da79e32f50b0f3637b6c8e4fc3b3a2c6.ppt
- Количество слайдов: 59
 Migraine & Chronic Daily Headache Vincent T. Martin, MD, FACP, FAHS Co-director, Headache & Facial Pain Clinic Professor of Medicine Department of Internal Medicine University of Cincinnati
Migraine & Chronic Daily Headache Vincent T. Martin, MD, FACP, FAHS Co-director, Headache & Facial Pain Clinic Professor of Medicine Department of Internal Medicine University of Cincinnati
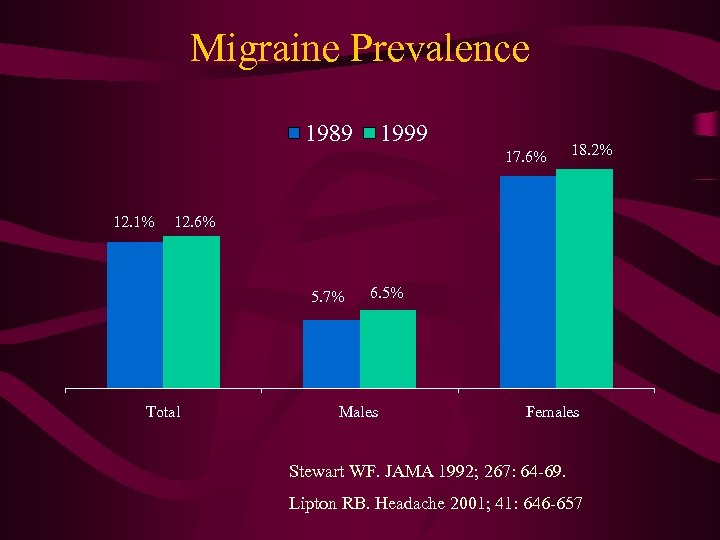 Migraine Prevalence 1989 1999 17. 6% 12. 1% 18. 2% 12. 6% 5. 7% Total 6. 5% Males Females Stewart WF. JAMA 1992; 267: 64 -69. Lipton RB. Headache 2001; 41: 646 -657
Migraine Prevalence 1989 1999 17. 6% 12. 1% 18. 2% 12. 6% 5. 7% Total 6. 5% Males Females Stewart WF. JAMA 1992; 267: 64 -69. Lipton RB. Headache 2001; 41: 646 -657
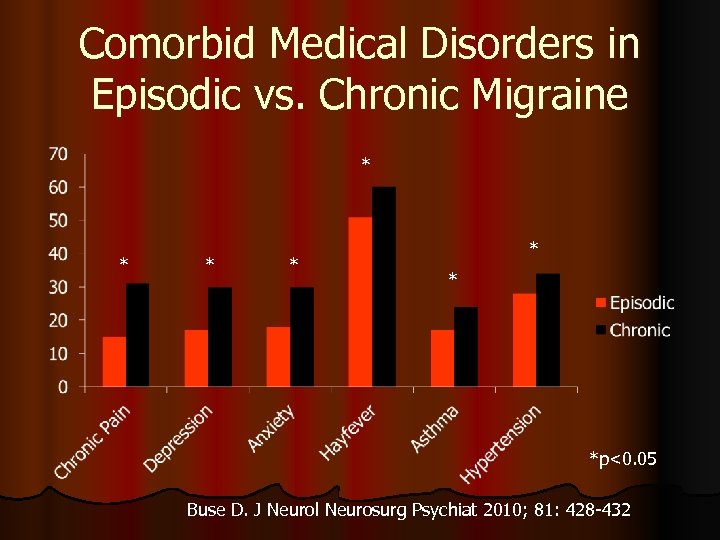 Comorbid Medical Disorders in Episodic vs. Chronic Migraine * * * *p<0. 05 Buse D. J Neurol Neurosurg Psychiat 2010; 81: 428 -432
Comorbid Medical Disorders in Episodic vs. Chronic Migraine * * * *p<0. 05 Buse D. J Neurol Neurosurg Psychiat 2010; 81: 428 -432
 Migraine Diagnosis
Migraine Diagnosis
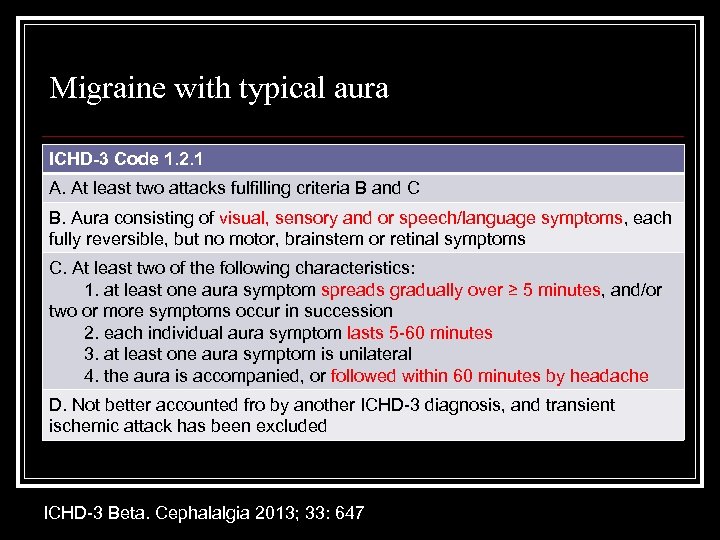 Migraine with typical aura ICHD-3 Code 1. 2. 1 A. At least two attacks fulfilling criteria B and C B. Aura consisting of visual, sensory and or speech/language symptoms, each fully reversible, but no motor, brainstem or retinal symptoms C. At least two of the following characteristics: 1. at least one aura symptom spreads gradually over ≥ 5 minutes, and/or two or more symptoms occur in succession 2. each individual aura symptom lasts 5 -60 minutes 3. at least one aura symptom is unilateral 4. the aura is accompanied, or followed within 60 minutes by headache D. Not better accounted fro by another ICHD-3 diagnosis, and transient ischemic attack has been excluded ICHD-3 Beta. Cephalalgia 2013; 33: 647
Migraine with typical aura ICHD-3 Code 1. 2. 1 A. At least two attacks fulfilling criteria B and C B. Aura consisting of visual, sensory and or speech/language symptoms, each fully reversible, but no motor, brainstem or retinal symptoms C. At least two of the following characteristics: 1. at least one aura symptom spreads gradually over ≥ 5 minutes, and/or two or more symptoms occur in succession 2. each individual aura symptom lasts 5 -60 minutes 3. at least one aura symptom is unilateral 4. the aura is accompanied, or followed within 60 minutes by headache D. Not better accounted fro by another ICHD-3 diagnosis, and transient ischemic attack has been excluded ICHD-3 Beta. Cephalalgia 2013; 33: 647
 Other Auras 1. 2. 1. 1 Typical aura without headache • No headache follows the aura within 60 minutes 1. 22 Migraine with brainstem aura (≥ 2 symptoms) • Dysarthria • Vertigo • Hypacusis • Diplopia • Ataxia • Decreased Level of Consciousness ICHD-3 Beta. Cephalalgia 2013; 33: 647 -650 1. 2. 3 Hemiplegic Migraine • Fully reversible motor weakness (and) • Fully reversible visual, sensory and/or speech language symptoms 1. 2. 4 Retinal migraine • Reversible monocular scintillations, scotoma or blindness
Other Auras 1. 2. 1. 1 Typical aura without headache • No headache follows the aura within 60 minutes 1. 22 Migraine with brainstem aura (≥ 2 symptoms) • Dysarthria • Vertigo • Hypacusis • Diplopia • Ataxia • Decreased Level of Consciousness ICHD-3 Beta. Cephalalgia 2013; 33: 647 -650 1. 2. 3 Hemiplegic Migraine • Fully reversible motor weakness (and) • Fully reversible visual, sensory and/or speech language symptoms 1. 2. 4 Retinal migraine • Reversible monocular scintillations, scotoma or blindness
 Migraine without aura ICHD-3 Code 1. 1 A. At least five attacks fulfilling criteria B-D B. Headache attacks lasting 4 -72 hours (untreated or unsuccessfully treated) C. Headache has at least two of the following four characteristics: 1. unilateral location 2. pulsating quality 3. moderate to severe pain intensity 4. aggravation by or causing avoidance of routine physical activity (walking or climbing stairs) D. During the headache at least one of the following: 1. nausea and/or vomiting 2. photophobia and phonophobia (both) E. Not better accounted for by another ICHD-3 diagnosis ICHD-3 Beta. Cephalalgia 2013; 33: 644
Migraine without aura ICHD-3 Code 1. 1 A. At least five attacks fulfilling criteria B-D B. Headache attacks lasting 4 -72 hours (untreated or unsuccessfully treated) C. Headache has at least two of the following four characteristics: 1. unilateral location 2. pulsating quality 3. moderate to severe pain intensity 4. aggravation by or causing avoidance of routine physical activity (walking or climbing stairs) D. During the headache at least one of the following: 1. nausea and/or vomiting 2. photophobia and phonophobia (both) E. Not better accounted for by another ICHD-3 diagnosis ICHD-3 Beta. Cephalalgia 2013; 33: 644
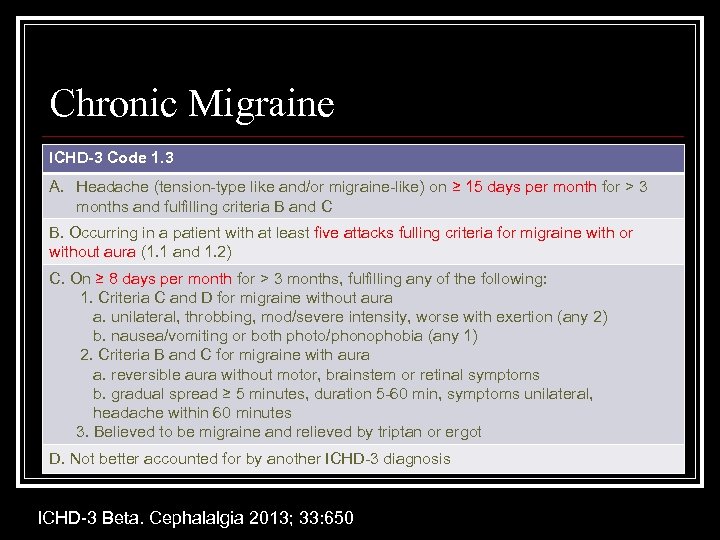 Chronic Migraine ICHD-3 Code 1. 3 A. Headache (tension-type like and/or migraine-like) on ≥ 15 days per month for > 3 months and fulfilling criteria B and C B. Occurring in a patient with at least five attacks fulling criteria for migraine with or without aura (1. 1 and 1. 2) C. On ≥ 8 days per month for > 3 months, fulfilling any of the following: 1. Criteria C and D for migraine without aura a. unilateral, throbbing, mod/severe intensity, worse with exertion (any 2) b. nausea/vomiting or both photo/phonophobia (any 1) 2. Criteria B and C for migraine with aura a. reversible aura without motor, brainstem or retinal symptoms b. gradual spread ≥ 5 minutes, duration 5 -60 min, symptoms unilateral, headache within 60 minutes 3. Believed to be migraine and relieved by triptan or ergot D. Not better accounted for by another ICHD-3 diagnosis ICHD-3 Beta. Cephalalgia 2013; 33: 650
Chronic Migraine ICHD-3 Code 1. 3 A. Headache (tension-type like and/or migraine-like) on ≥ 15 days per month for > 3 months and fulfilling criteria B and C B. Occurring in a patient with at least five attacks fulling criteria for migraine with or without aura (1. 1 and 1. 2) C. On ≥ 8 days per month for > 3 months, fulfilling any of the following: 1. Criteria C and D for migraine without aura a. unilateral, throbbing, mod/severe intensity, worse with exertion (any 2) b. nausea/vomiting or both photo/phonophobia (any 1) 2. Criteria B and C for migraine with aura a. reversible aura without motor, brainstem or retinal symptoms b. gradual spread ≥ 5 minutes, duration 5 -60 min, symptoms unilateral, headache within 60 minutes 3. Believed to be migraine and relieved by triptan or ergot D. Not better accounted for by another ICHD-3 diagnosis ICHD-3 Beta. Cephalalgia 2013; 33: 650
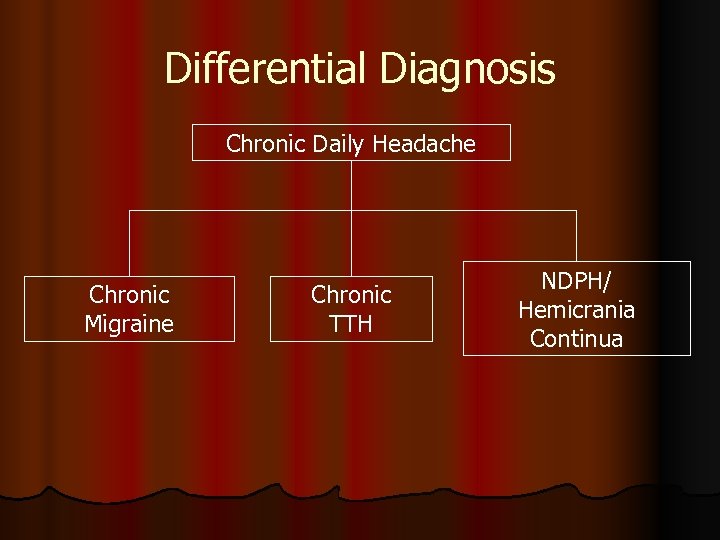 Differential Diagnosis Chronic Daily Headache Chronic Migraine Chronic TTH NDPH/ Hemicrania Continua
Differential Diagnosis Chronic Daily Headache Chronic Migraine Chronic TTH NDPH/ Hemicrania Continua
 Migraine Transformation
Migraine Transformation
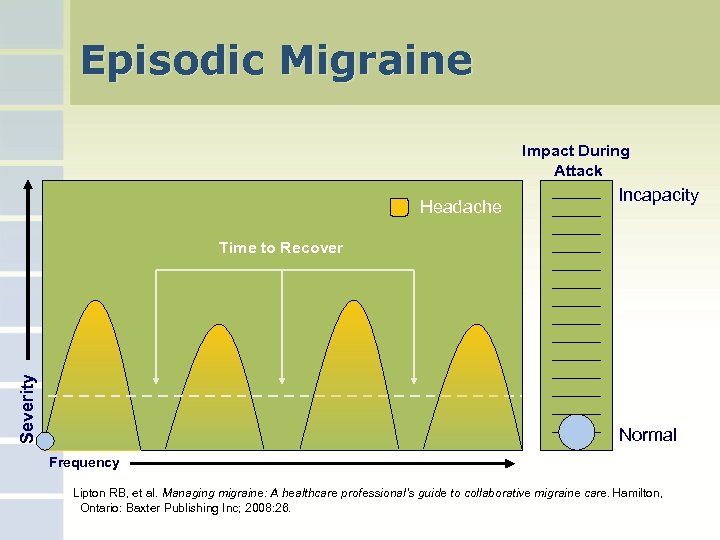 Episodic Migraine Impact During Attack Headache Incapacity Severity Time to Recover Normal Frequency Lipton RB, et al. Managing migraine: A healthcare professional’s guide to collaborative migraine care. Hamilton, Ontario: Baxter Publishing Inc; 2008: 26.
Episodic Migraine Impact During Attack Headache Incapacity Severity Time to Recover Normal Frequency Lipton RB, et al. Managing migraine: A healthcare professional’s guide to collaborative migraine care. Hamilton, Ontario: Baxter Publishing Inc; 2008: 26.
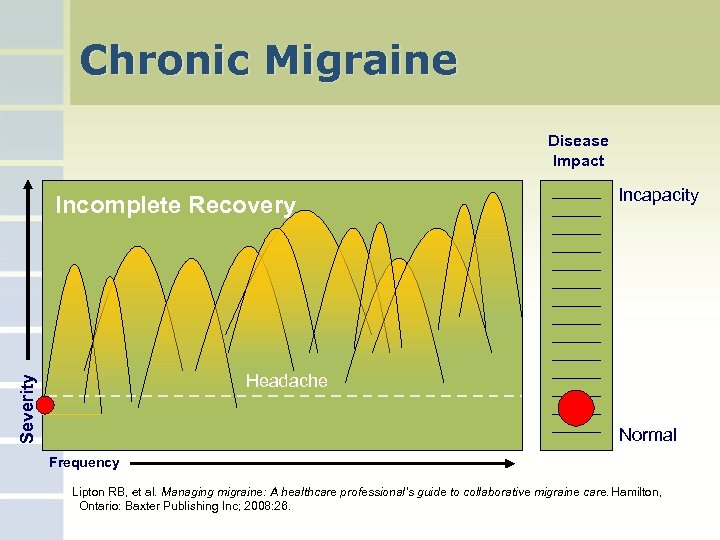 Chronic Migraine Disease Impact Incomplete Recovery Incapacity Severity Headache Normal Frequency Lipton RB, et al. Managing migraine: A healthcare professional’s guide to collaborative migraine care. Hamilton, Ontario: Baxter Publishing Inc; 2008: 26.
Chronic Migraine Disease Impact Incomplete Recovery Incapacity Severity Headache Normal Frequency Lipton RB, et al. Managing migraine: A healthcare professional’s guide to collaborative migraine care. Hamilton, Ontario: Baxter Publishing Inc; 2008: 26.
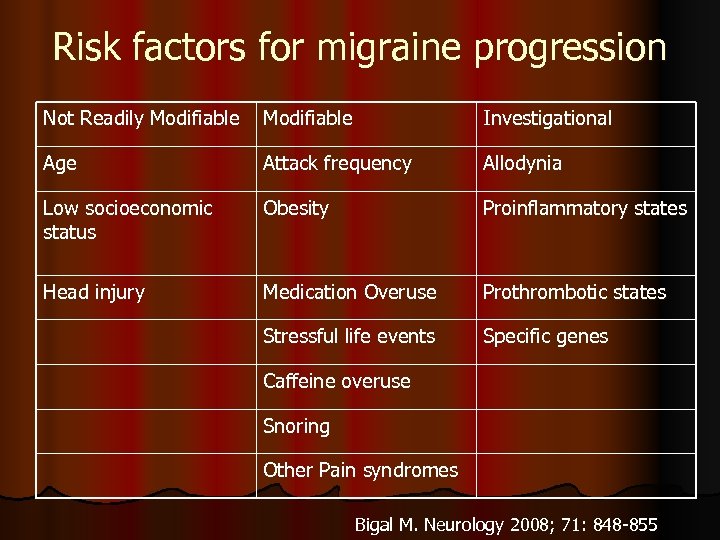 Risk factors for migraine progression Not Readily Modifiable Investigational Age Attack frequency Allodynia Low socioeconomic status Obesity Proinflammatory states Head injury Medication Overuse Prothrombotic states Stressful life events Specific genes Caffeine overuse Snoring Other Pain syndromes Bigal M. Neurology 2008; 71: 848 -855
Risk factors for migraine progression Not Readily Modifiable Investigational Age Attack frequency Allodynia Low socioeconomic status Obesity Proinflammatory states Head injury Medication Overuse Prothrombotic states Stressful life events Specific genes Caffeine overuse Snoring Other Pain syndromes Bigal M. Neurology 2008; 71: 848 -855
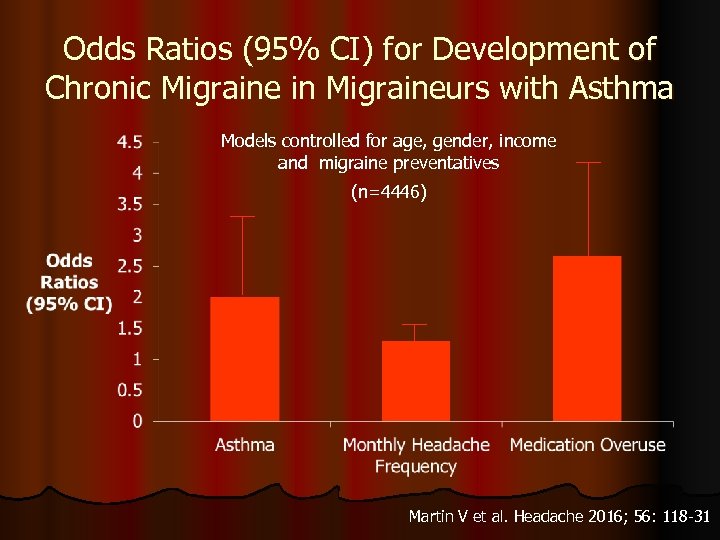 Odds Ratios (95% CI) for Development of Chronic Migraine in Migraineurs with Asthma Models controlled for age, gender, income and migraine preventatives (n=4446) Martin V et al. Headache 2016; 56: 118 -31
Odds Ratios (95% CI) for Development of Chronic Migraine in Migraineurs with Asthma Models controlled for age, gender, income and migraine preventatives (n=4446) Martin V et al. Headache 2016; 56: 118 -31
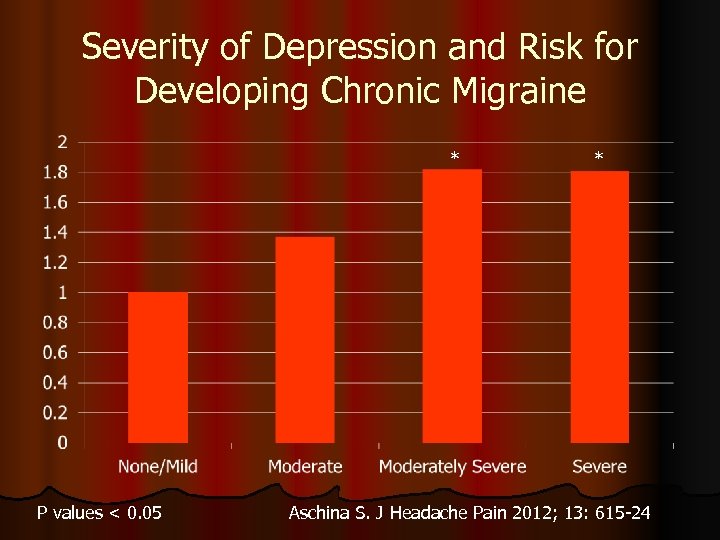 Severity of Depression and Risk for Developing Chronic Migraine * P values < 0. 05 * Aschina S. J Headache Pain 2012; 13: 615 -24
Severity of Depression and Risk for Developing Chronic Migraine * P values < 0. 05 * Aschina S. J Headache Pain 2012; 13: 615 -24
 Migraine Genetics
Migraine Genetics
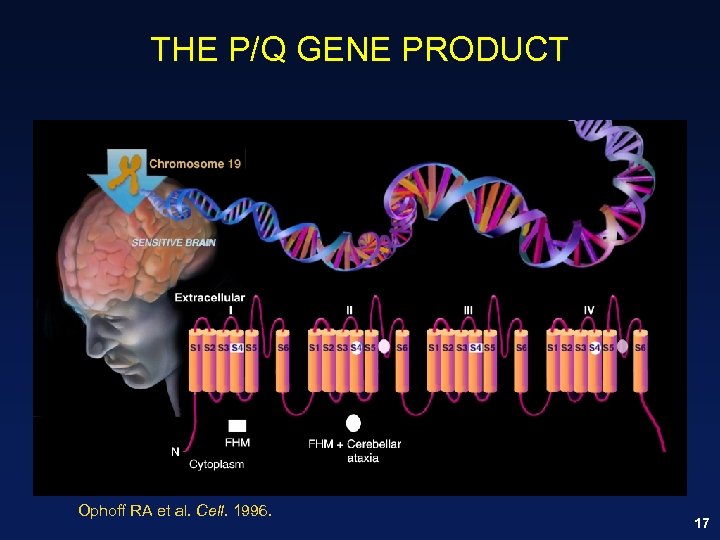 THE P/Q GENE PRODUCT Ophoff RA et al. Cell. 1996. 17
THE P/Q GENE PRODUCT Ophoff RA et al. Cell. 1996. 17
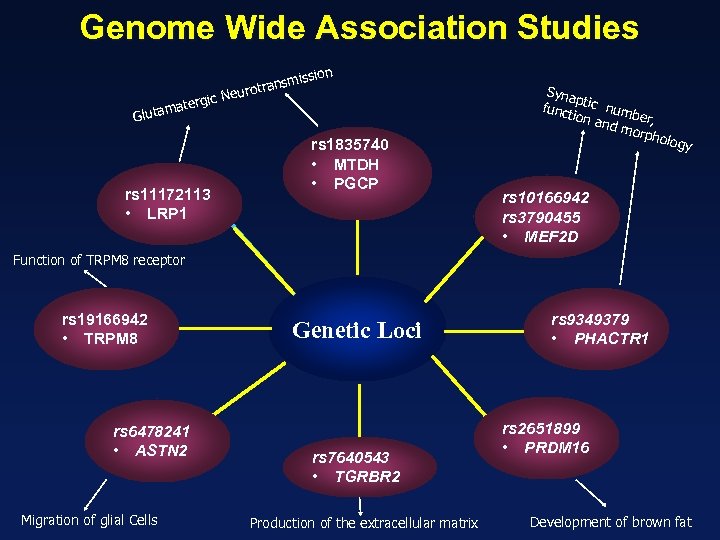 Genome Wide Association Studies ission ransm eurot rgic N mate Gluta rs 11172113 • LRP 1 rs 1835740 • MTDH • PGCP Syna p funct tic numb ion a e nd m r, orph olog y rs 10166942 rs 3790455 • MEF 2 D Function of TRPM 8 receptor rs 19166942 • TRPM 8 rs 6478241 • ASTN 2 Migration of glial Cells Genetic Loci rs 7640543 • TGRBR 2 Production of the extracellular matrix rs 9349379 • PHACTR 1 rs 2651899 • PRDM 16 Development of brown fat
Genome Wide Association Studies ission ransm eurot rgic N mate Gluta rs 11172113 • LRP 1 rs 1835740 • MTDH • PGCP Syna p funct tic numb ion a e nd m r, orph olog y rs 10166942 rs 3790455 • MEF 2 D Function of TRPM 8 receptor rs 19166942 • TRPM 8 rs 6478241 • ASTN 2 Migration of glial Cells Genetic Loci rs 7640543 • TGRBR 2 Production of the extracellular matrix rs 9349379 • PHACTR 1 rs 2651899 • PRDM 16 Development of brown fat
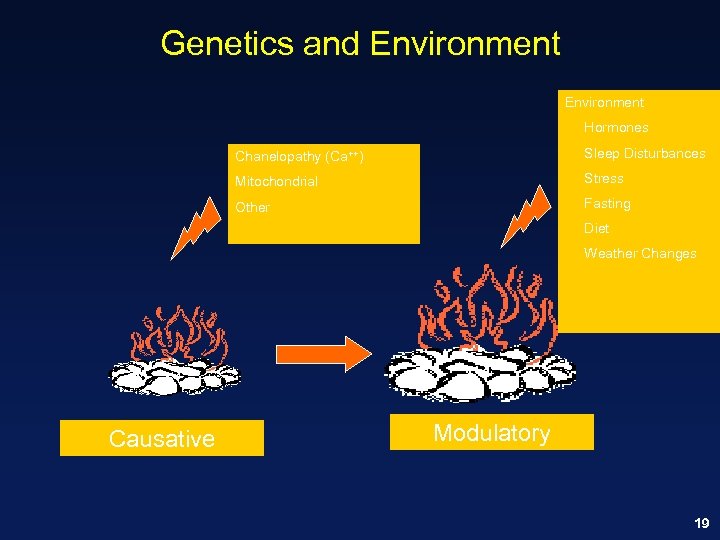 Genetics and Environment Hormones Chanelopathy (Ca++) Sleep Disturbances Mitochondrial Stress Other Fasting Diet Weather Changes Causative Modulatory 19
Genetics and Environment Hormones Chanelopathy (Ca++) Sleep Disturbances Mitochondrial Stress Other Fasting Diet Weather Changes Causative Modulatory 19
 Migraine Pathophysiology
Migraine Pathophysiology
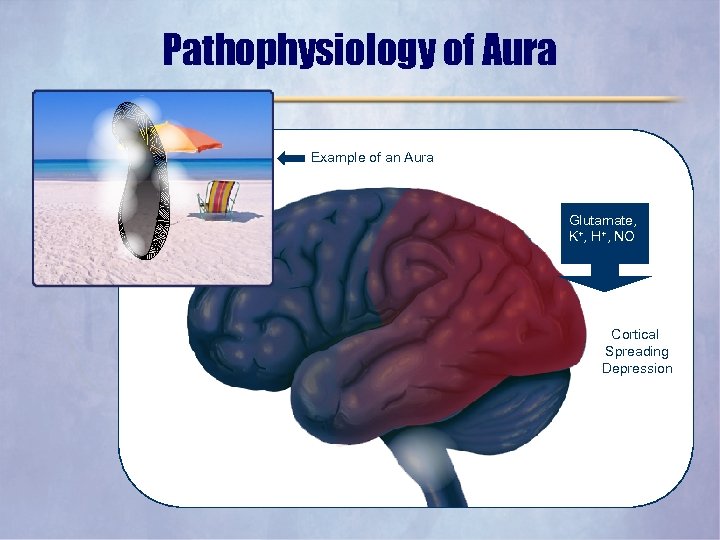 Pathophysiology of Aura Example of an Aura Glutamate, K+, H+, NO Cortical Spreading Depression
Pathophysiology of Aura Example of an Aura Glutamate, K+, H+, NO Cortical Spreading Depression
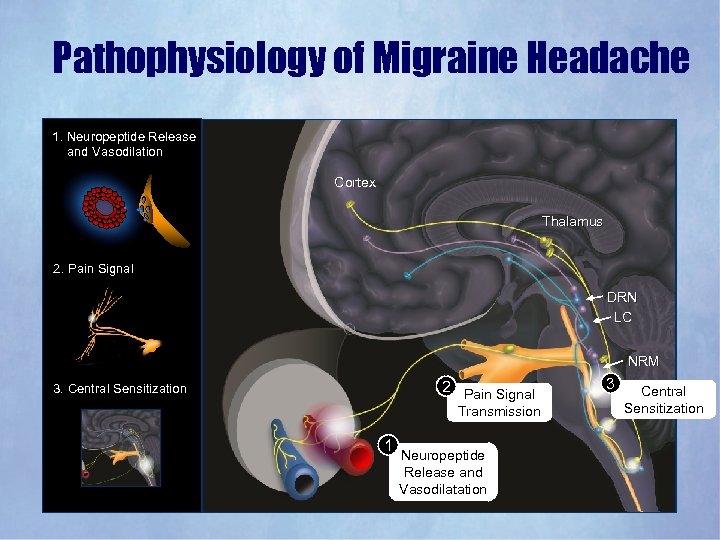 Pathophysiology of Migraine Headache 1. Neuropeptide Release and Vasodilation Cortex Thalamus 2. Pain Signal DRN LC NRM 2 Pain Signal 3. Central Sensitization Transmission 1 Neuropeptide Release and Vasodilatation 3 Central Sensitization
Pathophysiology of Migraine Headache 1. Neuropeptide Release and Vasodilation Cortex Thalamus 2. Pain Signal DRN LC NRM 2 Pain Signal 3. Central Sensitization Transmission 1 Neuropeptide Release and Vasodilatation 3 Central Sensitization
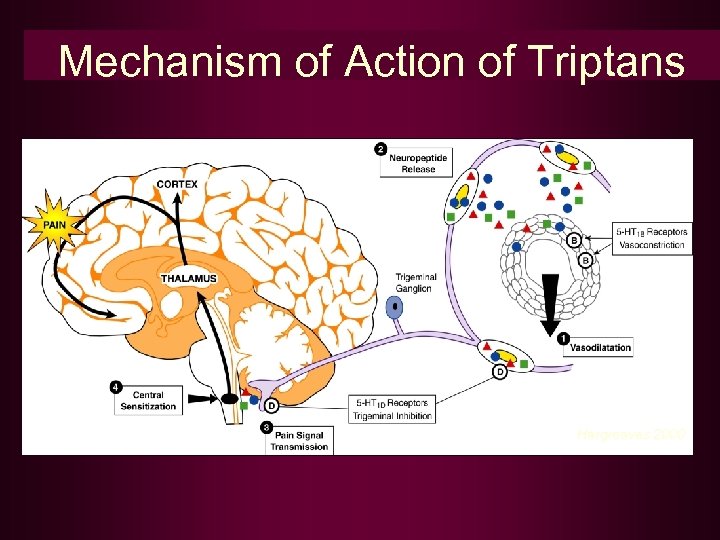 Mechanism of Action of Triptans Hargreaves 2000
Mechanism of Action of Triptans Hargreaves 2000
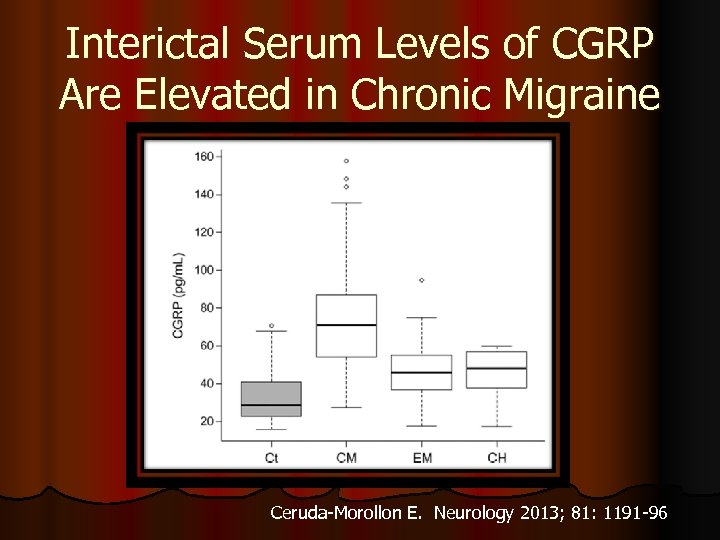 Interictal Serum Levels of CGRP Are Elevated in Chronic Migraine Ceruda-Morollon E. Neurology 2013; 81: 1191 -96
Interictal Serum Levels of CGRP Are Elevated in Chronic Migraine Ceruda-Morollon E. Neurology 2013; 81: 1191 -96
 Abortive Treatment of Migraine
Abortive Treatment of Migraine
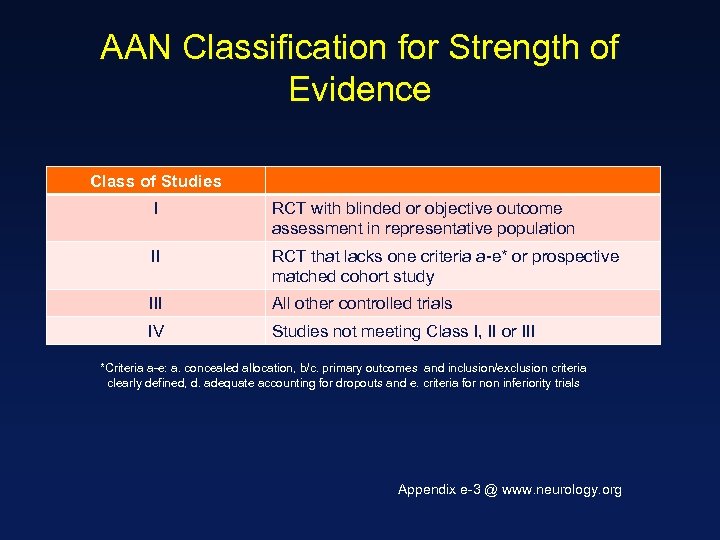 AAN Classification for Strength of Evidence Class of Studies I RCT with blinded or objective outcome assessment in representative population II RCT that lacks one criteria a-e* or prospective matched cohort study III All other controlled trials IV Studies not meeting Class I, II or III *Criteria a-e: a. concealed allocation, b/c. primary outcomes and inclusion/exclusion criteria clearly defined, d. adequate accounting for dropouts and e. criteria for non inferiority trials Appendix e-3 @ www. neurology. org
AAN Classification for Strength of Evidence Class of Studies I RCT with blinded or objective outcome assessment in representative population II RCT that lacks one criteria a-e* or prospective matched cohort study III All other controlled trials IV Studies not meeting Class I, II or III *Criteria a-e: a. concealed allocation, b/c. primary outcomes and inclusion/exclusion criteria clearly defined, d. adequate accounting for dropouts and e. criteria for non inferiority trials Appendix e-3 @ www. neurology. org
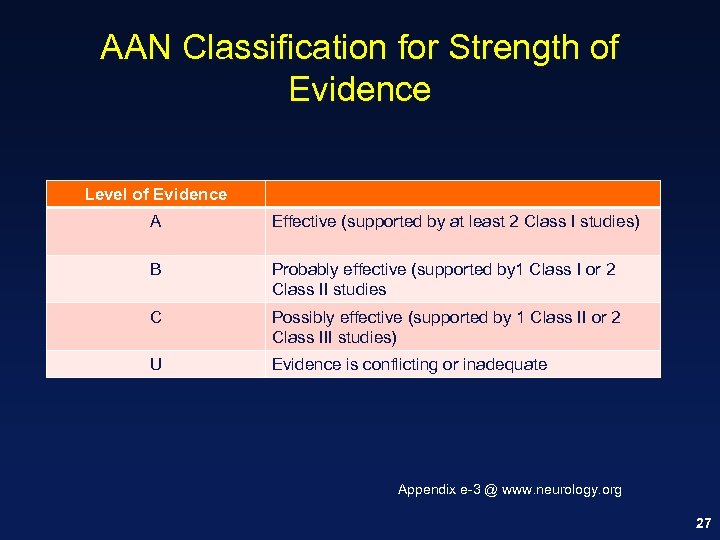 AAN Classification for Strength of Evidence Level of Evidence A Effective (supported by at least 2 Class I studies) B Probably effective (supported by 1 Class I or 2 Class II studies C Possibly effective (supported by 1 Class II or 2 Class III studies) U Evidence is conflicting or inadequate Appendix e-3 @ www. neurology. org 27
AAN Classification for Strength of Evidence Level of Evidence A Effective (supported by at least 2 Class I studies) B Probably effective (supported by 1 Class I or 2 Class II studies C Possibly effective (supported by 1 Class II or 2 Class III studies) U Evidence is conflicting or inadequate Appendix e-3 @ www. neurology. org 27
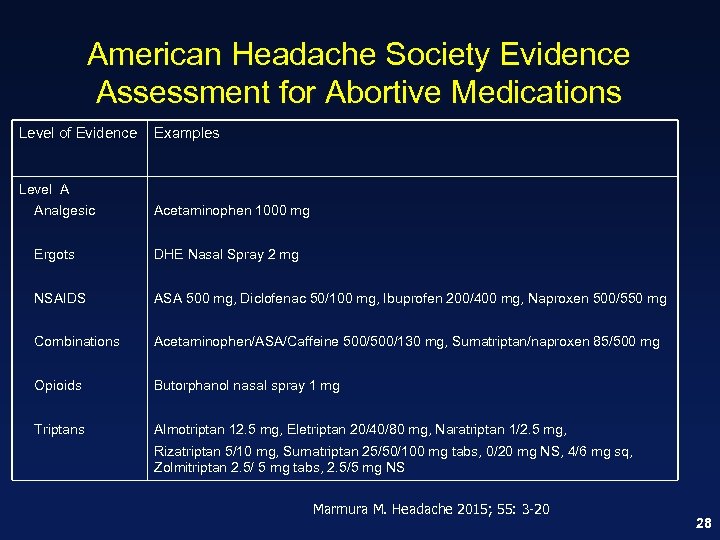 American Headache Society Evidence Assessment for Abortive Medications Level of Evidence Examples Level A Analgesic Acetaminophen 1000 mg Ergots DHE Nasal Spray 2 mg NSAIDS ASA 500 mg, Diclofenac 50/100 mg, Ibuprofen 200/400 mg, Naproxen 500/550 mg Combinations Acetaminophen/ASA/Caffeine 500/130 mg, Sumatriptan/naproxen 85/500 mg Opioids Butorphanol nasal spray 1 mg Triptans Almotriptan 12. 5 mg, Eletriptan 20/40/80 mg, Naratriptan 1/2. 5 mg, Rizatriptan 5/10 mg, Sumatriptan 25/50/100 mg tabs, 0/20 mg NS, 4/6 mg sq, Zolmitriptan 2. 5/ 5 mg tabs, 2. 5/5 mg NS Marmura M. Headache 2015; 55: 3 -20 28
American Headache Society Evidence Assessment for Abortive Medications Level of Evidence Examples Level A Analgesic Acetaminophen 1000 mg Ergots DHE Nasal Spray 2 mg NSAIDS ASA 500 mg, Diclofenac 50/100 mg, Ibuprofen 200/400 mg, Naproxen 500/550 mg Combinations Acetaminophen/ASA/Caffeine 500/130 mg, Sumatriptan/naproxen 85/500 mg Opioids Butorphanol nasal spray 1 mg Triptans Almotriptan 12. 5 mg, Eletriptan 20/40/80 mg, Naratriptan 1/2. 5 mg, Rizatriptan 5/10 mg, Sumatriptan 25/50/100 mg tabs, 0/20 mg NS, 4/6 mg sq, Zolmitriptan 2. 5/ 5 mg tabs, 2. 5/5 mg NS Marmura M. Headache 2015; 55: 3 -20 28
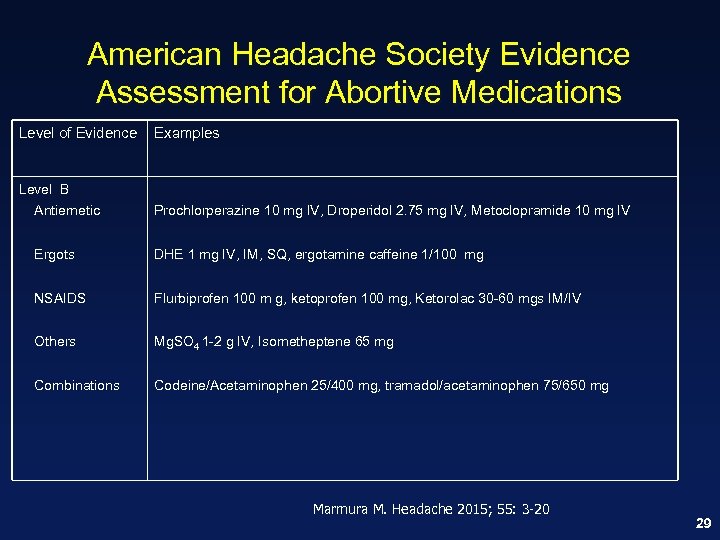 American Headache Society Evidence Assessment for Abortive Medications Level of Evidence Examples Level B Antiemetic Prochlorperazine 10 mg IV, Droperidol 2. 75 mg IV, Metoclopramide 10 mg IV Ergots DHE 1 mg IV, IM, SQ, ergotamine caffeine 1/100 mg NSAIDS Flurbiprofen 100 m g, ketoprofen 100 mg, Ketorolac 30 -60 mgs IM/IV Others Mg. SO 4 1 -2 g IV, Isometheptene 65 mg Combinations Codeine/Acetaminophen 25/400 mg, tramadol/acetaminophen 75/650 mg Marmura M. Headache 2015; 55: 3 -20 29
American Headache Society Evidence Assessment for Abortive Medications Level of Evidence Examples Level B Antiemetic Prochlorperazine 10 mg IV, Droperidol 2. 75 mg IV, Metoclopramide 10 mg IV Ergots DHE 1 mg IV, IM, SQ, ergotamine caffeine 1/100 mg NSAIDS Flurbiprofen 100 m g, ketoprofen 100 mg, Ketorolac 30 -60 mgs IM/IV Others Mg. SO 4 1 -2 g IV, Isometheptene 65 mg Combinations Codeine/Acetaminophen 25/400 mg, tramadol/acetaminophen 75/650 mg Marmura M. Headache 2015; 55: 3 -20 29
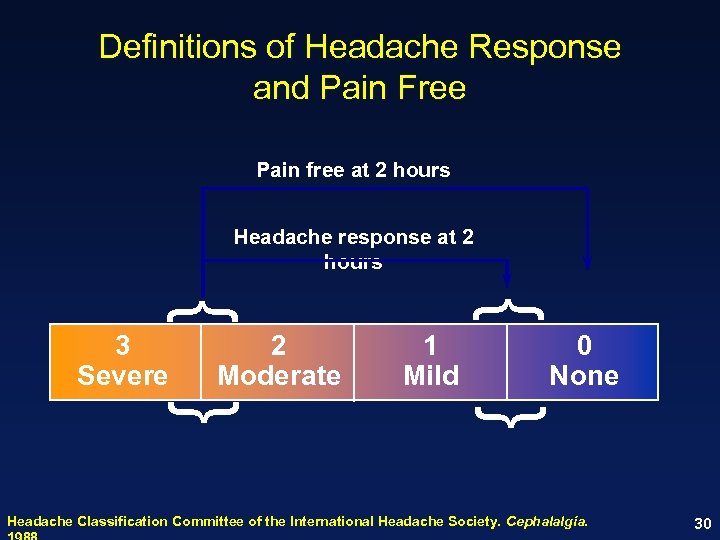 Definitions of Headache Response and Pain Free Pain free at 2 hours Headache response at 2 hours { { 1 Mild 0 None { 2 Moderate { 3 Severe Headache Classification Committee of the International Headache Society. Cephalalgia. 30
Definitions of Headache Response and Pain Free Pain free at 2 hours Headache response at 2 hours { { 1 Mild 0 None { 2 Moderate { 3 Severe Headache Classification Committee of the International Headache Society. Cephalalgia. 30
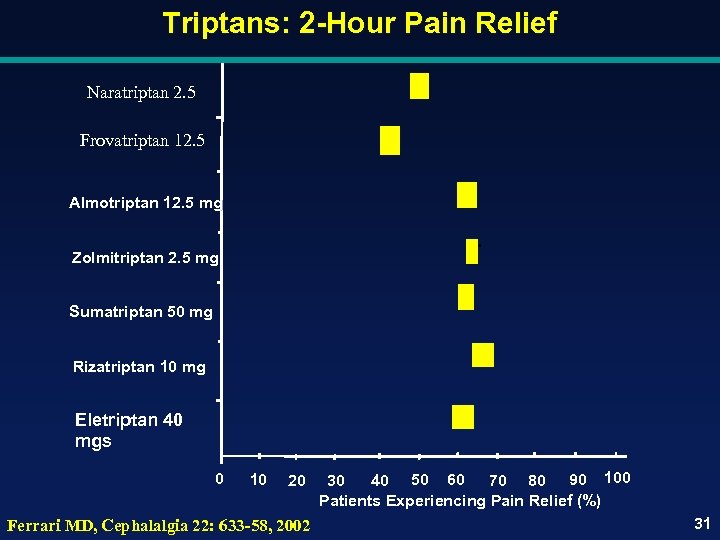 Triptans: 2 -Hour Pain Relief Naratriptan 2. 5 Frovatriptan 12. 5 Almotriptan 12. 5 mg Zolmitriptan 2. 5 mg Sumatriptan 50 mg Rizatriptan 10 mg Eletriptan 40 mgs 0 10 20 Ferrari MD, Cephalalgia 22: 633 -58, 2002 30 40 50 60 70 80 90 100 Patients Experiencing Pain Relief (%) 31
Triptans: 2 -Hour Pain Relief Naratriptan 2. 5 Frovatriptan 12. 5 Almotriptan 12. 5 mg Zolmitriptan 2. 5 mg Sumatriptan 50 mg Rizatriptan 10 mg Eletriptan 40 mgs 0 10 20 Ferrari MD, Cephalalgia 22: 633 -58, 2002 30 40 50 60 70 80 90 100 Patients Experiencing Pain Relief (%) 31
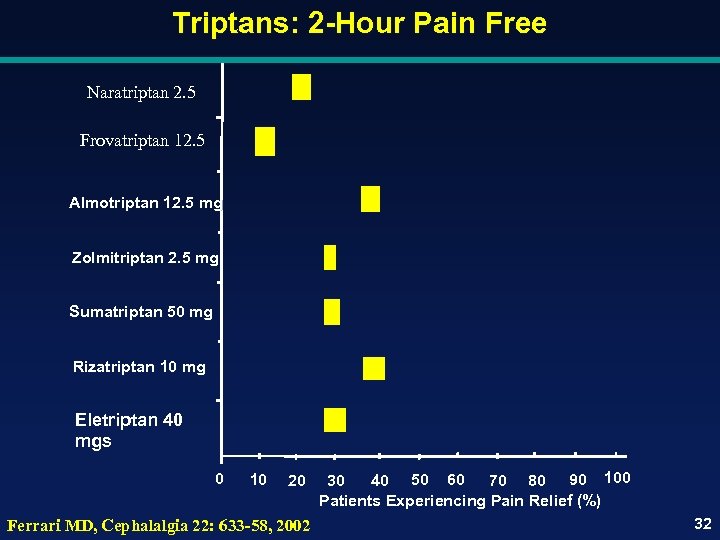 Triptans: 2 -Hour Pain Free Naratriptan 2. 5 Frovatriptan 12. 5 Almotriptan 12. 5 mg Zolmitriptan 2. 5 mg Sumatriptan 50 mg Rizatriptan 10 mg Eletriptan 40 mgs 0 10 20 Ferrari MD, Cephalalgia 22: 633 -58, 2002 30 40 50 60 70 80 90 100 Patients Experiencing Pain Relief (%) 32
Triptans: 2 -Hour Pain Free Naratriptan 2. 5 Frovatriptan 12. 5 Almotriptan 12. 5 mg Zolmitriptan 2. 5 mg Sumatriptan 50 mg Rizatriptan 10 mg Eletriptan 40 mgs 0 10 20 Ferrari MD, Cephalalgia 22: 633 -58, 2002 30 40 50 60 70 80 90 100 Patients Experiencing Pain Relief (%) 32
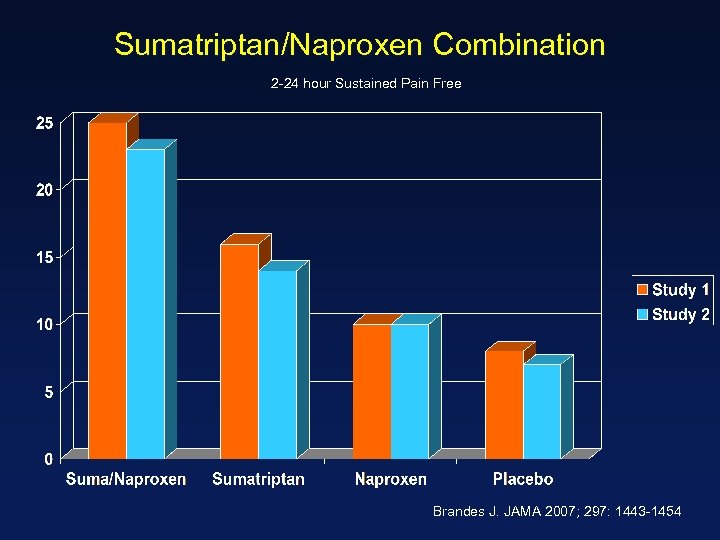 Sumatriptan/Naproxen Combination 2 -24 hour Sustained Pain Free Brandes J. JAMA 2007; 297: 1443 -1454
Sumatriptan/Naproxen Combination 2 -24 hour Sustained Pain Free Brandes J. JAMA 2007; 297: 1443 -1454
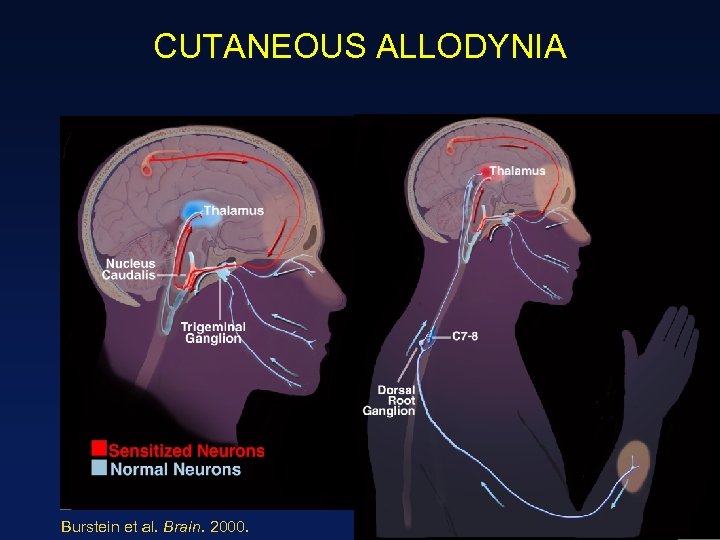 CUTANEOUS ALLODYNIA Burstein et al. Brain. 2000. 34
CUTANEOUS ALLODYNIA Burstein et al. Brain. 2000. 34
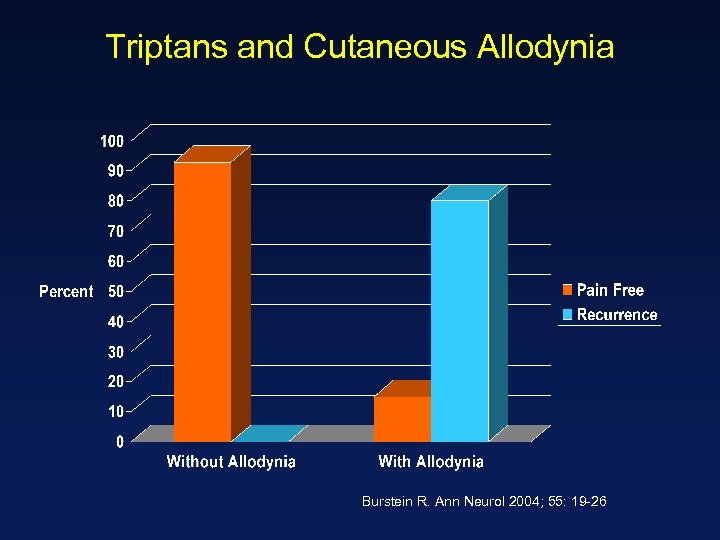 Triptans and Cutaneous Allodynia Burstein R. Ann Neurol 2004; 55: 19 -26
Triptans and Cutaneous Allodynia Burstein R. Ann Neurol 2004; 55: 19 -26
 Migraine Different Delivery Systems
Migraine Different Delivery Systems
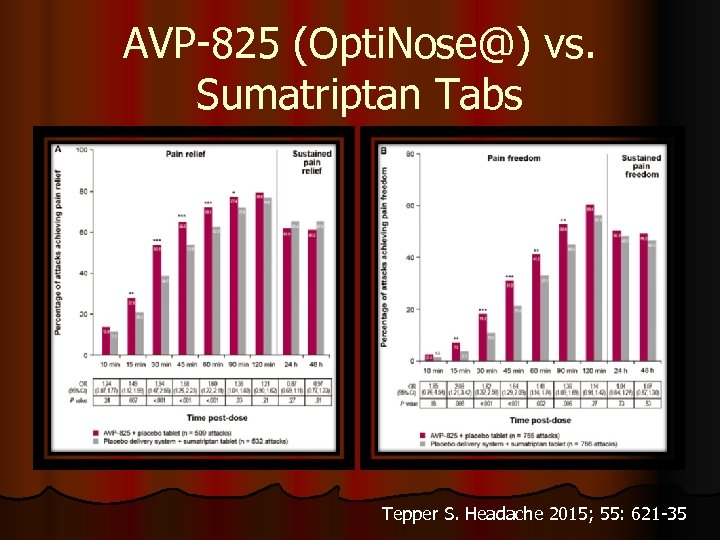 AVP-825 (Opti. Nose@) vs. Sumatriptan Tabs Tepper S. Headache 2015; 55: 621 -35
AVP-825 (Opti. Nose@) vs. Sumatriptan Tabs Tepper S. Headache 2015; 55: 621 -35
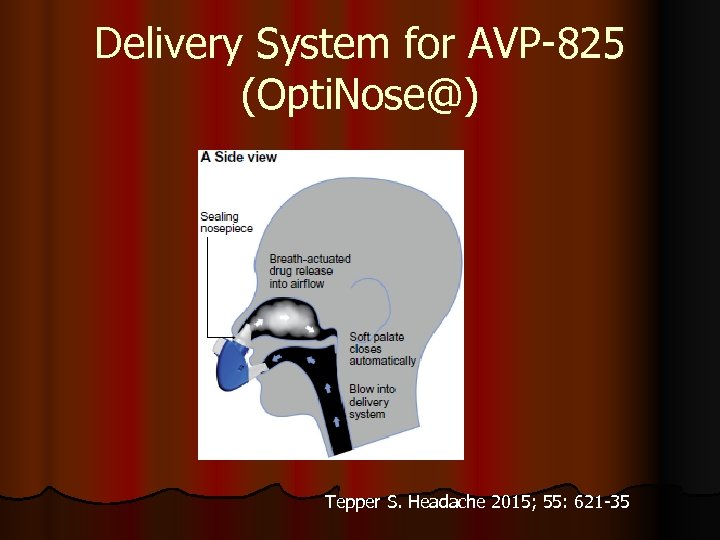 Delivery System for AVP-825 (Opti. Nose@) Tepper S. Headache 2015; 55: 621 -35
Delivery System for AVP-825 (Opti. Nose@) Tepper S. Headache 2015; 55: 621 -35
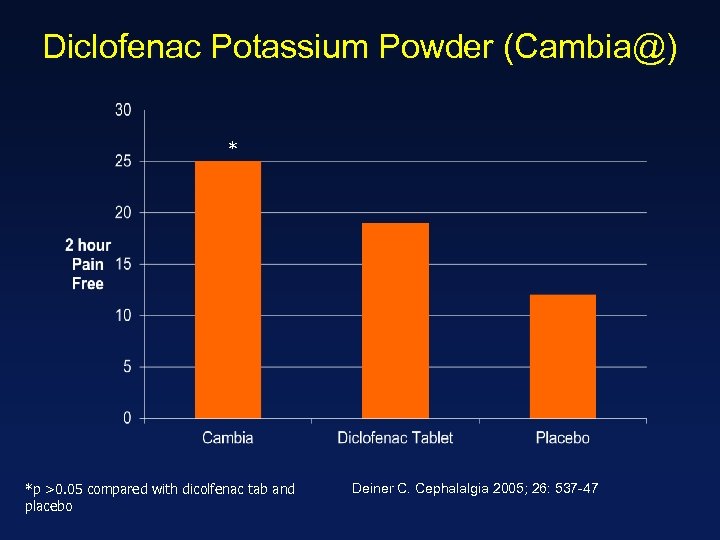 Diclofenac Potassium Powder (Cambia@) * *p >0. 05 compared with dicolfenac tab and placebo Deiner C. Cephalalgia 2005; 26: 537 -47
Diclofenac Potassium Powder (Cambia@) * *p >0. 05 compared with dicolfenac tab and placebo Deiner C. Cephalalgia 2005; 26: 537 -47
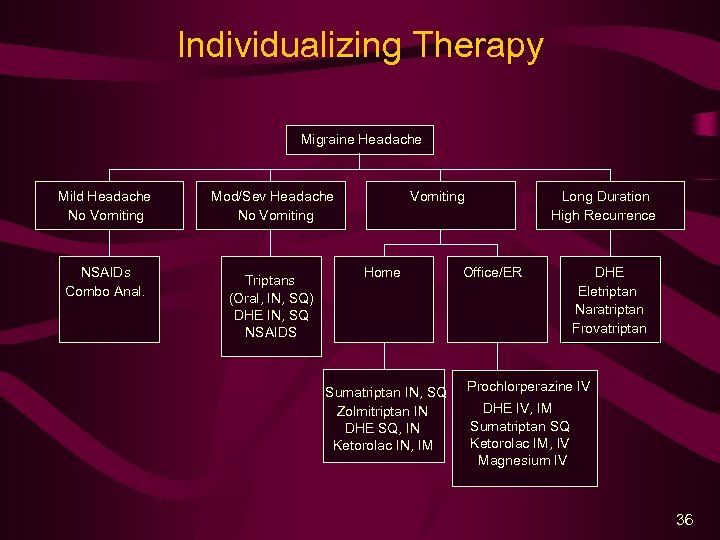 Individualizing Therapy Migraine Headache Mild Headache No Vomiting NSAIDs Combo Anal. Mod/Sev Headache No Vomiting Triptans (Oral, IN, SQ) DHE IN, SQ NSAIDS Vomiting Home Sumatriptan IN, SQ Zolmitriptan IN DHE SQ, IN Ketorolac IN, IM Long Duration High Recurrence Office/ER DHE Eletriptan Naratriptan Frovatriptan Prochlorperazine IV DHE IV, IM Sumatriptan SQ Ketorolac IM, IV Magnesium IV 36
Individualizing Therapy Migraine Headache Mild Headache No Vomiting NSAIDs Combo Anal. Mod/Sev Headache No Vomiting Triptans (Oral, IN, SQ) DHE IN, SQ NSAIDS Vomiting Home Sumatriptan IN, SQ Zolmitriptan IN DHE SQ, IN Ketorolac IN, IM Long Duration High Recurrence Office/ER DHE Eletriptan Naratriptan Frovatriptan Prochlorperazine IV DHE IV, IM Sumatriptan SQ Ketorolac IM, IV Magnesium IV 36
 Migraine Preventive Medications
Migraine Preventive Medications
 Classification of Migraine Preventatives Level of Evidence Examples Level A Antiepileptic Drugs Topiramate, Divalproex Sodium , Sodium Valproate Beta Blockers Propranolol, Metoprolol, Timolol Level B Antidepressants Amitriptyline, Venlafaxine Beta Blockers Atenolol, Nadolol Level C ACE Inhibitors Lisinopril ARBs Candesartan α-Agonists Clonidine, Guanfacine Antiepileptic Drugs Carbamazepine Beta-blockers Nebivolol Antihistamines Cyproheptadine Silberstein S. Neurology 2012; 78: 1337 -1345 42
Classification of Migraine Preventatives Level of Evidence Examples Level A Antiepileptic Drugs Topiramate, Divalproex Sodium , Sodium Valproate Beta Blockers Propranolol, Metoprolol, Timolol Level B Antidepressants Amitriptyline, Venlafaxine Beta Blockers Atenolol, Nadolol Level C ACE Inhibitors Lisinopril ARBs Candesartan α-Agonists Clonidine, Guanfacine Antiepileptic Drugs Carbamazepine Beta-blockers Nebivolol Antihistamines Cyproheptadine Silberstein S. Neurology 2012; 78: 1337 -1345 42
 Classification of Complimentary Therapies Level of Evidence Examples Level A Herbal Petasites Level B NSAIDS Fenoprofen, Ibuprofen, Ketoprofen, Naprosyn, Naproxen Soduim Herbal, Vitamins Magnesium, Feverfew, Riboflavin Anti-histamines Histamine SC Level C NSAIDS Flurbiprofen, Mefenamic Acid Herbal, Vitamins Co-Q 10 Hormonal Estrogen Holland S. Neurology 2012; 78: 1346 -1353 43
Classification of Complimentary Therapies Level of Evidence Examples Level A Herbal Petasites Level B NSAIDS Fenoprofen, Ibuprofen, Ketoprofen, Naprosyn, Naproxen Soduim Herbal, Vitamins Magnesium, Feverfew, Riboflavin Anti-histamines Histamine SC Level C NSAIDS Flurbiprofen, Mefenamic Acid Herbal, Vitamins Co-Q 10 Hormonal Estrogen Holland S. Neurology 2012; 78: 1346 -1353 43
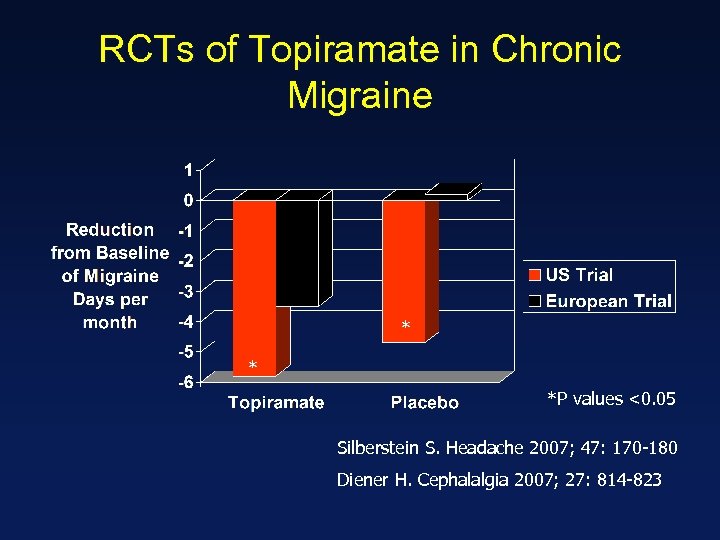 RCTs of Topiramate in Chronic Migraine * * *P values <0. 05 Silberstein S. Headache 2007; 47: 170 -180 Diener H. Cephalalgia 2007; 27: 814 -823
RCTs of Topiramate in Chronic Migraine * * *P values <0. 05 Silberstein S. Headache 2007; 47: 170 -180 Diener H. Cephalalgia 2007; 27: 814 -823
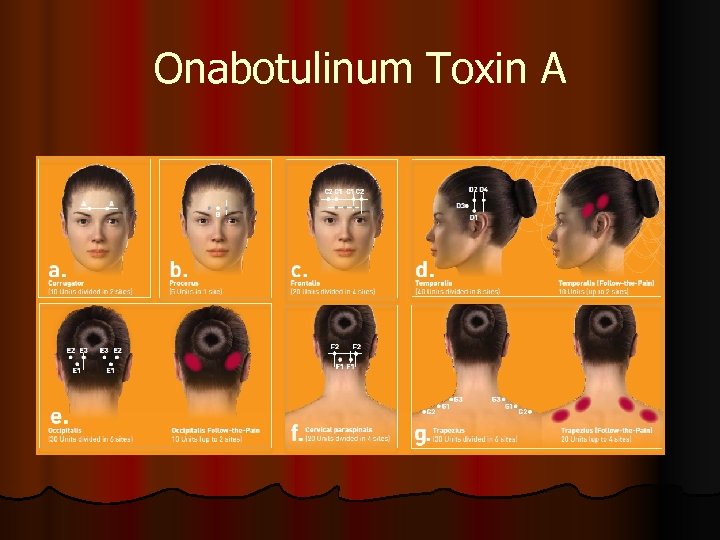 Onabotulinum Toxin A
Onabotulinum Toxin A
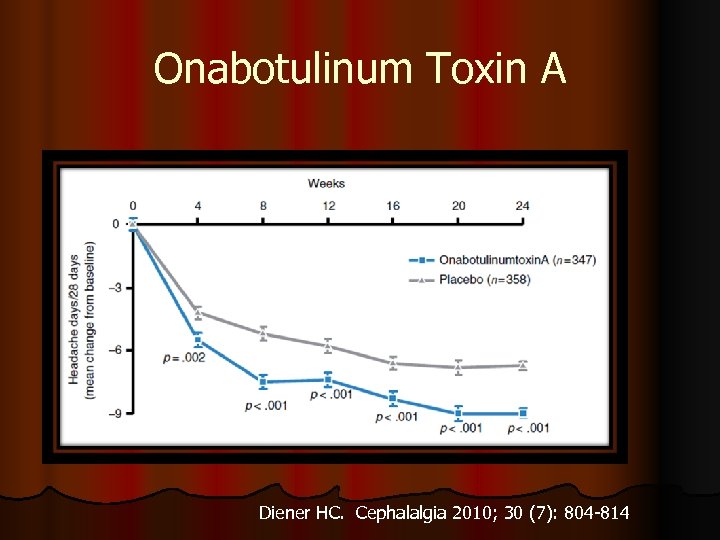 Onabotulinum Toxin A Diener HC. Cephalalgia 2010; 30 (7): 804 -814
Onabotulinum Toxin A Diener HC. Cephalalgia 2010; 30 (7): 804 -814
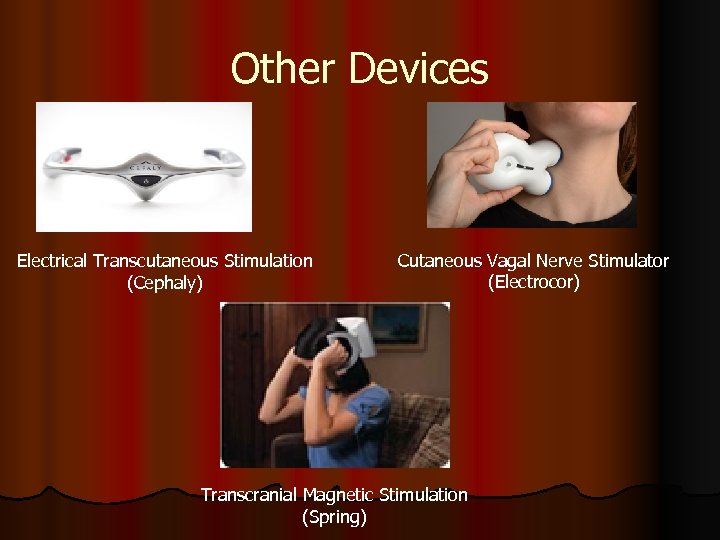 Other Devices Electrical Transcutaneous Stimulation (Cephaly) Cutaneous Vagal Nerve Stimulator (Electrocor) Transcranial Magnetic Stimulation (Spring)
Other Devices Electrical Transcutaneous Stimulation (Cephaly) Cutaneous Vagal Nerve Stimulator (Electrocor) Transcranial Magnetic Stimulation (Spring)
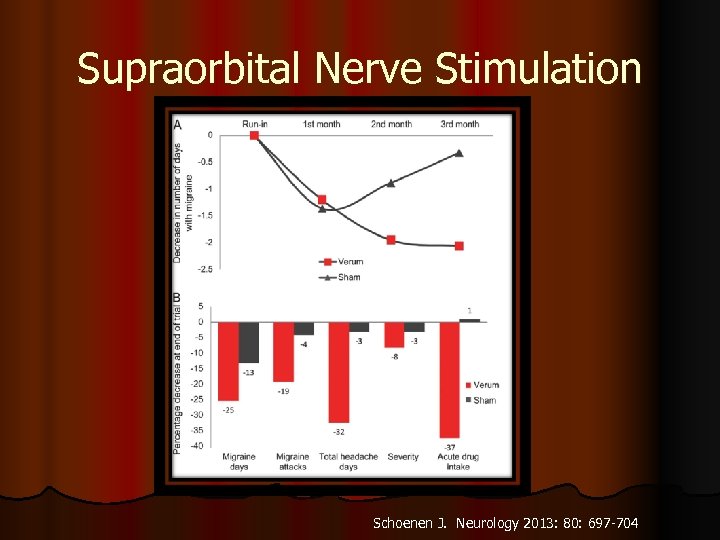 Supraorbital Nerve Stimulation Schoenen J. Neurology 2013: 80: 697 -704
Supraorbital Nerve Stimulation Schoenen J. Neurology 2013: 80: 697 -704
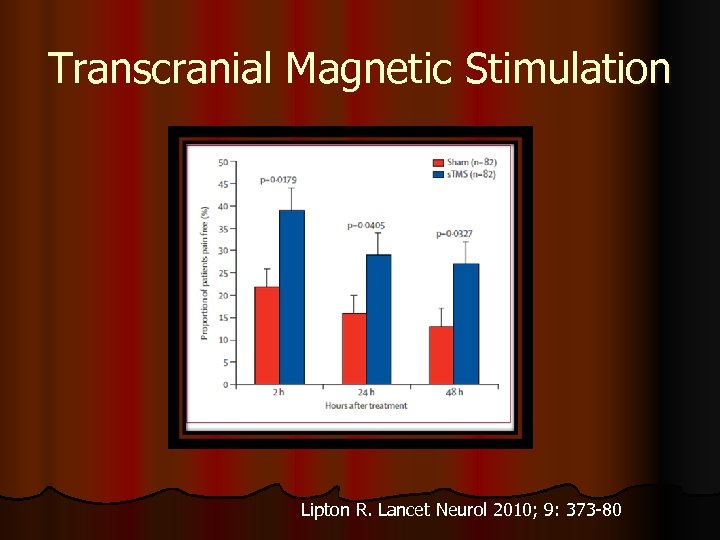 Transcranial Magnetic Stimulation Lipton R. Lancet Neurol 2010; 9: 373 -80
Transcranial Magnetic Stimulation Lipton R. Lancet Neurol 2010; 9: 373 -80
 Migraine Non FDA Approved Therapies
Migraine Non FDA Approved Therapies
 Occipital Nerve Stimulation http: //www. gizmodo. it/wp-content/uploads/2013/04/neurostimolazione. jpg
Occipital Nerve Stimulation http: //www. gizmodo. it/wp-content/uploads/2013/04/neurostimolazione. jpg
 Randomized Controlled Trials l Results mixed l ONSTIM Study l 39% in ONS group had a 50% reduction in headache frequency compared with 6% in sham group (p<0. 03) l PRISM l Did not meet primary endpoint of reduction in headache days l Silberstein study l Did not meet primary outcome measure (50% ↓ headache frequency l Significantly greater reduction in the frequency of headache days than sham ONS (-5. 4 days per month versus -. 08 days per month; p = 0. 008) Silberstein SD, Cephalalgia 2012; 32(16): 1165 -1179. Saper JR. Cephalalgia 2011; 31(3): 271 -285. Lipton R Cephalalgia 2009; 39: 30 [abstract].
Randomized Controlled Trials l Results mixed l ONSTIM Study l 39% in ONS group had a 50% reduction in headache frequency compared with 6% in sham group (p<0. 03) l PRISM l Did not meet primary endpoint of reduction in headache days l Silberstein study l Did not meet primary outcome measure (50% ↓ headache frequency l Significantly greater reduction in the frequency of headache days than sham ONS (-5. 4 days per month versus -. 08 days per month; p = 0. 008) Silberstein SD, Cephalalgia 2012; 32(16): 1165 -1179. Saper JR. Cephalalgia 2011; 31(3): 271 -285. Lipton R Cephalalgia 2009; 39: 30 [abstract].
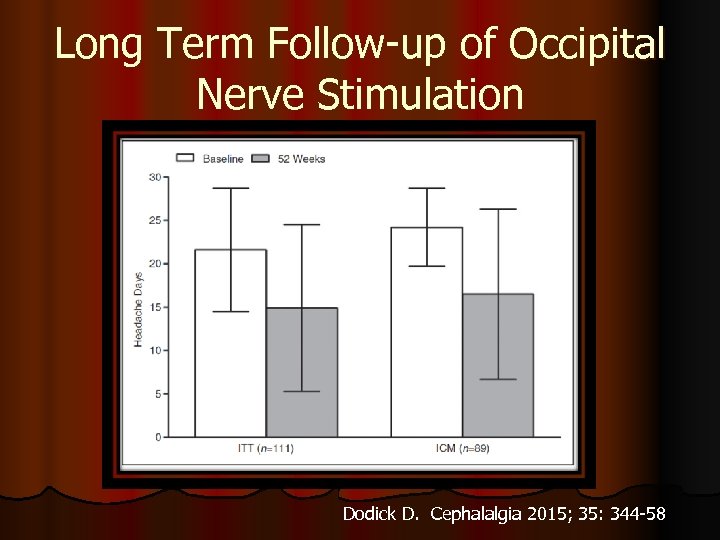 Long Term Follow-up of Occipital Nerve Stimulation Dodick D. Cephalalgia 2015; 35: 344 -58
Long Term Follow-up of Occipital Nerve Stimulation Dodick D. Cephalalgia 2015; 35: 344 -58
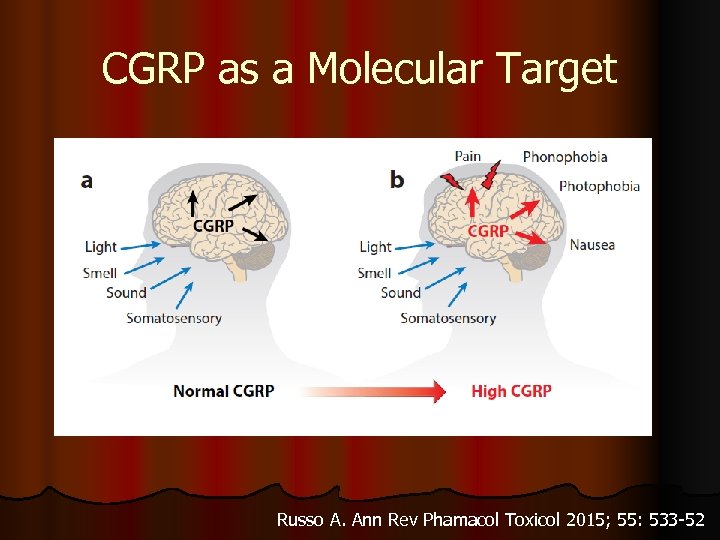 CGRP as a Molecular Target Russo A. Ann Rev Phamacol Toxicol 2015; 55: 533 -52
CGRP as a Molecular Target Russo A. Ann Rev Phamacol Toxicol 2015; 55: 533 -52
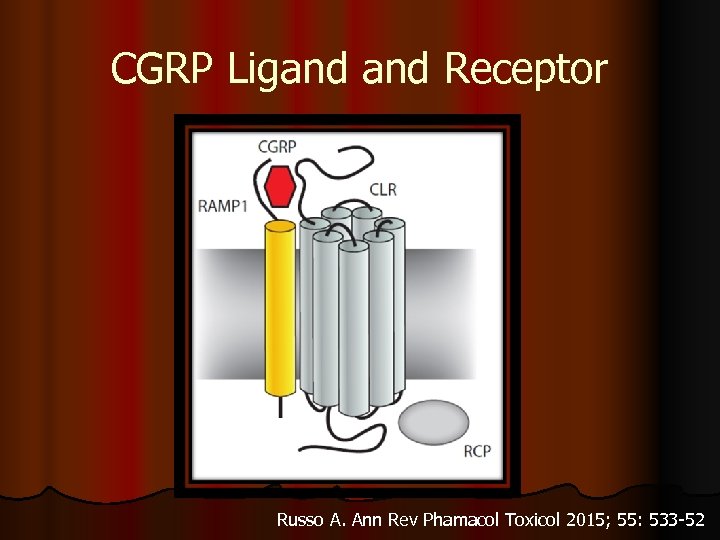 CGRP Ligand Receptor Russo A. Ann Rev Phamacol Toxicol 2015; 55: 533 -52
CGRP Ligand Receptor Russo A. Ann Rev Phamacol Toxicol 2015; 55: 533 -52
 CGRP Monoclonals Three monoclonal antibodies targeting CGRP (e. g. ALD 403, TEV-48125, LBR-101) and one directed against the CGRP receptor (AMG 334) l Advantages l Target specific l Long elimination half lives l Not metabolized to liver l Side effects related solely to targeting of CGRP l Administered parenterally every 2 weeks to 2 months l Bigal M. CNS Drugs March 2014
CGRP Monoclonals Three monoclonal antibodies targeting CGRP (e. g. ALD 403, TEV-48125, LBR-101) and one directed against the CGRP receptor (AMG 334) l Advantages l Target specific l Long elimination half lives l Not metabolized to liver l Side effects related solely to targeting of CGRP l Administered parenterally every 2 weeks to 2 months l Bigal M. CNS Drugs March 2014
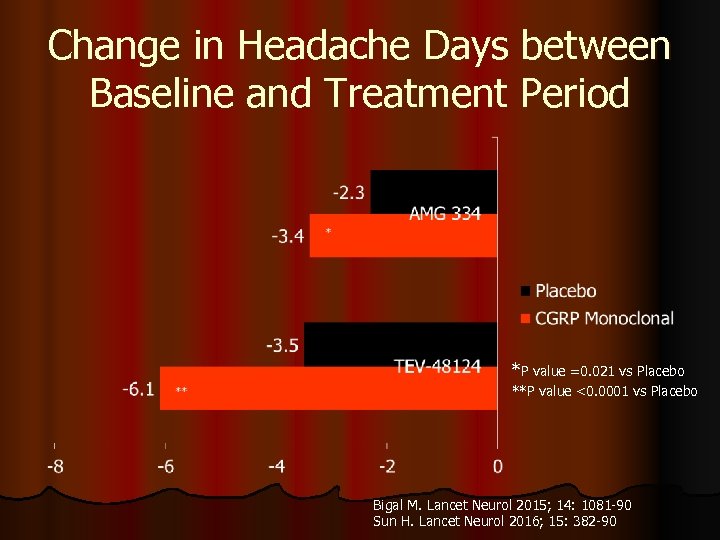 Change in Headache Days between Baseline and Treatment Period *P value =0. 021 vs Placebo **P value <0. 0001 vs Placebo Bigal M. Lancet Neurol 2015; 14: 1081 -90 Sun H. Lancet Neurol 2016; 15: 382 -90
Change in Headache Days between Baseline and Treatment Period *P value =0. 021 vs Placebo **P value <0. 0001 vs Placebo Bigal M. Lancet Neurol 2015; 14: 1081 -90 Sun H. Lancet Neurol 2016; 15: 382 -90
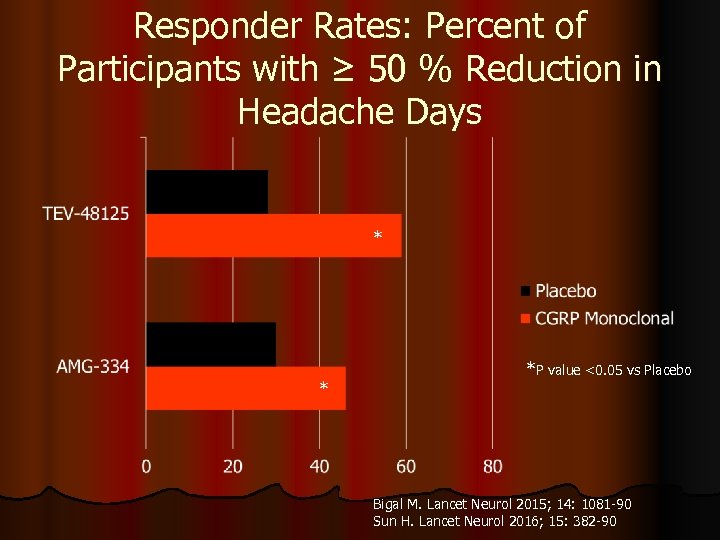 Responder Rates: Percent of Participants with ≥ 50 % Reduction in Headache Days * * *P value <0. 05 vs Placebo Bigal M. Lancet Neurol 2015; 14: 1081 -90 Sun H. Lancet Neurol 2016; 15: 382 -90
Responder Rates: Percent of Participants with ≥ 50 % Reduction in Headache Days * * *P value <0. 05 vs Placebo Bigal M. Lancet Neurol 2015; 14: 1081 -90 Sun H. Lancet Neurol 2016; 15: 382 -90
 Conclusions l Migraine is one of the more prevalent disorders encountered by primary care providers. l There a number of risk factors for transformation of episodic to chronic migraine. l Newer and established abortive and preventative therapies offer hope for migraine patients.
Conclusions l Migraine is one of the more prevalent disorders encountered by primary care providers. l There a number of risk factors for transformation of episodic to chronic migraine. l Newer and established abortive and preventative therapies offer hope for migraine patients.


