5d9c202d4245c71cc9d52c5a2ef953b2.ppt
- Количество слайдов: 30
 Millikan’s Oil Drop Experiment Dr. Frank Walmsley
Millikan’s Oil Drop Experiment Dr. Frank Walmsley
 Historical Setting Early 1900 s Rutherford had reported his alpha scattering experiments. Structure of atom known but not accepted by everyone. Charge on an alpha particle known. Experiments with cathode rays known including fact that rays are negatively charged.
Historical Setting Early 1900 s Rutherford had reported his alpha scattering experiments. Structure of atom known but not accepted by everyone. Charge on an alpha particle known. Experiments with cathode rays known including fact that rays are negatively charged.
 Historical Setting Needed to know charge on an electron. Millikan modified experiments tried by others and did very careful work. Used nonvolatile oil rather than volatile water. Corrected equations for rate of fall of drop – determined this experimentally
Historical Setting Needed to know charge on an electron. Millikan modified experiments tried by others and did very careful work. Used nonvolatile oil rather than volatile water. Corrected equations for rate of fall of drop – determined this experimentally
 Historical Setting Robert Millikan Received Nobel Prize for this work Work done at University of Chicago Became first president of Californa Institute of Technology
Historical Setting Robert Millikan Received Nobel Prize for this work Work done at University of Chicago Became first president of Californa Institute of Technology
 Electron Charge Note that the charge on the electron is NOT -1. It is one unit of negative charge. Current value: 1. 602 176 487 x 10 -19 C Millikan’s value: 1. 5924 x 10 -19 C
Electron Charge Note that the charge on the electron is NOT -1. It is one unit of negative charge. Current value: 1. 602 176 487 x 10 -19 C Millikan’s value: 1. 5924 x 10 -19 C
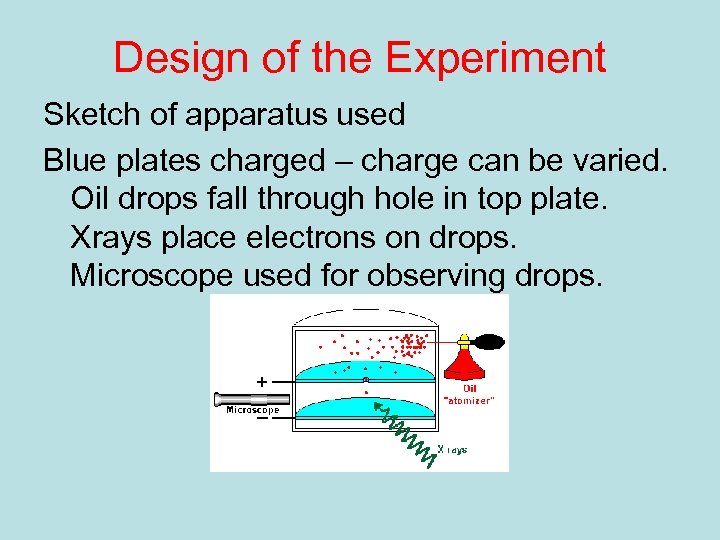 Design of the Experiment Sketch of apparatus used Blue plates charged – charge can be varied. Oil drops fall through hole in top plate. Xrays place electrons on drops. Microscope used for observing drops.
Design of the Experiment Sketch of apparatus used Blue plates charged – charge can be varied. Oil drops fall through hole in top plate. Xrays place electrons on drops. Microscope used for observing drops.
 Design of the Experiment Photo of actual apparatus used in the experiments.
Design of the Experiment Photo of actual apparatus used in the experiments.
 Design of the Experiments Time of fall of oil drop in the absence of field measured between two fixed points. Drop falls due to gravity. Electric field needed to hold drop steady between the two plates measured. When drop is suspended, force due to gravity equals force due to electrical attraction to + plate.
Design of the Experiments Time of fall of oil drop in the absence of field measured between two fixed points. Drop falls due to gravity. Electric field needed to hold drop steady between the two plates measured. When drop is suspended, force due to gravity equals force due to electrical attraction to + plate.
 Design of the Experiment Information needed Density of air Viscosity of air Density of oil Equation for rate of fall Strength of electric field Temperature Air Pressure
Design of the Experiment Information needed Density of air Viscosity of air Density of oil Equation for rate of fall Strength of electric field Temperature Air Pressure
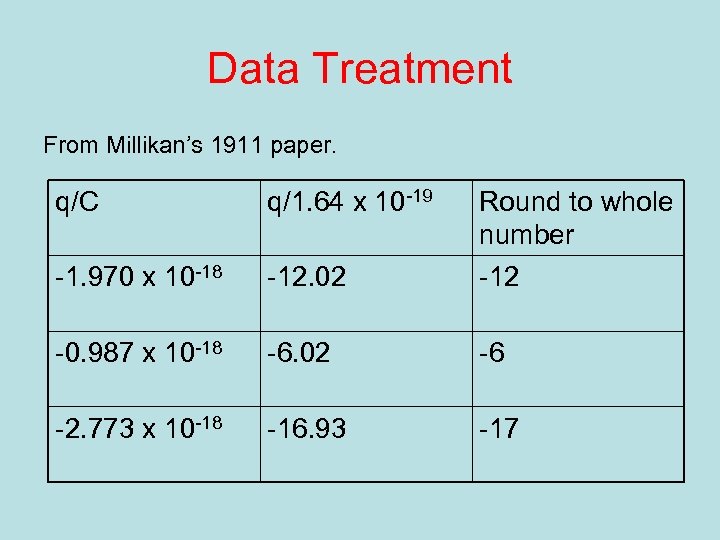 Data Treatment From Millikan’s 1911 paper. q/C q/1. 64 x 10 -19 Round to whole number -1. 970 x 10 -18 -12. 02 -12 -0. 987 x 10 -18 -6. 02 -6 -2. 773 x 10 -18 -16. 93 -17
Data Treatment From Millikan’s 1911 paper. q/C q/1. 64 x 10 -19 Round to whole number -1. 970 x 10 -18 -12. 02 -12 -0. 987 x 10 -18 -6. 02 -6 -2. 773 x 10 -18 -16. 93 -17
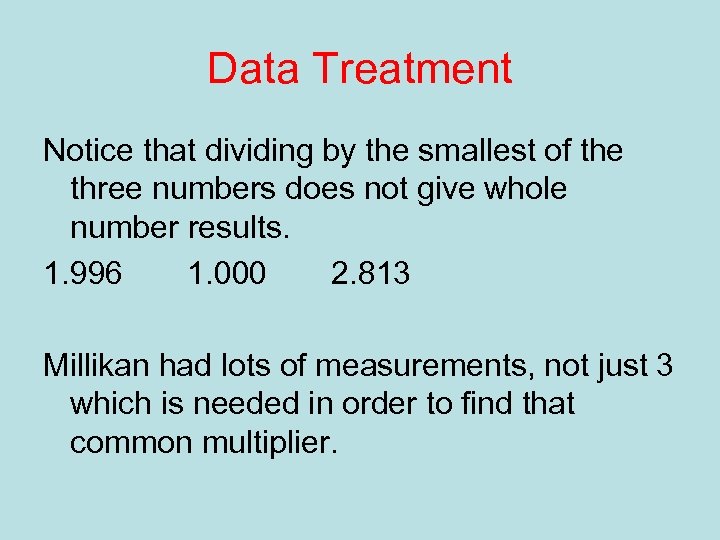 Data Treatment Notice that dividing by the smallest of the three numbers does not give whole number results. 1. 996 1. 000 2. 813 Millikan had lots of measurements, not just 3 which is needed in order to find that common multiplier.
Data Treatment Notice that dividing by the smallest of the three numbers does not give whole number results. 1. 996 1. 000 2. 813 Millikan had lots of measurements, not just 3 which is needed in order to find that common multiplier.
 Data Treatment Millikan did not know how many electrons had been added to the drop being measured. How do you find the lowest common multiplier? Let’s illustrate with 3 x 5 file cards in envelopes not knowing how many cards are in each envelope.
Data Treatment Millikan did not know how many electrons had been added to the drop being measured. How do you find the lowest common multiplier? Let’s illustrate with 3 x 5 file cards in envelopes not knowing how many cards are in each envelope.
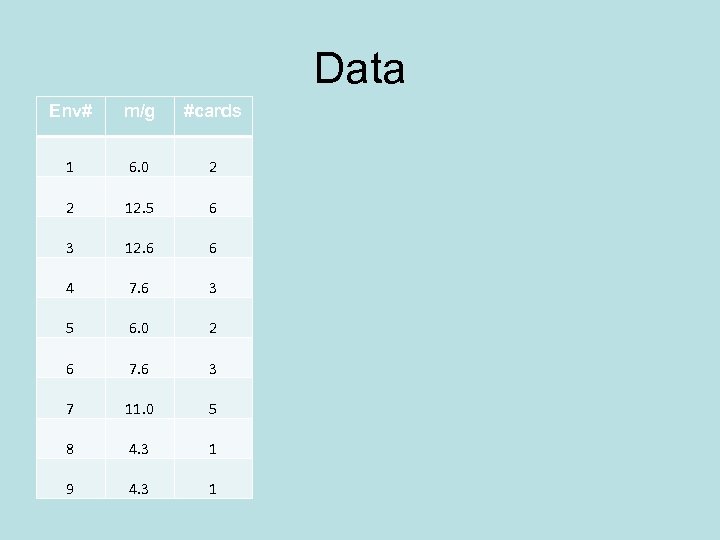 Data Env# m/g #cards 1 6. 0 2 2 12. 5 6 3 12. 6 6 4 7. 6 3 5 6. 0 2 6 7. 6 3 7 11. 0 5 8 4. 3 1 9 4. 3 1
Data Env# m/g #cards 1 6. 0 2 2 12. 5 6 3 12. 6 6 4 7. 6 3 5 6. 0 2 6 7. 6 3 7 11. 0 5 8 4. 3 1 9 4. 3 1
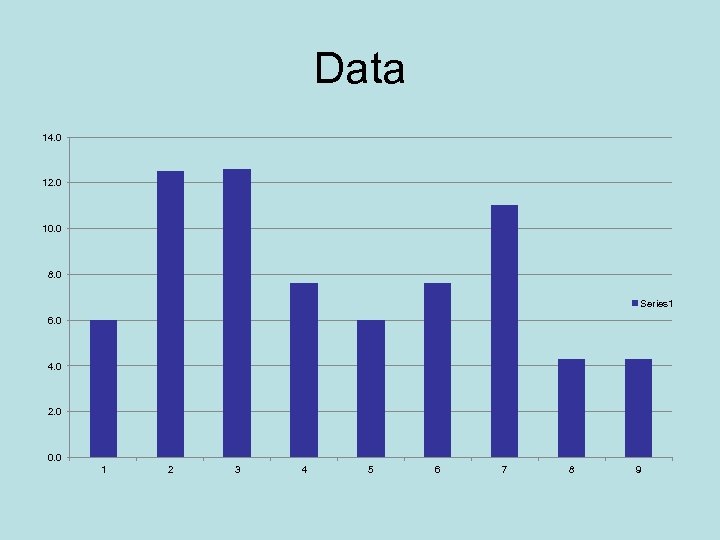 Data 14. 0 12. 0 10. 0 8. 0 Series 1 6. 0 4. 0 2. 0 0. 0 1 2 3 4 5 6 7 8 9
Data 14. 0 12. 0 10. 0 8. 0 Series 1 6. 0 4. 0 2. 0 0. 0 1 2 3 4 5 6 7 8 9
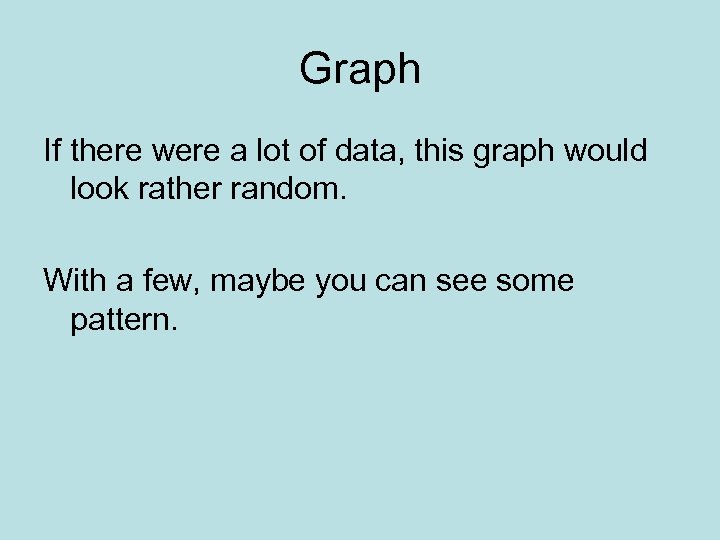 Graph If there were a lot of data, this graph would look rather random. With a few, maybe you can see some pattern.
Graph If there were a lot of data, this graph would look rather random. With a few, maybe you can see some pattern.
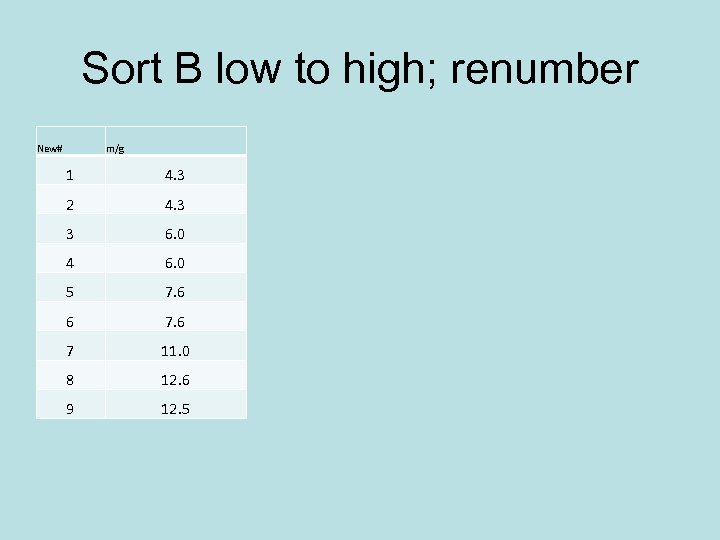 Sort B low to high; renumber New# m/g 1 4. 3 2 4. 3 3 6. 0 4 6. 0 5 7. 6 6 7 11. 0 8 12. 6 9 12. 5
Sort B low to high; renumber New# m/g 1 4. 3 2 4. 3 3 6. 0 4 6. 0 5 7. 6 6 7 11. 0 8 12. 6 9 12. 5
 Data 14. 0 12. 0 10. 0 8. 0 Series 1 6. 0 4. 0 2. 0 0. 0 1 2 3 4 5 6 7 8 9
Data 14. 0 12. 0 10. 0 8. 0 Series 1 6. 0 4. 0 2. 0 0. 0 1 2 3 4 5 6 7 8 9
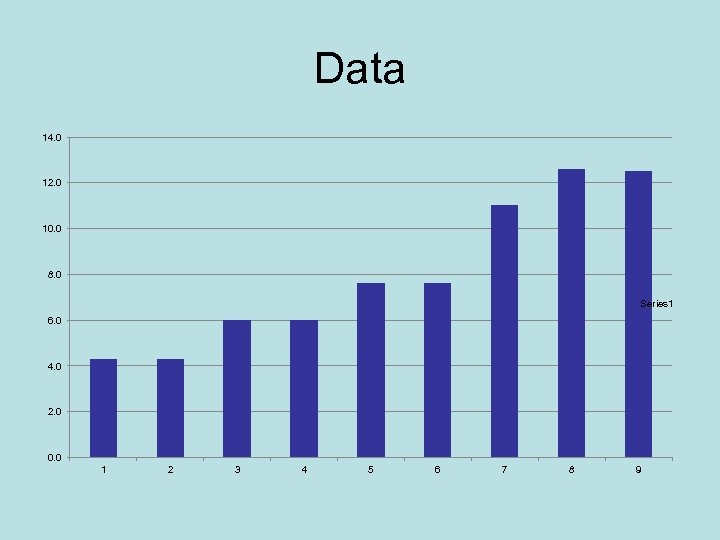
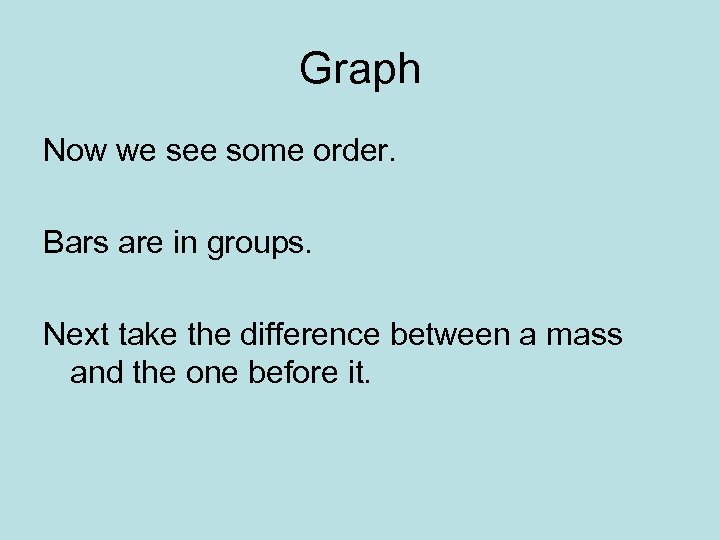 Graph Now we see some order. Bars are in groups. Next take the difference between a mass and the one before it.
Graph Now we see some order. Bars are in groups. Next take the difference between a mass and the one before it.
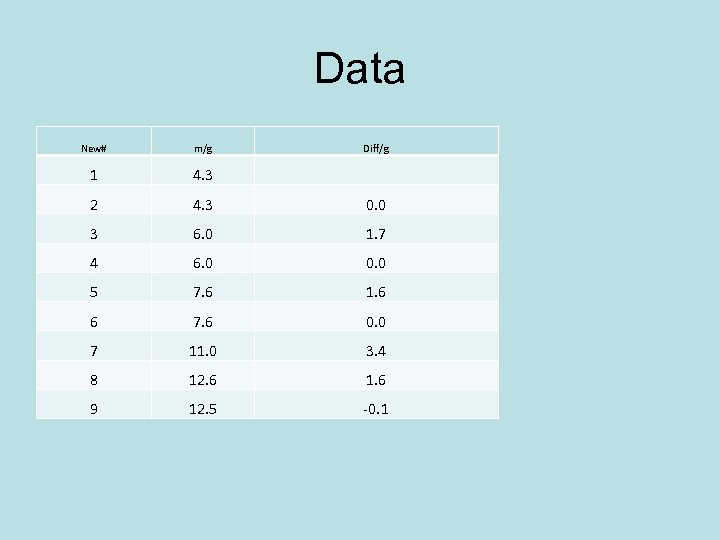 Data New# m/g Diff/g 1 4. 3 2 4. 3 0. 0 3 6. 0 1. 7 4 6. 0 0. 0 5 7. 6 1. 6 6 7. 6 0. 0 7 11. 0 3. 4 8 12. 6 1. 6 9 12. 5 -0. 1
Data New# m/g Diff/g 1 4. 3 2 4. 3 0. 0 3 6. 0 1. 7 4 6. 0 0. 0 5 7. 6 1. 6 6 7. 6 0. 0 7 11. 0 3. 4 8 12. 6 1. 6 9 12. 5 -0. 1
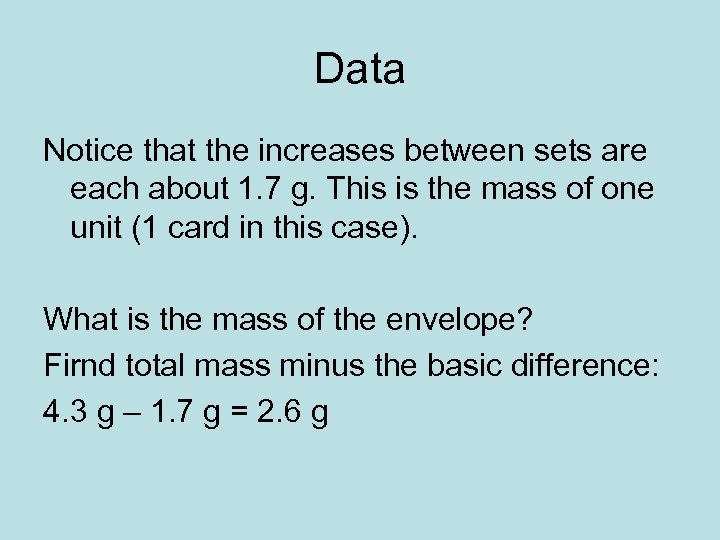 Data Notice that the increases between sets are each about 1. 7 g. This is the mass of one unit (1 card in this case). What is the mass of the envelope? Firnd total mass minus the basic difference: 4. 3 g – 1. 7 g = 2. 6 g
Data Notice that the increases between sets are each about 1. 7 g. This is the mass of one unit (1 card in this case). What is the mass of the envelope? Firnd total mass minus the basic difference: 4. 3 g – 1. 7 g = 2. 6 g
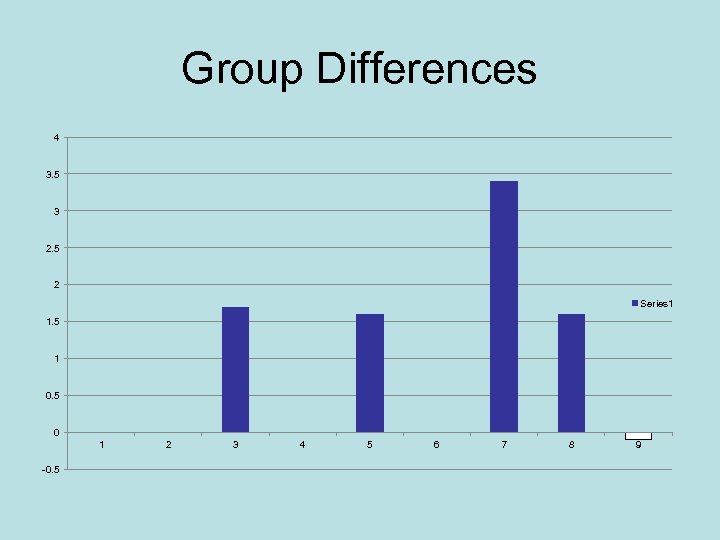 Group Differences 4 3. 5 3 2. 5 2 Series 1 1. 5 1 0. 5 0 1 -0. 5 2 3 4 5 6 7 8 9
Group Differences 4 3. 5 3 2. 5 2 Series 1 1. 5 1 0. 5 0 1 -0. 5 2 3 4 5 6 7 8 9
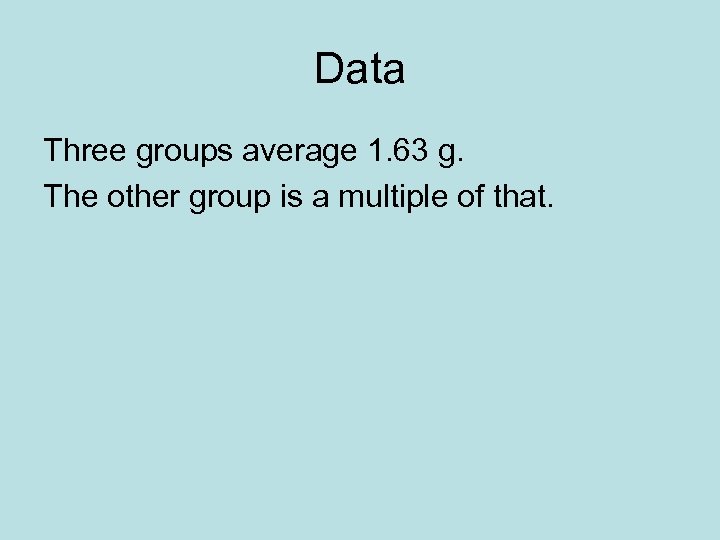 Data Three groups average 1. 63 g. The other group is a multiple of that.
Data Three groups average 1. 63 g. The other group is a multiple of that.
 Millikan did not have MSExcel and had lots of points. His notebooks are available on the web if you want to try to find how he did it.
Millikan did not have MSExcel and had lots of points. His notebooks are available on the web if you want to try to find how he did it.
 Experimental Results Showed that the charges were multiples of the minimum basic charge of one electron. Determined the value of that charge. This led to more accurate values for other constants such as Avogadro’s Number.
Experimental Results Showed that the charges were multiples of the minimum basic charge of one electron. Determined the value of that charge. This led to more accurate values for other constants such as Avogadro’s Number.
 Experimental Results A possibility: Millikan showed that all electrons had a -1 charge – no fractional charges. Except: he did have ONE measurement with a -1/3 charge which is the charge on a quark.
Experimental Results A possibility: Millikan showed that all electrons had a -1 charge – no fractional charges. Except: he did have ONE measurement with a -1/3 charge which is the charge on a quark.
 Experimental Results If he really did, then he does not get credit because experiments must be reproducible, not only by the original experimenter but, by others
Experimental Results If he really did, then he does not get credit because experiments must be reproducible, not only by the original experimenter but, by others
 . Other experiments done in the same manner (and other methods) have not revealed any more fractional charges on electrons. In 2004, tens of millions of measurements using Millikan’s method in an automated system failed to find any fractional charges.
. Other experiments done in the same manner (and other methods) have not revealed any more fractional charges on electrons. In 2004, tens of millions of measurements using Millikan’s method in an automated system failed to find any fractional charges.
 Was Millikan Dishonest? Millikan did not use all his data points in his published articles. Millikan said in his articles that he did use all points. How can both be true?
Was Millikan Dishonest? Millikan did not use all his data points in his published articles. Millikan said in his articles that he did use all points. How can both be true?
 Was Millikan Dishonest? It is true that Millikan selectively chose points to report. He discarded drops that were too small because they were too affected by bombardment from air molecules. He discarded drops that were too large because they did not obey the equations for rate of fall. He discarded drops that had only one measurement – that is, did not have additional measurements with fewer or greater numbers of electrons.
Was Millikan Dishonest? It is true that Millikan selectively chose points to report. He discarded drops that were too small because they were too affected by bombardment from air molecules. He discarded drops that were too large because they did not obey the equations for rate of fall. He discarded drops that had only one measurement – that is, did not have additional measurements with fewer or greater numbers of electrons.
 Was Millikan Dishonest? It appears that what he meant (and did not say clearly) was: He used all drops that met his criteria for being reliable. If he had used all drops, the results would have been essentially the same.
Was Millikan Dishonest? It appears that what he meant (and did not say clearly) was: He used all drops that met his criteria for being reliable. If he had used all drops, the results would have been essentially the same.


