15-Classification.ppt
- Количество слайдов: 37
 Mineral classes At the highest level, mineral species can be classified primarily on the main anion (O 2−, S 2− etc. ), and anionic complex [(OH)−, (SO 4)2−, (CO 2)2−, (PO 4)3−, (Bx. Oy. Z)−, (Six. Oy. Z)− etc. ) or lack of an anion (native elements) to form classes. That is minerals are classified by chemical composition.
Mineral classes At the highest level, mineral species can be classified primarily on the main anion (O 2−, S 2− etc. ), and anionic complex [(OH)−, (SO 4)2−, (CO 2)2−, (PO 4)3−, (Bx. Oy. Z)−, (Six. Oy. Z)− etc. ) or lack of an anion (native elements) to form classes. That is minerals are classified by chemical composition.
 Broadest division of minerals 1. Native elements (with the exception of gases) make up about 2. 20 minerals. These are further divided into metals, semi-metals, and 3. non-metals. Examples of the first are Au, Ag, Cu, Pt, Pd and Fe; of the second are As, Sb, Bi, Se, and Te and of the last: S and C. 2. Sulfides, including sulfarsenides, arsenides and tellurides. Here As replaces S and acts as an anion. 3. Sulfosalts: approximately 100 species. In these minerals, As and Sb play a role more akin to metals than anions. Many important Ag minerals are sulfosalts. 4. Oxides: a) simple and multiple where O combines with one or metals (cations) and b) hydroxides with OH- group and H 2 O molecules. 5. Halides: these minerals have Cl, Br, F, and I as anions. 6. Carbonates: based on the CO 3 radical. 7. Nitrates: (NO 3)-1, only 7 known minerals 8. Borates: (BO 3)-3 can form polymers--about 100 known minerals. 9. Phosphates (PO 4)-3 are usually hydrous as well. 10. Sulfates (SO 4)-2 can be hydrous or anhydrous • Tungstates (WO 4)-2 I mentioned the minerals scheelite and wolframite, 2. which are both ore minerals for W. 12. Silicates (Si. O 4)-4
Broadest division of minerals 1. Native elements (with the exception of gases) make up about 2. 20 minerals. These are further divided into metals, semi-metals, and 3. non-metals. Examples of the first are Au, Ag, Cu, Pt, Pd and Fe; of the second are As, Sb, Bi, Se, and Te and of the last: S and C. 2. Sulfides, including sulfarsenides, arsenides and tellurides. Here As replaces S and acts as an anion. 3. Sulfosalts: approximately 100 species. In these minerals, As and Sb play a role more akin to metals than anions. Many important Ag minerals are sulfosalts. 4. Oxides: a) simple and multiple where O combines with one or metals (cations) and b) hydroxides with OH- group and H 2 O molecules. 5. Halides: these minerals have Cl, Br, F, and I as anions. 6. Carbonates: based on the CO 3 radical. 7. Nitrates: (NO 3)-1, only 7 known minerals 8. Borates: (BO 3)-3 can form polymers--about 100 known minerals. 9. Phosphates (PO 4)-3 are usually hydrous as well. 10. Sulfates (SO 4)-2 can be hydrous or anhydrous • Tungstates (WO 4)-2 I mentioned the minerals scheelite and wolframite, 2. which are both ore minerals for W. 12. Silicates (Si. O 4)-4
 Dana Classification - principles The recent Dana classification number used in the database presented in the website « Webmineral » is based on Dana's New Mineralogy, Eighth Edition, by Richard V. Gaines, H. Catherine Skinner, Eugene E. Foord, Brian Mason, and Abraham Rosenzweig, with sections by Vandall T. King, Illustrations by Eric Dowty, (ISBN: 047119310 -0) Copyright © 1997, John Wiley & Sons, Inc. The material is used by permission of John Wiley & Sons, Inc.
Dana Classification - principles The recent Dana classification number used in the database presented in the website « Webmineral » is based on Dana's New Mineralogy, Eighth Edition, by Richard V. Gaines, H. Catherine Skinner, Eugene E. Foord, Brian Mason, and Abraham Rosenzweig, with sections by Vandall T. King, Illustrations by Eric Dowty, (ISBN: 047119310 -0) Copyright © 1997, John Wiley & Sons, Inc. The material is used by permission of John Wiley & Sons, Inc.
 Dana Classification - Principles "The first entry for each mineral species is a number containing four parts separated by periods. It represents a hierarchical system parallel to that of Linneaus but based on a combination of chemistry and crystal structure of the minerals. These numbers facilitate insertion and addition of a new species into a list emphasizing close chemical and structural affiliations, an advantage since each mineral is known by a different, and not necessarily related, name. "
Dana Classification - Principles "The first entry for each mineral species is a number containing four parts separated by periods. It represents a hierarchical system parallel to that of Linneaus but based on a combination of chemistry and crystal structure of the minerals. These numbers facilitate insertion and addition of a new species into a list emphasizing close chemical and structural affiliations, an advantage since each mineral is known by a different, and not necessarily related, name. "
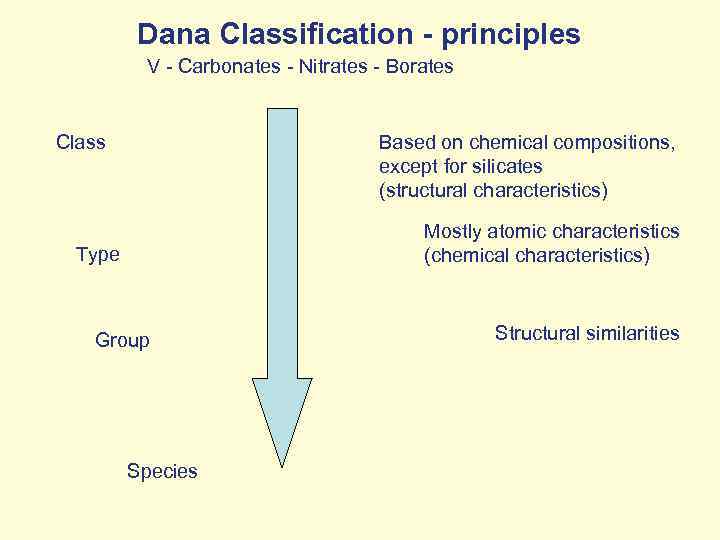 Dana Classification - principles V - Carbonates - Nitrates - Borates Class Based on chemical compositions, except for silicates (structural characteristics) Mostly atomic characteristics (chemical characteristics) Type Group Species Structural similarities
Dana Classification - principles V - Carbonates - Nitrates - Borates Class Based on chemical compositions, except for silicates (structural characteristics) Mostly atomic characteristics (chemical characteristics) Type Group Species Structural similarities
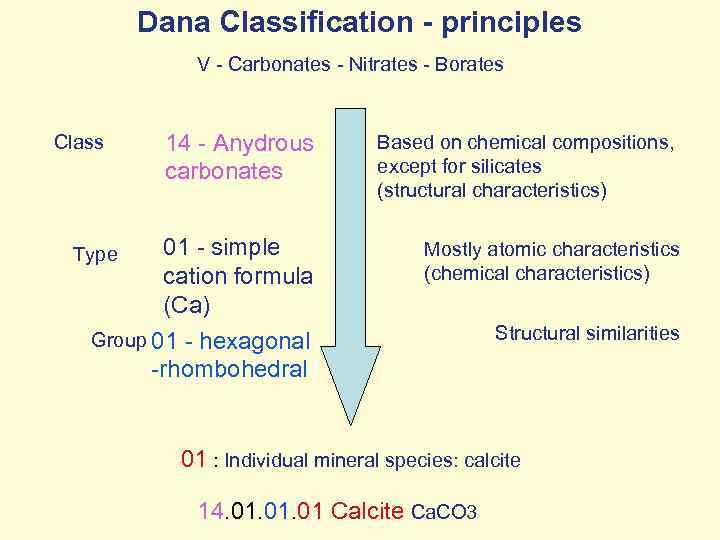 Dana Classification - principles V - Carbonates - Nitrates - Borates 14 - Anydrous carbonates Based on chemical compositions, except for silicates (structural characteristics) 01 - simple cation formula (Ca) Mostly atomic characteristics (chemical characteristics) Group 01 - hexagonal Structural similarities Class Type -rhombohedral 01 : Individual mineral species: calcite 14. 01. 01 Calcite Ca. CO 3
Dana Classification - principles V - Carbonates - Nitrates - Borates 14 - Anydrous carbonates Based on chemical compositions, except for silicates (structural characteristics) 01 - simple cation formula (Ca) Mostly atomic characteristics (chemical characteristics) Group 01 - hexagonal Structural similarities Class Type -rhombohedral 01 : Individual mineral species: calcite 14. 01. 01 Calcite Ca. CO 3
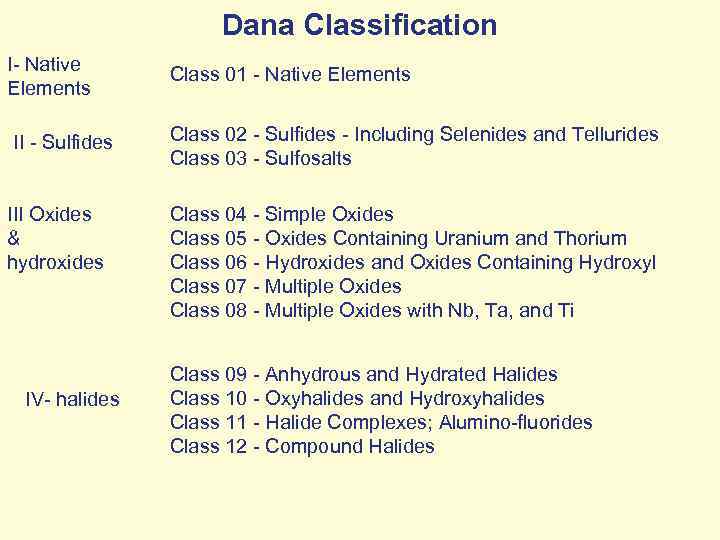 Dana Classification I- Native Elements Class 01 - Native Elements II - Sulfides Class 02 - Sulfides - Including Selenides and Tellurides Class 03 - Sulfosalts III Oxides & hydroxides Class 04 - Simple Oxides Class 05 - Oxides Containing Uranium and Thorium Class 06 - Hydroxides and Oxides Containing Hydroxyl Class 07 - Multiple Oxides Class 08 - Multiple Oxides with Nb, Ta, and Ti IV- halides Class 09 - Anhydrous and Hydrated Halides Class 10 - Oxyhalides and Hydroxyhalides Class 11 - Halide Complexes; Alumino-fluorides Class 12 - Compound Halides
Dana Classification I- Native Elements Class 01 - Native Elements II - Sulfides Class 02 - Sulfides - Including Selenides and Tellurides Class 03 - Sulfosalts III Oxides & hydroxides Class 04 - Simple Oxides Class 05 - Oxides Containing Uranium and Thorium Class 06 - Hydroxides and Oxides Containing Hydroxyl Class 07 - Multiple Oxides Class 08 - Multiple Oxides with Nb, Ta, and Ti IV- halides Class 09 - Anhydrous and Hydrated Halides Class 10 - Oxyhalides and Hydroxyhalides Class 11 - Halide Complexes; Alumino-fluorides Class 12 - Compound Halides
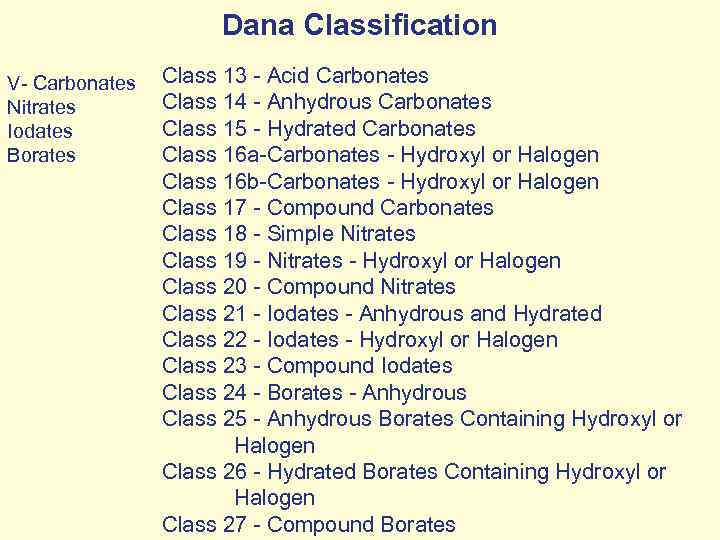 Dana Classification V- Carbonates Nitrates Iodates Borates Class 13 - Acid Carbonates Class 14 - Anhydrous Carbonates Class 15 - Hydrated Carbonates Class 16 a-Carbonates - Hydroxyl or Halogen Class 16 b-Carbonates - Hydroxyl or Halogen Class 17 - Compound Carbonates Class 18 - Simple Nitrates Class 19 - Nitrates - Hydroxyl or Halogen Class 20 - Compound Nitrates Class 21 - Iodates - Anhydrous and Hydrated Class 22 - Iodates - Hydroxyl or Halogen Class 23 - Compound Iodates Class 24 - Borates - Anhydrous Class 25 - Anhydrous Borates Containing Hydroxyl or Halogen Class 26 - Hydrated Borates Containing Hydroxyl or Halogen Class 27 - Compound Borates
Dana Classification V- Carbonates Nitrates Iodates Borates Class 13 - Acid Carbonates Class 14 - Anhydrous Carbonates Class 15 - Hydrated Carbonates Class 16 a-Carbonates - Hydroxyl or Halogen Class 16 b-Carbonates - Hydroxyl or Halogen Class 17 - Compound Carbonates Class 18 - Simple Nitrates Class 19 - Nitrates - Hydroxyl or Halogen Class 20 - Compound Nitrates Class 21 - Iodates - Anhydrous and Hydrated Class 22 - Iodates - Hydroxyl or Halogen Class 23 - Compound Iodates Class 24 - Borates - Anhydrous Class 25 - Anhydrous Borates Containing Hydroxyl or Halogen Class 26 - Hydrated Borates Containing Hydroxyl or Halogen Class 27 - Compound Borates
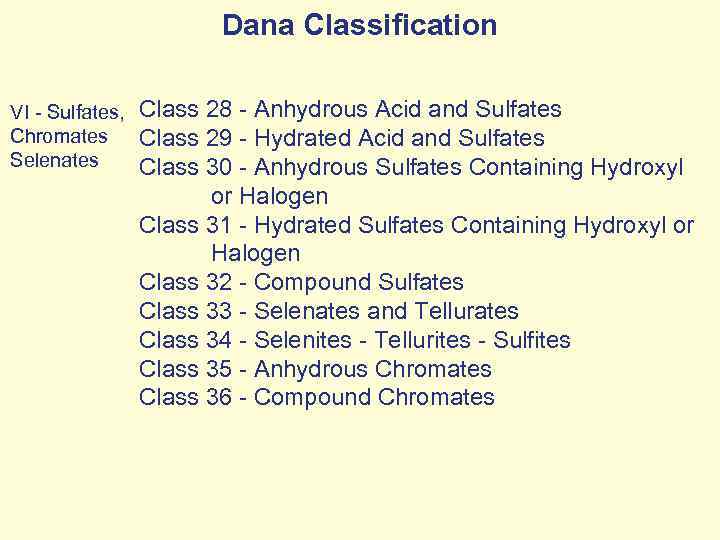 Dana Classification VI - Sulfates, Chromates Selenates Class 28 - Anhydrous Acid and Sulfates Class 29 - Hydrated Acid and Sulfates Class 30 - Anhydrous Sulfates Containing Hydroxyl or Halogen Class 31 - Hydrated Sulfates Containing Hydroxyl or Halogen Class 32 - Compound Sulfates Class 33 - Selenates and Tellurates Class 34 - Selenites - Tellurites - Sulfites Class 35 - Anhydrous Chromates Class 36 - Compound Chromates
Dana Classification VI - Sulfates, Chromates Selenates Class 28 - Anhydrous Acid and Sulfates Class 29 - Hydrated Acid and Sulfates Class 30 - Anhydrous Sulfates Containing Hydroxyl or Halogen Class 31 - Hydrated Sulfates Containing Hydroxyl or Halogen Class 32 - Compound Sulfates Class 33 - Selenates and Tellurates Class 34 - Selenites - Tellurites - Sulfites Class 35 - Anhydrous Chromates Class 36 - Compound Chromates
 Dana Classification VII - Phosphates Arsenates Vanadates Class 37 - Anhydrous Acid Phosphates Class 38 - Anhydrous Phosphates Class 39 - Hydrated Acid Phosphates Class 40 - Hydrated Phosphates Class 41 - Anhydrous Phosphates Containing Hydroxyl or Halogen Class 42 - Hydrated Phosphates Containing Hydroxyl or Halogen Class 43 - Compound Phosphates Class 44 - Antimonates Class 45 - Acid and normal Antimonites, Arsenites and Phosphites Class 46 - Basic or Halogen-Containing Antimonites, Arsenites and Phosphites Class 47 - Vanadium Oxysalts Class 48 - Anhydrous Molybdates and Tungstates Class 49 - Basic and Hydrated Molybdates and Tungstates
Dana Classification VII - Phosphates Arsenates Vanadates Class 37 - Anhydrous Acid Phosphates Class 38 - Anhydrous Phosphates Class 39 - Hydrated Acid Phosphates Class 40 - Hydrated Phosphates Class 41 - Anhydrous Phosphates Containing Hydroxyl or Halogen Class 42 - Hydrated Phosphates Containing Hydroxyl or Halogen Class 43 - Compound Phosphates Class 44 - Antimonates Class 45 - Acid and normal Antimonites, Arsenites and Phosphites Class 46 - Basic or Halogen-Containing Antimonites, Arsenites and Phosphites Class 47 - Vanadium Oxysalts Class 48 - Anhydrous Molybdates and Tungstates Class 49 - Basic and Hydrated Molybdates and Tungstates
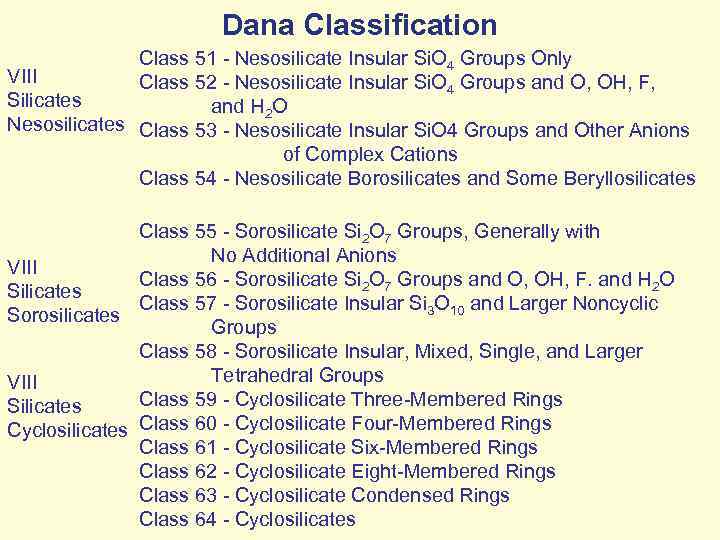 Dana Classification Class 51 - Nesosilicate Insular Si. O 4 Groups Only VIII Class 52 - Nesosilicate Insular Si. O 4 Groups and O, OH, F, Silicates and H 2 O Nesosilicates Class 53 - Nesosilicate Insular Si. O 4 Groups and Other Anions of Complex Cations Class 54 - Nesosilicate Borosilicates and Some Beryllosilicates Class 55 - Sorosilicate Si 2 O 7 Groups, Generally with No Additional Anions VIII Class 56 - Sorosilicate Si 2 O 7 Groups and O, OH, F. and H 2 O Silicates Class 57 - Sorosilicate Insular Si 3 O 10 and Larger Noncyclic Sorosilicates Groups Class 58 - Sorosilicate Insular, Mixed, Single, and Larger Tetrahedral Groups VIII Class 59 - Cyclosilicate Three-Membered Rings Silicates Cyclosilicates Class 60 - Cyclosilicate Four-Membered Rings Class 61 - Cyclosilicate Six-Membered Rings Class 62 - Cyclosilicate Eight-Membered Rings Class 63 - Cyclosilicate Condensed Rings Class 64 - Cyclosilicates
Dana Classification Class 51 - Nesosilicate Insular Si. O 4 Groups Only VIII Class 52 - Nesosilicate Insular Si. O 4 Groups and O, OH, F, Silicates and H 2 O Nesosilicates Class 53 - Nesosilicate Insular Si. O 4 Groups and Other Anions of Complex Cations Class 54 - Nesosilicate Borosilicates and Some Beryllosilicates Class 55 - Sorosilicate Si 2 O 7 Groups, Generally with No Additional Anions VIII Class 56 - Sorosilicate Si 2 O 7 Groups and O, OH, F. and H 2 O Silicates Class 57 - Sorosilicate Insular Si 3 O 10 and Larger Noncyclic Sorosilicates Groups Class 58 - Sorosilicate Insular, Mixed, Single, and Larger Tetrahedral Groups VIII Class 59 - Cyclosilicate Three-Membered Rings Silicates Cyclosilicates Class 60 - Cyclosilicate Four-Membered Rings Class 61 - Cyclosilicate Six-Membered Rings Class 62 - Cyclosilicate Eight-Membered Rings Class 63 - Cyclosilicate Condensed Rings Class 64 - Cyclosilicates
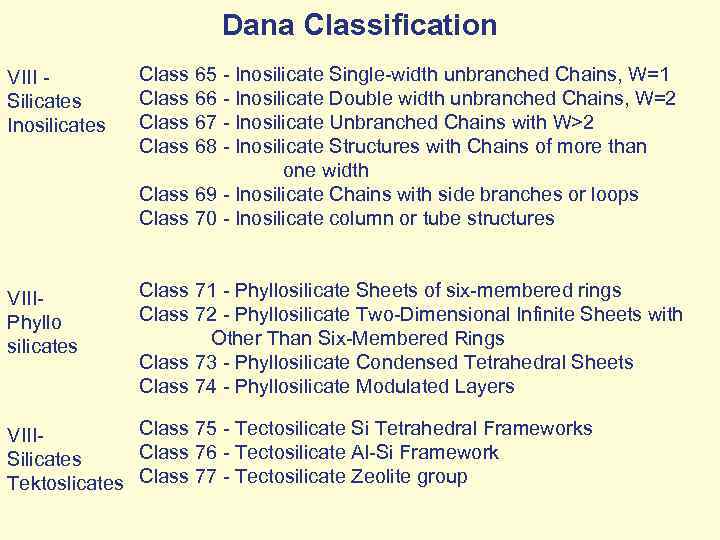 Dana Classification VIII - Silicates Inosilicates Class 65 - Inosilicate Single-width unbranched Chains, W=1 Class 66 - Inosilicate Double width unbranched Chains, W=2 Class 67 - Inosilicate Unbranched Chains with W>2 Class 68 - Inosilicate Structures with Chains of more than one width Class 69 - Inosilicate Chains with side branches or loops Class 70 - Inosilicate column or tube structures VIII- Phyllo silicates Class 71 - Phyllosilicate Sheets of six-membered rings Class 72 - Phyllosilicate Two-Dimensional Infinite Sheets with Other Than Six-Membered Rings Class 73 - Phyllosilicate Condensed Tetrahedral Sheets Class 74 - Phyllosilicate Modulated Layers Class 75 - Tectosilicate Si Tetrahedral Frameworks VIII- Class 76 - Tectosilicate Al-Si Framework Silicates Tektoslicates Class 77 - Tectosilicate Zeolite group
Dana Classification VIII - Silicates Inosilicates Class 65 - Inosilicate Single-width unbranched Chains, W=1 Class 66 - Inosilicate Double width unbranched Chains, W=2 Class 67 - Inosilicate Unbranched Chains with W>2 Class 68 - Inosilicate Structures with Chains of more than one width Class 69 - Inosilicate Chains with side branches or loops Class 70 - Inosilicate column or tube structures VIII- Phyllo silicates Class 71 - Phyllosilicate Sheets of six-membered rings Class 72 - Phyllosilicate Two-Dimensional Infinite Sheets with Other Than Six-Membered Rings Class 73 - Phyllosilicate Condensed Tetrahedral Sheets Class 74 - Phyllosilicate Modulated Layers Class 75 - Tectosilicate Si Tetrahedral Frameworks VIII- Class 76 - Tectosilicate Al-Si Framework Silicates Tektoslicates Class 77 - Tectosilicate Zeolite group
 Dana Classification IX Organic Minerals Class 50 - Salts of Organic Acids and Hydrocarbons
Dana Classification IX Organic Minerals Class 50 - Salts of Organic Acids and Hydrocarbons
 Mineral database http: //webmineral. com/danaclass. shtml#. UJZv. Lo. XPMt. V Give the different classes of minerals as reported before. At the bottom of the page, you have a link towards the list of minerals belonging to the different dana classes « Complete Listing -(1, 070 Kb) Classes 1 -78 » http: //webmineral. com/dana. php#. UJZv. AIXPMt. V Example
Mineral database http: //webmineral. com/danaclass. shtml#. UJZv. Lo. XPMt. V Give the different classes of minerals as reported before. At the bottom of the page, you have a link towards the list of minerals belonging to the different dana classes « Complete Listing -(1, 070 Kb) Classes 1 -78 » http: //webmineral. com/dana. php#. UJZv. AIXPMt. V Example
 Example 01 Native Elements Space Group in Red Point Group in Green * - Not IMA Approved. ! - Dana Number Added or Changed. ? - IMA Discredited Mineral Name. 01. 00 Native Elements Without Dana Classification Numbers 01. 00 Alloys 01. 00. 00 Antitaenite* gamma-Fe 3 Ni Unk Cubic 01. 00. 00 Tongxinite* Cu 2 Zn Unk Cubic 01. 00 Amalgam Alloys 01. 00. 00 Goldamalgam* (Au, Ag)Hg I m 3 m 4/m 3 2/m 01. 00 Metals 01. 00. 00 Tantalum* Ta I m 3 m 4/m 3 2/m 01. 00. 00 Titanium* Ti P 63/mmc 6/m 2/m 01. 00 Platinum Group 01. 00. 00 Rhenium* Re P 63/mmc 6/m 2/m 01. 00 Silicide 01. 00. 00 IMA 2007 -036 Ti. Fe. Si 2 Pbam 2/m 2/m
Example 01 Native Elements Space Group in Red Point Group in Green * - Not IMA Approved. ! - Dana Number Added or Changed. ? - IMA Discredited Mineral Name. 01. 00 Native Elements Without Dana Classification Numbers 01. 00 Alloys 01. 00. 00 Antitaenite* gamma-Fe 3 Ni Unk Cubic 01. 00. 00 Tongxinite* Cu 2 Zn Unk Cubic 01. 00 Amalgam Alloys 01. 00. 00 Goldamalgam* (Au, Ag)Hg I m 3 m 4/m 3 2/m 01. 00 Metals 01. 00. 00 Tantalum* Ta I m 3 m 4/m 3 2/m 01. 00. 00 Titanium* Ti P 63/mmc 6/m 2/m 01. 00 Platinum Group 01. 00. 00 Rhenium* Re P 63/mmc 6/m 2/m 01. 00 Silicide 01. 00. 00 IMA 2007 -036 Ti. Fe. Si 2 Pbam 2/m 2/m
 Example - General IMA 2007 -036 Information Chemical Formula: Ti. Fe. Si 2 Composition: Molecular Weight = 159. 90 gm Titanium 29. 94 % Ti Iron 34. 93 % Fe Silicon 35. 13 % Si ______ 100. 00 % Empirical Formula: Ti. Fe 2+Si 2 IMA Status: Proposed IMA 2007 Locality: Luobusa mine, Qusong County, Tibet, China Link to Min. Dat. org Location Data. Name Origin: Commission on New Minerals, Nomenclature and Classification (CNMNC)
Example - General IMA 2007 -036 Information Chemical Formula: Ti. Fe. Si 2 Composition: Molecular Weight = 159. 90 gm Titanium 29. 94 % Ti Iron 34. 93 % Fe Silicon 35. 13 % Si ______ 100. 00 % Empirical Formula: Ti. Fe 2+Si 2 IMA Status: Proposed IMA 2007 Locality: Luobusa mine, Qusong County, Tibet, China Link to Min. Dat. org Location Data. Name Origin: Commission on New Minerals, Nomenclature and Classification (CNMNC)
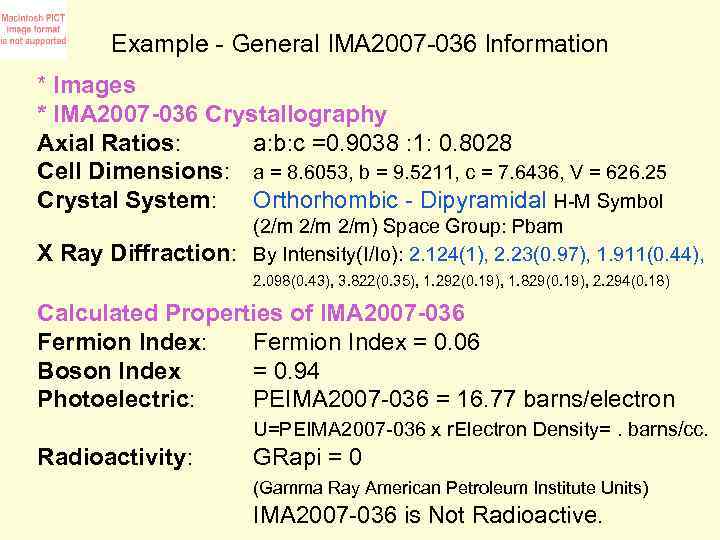 Example - General IMA 2007 -036 Information * Images * IMA 2007 -036 Crystallography Axial Ratios: a: b: c =0. 9038 : 1: 0. 8028 Cell Dimensions: a = 8. 6053, b = 9. 5211, c = 7. 6436, V = 626. 25 Crystal System: Orthorhombic - Dipyramidal H-M Symbol X Ray Diffraction: (2/m 2/m) Space Group: Pbam By Intensity(I/Io): 2. 124(1), 2. 23(0. 97), 1. 911(0. 44), 2. 098(0. 43), 3. 822(0. 35), 1. 292(0. 19), 1. 829(0. 19), 2. 294(0. 18) Calculated Properties of IMA 2007 -036 Fermion Index: Fermion Index = 0. 06 Boson Index = 0. 94 Photoelectric: PEIMA 2007 -036 = 16. 77 barns/electron U=PEIMA 2007 -036 x r. Electron Density=. barns/cc. Radioactivity: GRapi = 0 (Gamma Ray American Petroleum Institute Units) IMA 2007 -036 is Not Radioactive.
Example - General IMA 2007 -036 Information * Images * IMA 2007 -036 Crystallography Axial Ratios: a: b: c =0. 9038 : 1: 0. 8028 Cell Dimensions: a = 8. 6053, b = 9. 5211, c = 7. 6436, V = 626. 25 Crystal System: Orthorhombic - Dipyramidal H-M Symbol X Ray Diffraction: (2/m 2/m) Space Group: Pbam By Intensity(I/Io): 2. 124(1), 2. 23(0. 97), 1. 911(0. 44), 2. 098(0. 43), 3. 822(0. 35), 1. 292(0. 19), 1. 829(0. 19), 2. 294(0. 18) Calculated Properties of IMA 2007 -036 Fermion Index: Fermion Index = 0. 06 Boson Index = 0. 94 Photoelectric: PEIMA 2007 -036 = 16. 77 barns/electron U=PEIMA 2007 -036 x r. Electron Density=. barns/cc. Radioactivity: GRapi = 0 (Gamma Ray American Petroleum Institute Units) IMA 2007 -036 is Not Radioactive.
 Example - General IMA 2007 -036 Information * Images *IMA 2007 -036 Crystallography Axial Ratios: a: b: c =0. 9038: 1: 0. 8028 Cell Dimensions: a = 8. 6053, b = 9. 5211, c = 7. 6436, V = 626. 25 Crystal System: Orthorhombic - Dipyramidal H-M Symbol X Ray Diffraction: (2/m 2/m) Space Group: Pbam By Intensity(I/Io): 2. 124(1), 2. 23(0. 97), 1. 911(0. 44), 2. 098(0. 43), 3. 822(0. 35), 1. 292(0. 19), 1. 829(0. 19), 2. 294(0. 18) Calculated Properties of IMA 2007 -036 Fermion Index: Fermion Index = 0. 06 Boson Index = 0. 94 Photoelectric: PEIMA 2007 -036 = 16. 77 barns/electron U=PEIMA 2007 -036 x r Electron Density=. barns/cc. Radioactivity: GRapi = 0 (Gamma Ray American Petroleum Institute Units) IMA 2007 -036 is Not Radioactive.
Example - General IMA 2007 -036 Information * Images *IMA 2007 -036 Crystallography Axial Ratios: a: b: c =0. 9038: 1: 0. 8028 Cell Dimensions: a = 8. 6053, b = 9. 5211, c = 7. 6436, V = 626. 25 Crystal System: Orthorhombic - Dipyramidal H-M Symbol X Ray Diffraction: (2/m 2/m) Space Group: Pbam By Intensity(I/Io): 2. 124(1), 2. 23(0. 97), 1. 911(0. 44), 2. 098(0. 43), 3. 822(0. 35), 1. 292(0. 19), 1. 829(0. 19), 2. 294(0. 18) Calculated Properties of IMA 2007 -036 Fermion Index: Fermion Index = 0. 06 Boson Index = 0. 94 Photoelectric: PEIMA 2007 -036 = 16. 77 barns/electron U=PEIMA 2007 -036 x r Electron Density=. barns/cc. Radioactivity: GRapi = 0 (Gamma Ray American Petroleum Institute Units) IMA 2007 -036 is Not Radioactive.
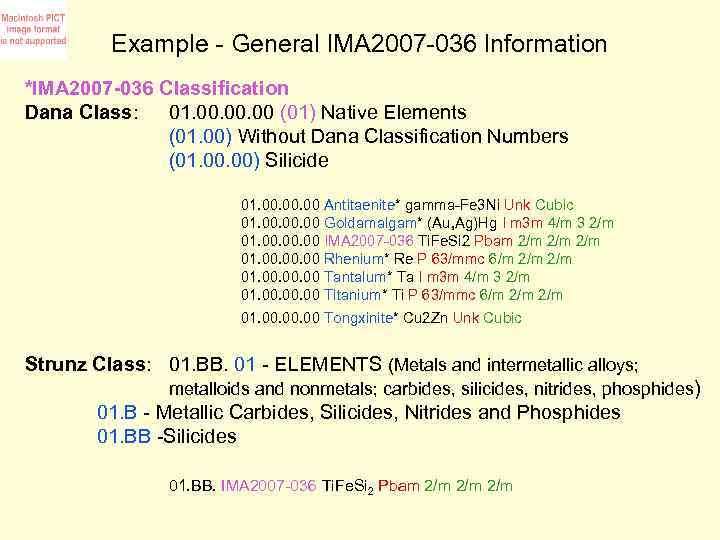 Example - General IMA 2007 -036 Information *IMA 2007 -036 Classification Dana Class: 01. 00. 00 (01) Native Elements (01. 00) Without Dana Classification Numbers (01. 00) Silicide 01. 00. 00 Antitaenite* gamma-Fe 3 Ni Unk Cubic 01. 00. 00 Goldamalgam* (Au, Ag)Hg I m 3 m 4/m 3 2/m 01. 00. 00 IMA 2007 -036 Ti. Fe. Si 2 Pbam 2/m 2/m 01. 00. 00 Rhenium* Re P 63/mmc 6/m 2/m 01. 00. 00 Tantalum* Ta I m 3 m 4/m 3 2/m 01. 00. 00 Titanium* Ti P 63/mmc 6/m 2/m 01. 00. 00 Tongxinite* Cu 2 Zn Unk Cubic Strunz Class: 01. BB. 01 - ELEMENTS (Metals and intermetallic alloys; metalloids and nonmetals; carbides, silicides, nitrides, phosphides) 01. B - Metallic Carbides, Silicides, Nitrides and Phosphides 01. BB -Silicides 01. BB. IMA 2007 -036 Ti. Fe. Si 2 Pbam 2/m 2/m
Example - General IMA 2007 -036 Information *IMA 2007 -036 Classification Dana Class: 01. 00. 00 (01) Native Elements (01. 00) Without Dana Classification Numbers (01. 00) Silicide 01. 00. 00 Antitaenite* gamma-Fe 3 Ni Unk Cubic 01. 00. 00 Goldamalgam* (Au, Ag)Hg I m 3 m 4/m 3 2/m 01. 00. 00 IMA 2007 -036 Ti. Fe. Si 2 Pbam 2/m 2/m 01. 00. 00 Rhenium* Re P 63/mmc 6/m 2/m 01. 00. 00 Tantalum* Ta I m 3 m 4/m 3 2/m 01. 00. 00 Titanium* Ti P 63/mmc 6/m 2/m 01. 00. 00 Tongxinite* Cu 2 Zn Unk Cubic Strunz Class: 01. BB. 01 - ELEMENTS (Metals and intermetallic alloys; metalloids and nonmetals; carbides, silicides, nitrides, phosphides) 01. B - Metallic Carbides, Silicides, Nitrides and Phosphides 01. BB -Silicides 01. BB. IMA 2007 -036 Ti. Fe. Si 2 Pbam 2/m 2/m
 Example - General IMA 2007 -036 Information Other IMA 2007 -036 Information See Also: Links to other databases for IMA 2007 -036 : 1 - Am. Min. Crystal Structure Database 2 - Athena 3 - Google Images 4 - Google Scholar 5 - Min. DAT 6 - Mineralienatlas (Deutsch) 7 - Online Mineral Museum 8 - Ruff. Info
Example - General IMA 2007 -036 Information Other IMA 2007 -036 Information See Also: Links to other databases for IMA 2007 -036 : 1 - Am. Min. Crystal Structure Database 2 - Athena 3 - Google Images 4 - Google Scholar 5 - Min. DAT 6 - Mineralienatlas (Deutsch) 7 - Online Mineral Museum 8 - Ruff. Info
 Example - General IMA 2007 -036 Information 5 - Min. DAT http: //www. mindat. org/min-39545. html Synonym of: Zangboite This page provides mineralogical data about IMA 2007 -036.
Example - General IMA 2007 -036 Information 5 - Min. DAT http: //www. mindat. org/min-39545. html Synonym of: Zangboite This page provides mineralogical data about IMA 2007 -036.
 Strunz classification Hugo Strunz introduced a chemical-structural classification of the entire domain of minerals (Mineralogische Tabellen, 1941), followed by A. S. Povarennykh with a modified classification (1966 in Russian, 1972 in English). The chemical-structural classification of H. Strunz has gone through a number of editions, and is currently in the process of being refined in the light of recent crystal-structure determinations (Nickel-Strunz Version 10) by the late Ernest H. Nickel and others.
Strunz classification Hugo Strunz introduced a chemical-structural classification of the entire domain of minerals (Mineralogische Tabellen, 1941), followed by A. S. Povarennykh with a modified classification (1966 in Russian, 1972 in English). The chemical-structural classification of H. Strunz has gone through a number of editions, and is currently in the process of being refined in the light of recent crystal-structure determinations (Nickel-Strunz Version 10) by the late Ernest H. Nickel and others.
 Strunz classification (http: //www. webmineral. com/strunz. shtml#. UJPQho. XPNpg) The current scheme divides minerals into ten classes, which are further divided into divisions, families and groups according to chemical composition and crystal structure. IMA/CNMNC proposes a new hierarchical scheme (Mills et al. 2009), using the Nickel-Strunz classes (10 ed):
Strunz classification (http: //www. webmineral. com/strunz. shtml#. UJPQho. XPNpg) The current scheme divides minerals into ten classes, which are further divided into divisions, families and groups according to chemical composition and crystal structure. IMA/CNMNC proposes a new hierarchical scheme (Mills et al. 2009), using the Nickel-Strunz classes (10 ed):
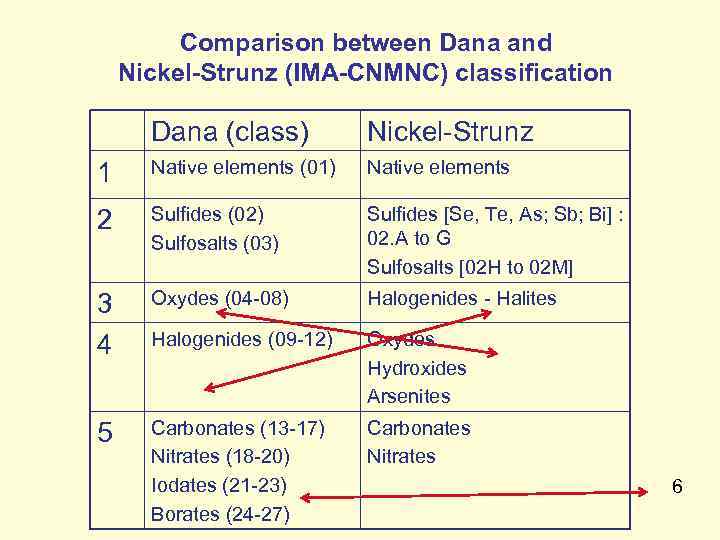 Comparison between Dana and Nickel-Strunz (IMA-CNMNC) classification Dana (class) Nickel-Strunz 1 Native elements (01) Native elements 2 Sulfides (02) Sulfosalts (03) Sulfides [Se, Te, As; Sb; Bi] : 02. A to G Sulfosalts [02 H to 02 M] 3 4 Oxydes (04 -08) Halogenides - Halites Halogenides (09 -12) Oxydes Hydroxides Arsenites 5 Carbonates (13 -17) Nitrates (18 -20) Iodates (21 -23) Borates (24 -27) Carbonates Nitrates 6
Comparison between Dana and Nickel-Strunz (IMA-CNMNC) classification Dana (class) Nickel-Strunz 1 Native elements (01) Native elements 2 Sulfides (02) Sulfosalts (03) Sulfides [Se, Te, As; Sb; Bi] : 02. A to G Sulfosalts [02 H to 02 M] 3 4 Oxydes (04 -08) Halogenides - Halites Halogenides (09 -12) Oxydes Hydroxides Arsenites 5 Carbonates (13 -17) Nitrates (18 -20) Iodates (21 -23) Borates (24 -27) Carbonates Nitrates 6
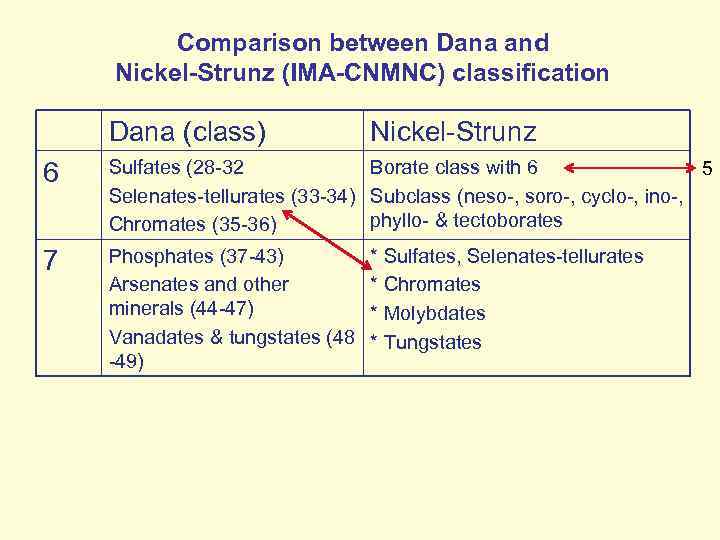 Comparison between Dana and Nickel-Strunz (IMA-CNMNC) classification Dana (class) Nickel-Strunz 6 Sulfates (28 -32 Borate class with 6 5 Selenates-tellurates (33 -34) Subclass (neso-, soro-, cyclo-, ino-, phyllo- & tectoborates Chromates (35 -36) 7 Phosphates (37 -43) Arsenates and other minerals (44 -47) Vanadates & tungstates (48 -49) * Sulfates, Selenates-tellurates * Chromates * Molybdates * Tungstates
Comparison between Dana and Nickel-Strunz (IMA-CNMNC) classification Dana (class) Nickel-Strunz 6 Sulfates (28 -32 Borate class with 6 5 Selenates-tellurates (33 -34) Subclass (neso-, soro-, cyclo-, ino-, phyllo- & tectoborates Chromates (35 -36) 7 Phosphates (37 -43) Arsenates and other minerals (44 -47) Vanadates & tungstates (48 -49) * Sulfates, Selenates-tellurates * Chromates * Molybdates * Tungstates
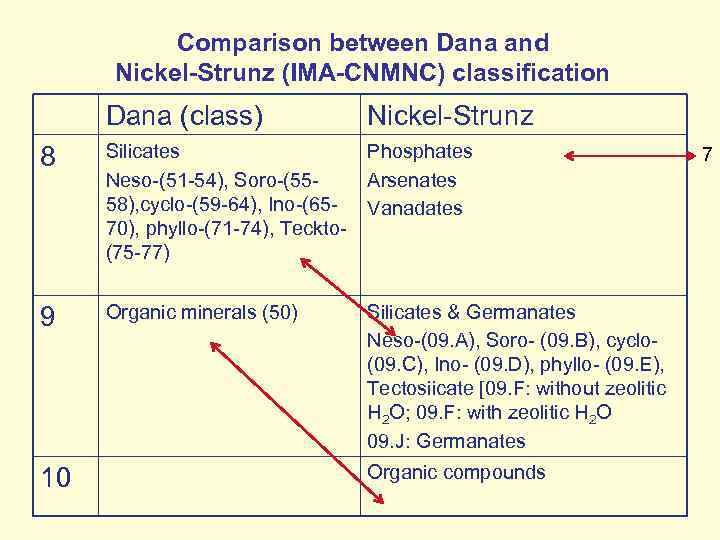 Comparison between Dana and Nickel-Strunz (IMA-CNMNC) classification Dana (class) Nickel-Strunz 8 Silicates Neso-(51 -54), Soro-(5558), cyclo-(59 -64), Ino-(6570), phyllo-(71 -74), Teckto(75 -77) Phosphates Arsenates Vanadates 9 Organic minerals (50) Silicates & Germanates Neso-(09. A), Soro- (09. B), cyclo- (09. C), Ino- (09. D), phyllo- (09. E), Tectosiicate [09. F: without zeolitic H 2 O; 09. F: with zeolitic H 2 O 09. J: Germanates 10 Organic compounds 7
Comparison between Dana and Nickel-Strunz (IMA-CNMNC) classification Dana (class) Nickel-Strunz 8 Silicates Neso-(51 -54), Soro-(5558), cyclo-(59 -64), Ino-(6570), phyllo-(71 -74), Teckto(75 -77) Phosphates Arsenates Vanadates 9 Organic minerals (50) Silicates & Germanates Neso-(09. A), Soro- (09. B), cyclo- (09. C), Ino- (09. D), phyllo- (09. E), Tectosiicate [09. F: without zeolitic H 2 O; 09. F: with zeolitic H 2 O 09. J: Germanates 10 Organic compounds 7
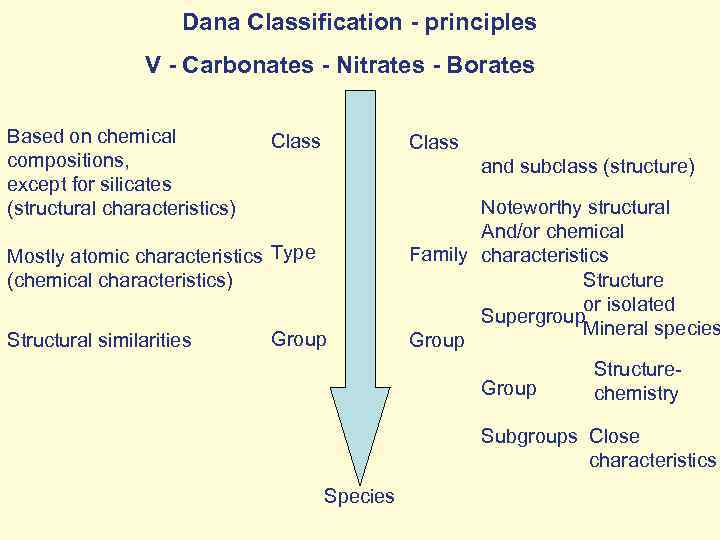 Dana Classification - principles V - Carbonates - Nitrates - Borates Based on chemical compositions, except for silicates (structural characteristics) Class and subclass (structure) Mostly atomic characteristics Type (chemical characteristics) Structural similarities Group Noteworthy structural And/or chemical Family characteristics Structure or isolated Supergroup Mineral species Group Structure. Group chemistry Subgroups Close characteristics Species
Dana Classification - principles V - Carbonates - Nitrates - Borates Based on chemical compositions, except for silicates (structural characteristics) Class and subclass (structure) Mostly atomic characteristics Type (chemical characteristics) Structural similarities Group Noteworthy structural And/or chemical Family characteristics Structure or isolated Supergroup Mineral species Group Structure. Group chemistry Subgroups Close characteristics Species
 Mineral Nomenclature Other comments • Individual minerals are known as species, such as forsterite or fayalite of the olivine series • A species may have varieties, such as Iceland Spar, tufa, or travertine of the mineral calcite
Mineral Nomenclature Other comments • Individual minerals are known as species, such as forsterite or fayalite of the olivine series • A species may have varieties, such as Iceland Spar, tufa, or travertine of the mineral calcite
 A hierarchical schema for mineral subdivisions Mineral class Mineral subclass Mineral family Mineral supergroup Mineral group(s) Mineral subgroup or mineral series
A hierarchical schema for mineral subdivisions Mineral class Mineral subclass Mineral family Mineral supergroup Mineral group(s) Mineral subgroup or mineral series
 Mineral subclasses apply to the borate and silicate classes, where the configuration and bonding of tetrahedra are used to group structurally similar minerals. The subclasses are: neso-, soro-, cyclo-, ino-, phyllo-and tectosilicate (silicates). Traditionally the borates are divided into monoborates, diborates, triborates, tetraborates etc. (e. g. Strunz & Nickel, 2001), however, enough structural data is known to base classification of borates on the polymerisation of the borate anion.
Mineral subclasses apply to the borate and silicate classes, where the configuration and bonding of tetrahedra are used to group structurally similar minerals. The subclasses are: neso-, soro-, cyclo-, ino-, phyllo-and tectosilicate (silicates). Traditionally the borates are divided into monoborates, diborates, triborates, tetraborates etc. (e. g. Strunz & Nickel, 2001), however, enough structural data is known to base classification of borates on the polymerisation of the borate anion.
 Mineral family Mineral families apply to groups and/or supergroups having similar structural and/or chemical features that make them unique. A mineral family can also consist of two or more supergroups. An example of a mineral family established on the basis of structural criteria is the zeolite family, where all members are characterised by their framework structures containing cavities, but individual minerals themselves may also belong to different groups (and supergroups). The feldspathoid family also belongs to this type of ‘structural’ family. Other families are defined on the basis of chemical features, as for example the pyrite–marcasite family (which would consist of the pyrite and marcasite supergroups).
Mineral family Mineral families apply to groups and/or supergroups having similar structural and/or chemical features that make them unique. A mineral family can also consist of two or more supergroups. An example of a mineral family established on the basis of structural criteria is the zeolite family, where all members are characterised by their framework structures containing cavities, but individual minerals themselves may also belong to different groups (and supergroups). The feldspathoid family also belongs to this type of ‘structural’ family. Other families are defined on the basis of chemical features, as for example the pyrite–marcasite family (which would consist of the pyrite and marcasite supergroups).
 Mineral supergroup A mineral supergroup consists of two or more groups which have essentially the same structure and composed of chemically similar elements. Generally, a supergroup will contain members from the same mineral class (e. g. the epidote supergroup), however in rare cases a supergroup may also contain groups belonging to different classes, as for example in the alunite supergroup. A supergroup may also contain isolated mineral species which do not belong to any mineral group, as for example vanadinite, which is the only vanadate in the apatite supergroup.
Mineral supergroup A mineral supergroup consists of two or more groups which have essentially the same structure and composed of chemically similar elements. Generally, a supergroup will contain members from the same mineral class (e. g. the epidote supergroup), however in rare cases a supergroup may also contain groups belonging to different classes, as for example in the alunite supergroup. A supergroup may also contain isolated mineral species which do not belong to any mineral group, as for example vanadinite, which is the only vanadate in the apatite supergroup.
 Mineral group A mineral group consists of two or more minerals with the same or essentially the same structure, and composed of chemically similar elements.
Mineral group A mineral group consists of two or more minerals with the same or essentially the same structure, and composed of chemically similar elements.
 Mineral subgroup or mineral series A mineral subgroup or mineral series should be used for minerals of a homologous series (e. g. , the lillianite and pavonite series and other sulphosalt series, Moëlo et al. , 2008) or polysomatic series (e. g. , biopyriboles and heterophyllosilicates, Ferraris et al. , 2008), where they do not meet the strict definition of a mineral group.
Mineral subgroup or mineral series A mineral subgroup or mineral series should be used for minerals of a homologous series (e. g. , the lillianite and pavonite series and other sulphosalt series, Moëlo et al. , 2008) or polysomatic series (e. g. , biopyriboles and heterophyllosilicates, Ferraris et al. , 2008), where they do not meet the strict definition of a mineral group.
 Mineral group definition Compositional aspects of a mineral group “Chemically similar elements” is taken to mean elements that have similar crystal-chemical behaviour. Thus, isoconfigurational minerals composed of elements with dissimilar crystal-chemical behaviour, such as galena, periclase and halite, are not to be regarded as belonging to the same group. Unoccupied structural sites are to be treated in the same way as chemical elements for the purpose of group placement.
Mineral group definition Compositional aspects of a mineral group “Chemically similar elements” is taken to mean elements that have similar crystal-chemical behaviour. Thus, isoconfigurational minerals composed of elements with dissimilar crystal-chemical behaviour, such as galena, periclase and halite, are not to be regarded as belonging to the same group. Unoccupied structural sites are to be treated in the same way as chemical elements for the purpose of group placement.
 Structural aspects of a mineral group (1) The expression “the same structure” means isotypic structures, i. e. , structures belonging to one structural type. Crystal structures regarded as being ‘essentially the same’ can be encompassed by the term ‘homeotypic’. As defined by the IUCr, “two structures are considered as homeotypic if all essential features of topology are preserved between them” (Lima-de-Faria et al. , 1990). In particular, homeotypic structures do not necessarily have the same space group. Therefore crystallographic variants such as superstructures, substructures and differences in the ordering of atoms that may give rise to multiple cells and/or different space groups, are considered to be homeotypic (e. g. , as in the recently defined labuntsovite (Chukanov et al. , 2002) and eudialyte (Johnsen et al. , 2003) groups). Some polymorphs, such as triclinic and monoclinic feldspars, can be regarded as homeotypic and can therefore be included in a group; others, such as the carbon polymorphs diamond and graphite, are topologically too dissimilar (i. e. , they are not homeotypic) and should not belong to the same group.
Structural aspects of a mineral group (1) The expression “the same structure” means isotypic structures, i. e. , structures belonging to one structural type. Crystal structures regarded as being ‘essentially the same’ can be encompassed by the term ‘homeotypic’. As defined by the IUCr, “two structures are considered as homeotypic if all essential features of topology are preserved between them” (Lima-de-Faria et al. , 1990). In particular, homeotypic structures do not necessarily have the same space group. Therefore crystallographic variants such as superstructures, substructures and differences in the ordering of atoms that may give rise to multiple cells and/or different space groups, are considered to be homeotypic (e. g. , as in the recently defined labuntsovite (Chukanov et al. , 2002) and eudialyte (Johnsen et al. , 2003) groups). Some polymorphs, such as triclinic and monoclinic feldspars, can be regarded as homeotypic and can therefore be included in a group; others, such as the carbon polymorphs diamond and graphite, are topologically too dissimilar (i. e. , they are not homeotypic) and should not belong to the same group.
 Structural aspects of a mineral group (2) Homologous series (e. g. , lillianite and pavonite series), polysomatic series (e. g. , biopyriboles, heterophyllosilicates) and other structural categories that comprise modular structures (Ferraris et al. , 2008) go beyond the strict definition of a homeotype, and therefore are not to be regarded as groups. However, some mineral species in these categories may belong to groups if they meet the necessary criteria. Polytypic variations within mineral species, as defined in Guinier et al. (1984), are not regarded as comprising groups.
Structural aspects of a mineral group (2) Homologous series (e. g. , lillianite and pavonite series), polysomatic series (e. g. , biopyriboles, heterophyllosilicates) and other structural categories that comprise modular structures (Ferraris et al. , 2008) go beyond the strict definition of a homeotype, and therefore are not to be regarded as groups. However, some mineral species in these categories may belong to groups if they meet the necessary criteria. Polytypic variations within mineral species, as defined in Guinier et al. (1984), are not regarded as comprising groups.


