da850ca9e35052738c5fe32cbd2164b0.ppt
- Количество слайдов: 50

Microbiology: A Systems Approach, 2 nd ed. Chapter 17: Diagnosing Infections

17. 1 Preparation for the Survey of Microbial Diseases • Methods used to identify bacteria to the level of genus and species – Phenotypic methods • Morphology • Physiology or biochemistry – Immunologic method • Serological analysis – Genotypic techniques • More and more often used as a sole resource for identifying bacteria

Phenotypic Methods • • Microscopic morphology Macroscopic morphology Physiological/Biochemical characteristics Chemical analysis

Microscopic Morphology • • Cell shape and size Gram stain reaction Acid fast reaction Special structures

Macroscopic Morphology • Colony appearance • Speed of growth • Patterns of growth
Physiological/Biochemical Characteristics • Traditional mainstay of bacterial identification • Diagnostic tests for determining the presence of specific enzymes and assessing nutritional and metabolic activities • Examples – Fermentation of sugars – Capacity to metabolize complex polymers – Production of gas – Presence of enzymes – Sensitivity to antimicrobic drugs
Chemical Analysis • Analyzing the types of specific structural substances that the microorganism contains • Examples – Chemical composition of peptides in the cell wall – Lipids in membranes
Genotypic Methods • Primary advantage over phenotypic methods: actually culturing the microorganisms is not always necessary • Also are increasingly automated with results obtained very quickly

Immunologic Methods • Antibody response to antigens • Blood testing- often easier than testing for the microbe itself • Laboratory kits available for immediate identification of a number of pathogens

17. 2 On the Track of the Infectious Agent: Specimen Collection • The success of identification and treatment depends on how specimens are collected, handled, and stored • General aseptic procedures must be used
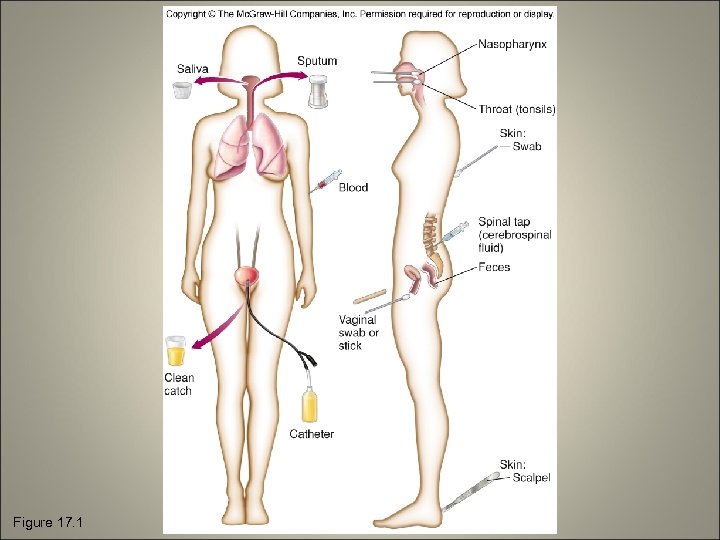
Figure 17. 1
Overview of Laboratory Techniques • Direct tests using microscopic, immunologic, or genetic methods • Cultivation, isolation, and identification of pathogens using a wide variety of general and specific tests • Results of specimen analysis entered in a summary patient chart
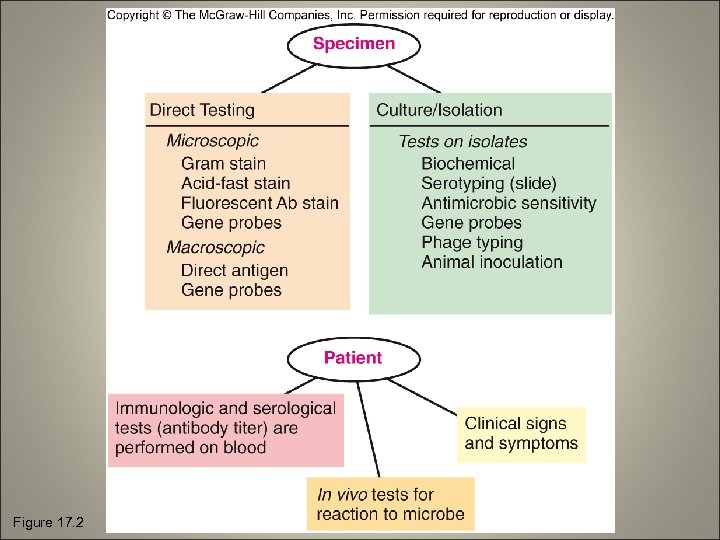
Figure 17. 2

Figure 17. 3

17. 3 Phenotypic Methods • Immediate Direct Examination of Specimen – Gram stain – Acid-fast stain – Direct fluorescence antibody (DFA) tests – Direct antigen testing
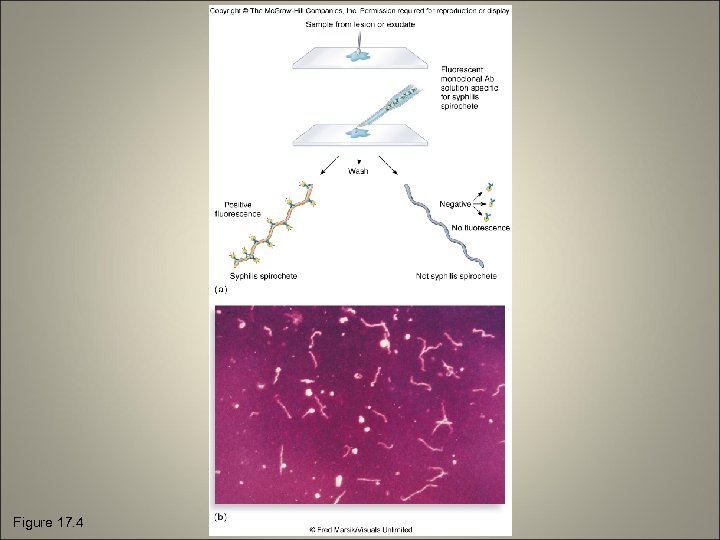
Figure 17. 4
Cultivation of Specimen • Isolation media • Biochemical testing – Carbohydrate fermentation (acid and/or gas) – Hydrolysis of gelatin, startch, and other polymers – Enzyme actions such as catalase, oxidase, and coagulase – By-products of metabolism
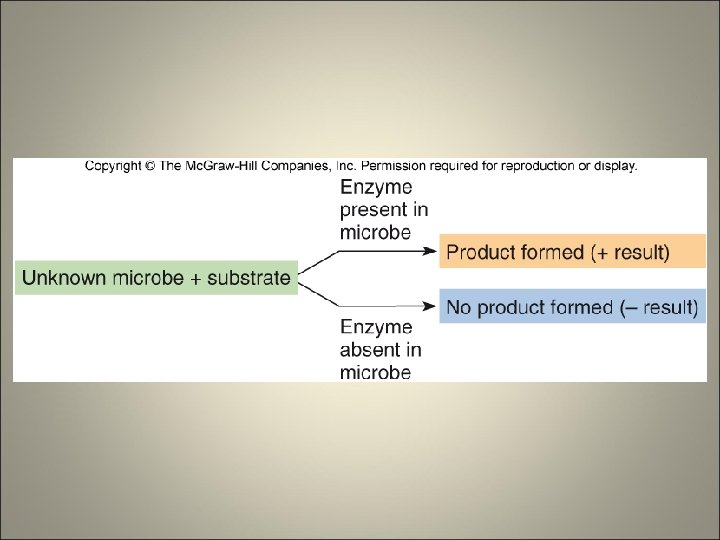
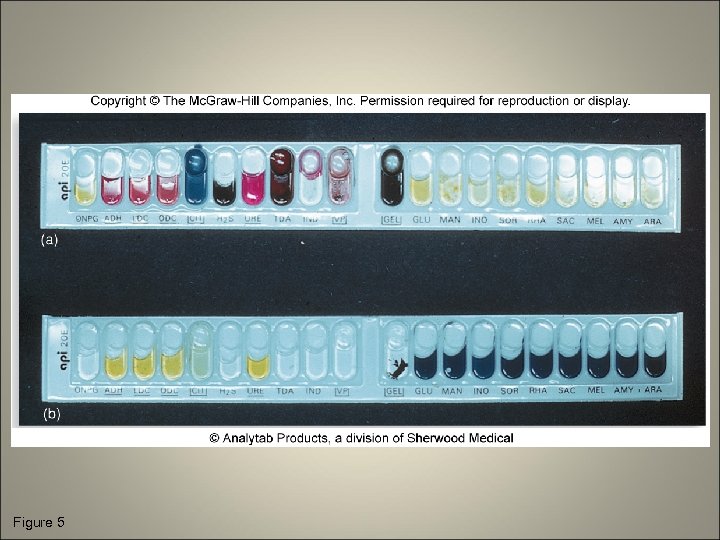
Figure 5
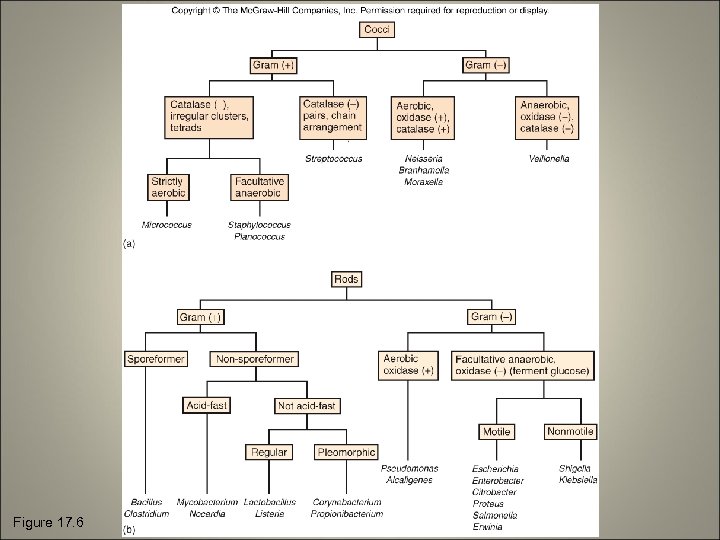
Figure 17. 6

Miscellaneous Tests • Phage typing • Animal inoculation • Antimicrobial sensitivity
Determining Clinical Significance of Cultures • Is an isolate clinically important? • How do you decide whether it is a contaminant or part of the normal biota? • Possible criteria – Number – Repeated isolation of a relatively pure culture of any microorganism
17. 4 Genotypic Methods • DNA Analysis Using Genetic Probes – Hybridization- can identify a bacterial species by analyzing segments of its DNA – Small fragments of single-stranded DNA or RNA called probes • Known to be complementary to the specific sequences of DNA from a particular microbe • Unknown test DNA from cells is bound to blotter paper • Add probes to blotter • Observe for signs that the probes have become fixed to the test DNA
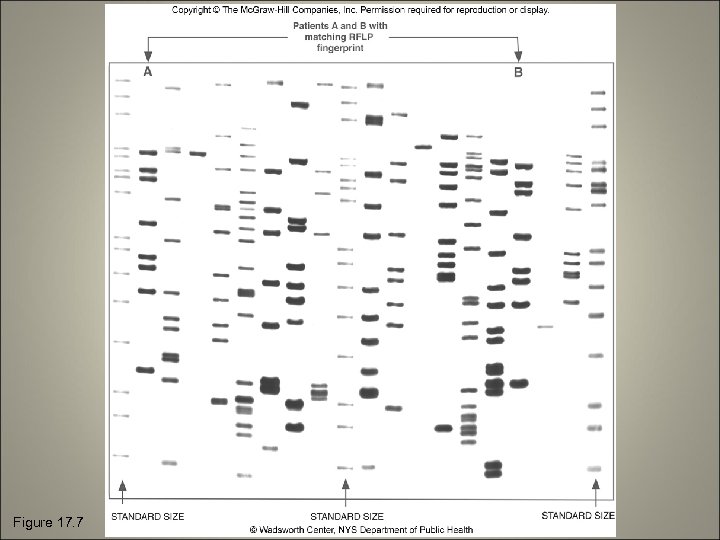
Figure 17. 7
Nucleic Acid Sequencing and r. RNA Analysis • Comparison of the sequence of nitrogen bases in r. RNA • Effective for differentiating general group differences • Can be fine-tuned to identify at the species level
Polymerase Chain Reaction • Rapid identification of pathogens • Developed for a wide variety of bacteria, viruses, protozoa, and fungi • Biosensor

17. 5 Immunologic Methods • Characteristics of antibodies can reveal the history of a patient’s contact with microorganisms or other antigens • Serological testing • Serology: the branch of immunology that traditionally deals with in vitro diagnostic testing of the serum
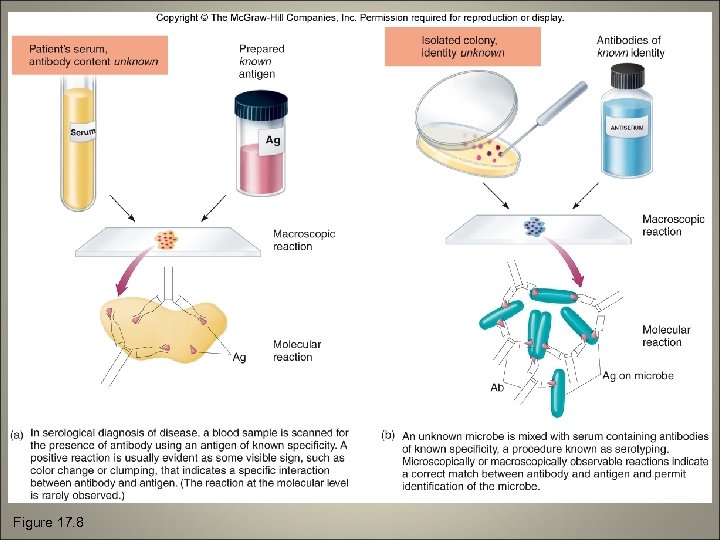
Figure 17. 8

General Features of Immune Testing • Strategies – Agglutination – Precipitation – Immunodiffusion – Complement fixation – Fluorescent antibody tests – Immunoassay tests • Specificity and sensitivity
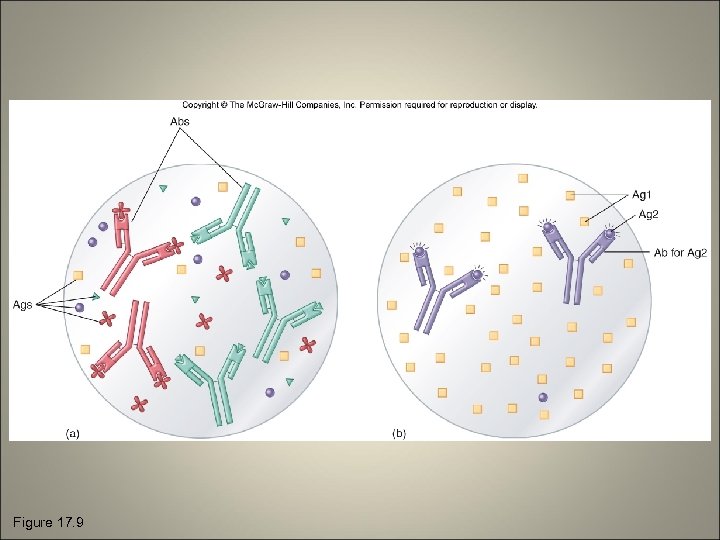
Figure 17. 9
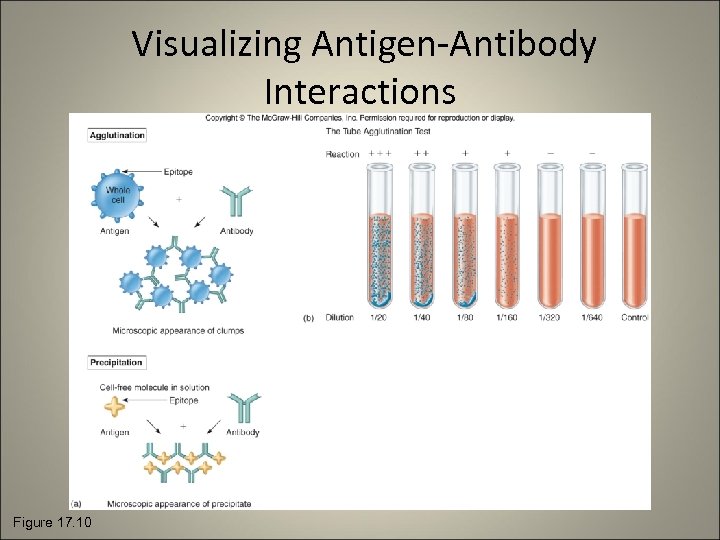
Visualizing Antigen-Antibody Interactions Figure 17. 10
Agglutination and Precipitation Reactions • Agglutination: antigens are whole cells such as red blood cells or bacteria with determinant groups on the surface • Precipitation: the antigen is a soluble molecule
Agglutination Testing • Antibodies cross-link the antigens to form visible clumps • Performed routinely to determine ABO and Rh blood types • Widal test: tube agglutination test for diagnosing salmonelloses and undulant fever • Rapid plasma regain (RPR) test: tests for antibodies to syph 8 ilis • Weil-Felix reaction: diagnoses ricketsial infections • Latex agglutination tests: tiny latex beads with antigens affixed

Precipitation Tests • The soluble antigen is precipitated by an antibody • Reaction is observable as a cloudy or opaque zone at the point of contact • VDRL (Veneral Disease Research Lab) test • Double diffusion (Ouchterlony) method • Immunoelectrophoresis
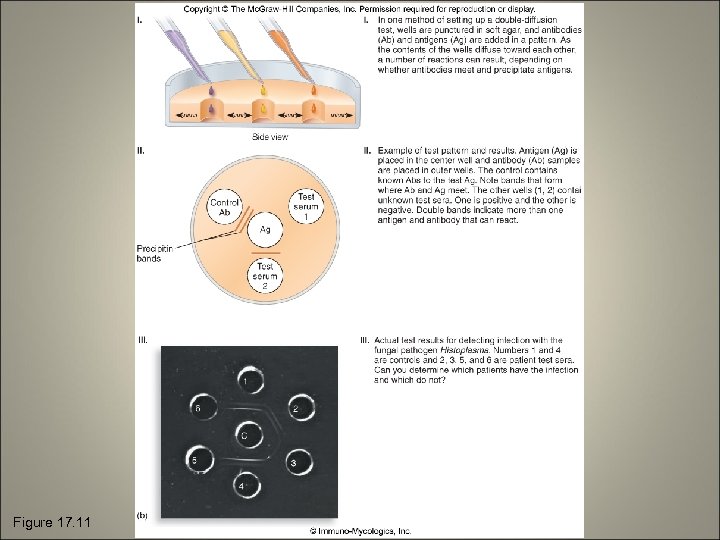
Figure 17. 11
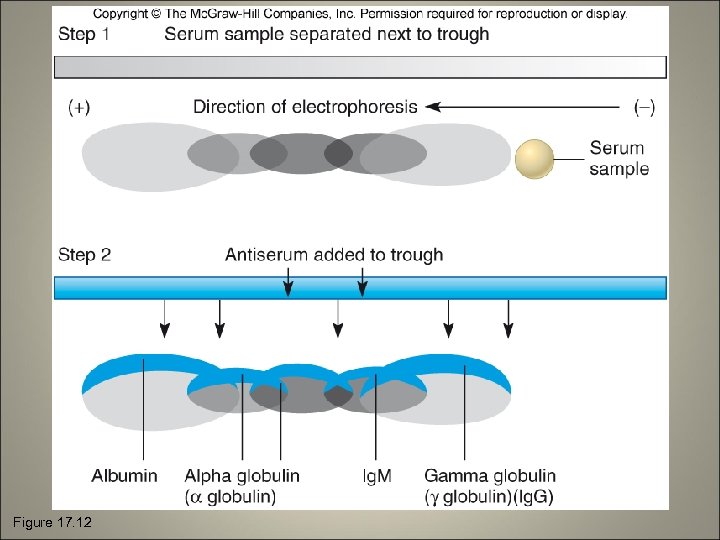
Figure 17. 12
The Western Blot for Detecting Proteins • Involves electrophoretic separation of proteins followed by an immunoassay to detect those proteins • Counterpart of the Southern blot test • Test material is electrophoresed in a gel to separate out particular bands • Gel transferred to a special blotter that binds the reactants in place • Blot developed by incubating it with a solution of antigen or antibody labeled with radioactive, fluorescent, or luminescent labels
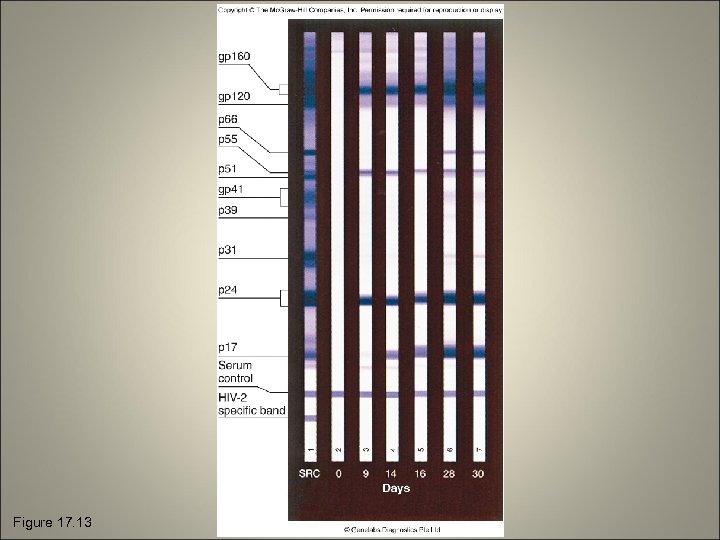
Figure 17. 13

Complement Fixation • Lysin or cytolysin: an antibody that requires complement to complete the lysis of its antigenic target cell
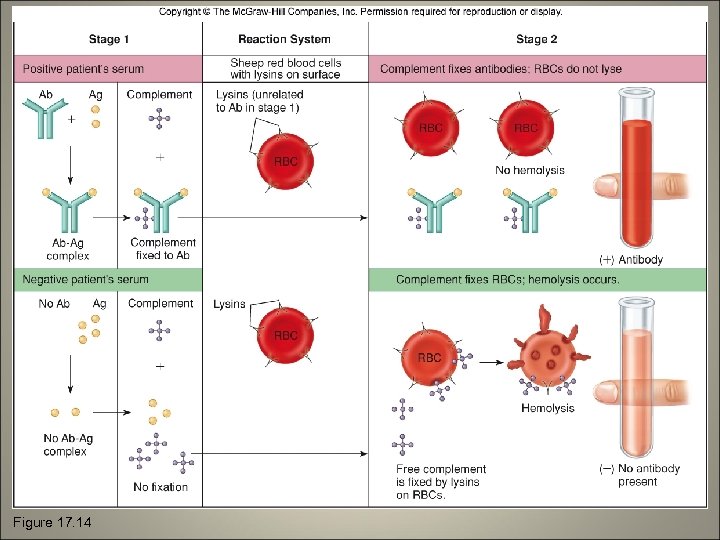
Figure 17. 14

Miscellaneous Serological Tests • • Treponema pallidum immobilization (TPI) test Toxin neutralization tests Serotyping Quellung test
Flurorescent Antibodies and Immunofluorescence Testing • Direct testing: an unknown test specimen or antigen is fixed to a slide and exposed to a fluorescent antibody solution of known composition • Indirect testing: the fluorescent antibodies are antibodies made to react with the Fc region of another antibody
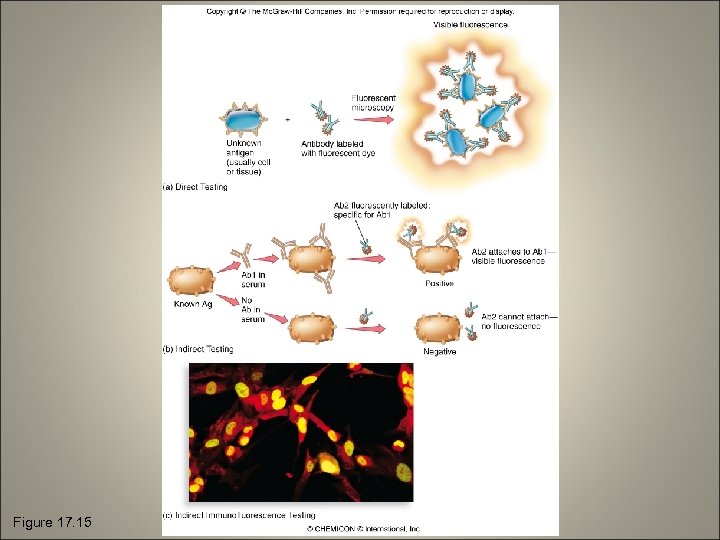
Figure 17. 15
Immunoassays • Extremely sensitive methods that permit rapid and accurate measurement of trace antigen or antibody • Radioactive isotope labels • Enzyme labels • Sensitive electronic sensors

Radioimmunoassay (RIA) • Antibodies or antigens labeled with a radioactive isotope used to pinpoint minute amounts of a corresponding antigen or antibody • Compare the amount of radioactivity present in a sample before and after incubation with a known, labeled antigen or antibody • Large amounts of a bound radioactive component indicate that the unknown test substance was not present • Radioimmunosorbent test (RIST) • Radioallergosorbent test (RAST)

Enzyme-Linked Immunosorbent Assay (ELISA) • Enzyme-antibody complex that can be used as a color tracer for antigen-antibody reactions • Indirect • Direct
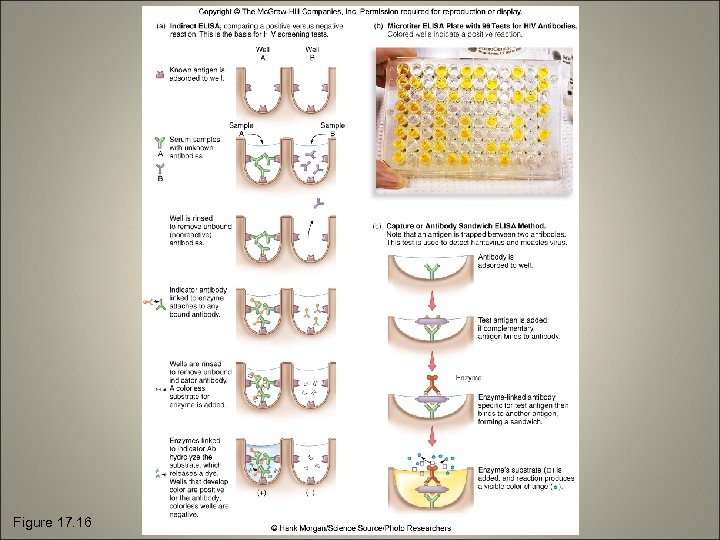
Figure 17. 16
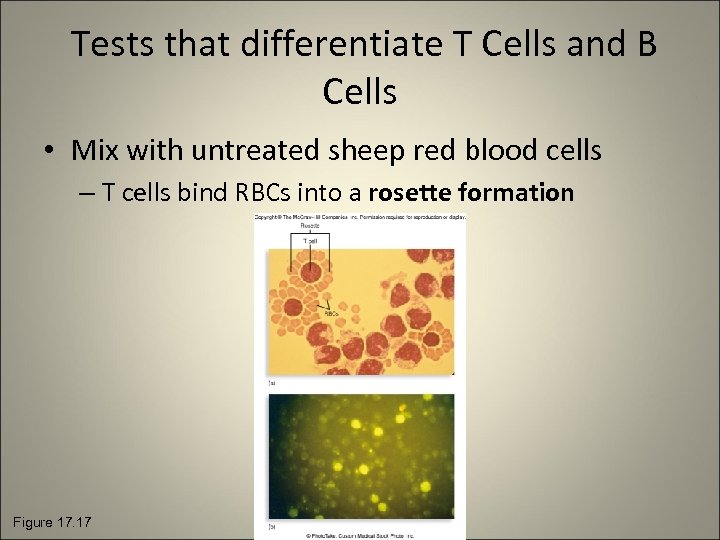
Tests that differentiate T Cells and B Cells • Mix with untreated sheep red blood cells – T cells bind RBCs into a rosette formation Figure 17. 17

In Vivo Testing • Tuberculin test • Other diagnostic skin tests
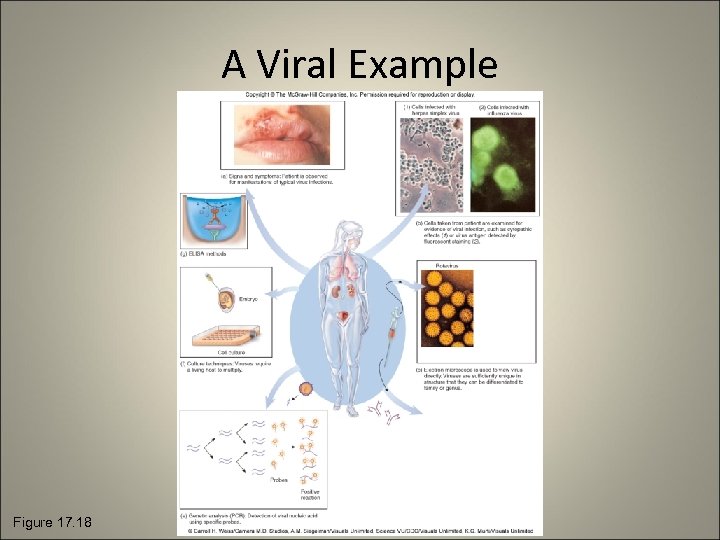
A Viral Example Figure 17. 18
da850ca9e35052738c5fe32cbd2164b0.ppt