 0, 001 0, 2: 10 0, 001 0, 3: 10 0, 002 0, 1: 10 0, 002 0, 2: 10 0, 002 0, 3: 10 Mean of size distribution in dependence of stirring time and regime 3 min. 40 -20 5 min. 40 -20 217, 4 641, 7 697, 2 642, 5 640, 4 681, 3 234, 5 533, 9 611, 6 349, 2 537, 5 638, 2 255, 1 559, 5 660, 2 556, 7 642, 1 811, 4 Table 1 –Regime for primary emulsion 317, 3 550, 5 651, 3 464, 3 604, 3 571, 8

CONCLUSION In diploma work we have studied insulin encapsulation method by polumeric composition of Chitosan/Xanthan. Novel approach to encapsulation of insulin was developed. The droplets of an w/o emulsion stabilized by polyelectrolyte-surfactant complexes were used as initial carriers for the active agent and simultaneously as liquid templates for the microcapsules build up using the layer-by-layer polyelectrolyte deposition. The microencapsulation approach developed is straightforward and economic and could be used for the encapsulation of bioactive ingredients for pharmaceulticals and food applications. However, for oral insulin to become reality for patients there a number of important challenges that need to be addressed. First, long-term efficacy and safety need to be demonstrated through adequately powered studies in different patient populations across the diabetes spectrum. Reproducible absorption of the drug and understanding of meal related absorption are clearly important goals for developing a drug that needs to be administered lifelong. Second, clinical studies need to demonstrate superiority over injectable insulin formulations and including improved hypoglycaemic profile, reduced weight gain and better disease progression outcome in long-term studies. Third, one of the important unknowns of long-term insulin delivery through oral route is its potential for inducing mitogenic changes in the gutmucosa. Insulin being a growth promoter has always been under scrutiny of the regulators for this potential toxicity issue. This would need to be addressed in long-term toxicology studies. Finally, the success of oral insulin depends on the ability to manufacture insulin both in sufficient quantities for oral delivery as well as efficiently in a cost-conscious pharmaceutical marketplace. If all the issues involved are successfully addressed, a treatment paradigmchanging oral insulin therapy may be the result.

Thank you for attention!!!
инкапсуляция 01.04.2016 - копия.pptx