Microbial Technology.ppt
- Количество слайдов: 81
MICROBIAL TECHNOLOGY COURSE CONTENT L 1 Introduction to Microbial Based Industries L 2 & L 3 Isolation and Preservation of Industrial Microorganisms L 4 – L 6 Improvement of microbial productivities L 7 & L 8 Microbial Enzymes L 9 & L 10 Antibiotics L 11 – L 13 Production of organic solvents and organic acids and amino acids L 14 & L 15 Fermentation Processes for the production of physiologically active substances

FIT 3133 MICROBIAL TECHNOLOGY COURSE CONTENT (Contd) L 16 – L 18 Microbes in food and beverages industries L 19 Biotransformation of lignocellulolysic materials and degradation of xenobiotic compounds L 20 & L 21 Environmental Microbial Technology L 22 & L 23 Microbiology in the Mining and Petroleum Industry L 24 & L 25 Microbial Processes in Agriculture L 26 - L 28 Waste Treatment Systems for Fermentation Industries

INTRODUCTION TO MICROBIAL BASED INDUSTRIES
THE CONCEPT OF INDUSTRIAL PROCESSES……… TE VE LA R O NS FE PR IM RA NS OP T A L VE R T DE UP LE A SC ION T IZA IM T OP COMMERCIALIZATION

INTRODUCTION MICROORGANISMS AND MAN l All microorganisms are important for human, animal, palnt and the environment l Positive and negative impacts: saprophytes, symbiosis, commensalism, parasitism/pathogens l Exploitation of microorganisms for the production of metabolites which can be used for human consumption and providing solutions to problems faced by the ecosystem l Metabolites are produced as tools, weapons, defense mechanisms, nutritions, change environmental conditions, metabolic products or by products l Enhancing the productivities through Microbial Technology – improving biotransformation processes through manipulation of biochemical pathways of the system

MICROORGANISMS AND THE ENVIRONMENT • Microorganisms – ubiquitous in the environment • Soils – reservoir for microorganisms (due to high nutritional content of soils) • Bacteria, actinomycetes, fungi, algae (1 g of soil has several millions of bacteria/several thousands of fungi species) • In the environment – biogeochemical cycles • The role is to maintain the equilibrium of the elements (C, N, O, S, P) • Pollutants in the environment (affect the equilibrium) • Waste treatment systems (biological, physical and chemical methods) • Bioremediation
MICROORGANISMS AND DISEASES OF MAN, ANIMAL AND PLANTS • Normal microflora on human, animal and plants (non-pathogenic) • Opportunistics – health problems in individuals with low immunity • Natural defense mechanisms – in healthy individuals • Interferon, lymphokines, antibodies, antibiotics, white blood cells, phagocytes, inflammation • Diseases can be caused by bacteria, fungi, viruses and parasites • Microorganisms – wide spectrum of host in causing diseases, while other may be very specific (host specific) • Used of drugs and antibiotics to combat microorganisms causing various diseases • Drugs are also wide spectrum – majority are specific in their action
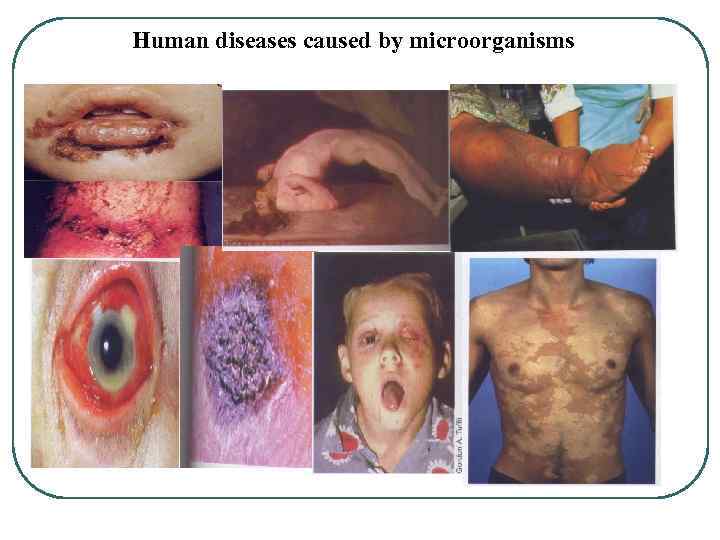
Human diseases caused by microorganisms

MICROORGANISMS AND MANUFACTURING INDUSTRIES • Microbial metabolites are products which have commercial potentials • Produced by fermentation processes using selected microorganisms on large scales • Enzymes, solvents, biofuel, biogas, organic acids, antibiotics, vitamins, insulin, hormone, pigments, amino acids etc. • Chemicals and drugs using animal and plant cells – tissue culture technology • Industrial production with downstream processes for large scale product extraction and development

MICROORGANISMS IN FOOD AND BEVERAGE INDUSTRIES • Wide applications of microorganisms in food and beverage industries • Areas involving microorganisms – food spoilage, food preservation, fermented food, new food with different nutritional and functional qualities. • Change in food – food spoilage or new flavours and quality of food • Food spoilage – putrefaction, fermentation and rancidity • Large scale of food and beverage production : such as wine and beer making, fermented food and microbial products as food supplements • Fermentation processes for large scale production • Food quality : control of microbial contamination

• Agriculture – one of the biggest area involving microorganisms • Spoilage of agricultural products, soil and water microbiology, biofertilizers and • plant pathology • Biocontrol of plant diseases – entomopathogens. Eg the use of Bacillus popillae and B. Thuringiensis, Phycomycetes, Deuteromycetes (Metarhizium sp. Conidiobolus coronotus and Aspergillus niger), virus, protozoa • Nitrogen fixation – Rhizobium sp. (produced by fermentation processes) • Plant from the family Leguminosae (symbiotic relationship as indicated by the presence of nodules • Wide species of bacteria and fungi can caused deiseases in plants and animals • Post harvest technology (prevention of microbial

FUTURE OF MICROBIAL INDUSTRIES • Microbial Biotechnology • Large variety of products from microbial processes with commercializable potentials • Application in various areas and industries, including manufacturing, services, medical, food, pharmaceutical, etc. • Modified and enhanced production systems – microbial technology : physiological modification and process control/scale up • Strengthening fundamental knowledge in understanding the physiology of metabolite hyperproduction and optimization of production system • Screening for new products (such as pharmaceutical
PROBLEMS RELATED TO MICROBIAL INDUSTRIES a. Microbial pathogenicity – spontaneous mutation resulted in the production of toxins (environmental factors) Eg vaccines production from pathogens, the use of GMOs, genetic alterations b. Microbes with biologically active products – products present in the environment which will change the microbial ecosystem c. The handling of large quantity of microorganisms as byproducts of fermentation processes d. Stability and the purity of potential producers e. Contamination of production system

ISOLATION AND PRESERVATION OF INDUSTRIAL MICROORGANISMS
• Isolation as the first step in obtaining the microorganisms capable of producing metabolites/products of commercial potentials • Pure isolates • General methods for isolation of microorganisms with the ability to produce metabolites of interest. Eg. i. Alkalophiles – medium with high p. H ii. Thermophiles – incubation at temperatures of >50 o. C iii. Anaerobes – incubation in oxygen free chamber • The use of liquid medium and solid media for microbial isolation
Random Isolation method • For metabolites – without specific method for detection of metabolites • Media used which will allow the microorganisms to express all the genetic information (C, N, P, O and S, vitamin, minerals and suitable p. H) Screening of microorganisms for the production of specific compounds • Primary screening – detection of products from a mixed population and selection is made based on the isolates showing the highest/maximum production. (spread plate or pour plate method) • Isolation to pure cultures and subcultures • Confirmation of production – stability of metabolite production • Preservation of microbial isolates
• Secondary screening - production potential reconfirm using different systems • Different media (normally liquid media) – qualitative analysis of productivity • Cultivation system - batch, fed batch or continuous system (shake flask or laboratory fermenter can be used) • To allow the isolates to express the capability of the genetic materials for the production of the metabolites • Optimization for production based on the governing parameters may be employed (p. H, temperature, oxygen concentration, inducer, aeration and agitation and other nutritional factors • Secondary screening provides the cultivation conditions and nutritional requirement for the scale up processes for mass production
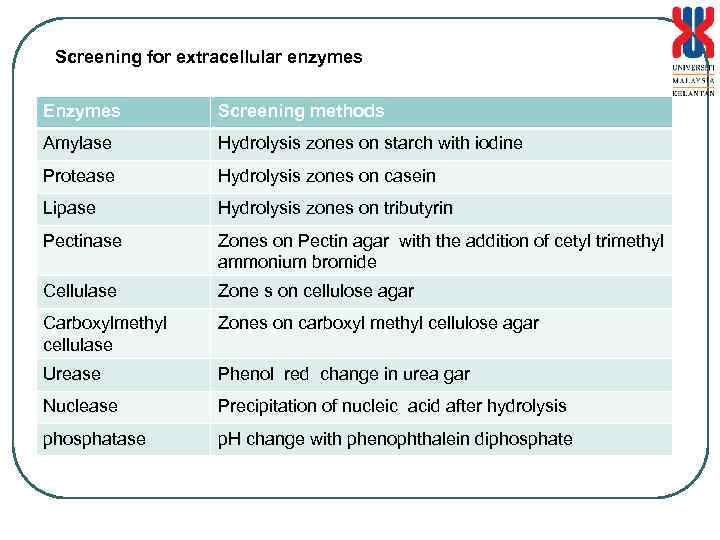
Screening for extracellular enzymes Enzymes Screening methods Amylase Hydrolysis zones on starch with iodine Protease Hydrolysis zones on casein Lipase Hydrolysis zones on tributyrin Pectinase Zones on Pectin agar with the addition of cetyl trimethyl ammonium bromide Cellulase Zone s on cellulose agar Carboxylmethyl cellulase Zones on carboxyl methyl cellulose agar Urease Phenol red change in urea gar Nuclease Precipitation of nucleic acid after hydrolysis phosphatase p. H change with phenophthalein diphosphate

Screening of antibiotic production Antibiotic – organic substances/compounds produced by microorganisms exhibiting anta gonist against the growth of microorganisms at low concentration • Produced mainly by actinomycetes from the genus Streptomyces, Nocardia and Micromonospora • However, Bacillus sp. or Penicillium sp. are 1. potential producers • Sources of antibiotic producers - soil • Screening method : plating method with test microorganisms such as Bacillus subtilis, Streptomyces viridochromogenes and Clostridium pasteurimum, . Other may include Staphylococcus aureus, Streptococcus faecalis, Proteus vulgaris, Klebsiella pneumonia, Candida albicans, Escherichia coli, Trichomonas vaginalis, Bacteroides fragilis and Trichophyton mentagrophytes
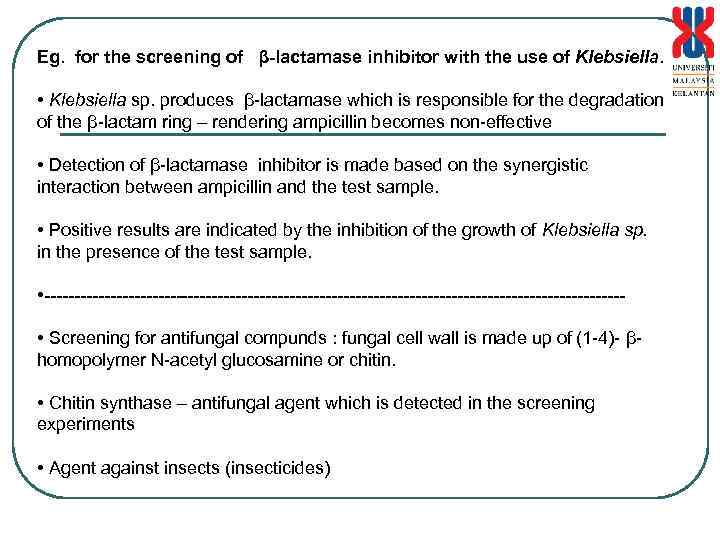
Eg. for the screening of β-lactamase inhibitor with the use of Klebsiella. • Klebsiella sp. produces β-lactamase which is responsible for the degradation of the β-lactam ring – rendering ampicillin becomes non-effective • Detection of β-lactamase inhibitor is made based on the synergistic interaction between ampicillin and the test sample. • Positive results are indicated by the inhibition of the growth of Klebsiella sp. in the presence of the test sample. • ------------------------------------------------ • Screening for antifungal compunds : fungal cell wall is made up of (1 -4)- βhomopolymer N-acetyl glucosamine or chitin. • Chitin synthase – antifungal agent which is detected in the screening experiments • Agent against insects (insecticides)
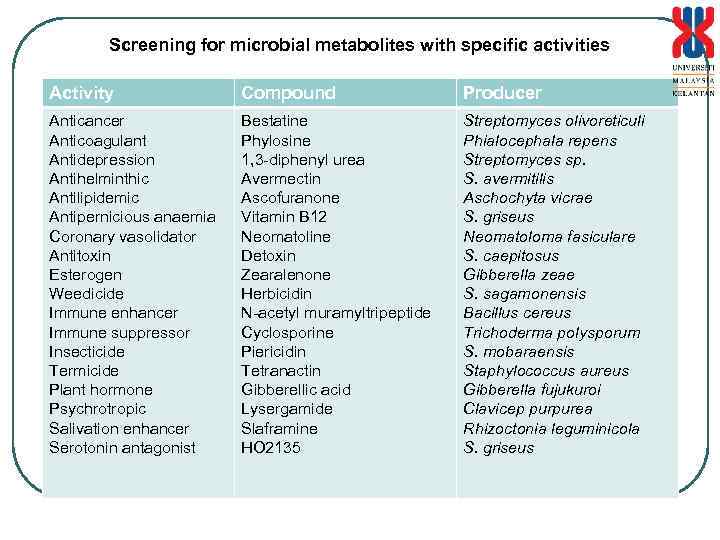
Screening for microbial metabolites with specific activities Activity Compound Producer Anticancer Anticoagulant Antidepression Antihelminthic Antilipidemic Antipernicious anaemia Coronary vasolidator Antitoxin Esterogen Weedicide Immune enhancer Immune suppressor Insecticide Termicide Plant hormone Psychrotropic Salivation enhancer Serotonin antagonist Bestatine Phylosine 1, 3 -diphenyl urea Avermectin Ascofuranone Vitamin B 12 Neomatoline Detoxin Zearalenone Herbicidin N-acetyl muramyltripeptide Cyclosporine Piericidin Tetranactin Gibberellic acid Lysergamide Slaframine HO 2135 Streptomyces olivoreticuli Phialocephala repens Streptomyces sp. S. avermitilis Aschochyta vicrae S. griseus Neomatoloma fasiculare S. caepitosus Gibberella zeae S. sagamonensis Bacillus cereus Trichoderma polysporum S. mobaraensis Staphylococcus aureus Gibberella fujukuroi Clavicep purpurea Rhizoctonia leguminicola S. griseus
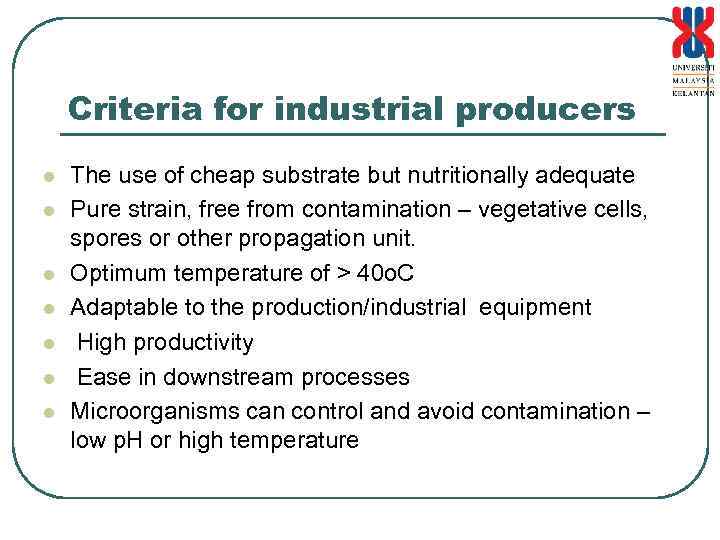
Criteria for industrial producers l l l l The use of cheap substrate but nutritionally adequate Pure strain, free from contamination – vegetative cells, spores or other propagation unit. Optimum temperature of > 40 o. C Adaptable to the production/industrial equipment High productivity Ease in downstream processes Microorganisms can control and avoid contamination – low p. H or high temperature

Storage and preservation of industrial microorganisms • Isolation of industrial microorganisms is a long and expensive process. • Microorganisms must be able to maintain its productivity • Isolates must be pure and contaminants free • Preservation becomes important to prevent genetic alteration, avoid contamination , able to survive/remain active over a long period of time • Subculturing over known period of time (however notrepeated subculturing to prevent strain degeneration) • Strain degeneration – indicated but reduce stability of isolates • Various methods of microbial staorage and preservation.
Storage under reduced temperature • Agar slants - in refrigerator (5 o. C) or freezer (-20 o. C) : subculturing every 6 months • Agar can be overlaid with sterile mineral oil which will be kept for a year • The oil will prevent evaporation, slows gaseous exchange and normally used to store bacteria, yeast and fungi without spores • Dry soil - for the storage of fungal spores. The soil acts as carrier. • Moist soil inoculated with spores, dried and stored as primary stock culture • fungal spores can be stored in distilled water and stored at 5 o. C – limited application for short period of time.
Storage under dehydration • Dehydration method – longer storage time by stopping the metabolic activities and inhibit cell multiplication • Temperature is reduced and water loss is complete. Eg freezing • Freezing – resulted destruction to cells. Therefore, to prevent : i. Temperature reduction at the rate of 1 o. C per min until – 20 o. C, and quickly to the storage temperature ii. Thawing must be done quickly using elevated temperatures • To increase survival rate, add glycerol, sugar or protectant substances with minimum electrolytes • Method – Liquid nitrogen (-150 o. C - -196 o. C) • Stationary phase cultures are used which are suspended in 10% glycerol and placed in ampoules and stored in liquid nitrogen. • Prior to storage, freeze the culture gradually

Lyophilization • Freeze drying method – freezing at -35 o. C, followed by drying in vacuum (200 µm. Hg) • Cells from the stationary phase of growth and suspended togther with the protectants such as milk, serum or sodium glutamate • Cells suspension placed in ampoules and placed under vacuum under sublimation completes. • Ampoules sealed and can be stored in refrigerator for almost 10 years • For use, ampoules are surface sterilized using alcohol or clorox. Break the ampoules at the top of the ampoules. A small volume of sterile distilled water is added in the ampoules and allowed for 30 min. • The cells can be grown on agar plates or added into 50 ml of liquid media using a 250 -ml conical flask. Observe the cell growth

IMPROVEMENT OF MICROBIAL PRODUCTIVITIES
Introduction Production of metabolites are normally low; therefore need to enhance productivity by either optimization of culture media and growth conditions Optimization of cultivation conditions • Industrial processes depend on the solution of problems at laboratory scale • Vessel/equipment for fermentation processes Equipment and Fermentation vessels : At Laboratory scale 1. Shake flask and agitation (types, design and the use of baffle) – Plug for flask – aeration conditions 2. Shaking (reciprocal and rotary) 3. Culture method (shaking on tray system, 15 – 30 o. C elevation for improved oxygen transfer) (Temperature control – using suitable chamber for temperature control)
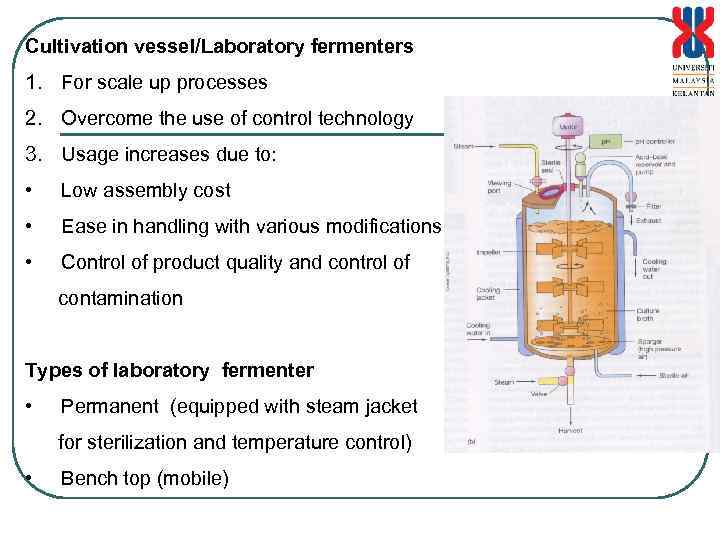
Cultivation vessel/Laboratory fermenters 1. For scale up processes 2. Overcome the use of control technology 3. Usage increases due to: • Low assembly cost • Ease in handling with various modifications • Control of product quality and control of contamination Types of laboratory fermenter • Permanent (equipped with steam jacket for sterilization and temperature control) • Bench top (mobile)
Requirement of Laboratory Fermenter 1. Possess geometric dimensions similar to that of industrial fermenter 2. 3. Flexibility for studies on the effect of parameter and fermentation variables 4. Small size but capable of controlling fermentation parameters. 5. Ease in assembling/cleaning/dissembling 6. Medium volume sufficient for sampling 7. Control equipments/accessories must be effective for consistent operation throughout fermentation 7. Avoid contamination or devise mechanism for prevention 8. Facilities for foam control must be equipped.
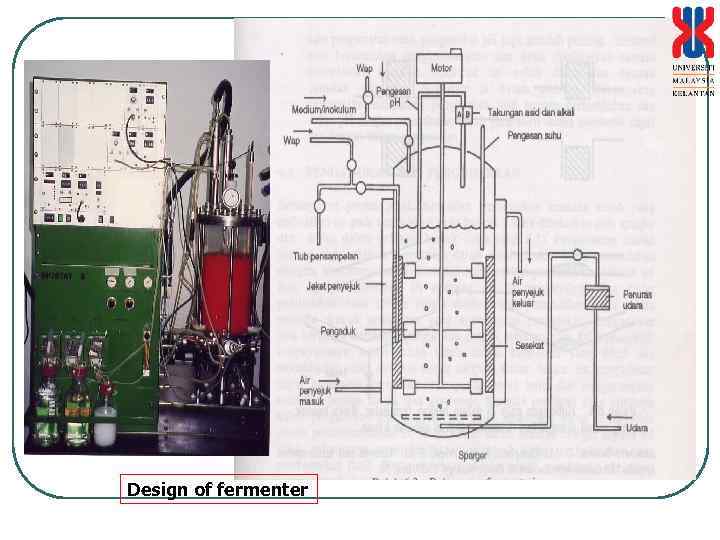
Design of fermenter
Industrial scale fermentation system Types (based on the media movement): 1. Mechanical (impeller) 2. Pneumatic (Air) 3. Hydrodynamic (Water) Considerations for industrial fermenter Operates aseptically: 1. Adequate agitation and aeration – based on oxygen requirements for fermentation 3. Temperature and p. H control system 4. Smooth inner surfaces with fixed geometric dimension 3. Suitable for various fermentation processes 4. Low labour 5. Good and efficient support services.
Piping system in fermenter
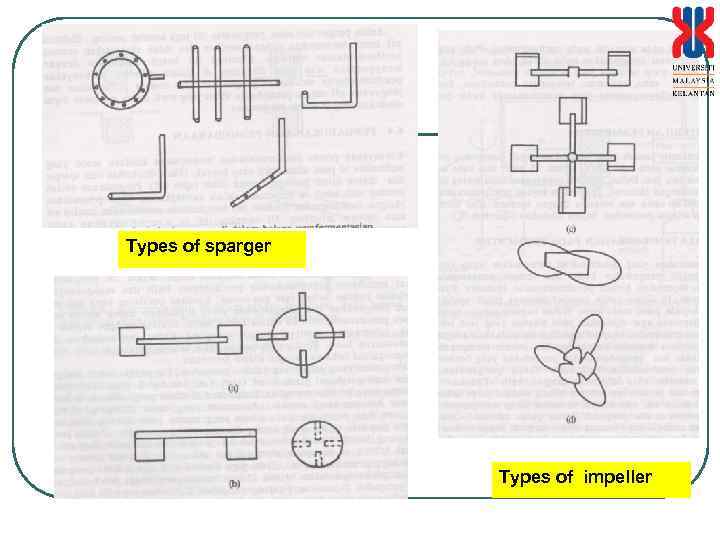
Types of sparger Types of impeller
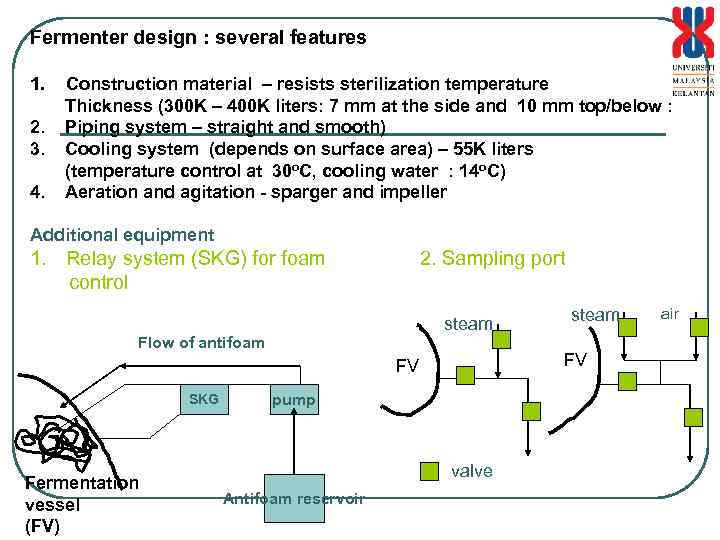
Fermenter design : several features 1. Construction material – resists sterilization temperature Thickness (300 K – 400 K liters: 7 mm at the side and 10 mm top/below : 2. Piping system – straight and smooth) 3. Cooling system (depends on surface area) – 55 K liters (temperature control at 30 o. C, cooling water : 14 o. C) 4. Aeration and agitation - sparger and impeller Additional equipment 1. Relay system (SKG) for foam 2. Sampling port control steam Flow of antifoam FV FV SKG Fermentation vessel (FV) pump valve Antifoam reservoir steam air

• FERMENTER DESIGNS IN SUBMERGED SYSTEMS
MODIFICATION OF GENE SYSTEM • Production of metabolites by microorganisms has limitation and is controlled by the genome • Therefore, to enhance the productivity, the genome need to be modified • During cell division, genetic alteration occurs continuously resulted weak progeny, although there are stronger ones • Selection of the stronger variants – high productivity (induced mutants or recombinants) • Mutants can be obtained via physical or chemical mutagenesis Eg: Physical mutagen – ultra violet radiation Chemical mutagen – N-methyl-N-nitrosoguanidine (NTG) • Recombinant DNA
Types of mutants a. Auxotroph mutants – exhibiting defect in the metabolic pathway and can be detected based on the growth on minimum medium. They lack amino acid, vitamin or nucleic acid component (purine and pirimidine) Random mutation • Modification of cellular genome which control the production of metabolites • Poor stability and depends on the environmental factors • Changes/mutation may result reduction in productivity b. Analogue resistant mutant - selection of mutants using metabolic analogues especially secondary metabolites. Increase in production because no phenomenon of feedback represssion. Eg production of pyrolnitrin from tryptophan. Analogue used are floro - and methyltryptophan. c. Mutant with low production of undesirable byproducts. Eg S. noursei produces cycloheximide and nystatin. However, mutant can be selected to only produce nystatin. d. Mutants that show resistance toward metabolites e. Mutant that alter cell permeability f. Mutants that are able to produce new products
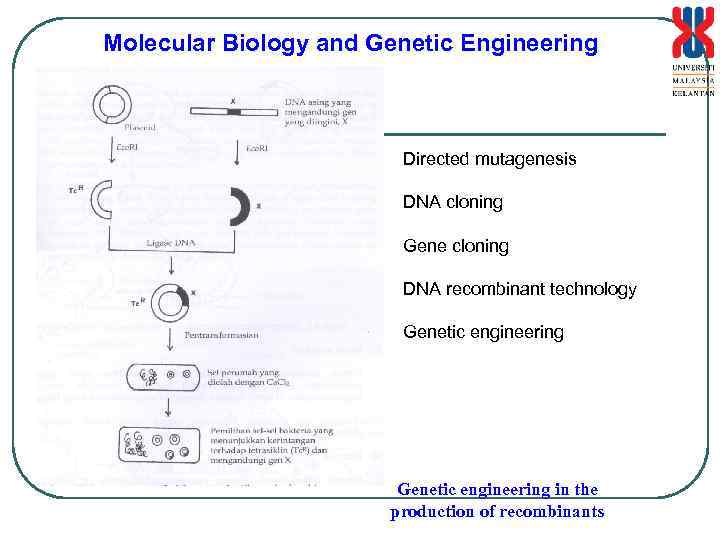
Molecular Biology and Genetic Engineering Directed mutagenesis DNA cloning Gene cloning DNA recombinant technology Genetic engineering in the production of recombinants

SEXUAL AND ASEXUAL REPRODUCTION • Filamentous fungi do not have complete sexual cycle • Neurospora crassa is a haploid and transferred via the hyphae fragment or asexual spores. • Sexual cycle involves 2 strains which can fuse to form the diploid stage (Heterokaryon) • Heterokaryon can undergo meiosis and form 4 haploid nucleus. And 2 stage mitosis to form 8 ascopores. - Parasexual • can be enhanced with the addition of p-florophenyl alanine and carabamate benzylmidazol. • S. cerevisiae has haploid stage which can undergo budding. Haploid cells can undergo fusion to form diploid , and with meiosis to form 4 haploid cells. Diploid cells can divide by mitosis and also undergo budding
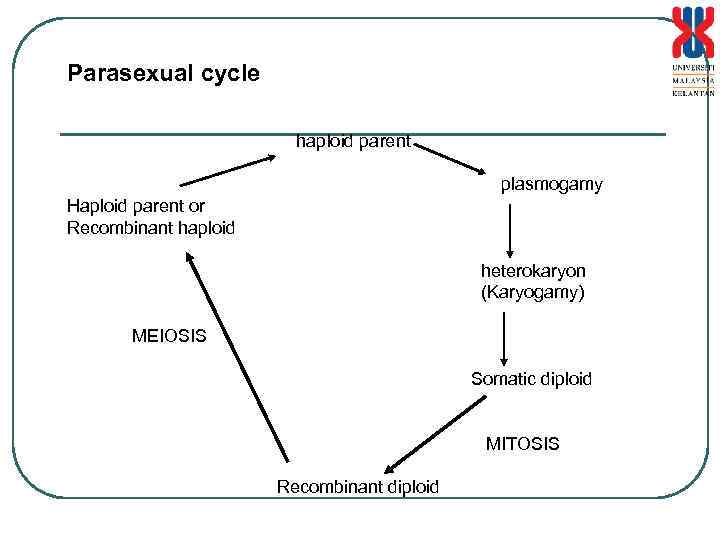
Parasexual cycle haploid parent plasmogamy Haploid parent or Recombinant haploid heterokaryon (Karyogamy) MEIOSIS Somatic diploid MITOSIS Recombinant diploid
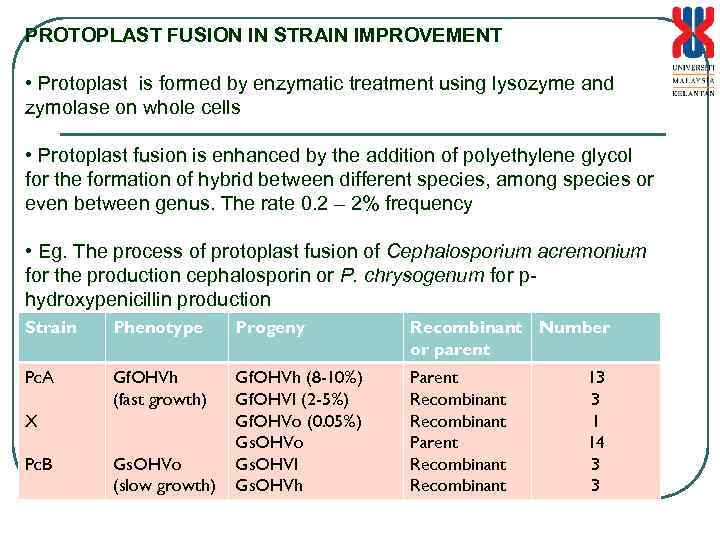
PROTOPLAST FUSION IN STRAIN IMPROVEMENT • Protoplast is formed by enzymatic treatment using lysozyme and zymolase on whole cells • Protoplast fusion is enhanced by the addition of polyethylene glycol for the formation of hybrid between different species, among species or even between genus. The rate 0. 2 – 2% frequency • Eg. The process of protoplast fusion of Cephalosporium acremonium for the production cephalosporin or P. chrysogenum for phydroxypenicillin production Strain Phenotype Progeny Recombinant Number or parent Pc. A Gf. OHVh (fast growth) Gf. OHVh (8 -10%) Gf. OHVI (2 -5%) Gf. OHVo (0. 05%) Gs. OHVo Gs. OHVI Gs. OHVh Parent Recombinant X Pc. B Gs. OHVo (slow growth) 13 3 1 14 3 3
MICROBIAL ENZYMES
Industrial processes for the production of enzymes • Enzymes are produced by living cells and acted specifically independence of the cells that produced the enzymes • Substrate spesificity – Enzyme classification (oxidoreductase, transferase, hydrolase, lyase, isomerase, ligase) Selection of microorganisms for industrial production of enzymes • • Extracellular enzymes are preferred High enzyme productivity Cheap substrates Activities of microorganisms directed to enzyme synthesis and low formation of side products. • Cheap extraction processes • Microorganisms do not produce toxins or compounds with antibiotic properties.
Cultivation techniques for enzyme production A) Submerged cultures (Liquid media) B) Solid cultures (solid state fermentation, SSF) Advantages of SSF (utilizing agrowastes as substrates: rice husks, palm kernel cake, sugar cane baggase, oil palm trunks etc. ) 1. 2. 3. 4. 5. 6. High volumetric productivities of enzymes Low power consumption Minimum process control High enzyme concentration Simple equipment for cultivation Ease in scale up processes

Types of SSF • Thin layer techniques (tray system with substrate thickness 5 – 7 cm) • Deep bed process (Substrate thickness 1 – 1. 5 m, with supply of air for sterilization and growth, mixing performed as required) INDUSTRIAL ENZYMES 1. Amylolytic enzymes • -amylase, glucoamylase, gluco-isomerase, pullulanase, iso-amylase • Starch degrading enzymes and main application in the production of sweetener • Production of -amylase by Bacillus subtilis and Aspergillus niger in medium containing 5 – 8% starch and yeast extract or malt extract as nitrogen source.
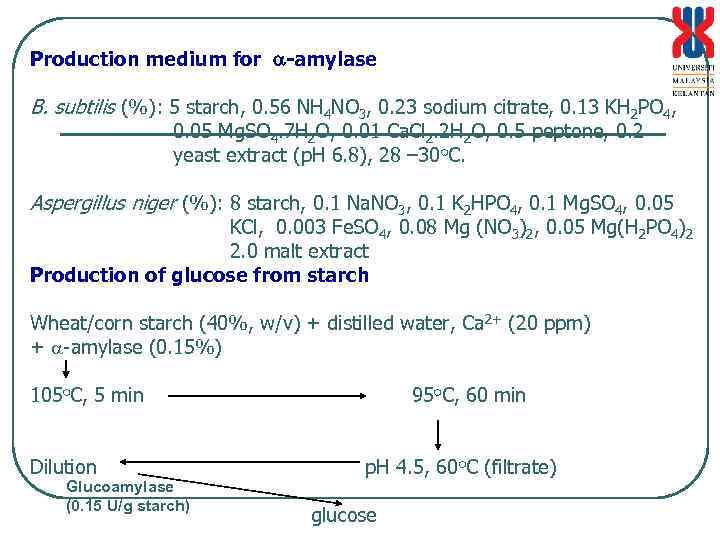
Production medium for -amylase B. subtilis (%): 5 starch, 0. 56 NH 4 NO 3, 0. 23 sodium citrate, 0. 13 KH 2 PO 4, 0. 05 Mg. SO 4. 7 H 2 O, 0. 01 Ca. Cl 2. 2 H 2 O, 0. 5 peptone, 0. 2 yeast extract (p. H 6. 8), 28 – 30 o. C. Aspergillus niger (%): 8 starch, 0. 1 Na. NO 3, 0. 1 K 2 HPO 4, 0. 1 Mg. SO 4, 0. 05 KCl, 0. 003 Fe. SO 4, 0. 08 Mg (NO 3)2, 0. 05 Mg(H 2 PO 4)2 2. 0 malt extract Production of glucose from starch Wheat/corn starch (40%, w/v) + distilled water, Ca 2+ (20 ppm) + -amylase (0. 15%) 105 o. C, 5 min Dilution Glucoamylase (0. 15 U/g starch) 95 o. C, 60 min p. H 4. 5, 60 o. C (filtrate) glucose
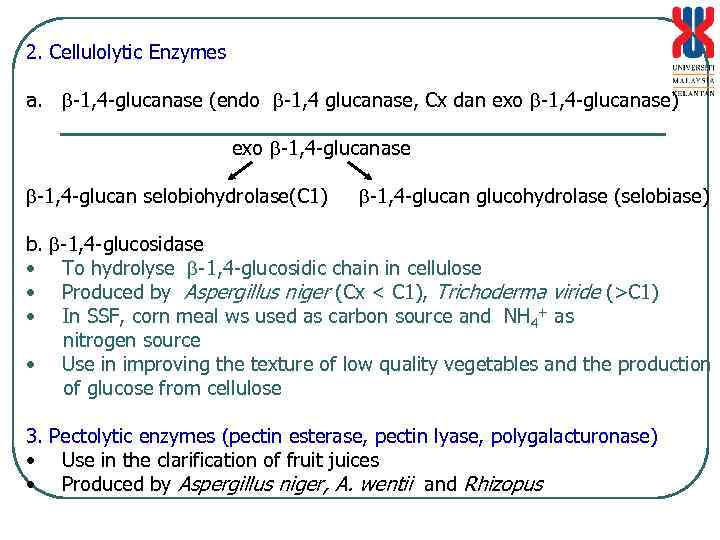
2. Cellulolytic Enzymes a. -1, 4 -glucanase (endo -1, 4 glucanase, Cx dan exo -1, 4 -glucanase) exo -1, 4 -glucanase -1, 4 -glucan selobiohydrolase(C 1) -1, 4 -glucan glucohydrolase (selobiase) -1, 4 -glucosidase To hydrolyse -1, 4 -glucosidic chain in cellulose Produced by Aspergillus niger (Cx < C 1), Trichoderma viride (>C 1) In SSF, corn meal ws used as carbon source and NH 4+ as nitrogen source • Use in improving the texture of low quality vegetables and the production of glucose from cellulose b. • • • 3. Pectolytic enzymes (pectin esterase, pectin lyase, polygalacturonase) • Use in the clarification of fruit juices • Produced by Aspergillus niger, A. wentii and Rhizopus
3. Lipolytic enzymes (lipase, esterase, lipoprotein lipase and phospholipase) • Glycerol ester hydrolase hydrolyses fats to fatty acids and glycerol • Produced by bacteria dan fungi/yeasts (Humicola, Aspergillus, Pseudomonas, Achromobacter, Rhizopus, Candida etc. ) • Oils or fats as inducers in production, although in the case of Penicillium roquefortii, oil inhibits lipase production.
4. Proteolytic enzymes (alkali, neutral and acid) • Second most important enzyme after amylolytic enzymes • • Used widely in detergency, diary, production of protein hydrolysate, pharmaceutical and leather industries • In detergency, alkali protease used was from Bacillus licheniformis and B. amyloliquefaciens • Protease production suppressed by NH 4+ and excessive amino acid • In detergent, enzyme is prepared in the microencapsulated form to prevent allergic effects. • Neutral protease less stable, thus limited applications. • Acidic protease (renin) important for cheese industry was produced by Mucor pusillus dan M. meihei.

ANTIBIOTICS
Production of antibiotic : Use of antibiotics (other than as therapeutic agents) 1. Anticancer (cytostasis agent) – adriamycin and mitramycin 2. In plant pathology: Selective at low concentration Low toxicity Degradable by soil microorganisms 3. Food preservatives Eg. Pimaricin (antifungal agent used for surface sterilization, tylosin against spores of Bacillus and nicin against spores of Clostridium. Chlortetracycline – for preservation of fish, meat and chicken at 5 ppm (in ice) or 10 ppm (submerged) 4. Inducer for growth of livestock (also used in veterinary medicine) – normally used 1 -10 mg/kg of feed. Eg. Enducidin, micamycin In veterinary medicine – coccidiostasis (monensin, lasalocida, and salinomycin). Other drugs include hygromycin B, thiostrepton and tylosin 5. Tool for biochemistry and molecular biology (DNA replication, transcription and cell wall synthesis)
Antibiotic Classification 1. -lactam (Penicillin, cephalosporin and cephamycin) Penicillin produced by P. chrysogenum is effective for gm tve bacteria which inhibit the synthesis of cell wall peptidolycan via its action on the enzyme transpeptidase and D-alanine carboxypeptidase 2. Amino acid/peptide (Actinomycin, D-cycloserine, Bacitracin) Bacitracin, a dodecapeptide (4 D and 4 L amino acid) is produced by B. licheniformis inhibits cell wall synthesis D-cycloserine (S. orchidaceus, S. lavendulae, S. garyphalus, S. roseochromogenes ) Polymyxin (Pseudomonas aeruginosa) 3. Carbohydrate (glycoside and sugar derivatives) Vancomycin - wide spectrum, contains vancosamine, effective against Staphylococcus Avilamycin – produced by S. viridochromogenes used as food additive 4. Aminoglycoside (Streptomycin) Inhibit the synthesis of protein via the binding of S 12 ribosome of the 30 S subunit 5. Tetracycline (with naphthacene ring) Chlortetracycline (S. aureofaciens) and tetracycline (S. viridifaciens) , wide spectrum against gram +ve and –ve, riketsia, mycoplasma, leptospira, spirochate, and chlamydia 6. Aromatic (Chloramphenicol (bakteriostat on protein synthesis ) and Griseofulvin) – Fungistat against chitinous cell wall
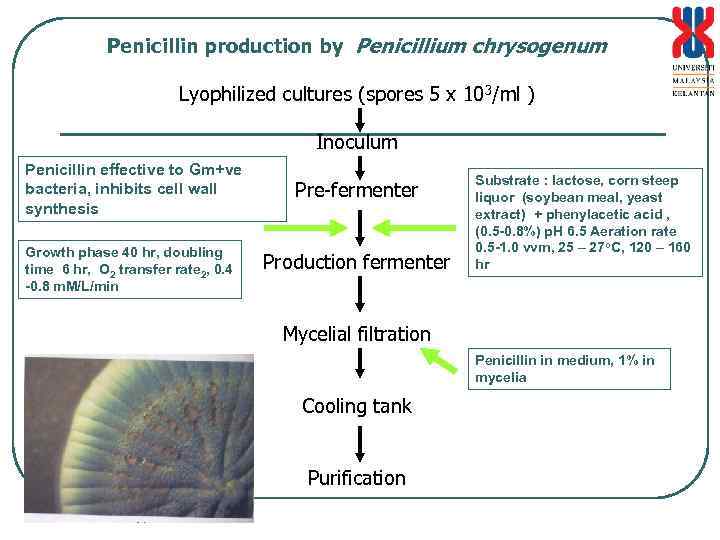
Penicillin production by Penicillium chrysogenum Lyophilized cultures (spores 5 x 103/ml ) Inoculum Penicillin effective to Gm+ve bacteria, inhibits cell wall synthesis Growth phase 40 hr, doubling time 6 hr, O 2 transfer rate 2, 0. 4 -0. 8 m. M/L/min Pre-fermenter Production fermenter Substrate : lactose, corn steep liquor (soybean meal, yeast extract) + phenylacetic acid , (0. 5 -0. 8%) p. H 6. 5 Aeration rate 0. 5 -1. 0 vvm, 25 – 27 o. C, 120 – 160 hr Mycelial filtration Penicillin in medium, 1% in mycelia Cooling tank Purification
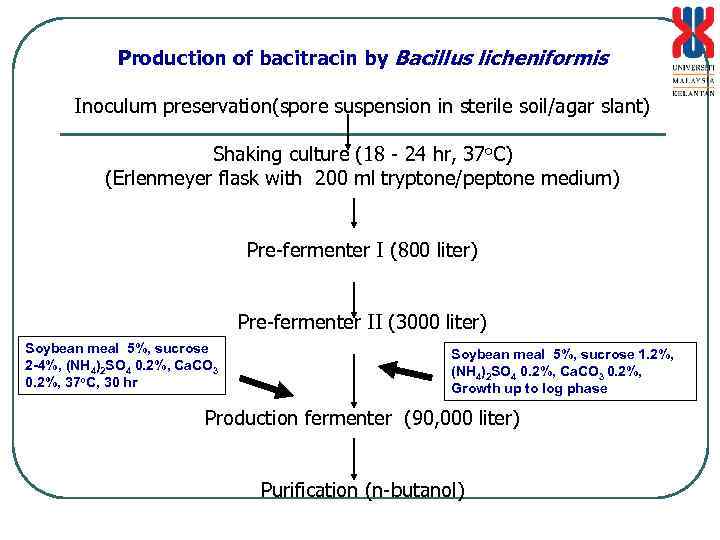
Production of bacitracin by Bacillus licheniformis Inoculum preservation(spore suspension in sterile soil/agar slant) Shaking culture (18 - 24 hr, 37 o. C) (Erlenmeyer flask with 200 ml tryptone/peptone medium) Pre-fermenter I (800 liter) Pre-fermenter II (3000 liter) Soybean meal 5%, sucrose 2 -4%, (NH 4)2 SO 4 0. 2%, Ca. CO 3 0. 2%, 37 o. C, 30 hr Soybean meal 5%, sucrose 1. 2%, (NH 4)2 SO 4 0. 2%, Ca. CO 3 0. 2%, Growth up to log phase Production fermenter (90, 000 liter) Purification (n-butanol)
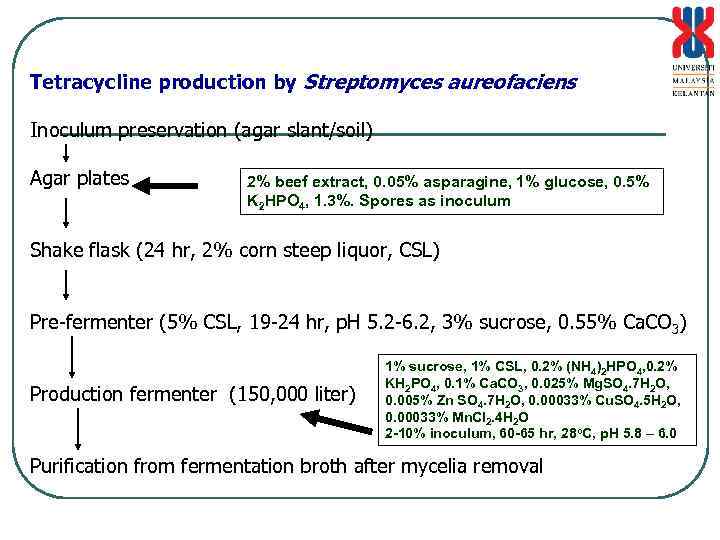
Tetracycline production by Streptomyces aureofaciens Inoculum preservation (agar slant/soil) Agar plates 2% beef extract, 0. 05% asparagine, 1% glucose, 0. 5% K 2 HPO 4, 1. 3%. Spores as inoculum Shake flask (24 hr, 2% corn steep liquor, CSL) Pre-fermenter (5% CSL, 19 -24 hr, p. H 5. 2 -6. 2, 3% sucrose, 0. 55% Ca. CO 3) Production fermenter (150, 000 liter) 1% sucrose, 1% CSL, 0. 2% (NH 4)2 HPO 4, 0. 2% KH 2 PO 4, 0. 1% Ca. CO 3, 0. 025% Mg. SO 4. 7 H 2 O, 0. 005% Zn SO 4. 7 H 2 O, 0. 00033% Cu. SO 4. 5 H 2 O, 0. 00033% Mn. Cl 2. 4 H 2 O 2 -10% inoculum, 60 -65 hr, 28 o. C, p. H 5. 8 – 6. 0 Purification from fermentation broth after mycelia removal

PRODUCTION OF ORGANIC SOLVENTS AND ORGANIC & AMINO ACIDS
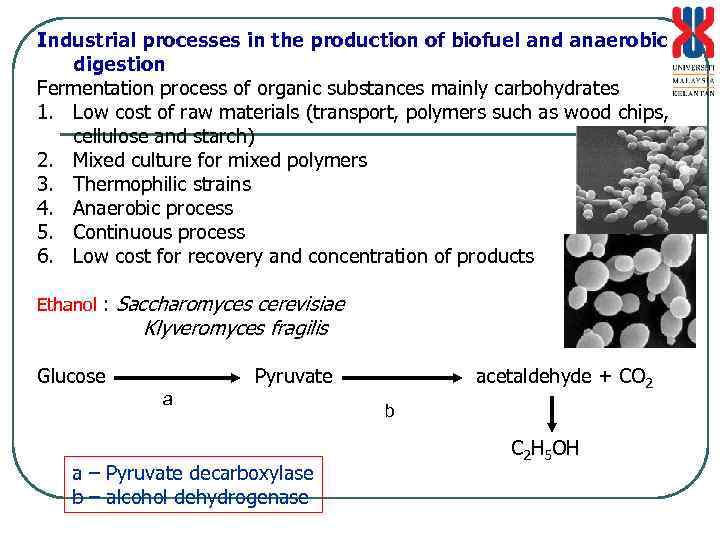
Industrial processes in the production of biofuel and anaerobic digestion Fermentation process of organic substances mainly carbohydrates 1. Low cost of raw materials (transport, polymers such as wood chips, cellulose and starch) 2. Mixed culture for mixed polymers 3. Thermophilic strains 4. Anaerobic process 5. Continuous process 6. Low cost for recovery and concentration of products Ethanol : Saccharomyces cerevisiae Klyveromyces fragilis Glucose Pyruvate a a – Pyruvate decarboxylase b – alcohol dehydrogenase acetaldehyde + CO 2 b C 2 H 5 OH
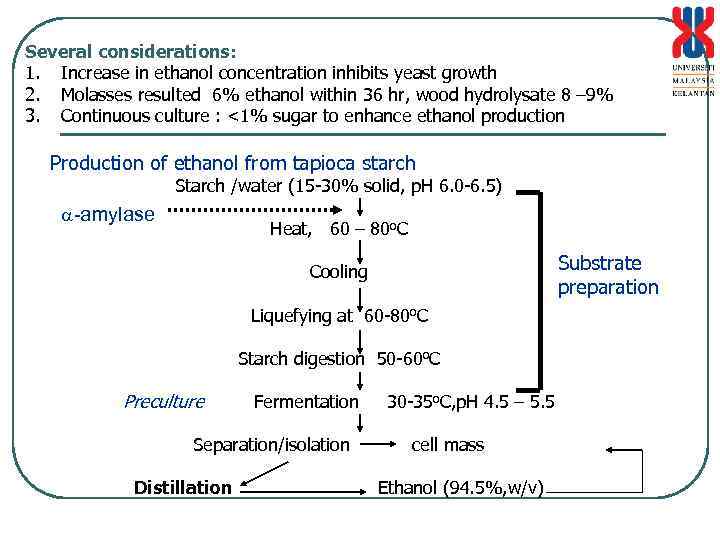
Several considerations: 1. Increase in ethanol concentration inhibits yeast growth 2. Molasses resulted 6% ethanol within 36 hr, wood hydrolysate 8 – 9% 3. Continuous culture : <1% sugar to enhance ethanol production Production of ethanol from tapioca starch Starch /water (15 -30% solid, p. H 6. 0 -6. 5) -amylase Heat, 60 – 80 o. C Substrate preparation Cooling Liquefying at 60 -80 o. C Starch digestion 50 -60 o. C Preculture Fermentation Separation/isolation Distillation 30 -35 o. C, p. H 4. 5 – 5. 5 cell mass Ethanol (94. 5%, w/v)
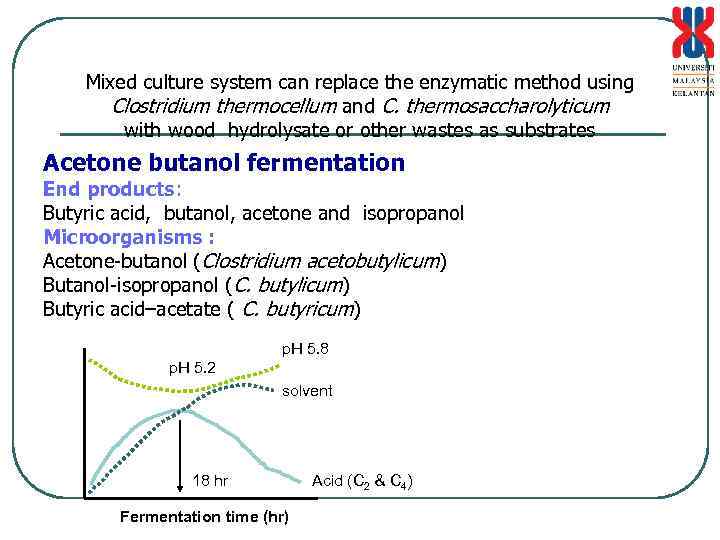
Mixed culture system can replace the enzymatic method using Clostridium thermocellum and C. thermosaccharolyticum with wood hydrolysate or other wastes as substrates Acetone butanol fermentation End products: Butyric acid, butanol, acetone and isopropanol Microorganisms : Acetone-butanol (Clostridium acetobutylicum) Butanol-isopropanol (C. butylicum) Butyric acid–acetate ( C. butyricum) p. H 5. 8 p. H 5. 2 solvent 18 hr Fermentation time (hr) Acid (C 2 & C 4)
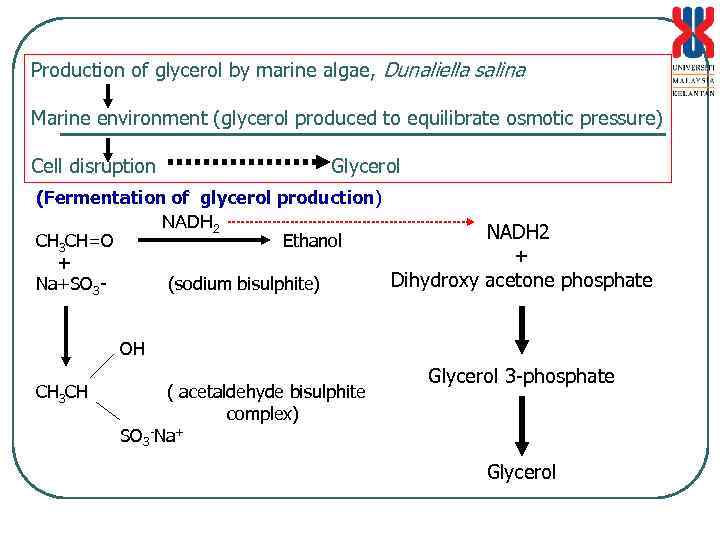
Production of glycerol by marine algae, Dunaliella salina Marine environment (glycerol produced to equilibrate osmotic pressure) Cell disruption Glycerol (Fermentation of glycerol production) NADH 2 CH 3 CH=O Ethanol + + Dihydroxy acetone phosphate Na+SO 3(sodium bisulphite) OH CH 3 CH ( acetaldehyde bisulphite complex) SO 3 -Na+ Glycerol 3 -phosphate Glycerol

• Hydrolytic and fermentative bacteria – obligate/facultative anaerobe 108 -109/ml sludge, rate limiting step • Acetogenic bacteria, obligate hydrogen producer – 4 x 106/ml sludge Synthrophobacter wolfei (butyrate to acetate) Synthrophobacter wolinii (propionate to acetate + H 2) • Methanogen – 106 -108/ml sludge, archaebacteria, coenzim F 420/F 430 Source of wastes for anaerobic digestion 1. Industrial wastes and food processing wastes 2. Animal feces and agrowastes/biomass 3. Domestic or municipal wastes Need to determine solid substance, organic content, and BOD/COD level Chemical composition (P, trace element, N source [C: N 40: 1], NH 3, SO 4 and heavy metal
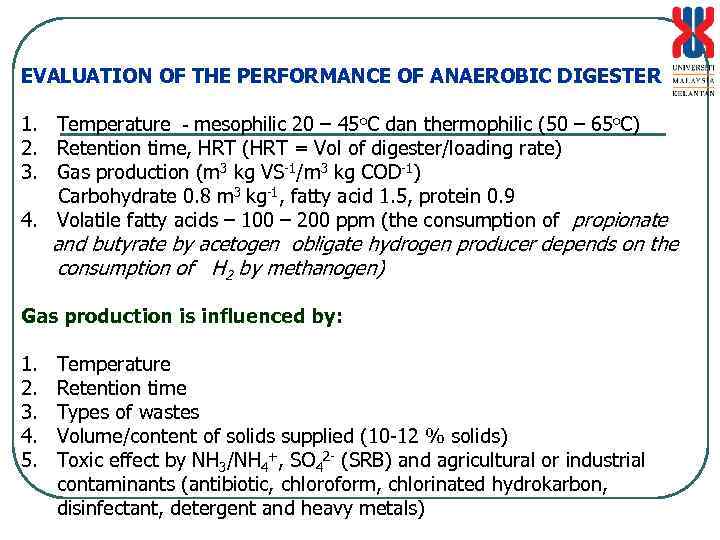
EVALUATION OF THE PERFORMANCE OF ANAEROBIC DIGESTER 1. Temperature - mesophilic 20 – 45 o. C dan thermophilic (50 – 65 o. C) 2. Retention time, HRT (HRT = Vol of digester/loading rate) 3. Gas production (m 3 kg VS-1/m 3 kg COD-1) Carbohydrate 0. 8 m 3 kg-1, fatty acid 1. 5, protein 0. 9 4. Volatile fatty acids – 100 – 200 ppm (the consumption of propionate and butyrate by acetogen obligate hydrogen producer depends on the consumption of H 2 by methanogen) Gas production is influenced by: 1. 2. 3. 4. 5. Temperature Retention time Types of wastes Volume/content of solids supplied (10 -12 % solids) Toxic effect by NH 3/NH 4+, SO 42 - (SRB) and agricultural or industrial contaminants (antibiotic, chloroform, chlorinated hydrokarbon, disinfectant, detergent and heavy metals)
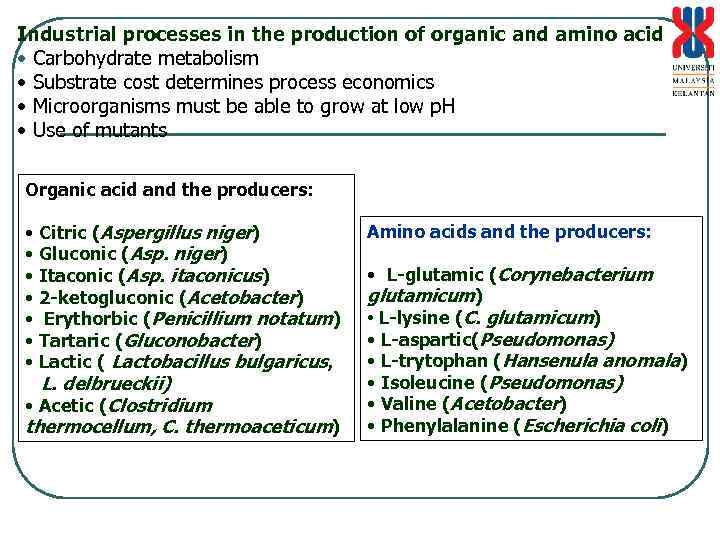
Industrial processes in the production of organic and amino acid • Carbohydrate metabolism • Substrate cost determines process economics • Microorganisms must be able to grow at low p. H • Use of mutants Organic acid and the producers: • Citric (Aspergillus niger) • Gluconic (Asp. niger) • Itaconic (Asp. itaconicus) • 2 -ketogluconic (Acetobacter) • Erythorbic (Penicillium notatum) • Tartaric (Gluconobacter) • Lactic ( Lactobacillus bulgaricus, L. delbrueckii) • Acetic (Clostridium thermocellum, C. thermoaceticum) Amino acids and the producers: • L-glutamic (Corynebacterium glutamicum) • L-lysine (C. glutamicum) • L-aspartic(Pseudomonas) • L-trytophan (Hansenula anomala) • Isoleucine (Pseudomonas) • Valine (Acetobacter) • Phenylalanine (Escherichia coli)
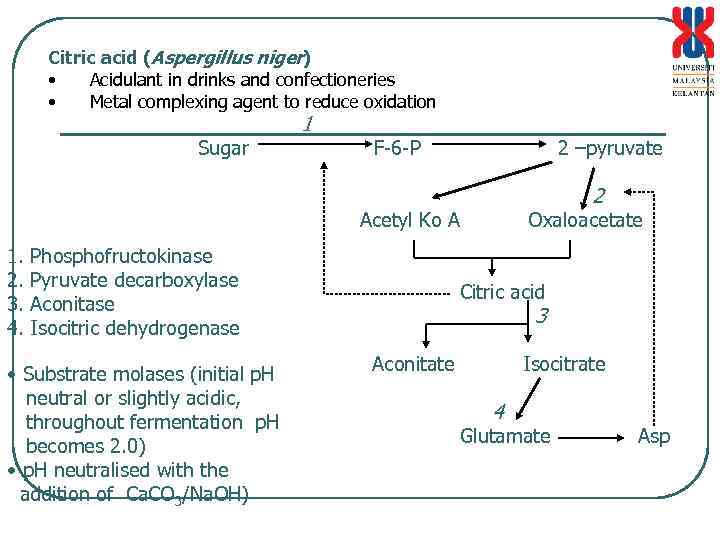
Citric acid (Aspergillus niger) • Acidulant in drinks and confectioneries • Metal complexing agent to reduce oxidation Sugar 1 F-6 -P 2 –pyruvate 2 Acetyl Ko A 1. 2. 3. 4. Phosphofructokinase Pyruvate decarboxylase Aconitase Isocitric dehydrogenase • Substrate molases (initial p. H neutral or slightly acidic, throughout fermentation p. H becomes 2. 0) • p. H neutralised with the addition of Ca. CO 3/Na. OH) Oxaloacetate Citric acid 3 Aconitate Isocitrate 4 Glutamate Asp
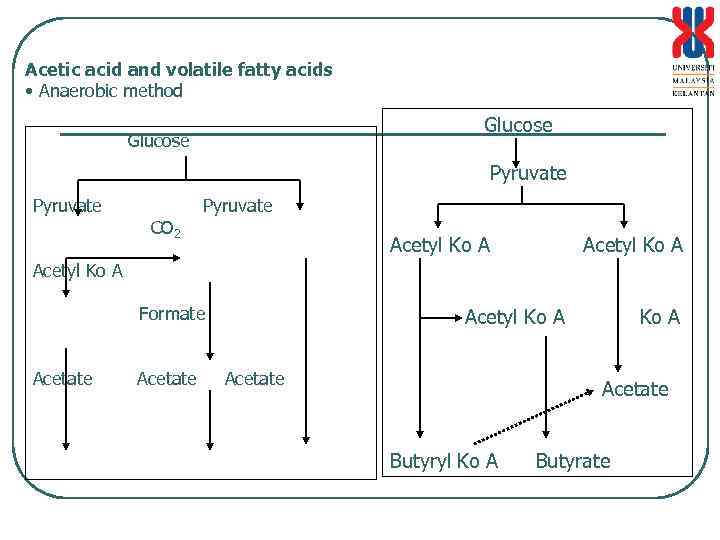
Acetic acid and volatile fatty acids • Anaerobic method Glucose Glucose Pyruvate CO 2 Pyruvate Acetyl Ko A Formate Acetate Acetyl Ko A Acetate Butyryl Ko A Butyrate
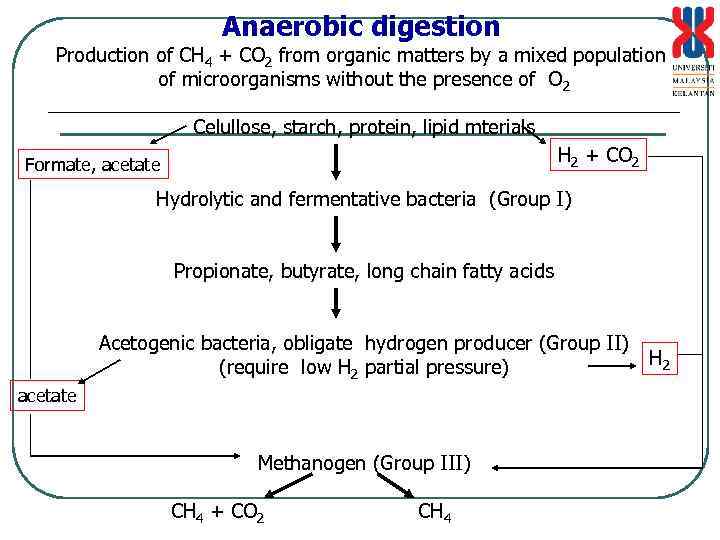
Anaerobic digestion Production of CH 4 + CO 2 from organic matters by a mixed population of microorganisms without the presence of O 2 _____________________________ Celullose, starch, protein, lipid mterials H 2 + CO 2 Formate, acetate Hydrolytic and fermentative bacteria (Group I) Propionate, butyrate, long chain fatty acids Acetogenic bacteria, obligate hydrogen producer (Group II) (require low H 2 partial pressure) acetate Methanogen (Group III) CH 4 + CO 2 CH 4 H 2
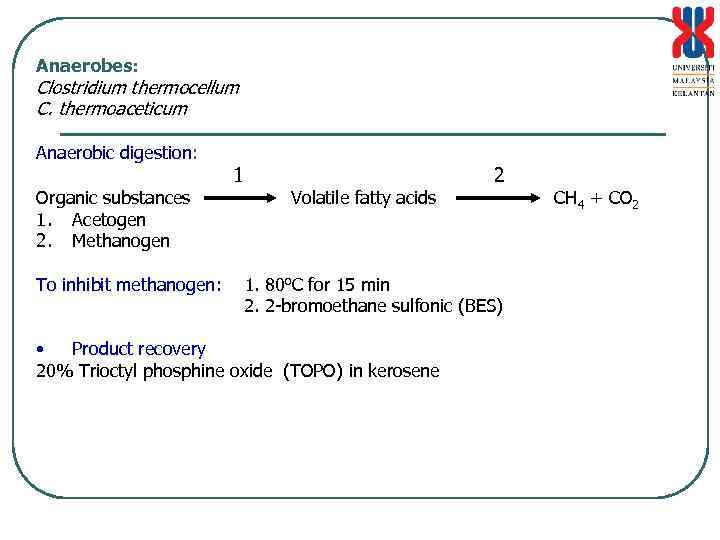
Anaerobes: Clostridium thermocellum C. thermoaceticum Anaerobic digestion: Organic substances 1. Acetogen 2. Methanogen To inhibit methanogen: 1 Volatile fatty acids 2 1. 80 o. C for 15 min 2. 2 -bromoethane sulfonic (BES) • Product recovery 20% Trioctyl phosphine oxide (TOPO) in kerosene CH 4 + CO 2
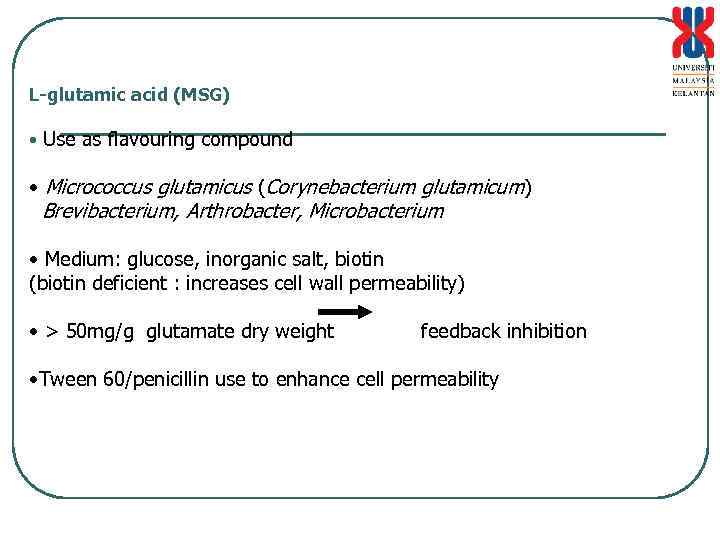
L-glutamic acid (MSG) • Use as flavouring compound • Micrococcus glutamicus (Corynebacterium glutamicum) Brevibacterium, Arthrobacter, Microbacterium • Medium: glucose, inorganic salt, biotin (biotin deficient : increases cell wall permeability) • > 50 mg/g glutamate dry weight feedback inhibition • Tween 60/penicillin use to enhance cell permeability
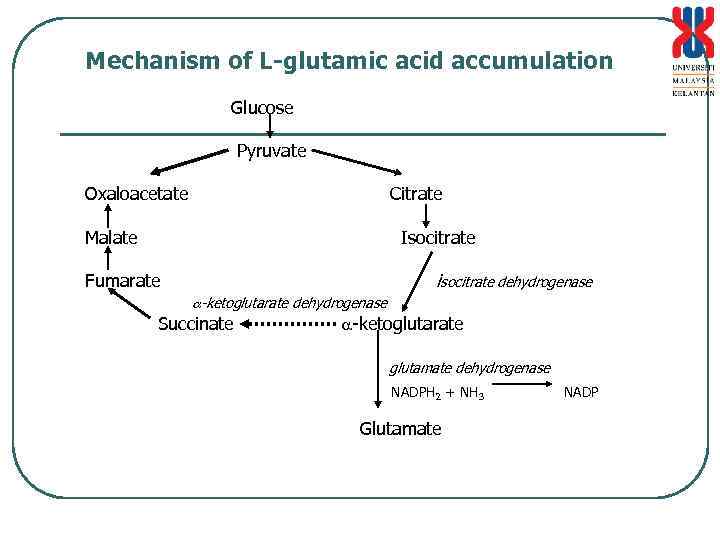
Mechanism of L-glutamic acid accumulation Glucose Pyruvate Oxaloacetate Citrate Malate Isocitrate isocitrate dehydrogenase Fumarate -ketoglutarate dehydrogenase Succinate -ketoglutarate glutamate dehydrogenase NADPH 2 + NH 3 Glutamate NADP
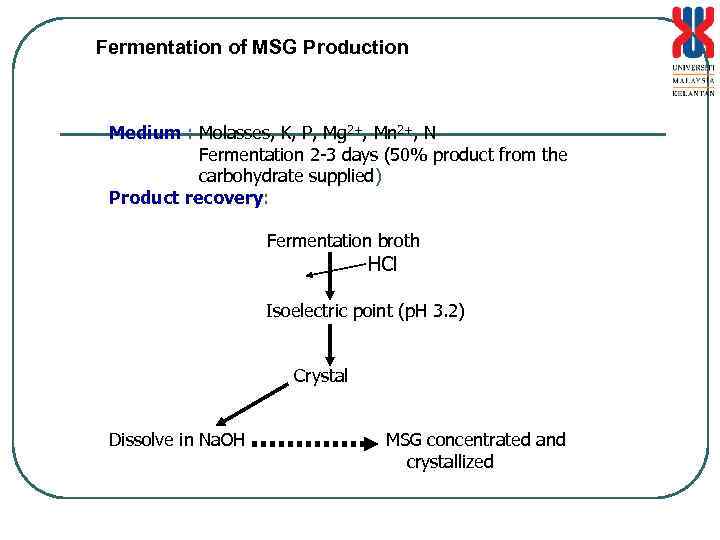
Fermentation of MSG Production Medium : Molasses, K, P, Mg 2+, Mn 2+, N Fermentation 2 -3 days (50% product from the carbohydrate supplied) Product recovery: Fermentation broth HCl Isoelectric point (p. H 3. 2) Crystal Dissolve in Na. OH MSG concentrated and crystallized

Aspartic acid (use as sweetener) • Brevibacterium or Corynebacterium using glucose • Fumarate aspartase Aspartate NH 4 Producer of aspartase: Escherichia coli, Bacteroides succinum, Serratia marcensens and Pseudomonas trifolii Production method • Cell washed with cetyl pyridinium bromide to increase cell permeability Immobilized cells – Acrylamide gel • Fumarate added in the fermentation process during the maximum production of aspartase.
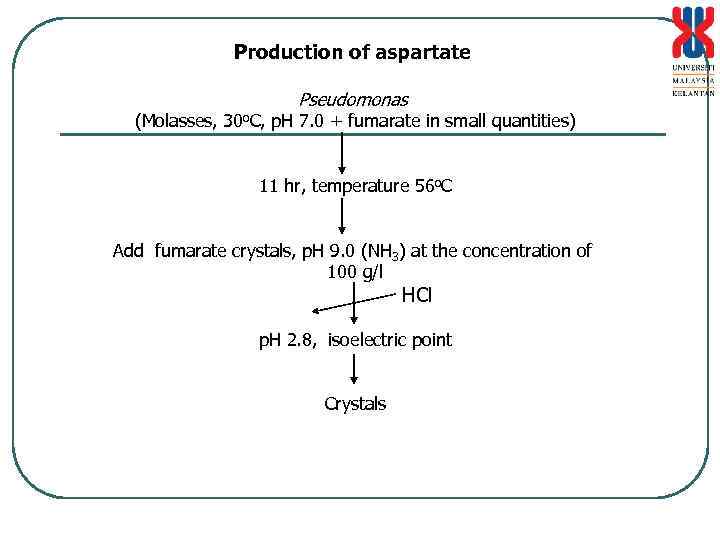
Production of aspartate Pseudomonas (Molasses, 30 o. C, p. H 7. 0 + fumarate in small quantities) 11 hr, temperature 56 o. C Add fumarate crystals, p. H 9. 0 (NH 3) at the concentration of 100 g/l HCl p. H 2. 8, isoelectric point Crystals

FERMENTATION PROCESSES FOR THE PRODUCTION OF PHYSIOLOGICALLY ACTIVE SUBSTANCES

Pigment and Vitamin i. -carotene • • Precursor for Vitamin A Used as colourant (Eg. in margarine) Isoprene derivatives with high unsaturation -carotene posseses the -ionone ring structure Process for the production of -carotene by Blakeslea trispora B. trispora NRRL 2456(+) B. trispora NRRL 2457(-) Inoculum: sterile soil Culture on agar slant (incubation 168 h, 27 o. C) Preculture (400 m. L)- 48 h, 26 o. C 1 g/L -ionone added in the production medium after 48 h Mixed culture(120 L) – inoculum 400 m. L, 40 h, 26 o. C, 170 rpm, 1 vvm Production culture(320 L) – inoculum 32 L, 185 h, 26 o. C, 210 rpm, 1. 3 vvm
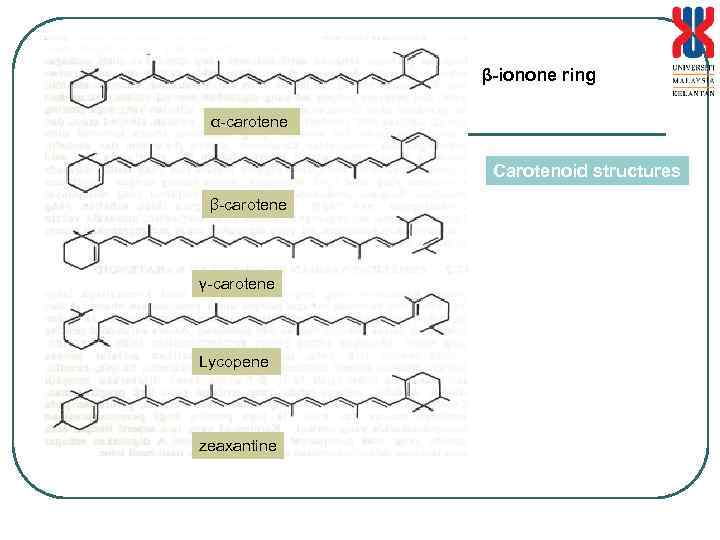
β-ionone ring α-carotene Carotenoid structures β-carotene γ-carotene Lycopene zeaxantine
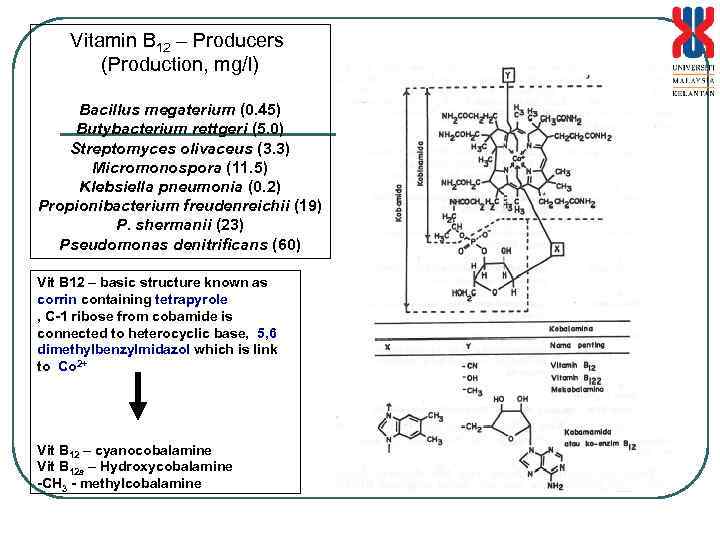
Vitamin B 12 – Producers (Production, mg/l) Bacillus megaterium (0. 45) Butybacterium rettgeri (5. 0) Streptomyces olivaceus (3. 3) Micromonospora (11. 5) Klebsiella pneumonia (0. 2) Propionibacterium freudenreichii (19) P. shermanii (23) Pseudomonas denitrificans (60) Vit B 12 – basic structure known as corrin containing tetrapyrole , C-1 ribose from cobamide is connected to heterocyclic base, 5, 6 dimethylbenzylmidazol which is link to Co 2+ Vit B 12 – cyanocobalamine Vit B 12 a – Hydroxycobalamine -CH 3 - methylcobalamine
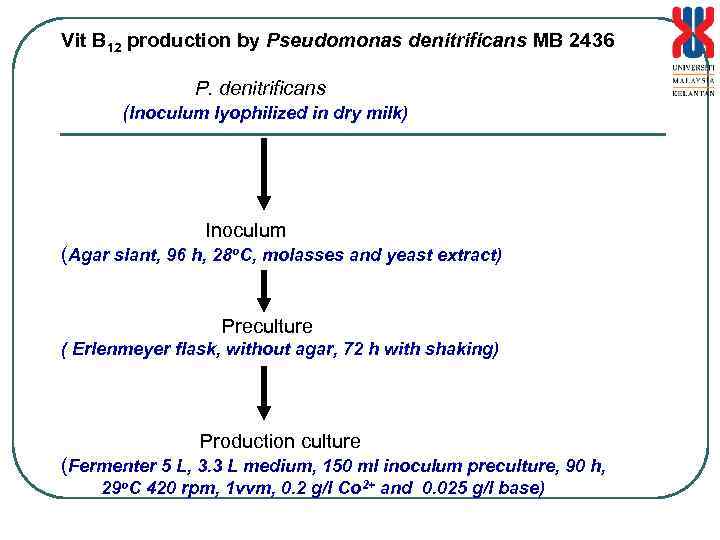
Vit B 12 production by Pseudomonas denitrificans MB 2436 P. denitrificans (Inoculum lyophilized in dry milk) Inoculum (Agar slant, 96 h, 28 o. C, molasses and yeast extract) Preculture ( Erlenmeyer flask, without agar, 72 h with shaking) Production culture (Fermenter 5 L, 3. 3 L medium, 150 ml inoculum preculture, 90 h, 29 o. C 420 rpm, 1 vvm, 0. 2 g/l Co 2+ and 0. 025 g/l base)
Riboflavine (Vitamin B 2) – Producers 1. Clostridium actobutylicum (97 mg/l) 2. Mycobacterium smegmatis (58 mg/l) 3. Mucocandida riboflavina (200 mg/l) 4. Candida flaneri (567 mg/l) 5. Eremothecium ashbyii (2480 mg/l) 6. Ashbya gossypii (6420 mg/l) Production medium: Corn steep liquour 2. 25%, Peptone 3. 5%, soy bean oil 4. 5%, inoculum 0. 75 – 2% (culture 24 - 48 h, 7 days, 0. 3 vvm, 28 o. C Ergot Alkaloid Produced by Clavicep purpurea grown on rye grass (ergot) • Enhances simpathetic nervous system and affected smooth muscles and antagonisme against adrenaline dan serotonin • Chronic poisoning – ergotisme (vomitting, convulsion dan gangrene. • 2 classes : Clavine alkaloid (95%) – with ergoline ring Lysergic acid alkaloid (Ergo) • Production in submerged system C. paspali - -hydroxy ethyl lysergamide, 8, 9 -lysergic acid, ergometerine C. fusiformis – Clavine (mainly ergoclavine) C. purpurea - Ergotamine, ergosin, ergocornin, ergocristine, ergocriptine
Vaccine production Immunity – antibody dan antigen - Active immunity and passive immunity 3 types of antigen: 1. Killed vaccine: Bordetella pertussi ( whooping cough) Mixed culture Salmonella typhi and S. paratyphi, polio salk vaccine 2. Bacterial toxoid – toxin which are deactivated with formalin such tetanus toxoid(Clostridium tetani) and diphtheria toxoid (Corynebacterium diptheriae) 3. Live vaccine – Vaccinia virus(cacar), polio vaccines, BCG (tuberculosis) dan Brucella obortus vaccines.
Production of polio salk vaccines Kidney, cleaned with Hank Solution Cut into fragments, washed with saline phosphate buffer solution Immersed in 0. 25% trypsin in Dulbecco Solution (PBS), p. H 7. 4 -8. 0, at trypsin optimum temperature Serial transfer at 30 o. C Cell suspension diluted 100 - 500 X using the medium in Roux bottle Cell layer incubated for 4 -6 days at 37 o. C Inoculate with virus, 4 days , 37 oc (cytopathic effect) Virus particles harvested Deactivated with formalin and stored at 4 o. C.
Microbial Technology.ppt