Microbial metabolism, enzimes and nutrition.ppt
- Количество слайдов: 102

Microbial physiology. Microbial metabolism. Enzymes. Nutrition. Bioenergetics. Bacterial growth and multiplication. Konrad T. By Juszkiewicz, MD, MPH
Microbial physiology. Microbial metabolism. Enzymes. Nutrition. Bioenergetics. Bacterial growth and multiplication.

Microbial metabolism the Greek metabole, meaning change. Metabolism - the sum of the biochemical reactions required for energy generation AND the use of energy to synthesize cell material from small molecules in the environment.

Why do we must know the metabolism of bacteria? Because we want to know how to inhibit or stop bacteria growth and want to control their metabolism.
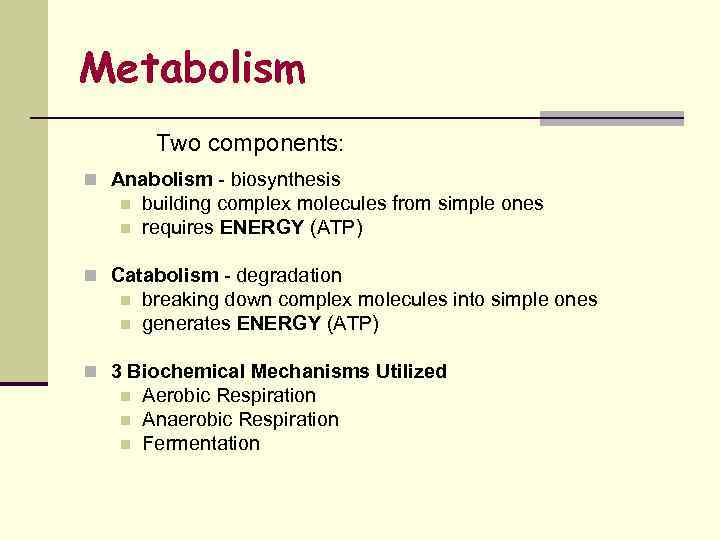
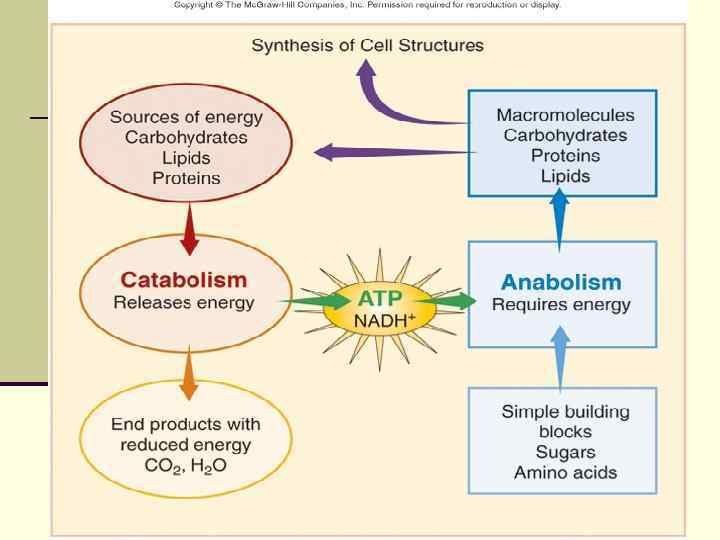
Metabolism Two components: n Anabolism - biosynthesis n n building complex molecules from simple ones requires ENERGY (ATP) n Catabolism - degradation n n breaking down complex molecules into simple ones generates ENERGY (ATP) n 3 Biochemical Mechanisms Utilized n n n Aerobic Respiration Anaerobic Respiration Fermentation
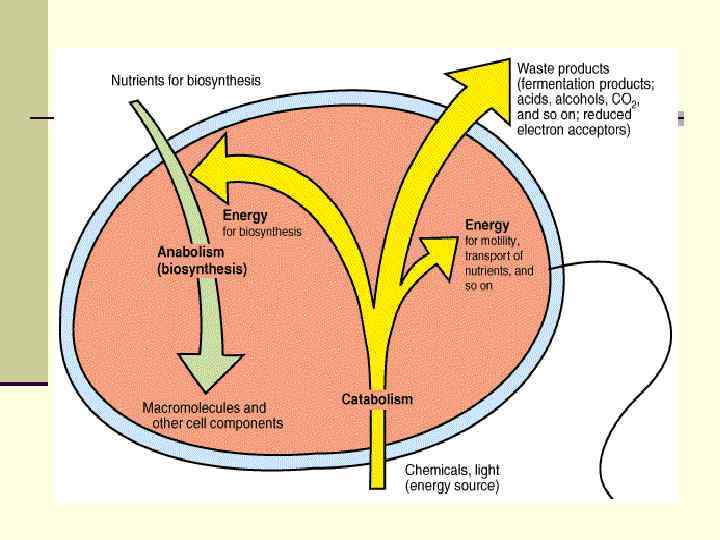
n Catabolic reactions or sequences produce energy as ATP adenosine triphosphate , which can be utilized in anabolic reactions to build cell material from nutrients in the environment.

METABOLIC DIVERSITY n Bacterial metabolism is classified into nutritional groups on the basis of three major criteria: 1. Source of energy, used for growth 2. Source of carbon, and 3. Sours of electron donors used for growth.
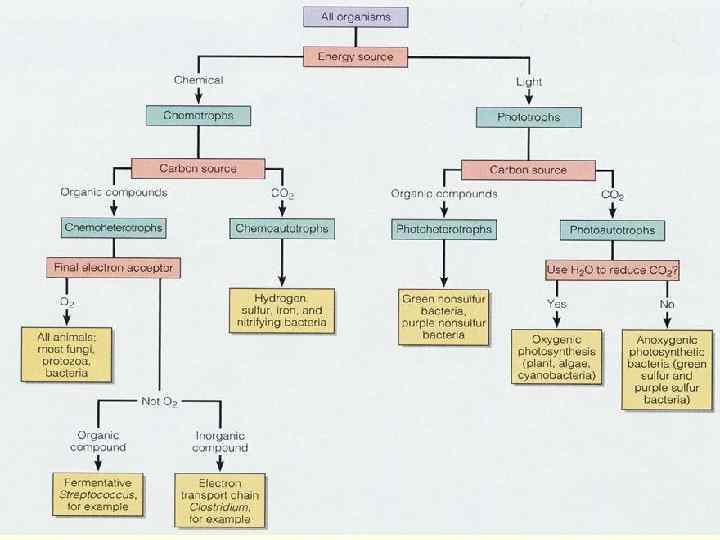

1. ENERGY SOURCE n a. Phototrophs —can use light energy n b. Chemotrophs —must obtain energy from oxidation-reduction of external chemical compounds
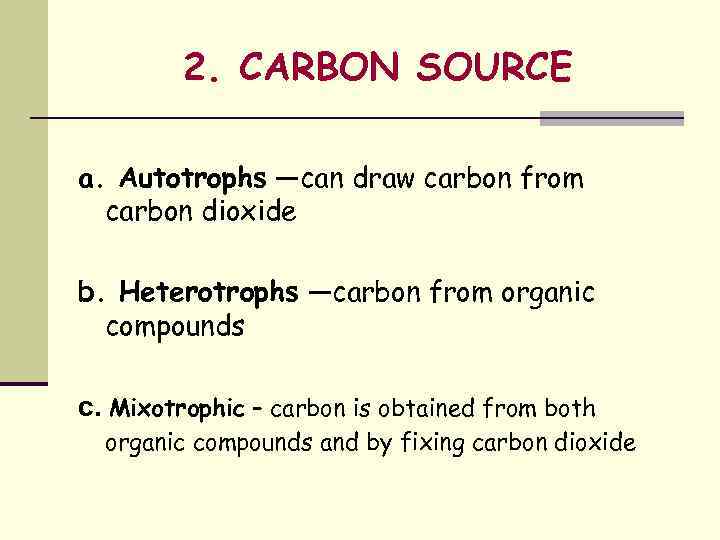
2. CARBON SOURCE a. Autotrophs —can draw carbon from carbon dioxide b. Heterotrophs —carbon from organic compounds c. Mixotrophic – carbon is obtained from both organic compounds and by fixing carbon dioxide

These requirements can be combined: 1. Photoautotrophs - light energy, carbon from 2. Photoheterotrophs —light energy, carbon from organic compounds 3. Chemoautotrophs —energy from chemical compounds, carbon from CO 2 4. Chemoheterotrophs —energy from chemical compounds, carbon from organic compounds
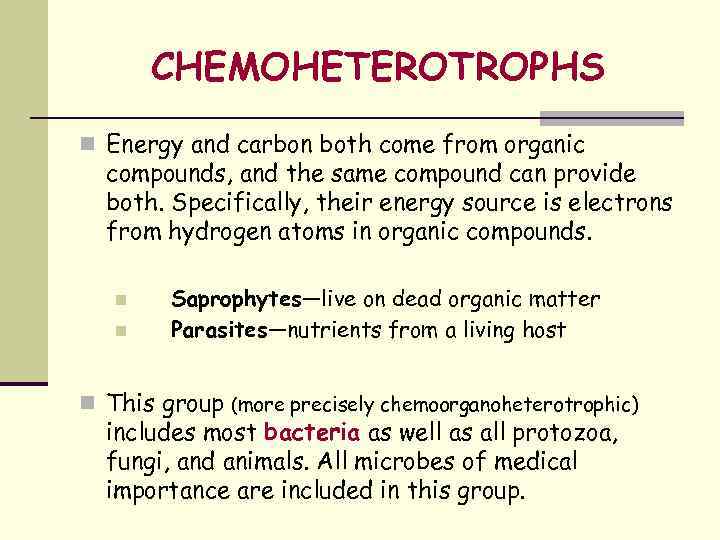
CHEMOHETEROTROPHS n Energy and carbon both come from organic compounds, and the same compound can provide both. Specifically, their energy source is electrons from hydrogen atoms in organic compounds. n n Saprophytes—live on dead organic matter Parasites—nutrients from a living host n This group (more precisely chemoorganoheterotrophic) includes most bacteria as well as all protozoa, fungi, and animals. All microbes of medical importance are included in this group.
Microbial physiology. Microbial metabolism. Bioenergetics. Enzymes. Nutrition. Bacterial growth and multiplication.
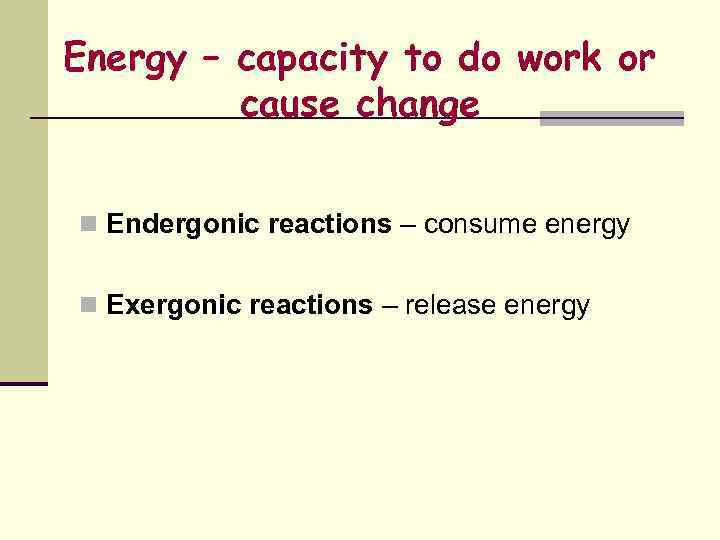
Energy – capacity to do work or cause change n Endergonic reactions – consume energy n Exergonic reactions – release energy
Energy Production n 3 Biochemical Mechanisms Utilized n n n Aerobic Respiration Anaerobic Respiration Fermentation
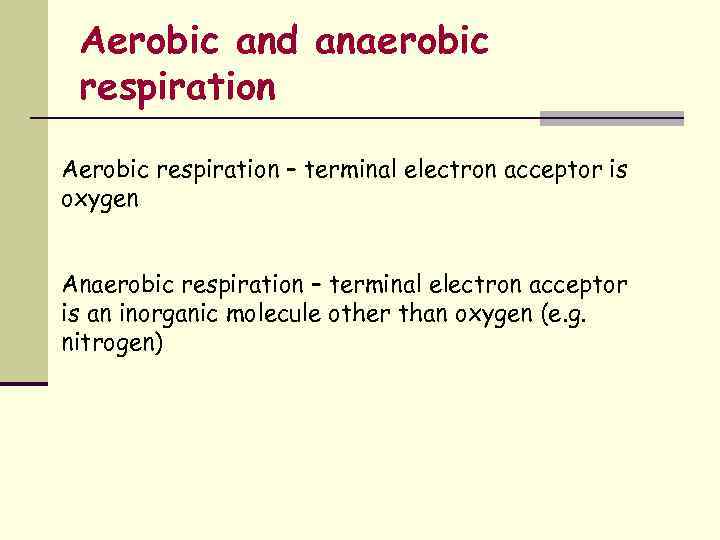
Aerobic and anaerobic respiration Aerobic respiration – terminal electron acceptor is oxygen Anaerobic respiration – terminal electron acceptor is an inorganic molecule other than oxygen (e. g. nitrogen)
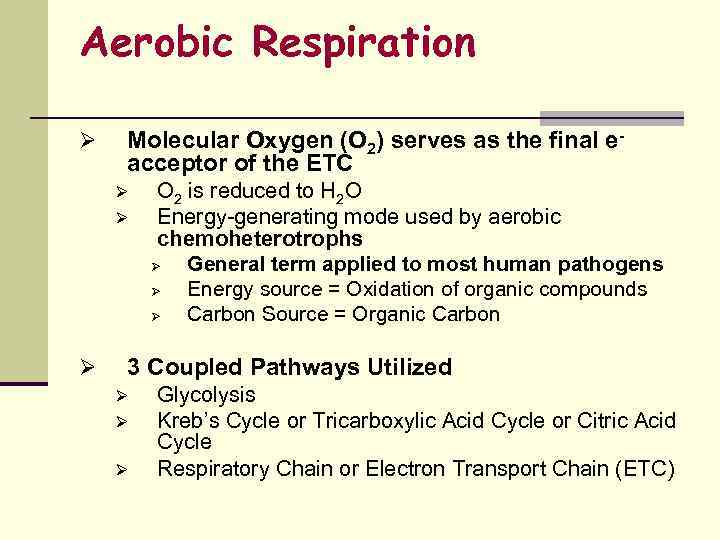
Aerobic Respiration Ø Molecular Oxygen (O 2) serves as the final eacceptor of the ETC Ø Ø Ø O 2 is reduced to H 2 O Energy-generating mode used by aerobic chemoheterotrophs Ø General term applied to most human pathogens Ø Energy source = Oxidation of organic compounds Ø Carbon Source = Organic Carbon 3 Coupled Pathways Utilized Ø Ø Ø Glycolysis Kreb’s Cycle or Tricarboxylic Acid Cycle or Citric Acid Cycle Respiratory Chain or Electron Transport Chain (ETC)
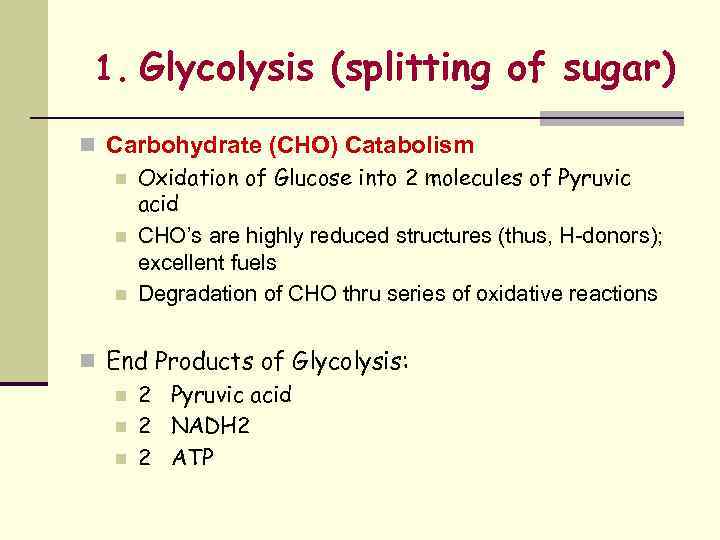
1. Glycolysis (splitting of sugar) n Carbohydrate (CHO) Catabolism n Oxidation of Glucose into 2 molecules of Pyruvic acid n CHO’s are highly reduced structures (thus, H-donors); excellent fuels n Degradation of CHO thru series of oxidative reactions n End Products of Glycolysis: n 2 Pyruvic acid n 2 NADH 2 n 2 ATP
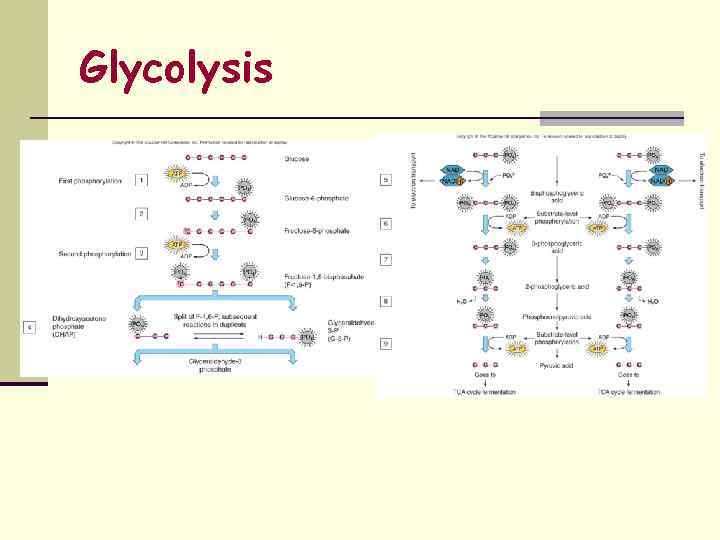
Glycolysis
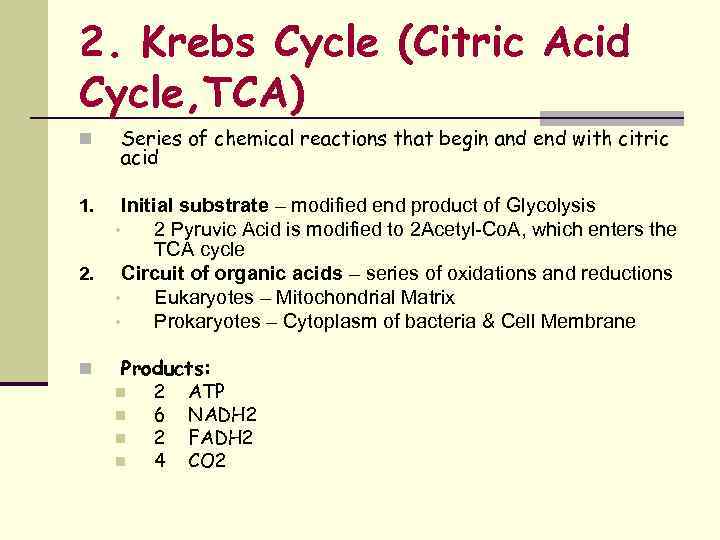
2. Krebs Cycle (Citric Acid Cycle, TCA) n Series of chemical reactions that begin and end with citric acid 1. Initial substrate – modified end product of Glycolysis • 2 Pyruvic Acid is modified to 2 Acetyl-Co. A, which enters the TCA cycle Circuit of organic acids – series of oxidations and reductions • Eukaryotes – Mitochondrial Matrix • Prokaryotes – Cytoplasm of bacteria & Cell Membrane 2. n Products: n n 2 6 2 4 ATP NADH 2 FADH 2 CO 2
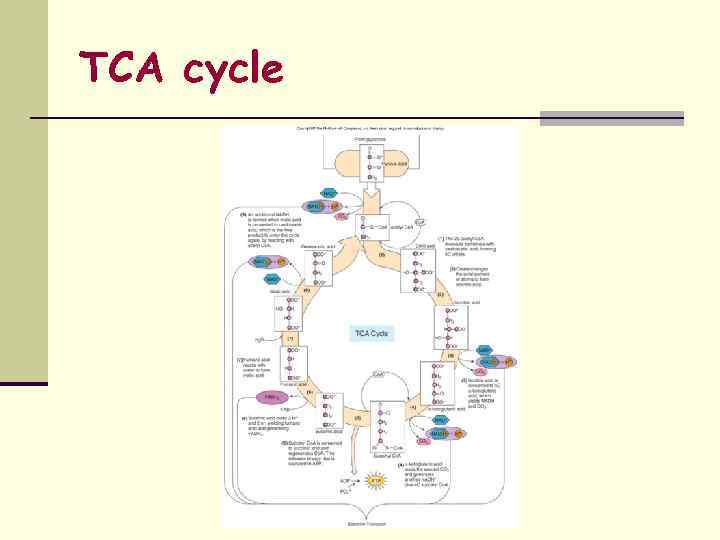
TCA cycle
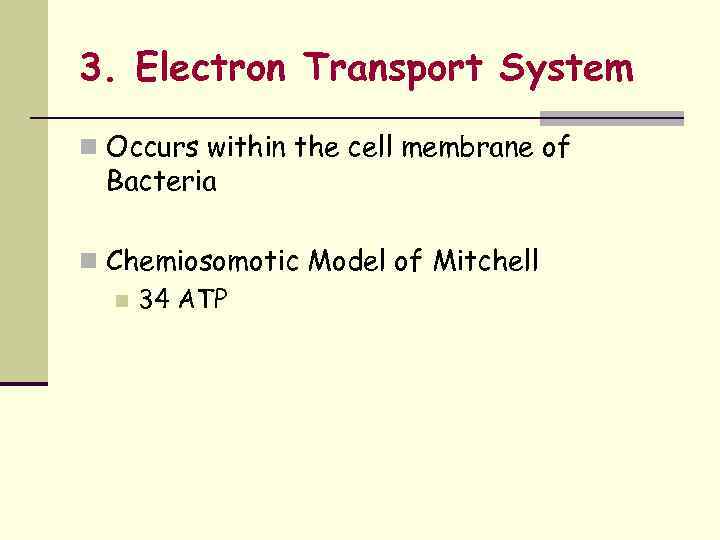
3. Electron Transport System n Occurs within the cell membrane of Bacteria n Chemiosomotic Model of Mitchell n 34 ATP
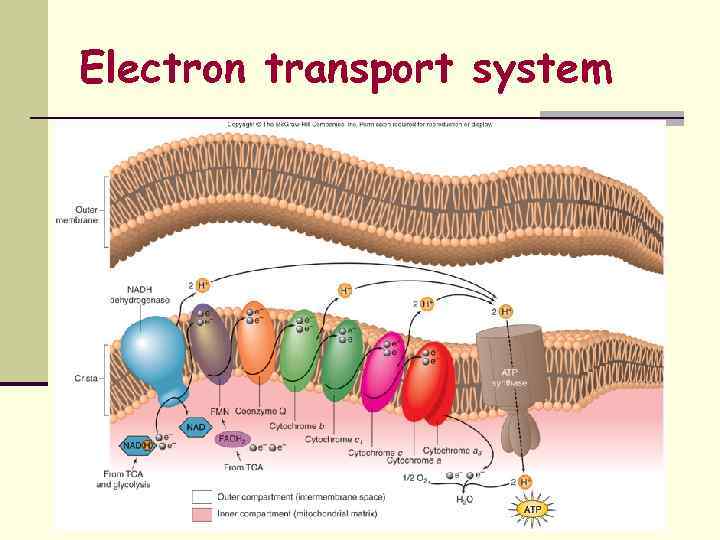
Electron transport system
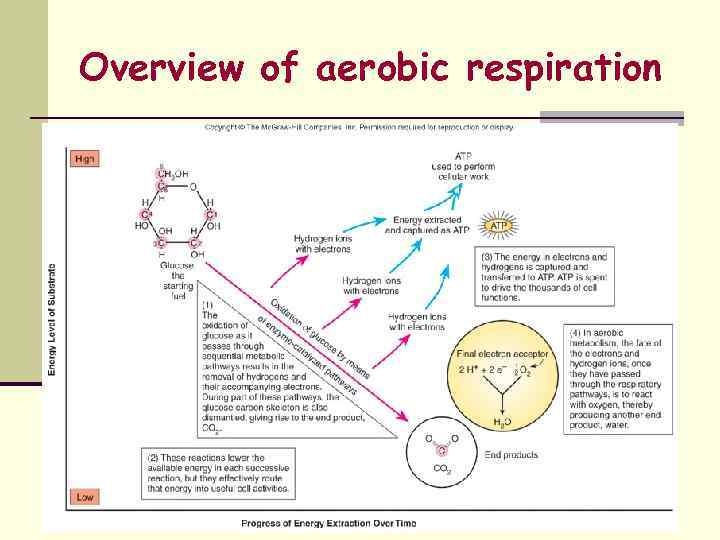
Overview of aerobic respiration

Anaerobic respiration Utilizes same 3 coupled pathways as Aerobic Respiration Used as an alternative to aerobic respiration Final electron acceptor something other than oxygen: NO 3 - : Pseudomonas, Bacillus. SO 4 -: Desulfovibrio CO 3 -: methanogens In Facultative organisms In Obligate anaerobes Lower production of ATP because only part of the TCA cycle and the electron transport chain operate.
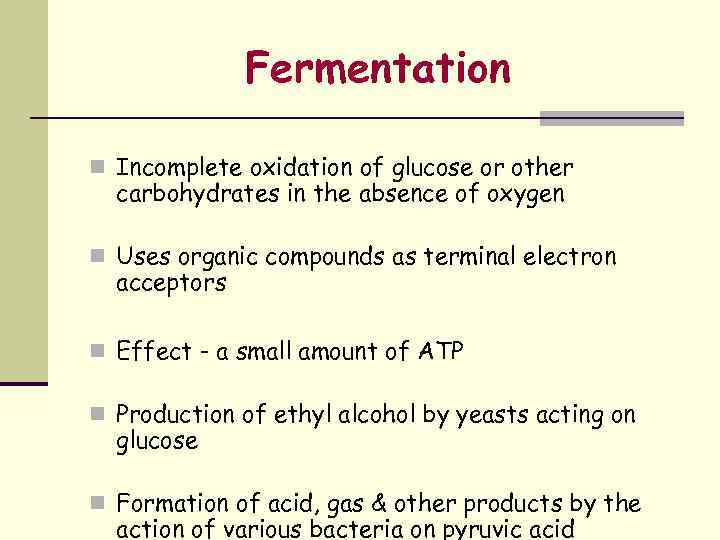
Fermentation n Incomplete oxidation of glucose or other carbohydrates in the absence of oxygen n Uses organic compounds as terminal electron acceptors n Effect - a small amount of ATP n Production of ethyl alcohol by yeasts acting on glucose n Formation of acid, gas & other products by the action of various bacteria on pyruvic acid
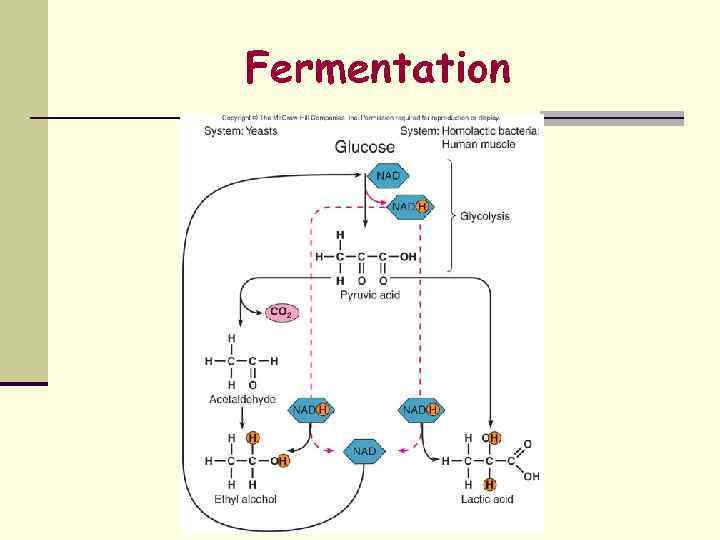
Fermentation

Fermentation may result in numerous end products 1. Type of organism 2. Original substrate 3. Enzymes that are present and active
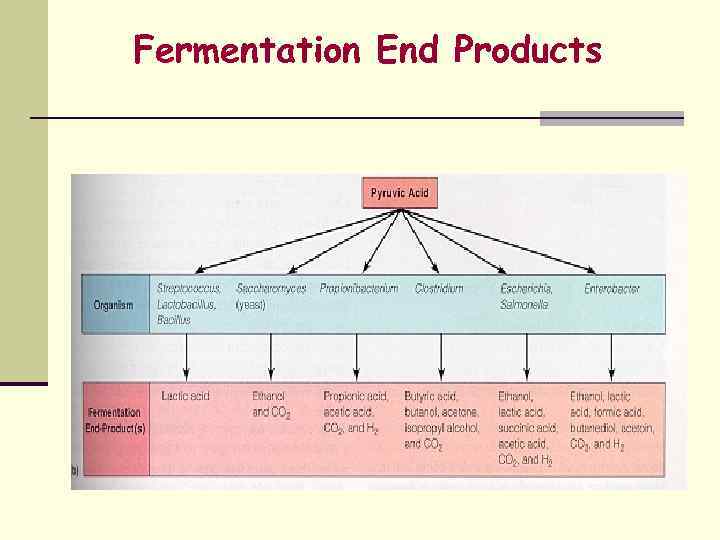
Fermentation End Products
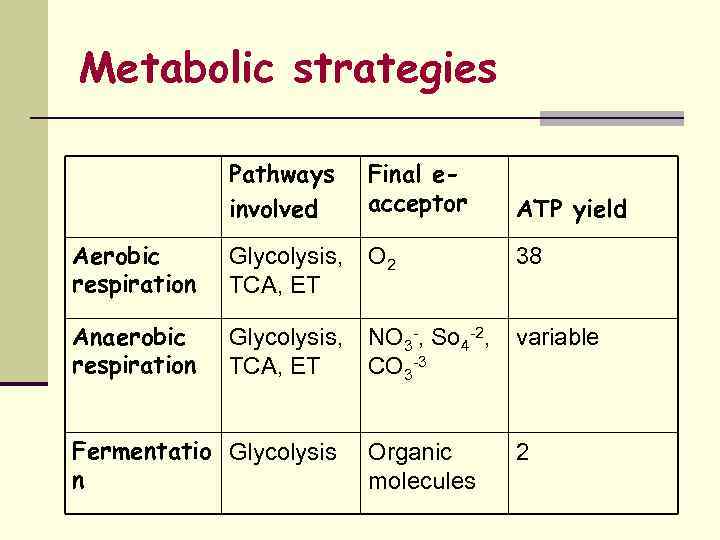
Metabolic strategies Pathways involved Final eacceptor ATP yield Aerobic respiration Glycolysis, O 2 TCA, ET Anaerobic respiration Glycolysis, NO 3 -, So 4 -2, variable TCA, ET CO 3 -3 Fermentatio Glycolysis n Organic molecules 38 2

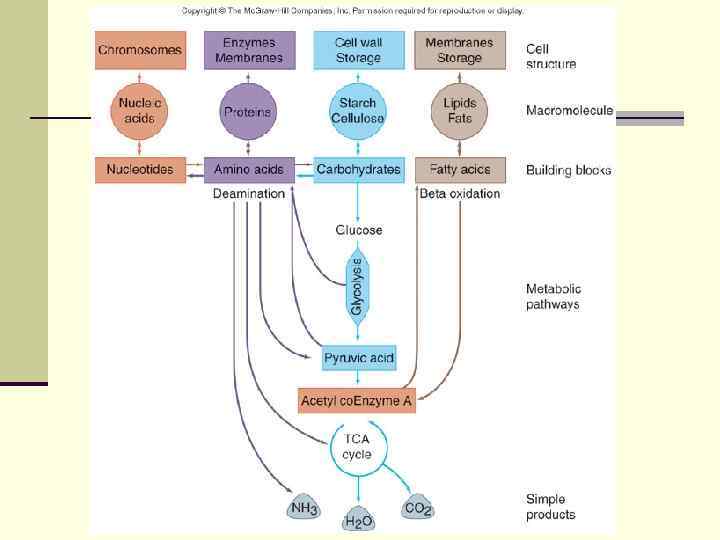
n Many pathways of metabolism are bi-directional or amphibolic n Metabolites can serve as building blocks or sources of energy n n n Pyruvic acid can be converted into amino acids through amination Amino acids can be converted into energy sources through deamination Glyceraldehyde-3 -phosphate can be converted into precursors for amino acids, carbohydrates and fats

Redox reactions n Always occur in pairs. n There is an electron donor and electron acceptor which constitute a redox pair. n Released energy can be captured to phosphorylate ADP or another compound.
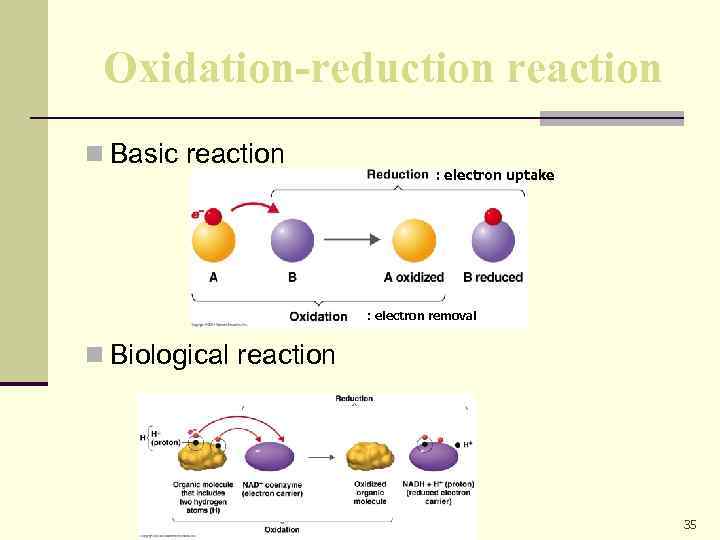
Oxidation-reduction reaction n Basic reaction : electron uptake : electron removal n Biological reaction 35
ATP n 3 part molecule consisting of n adenine – a nitrogenous base n ribose – a 5 -carbon sugar n 3 phosphate groups n Removal of the terminal phosphate releases energy n Adenosine Tri Phosphate ADP + energy + phosphate n ATP contains energy that can be easily released (high-energy or unstable energy bond) n Required for anabolic reactions n
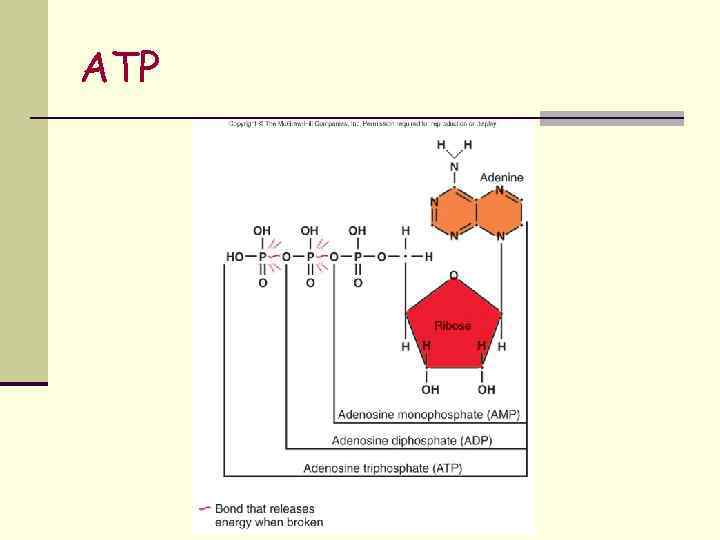
ATP
Formation of ATP 1. substrate-level phosphorylation 2. 3. oxidative phosphorylation, )reduced chemicals( Photophosphorylation )reduced chlorophyll molecules( Uses of ATP: n Energy for active transport n Energy for movement n Energy for synthesis of cellular components ALL SYNTHESIS REACTIONS INVOLVE USE OF ENERGY
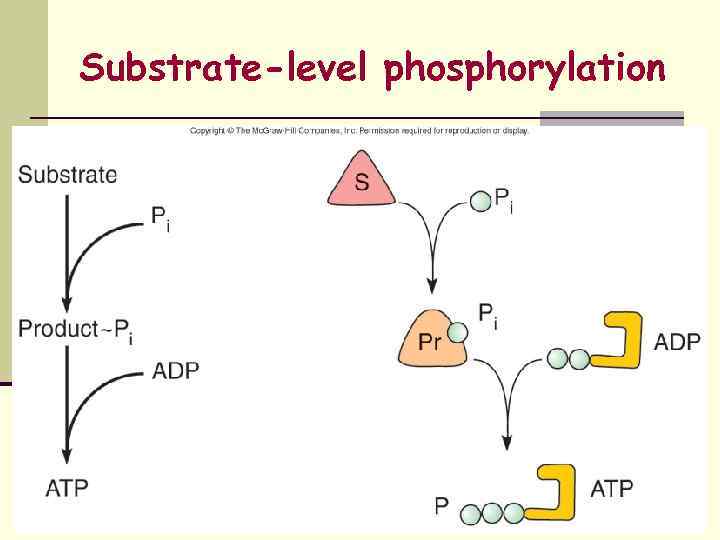
Substrate-level phosphorylation
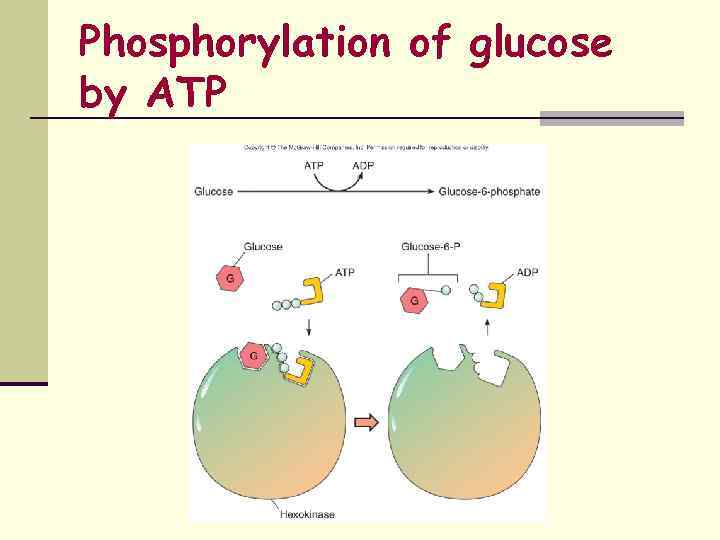
Phosphorylation of glucose by ATP
Lipid Metabolism n Lipids are essential to the structure and function of membranes n Lipids also function as energy reserves, which can be mobilized as sources of carbon n 90% of this lipid is “triacyglycerol” triacyglycerol lipase glycerol + 3 fatty acids n The major fatty acid metabolism is “β-oxidation”
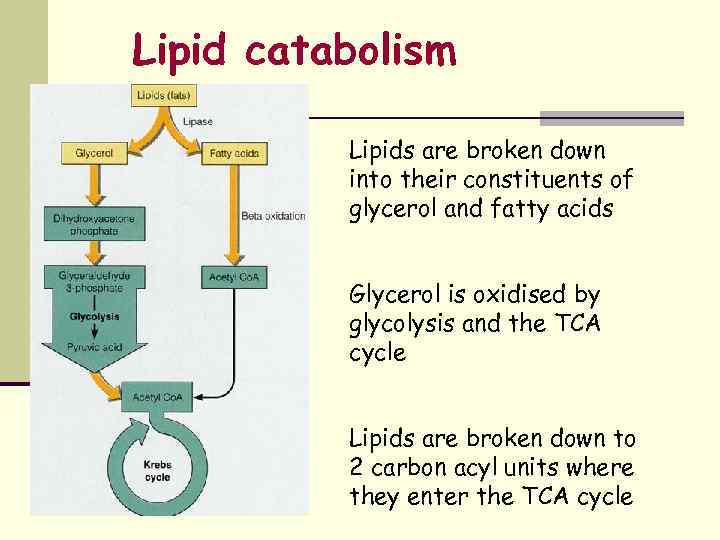
Lipid catabolism Lipids are broken down into their constituents of glycerol and fatty acids Glycerol is oxidised by glycolysis and the TCA cycle Lipids are broken down to 2 carbon acyl units where they enter the TCA cycle
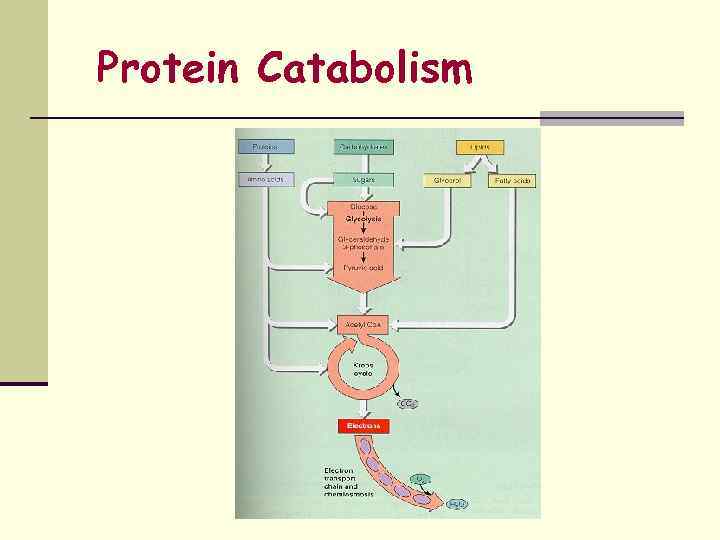
Protein Catabolism
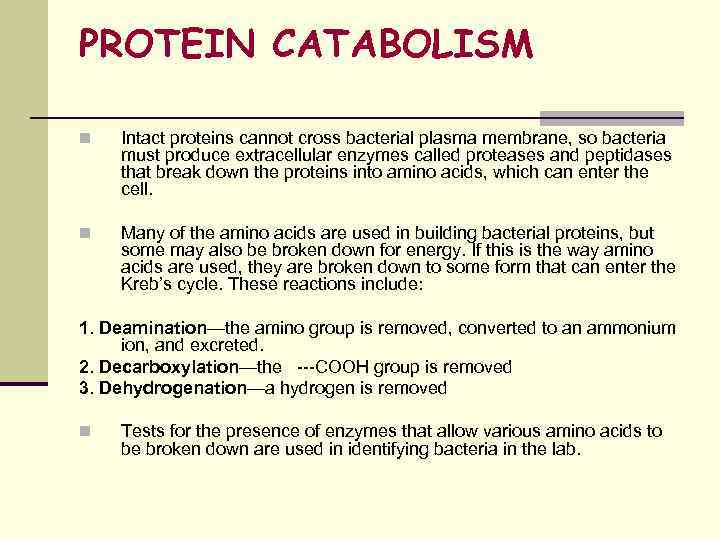
PROTEIN CATABOLISM n Intact proteins cannot cross bacterial plasma membrane, so bacteria must produce extracellular enzymes called proteases and peptidases that break down the proteins into amino acids, which can enter the cell. n Many of the amino acids are used in building bacterial proteins, but some may also be broken down for energy. If this is the way amino acids are used, they are broken down to some form that can enter the Kreb’s cycle. These reactions include: 1. Deamination—the amino group is removed, converted to an ammonium ion, and excreted. 2. Decarboxylation—the ---COOH group is removed 3. Dehydrogenation—a hydrogen is removed n Tests for the presence of enzymes that allow various amino acids to be broken down are used in identifying bacteria in the lab.
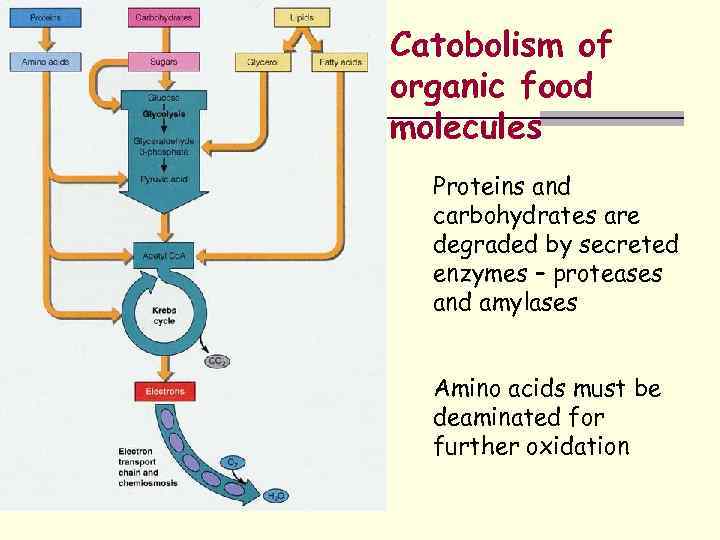
Catobolism of organic food molecules Proteins and carbohydrates are degraded by secreted enzymes – proteases and amylases Amino acids must be deaminated for further oxidation
Microbial physiology. Microbial metabolism. Enzymes. Bioenergetics. Nutrition. Bacterial growth and multiplication.
Growth and multiplication mode: Binary fission
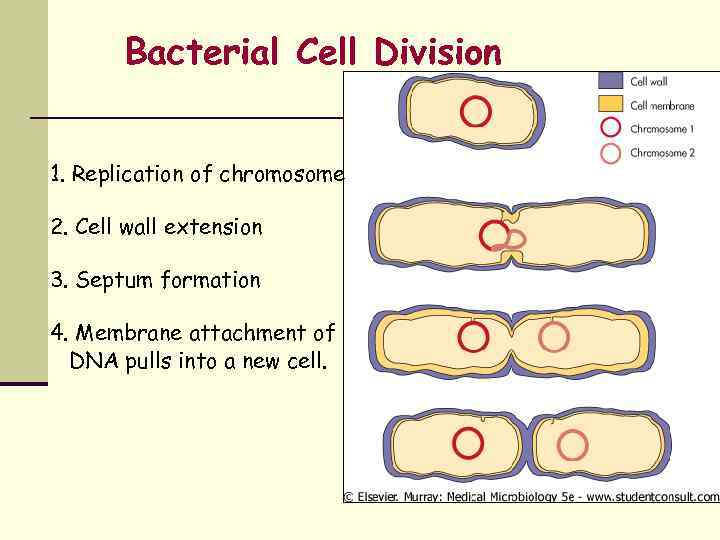
Bacterial Cell Division 1. Replication of chromosome 2. Cell wall extension 3. Septum formation 4. Membrane attachment of DNA pulls into a new cell.
Growth n It is an increase in all the cell components, which ends in multiplication of cell leading to an increase in population. n It involves - an increase in the size of the cell & an increase in the number of individual cells. n Bacteria divide by binary fission.
Generation time n Interval of time between two cell divisions OR n The time required for a bacterium to give rise to 2 daughter cells under optimum conditions n Also called population doubling time.
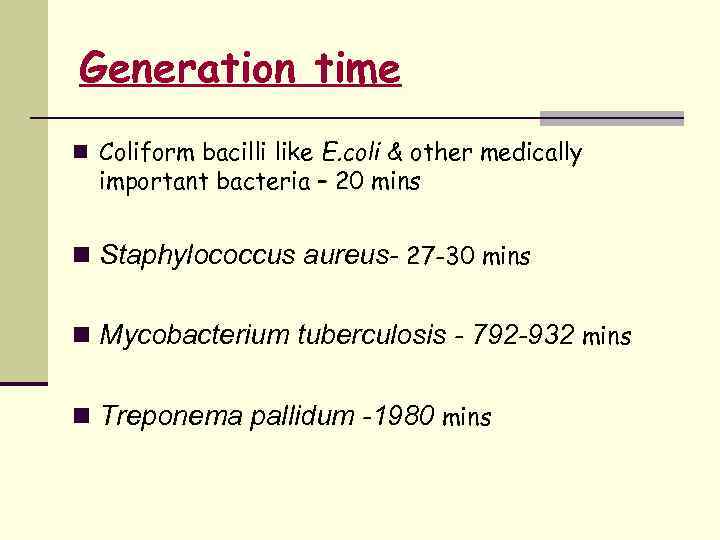
Generation time n Coliform bacilli like E. coli & other medically important bacteria – 20 mins n Staphylococcus aureus- 27 -30 mins n Mycobacterium tuberculosis - 792 -932 mins n Treponema pallidum -1980 mins
Growth form in Laboratory n Colony – formed by bacteria growing on solid media. (20 -30 cell divisions) n Each bacterial colony represents a clone of cells derived from a single parent cell. n Turbidity – liquid media - 107 -109 cells/ml n Biofilm formation – thin spread over an inert surface.
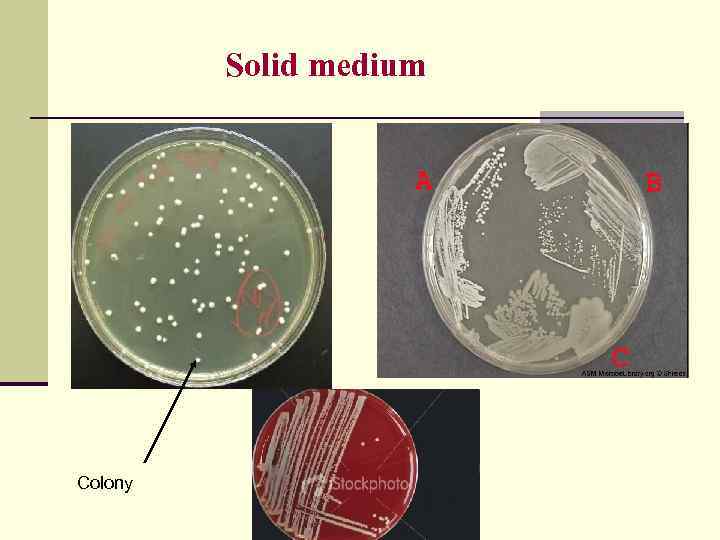
Solid medium Colony

Liquid medium
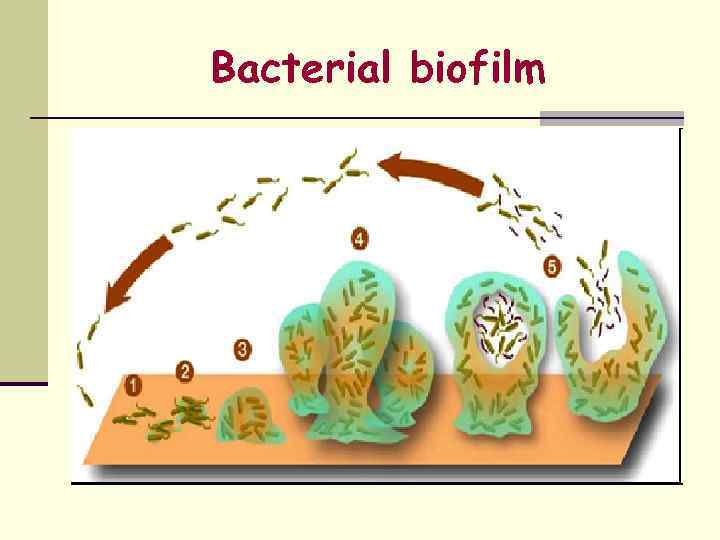
Bacterial biofilm
Bacterial counts n Cell Counts. . . many ways n 2 methods – Total cell count - Viable cell count
Total Count n Total number of cells in the sample = living + dead. Can be obtained by : n Direct counting under microscope using counting chambers. n Counting in an electronic device – Coulter counter.
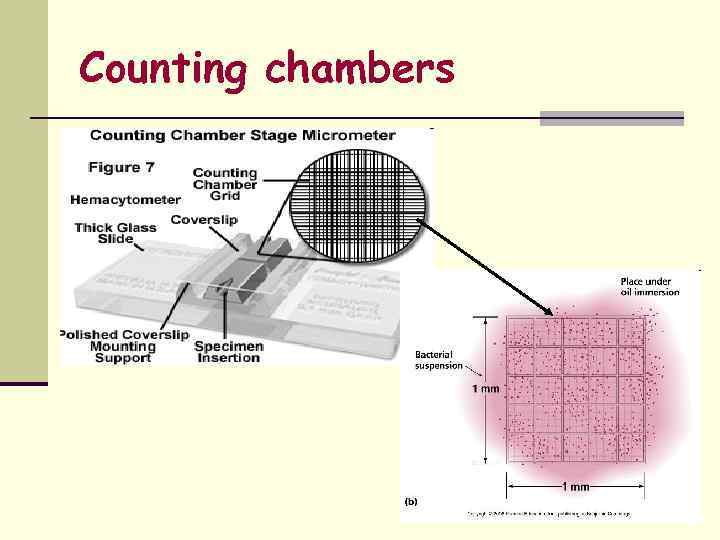
Counting chambers
Over method n Direct counting using stained smears - by spreading a known volume of culture over a measured area of slide. n Opacity measurements using an absorptiometer/ nephalometer. n Chemical assays of cell components.
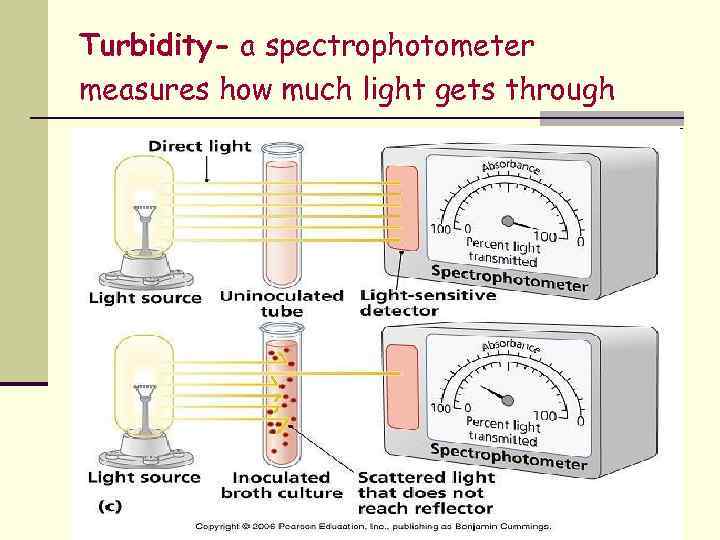
Turbidity- a spectrophotometer measures how much light gets through
Compared to known controls, Mac. Farland controls
Viable Cell Count n Measures the number of living cells. n Methods – Surface colony count n n n Dilution method Plating method Number of colonies that develop after incubation gives an estimate of the viable count.
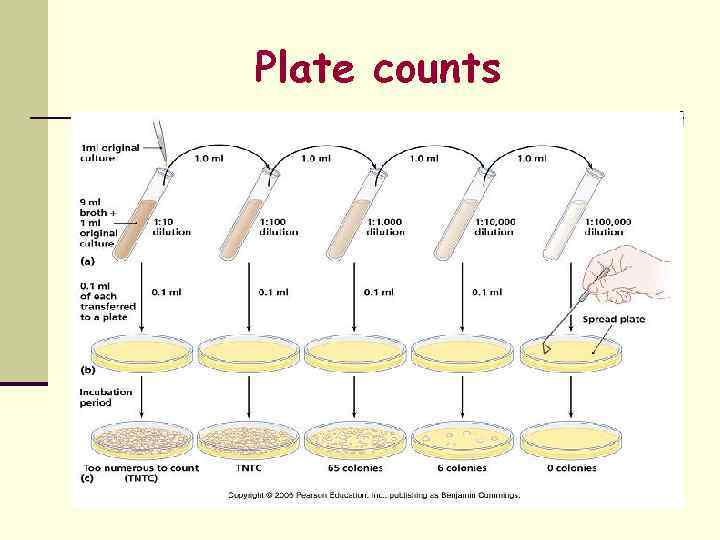
Plate counts
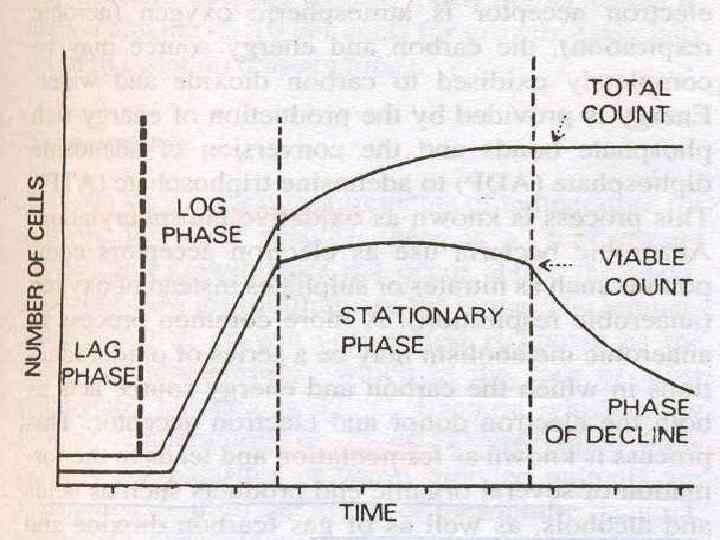
Bacterial Growth Curve n When a bacterium is added to a suitable liquid medium and incubated, its growth follows a definite course. n If bacteria counts are made at intervals after inoculation & plotted in relation to time, a growth curve is obtained. n Shows 4 phases : n Lag, n Log or Exponential, n Stationary n Decline.
Phases of Growth Curve n 1. Lag phase – No increase in number but there may be an increase in the size of the cell. n 2. Log OR Exponential phase – cells start dividing and their increases exponentially. number

Phases of Growth Curve n 3. Stationary phase – cell division stops due to depletion of nutrients & accumulation of toxic products. - equilibrium exists between dying cells and the newly formed cells, so viable count remains stationary n 4. Phase of Decline – population decreases due to the death of cells – autolytic enzymes.

Morphological & Physiological alterations during growth n Lag phase – maximum cell size towards the end of lag phase. n Log phase – smaller cells, stain uniformly n Stationary phase – irregular staining, sporulation and production of exotoxins & antibiotics n Phase of Decline –involution forms(with ageing)

Factors Affecting Bacterial Growth n Availability of Nutrients & H 2 O n Temperature n Atmosphere – O 2 & CO 2 n H-ion concentration n Moisture & drying n Osmotic effects n Radiation n Mechanical & sonic stress.
Bacterial Nutrition n Water constitutes 80% of the total weight of bacterial cells. n Proteins, polysaccharides, lipids, nucleic acids, mucopeptides & low molecular weight compounds make up the remaining 20%.

Moisture & Drying n Water – essential ingredient of bacterial protoplasm. Hence drying is lethal to cells. n Effect of drying varies : n n T. pallidum – highly sensitive Staphylococci sp– stand for months n Spores – resistant to desiccation, may survive for several decades.
Nutrients Functions – – Generation of energy Synthesis of cellular materials Essential nutrients (basic bioelements needed for bacterial – cell growth) – – – H 2 O: universal solvent; hydrolyzing agent Carbon: food & E* source; in form of prot. , sugar, lipid Nitrogen: for prot. syn; nucleic acid syn (purines & pyrimidines) Sulfur (sulfate): AA syn (i. e. , Cystine) Phosphate: key component of DNA & RNA, ATP, and inner & outer membrane phospholipids Minerals: assoc’d w/ PRO (i. e. , Fe: PRO); common component of enzymes.
Nutrients 2 types 1. Macronutrients – needed in large quantities for cellular metabolism & basic cell structure Ø 2. Micronutrients – needed in small quantities; more specialized (enzyme & pigment structure & function) Ø – C, H, O, N Mn, Zn Fastidious Bacteria: microbes that require other complex - nutrients/growth factors ( i. e. , Vitamins or AAs)
Temperature n Vary in the temperature requirements. n Temperature range – growth does not occur above the maximum or below the minimum. n Optimum Temperature – growth occurs best, 37ºC for most pathogenic bacteria.
Uptake of nutrients by bacteria o Passive diffusion o o o simple diffusion facilitated diffusion Active transport
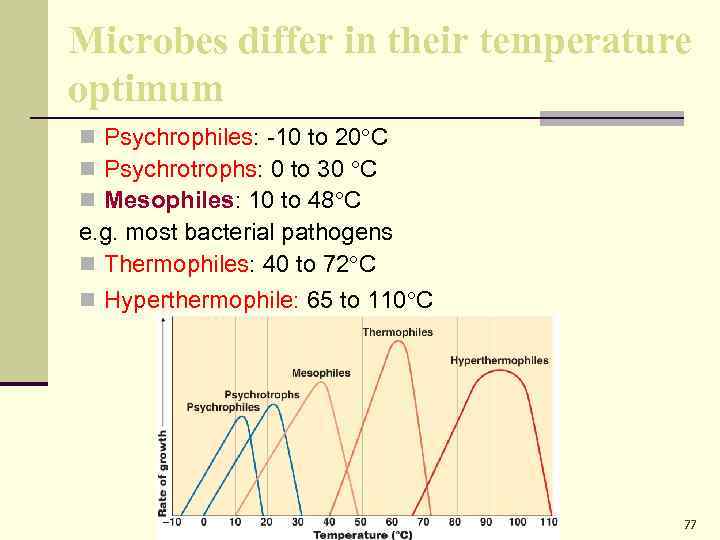
Microbes differ in their temperature optimum n Psychrophiles: -10 to 20 C n Psychrotrophs: 0 to 30 C n Mesophiles: 10 to 48 C e. g. most bacterial pathogens n Thermophiles: 40 to 72 C n Hyperthermophile: 65 to 110 C 77
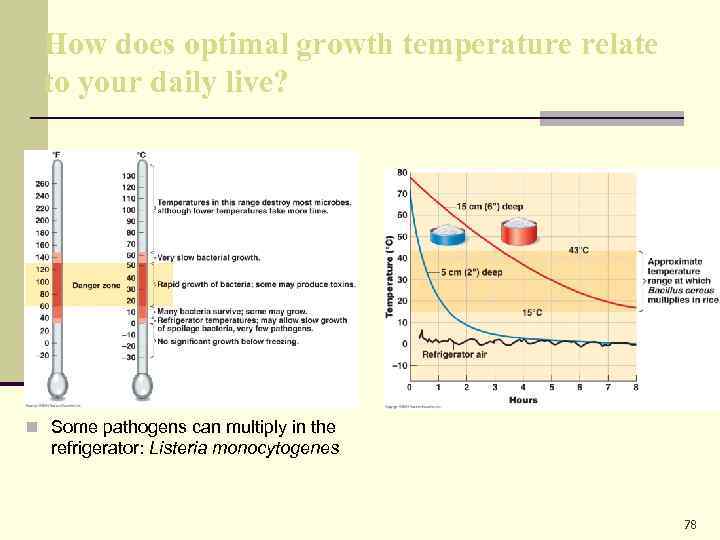
How does optimal growth temperature relate to your daily live? n Some pathogens can multiply in the refrigerator: Listeria monocytogenes 78
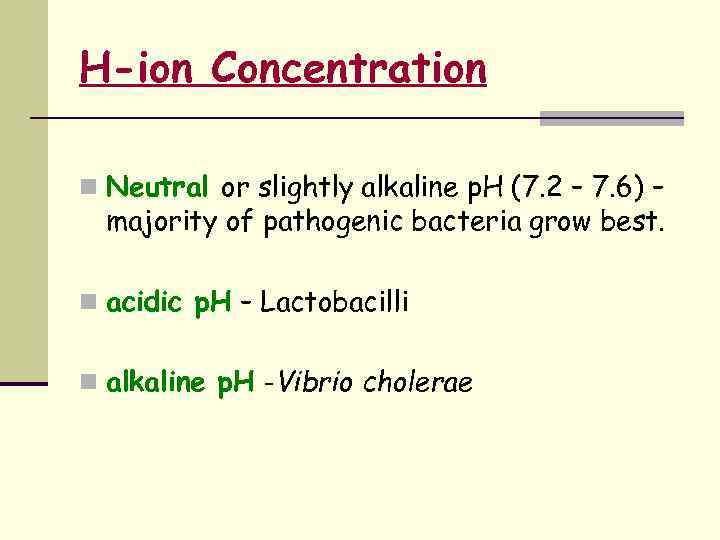
H-ion Concentration n Neutral or slightly alkaline p. H (7. 2 – 7. 6) – majority of pathogenic bacteria grow best. n acidic p. H – Lactobacilli n alkaline p. H -Vibrio cholerae
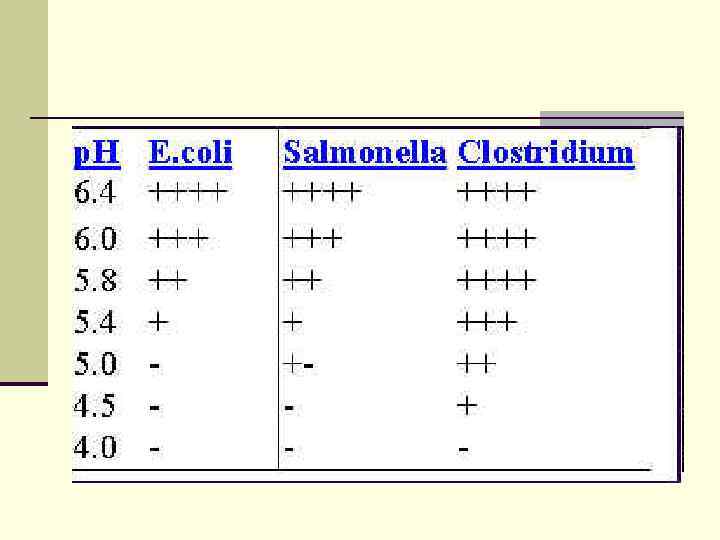
Osmotic Pressure or Osmolarity Ø Most bacteria require an isotonic environment or a hypotonic environment for optimum growth. Ø Osmotolerant - organisms that can grow at relatively high salt concentration (up tp 10%). Ø Halophiles - bacteria that require relatively high salt concentrations for growth, like some of the Archea that require sodium chloride concentrations of 20 % or higher.
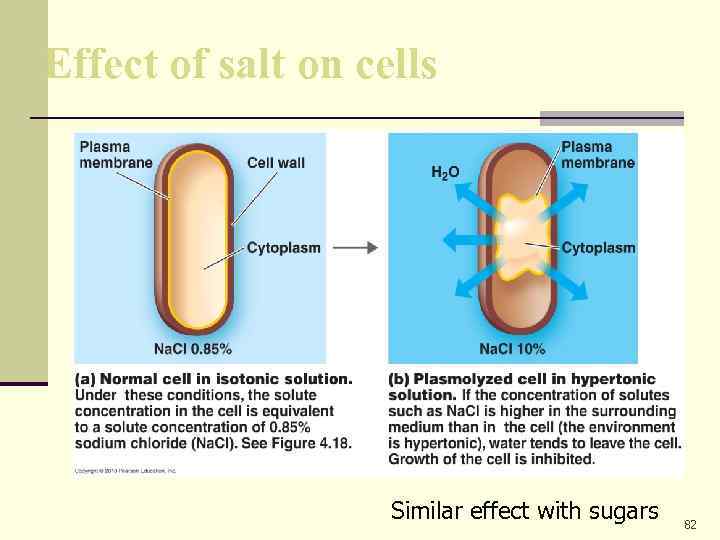
Effect of salt on cells Similar effect with sugars 82

Radiation, stress n Radiation n X rays & gamma rays exposure – lethal n Mechanical & Sonic Stress n May be ruptured by mechanical stress.
Growth Factors Some bacteria require certain organic compounds in minute quantities – Growth Factors OR Bacterial Vitamins. It can be : n Essential – when growth does not occur in their absence. n Accessory – when they enhance growth, without being absolutely necessary for it. n

Growth Factors Identical with eukaryotic nutrition n n n Vitamin B complex – thiamine riboflavine nicotinic acid pyridoxine folic acid & Vit. B 12
Presence or Absence of Gases n Primary gases = O 2, N 2, & CO 2 Ø O 2 - greatest impact on microbial growth (even if the microorganism does not require it) Ø Aerobic respiration – terminal electron acceptor is oxygen. Ø Anaerobic respiration – terminal electron acceptor is an inorganic molecule other than oxygen (e. g. nitrogen).
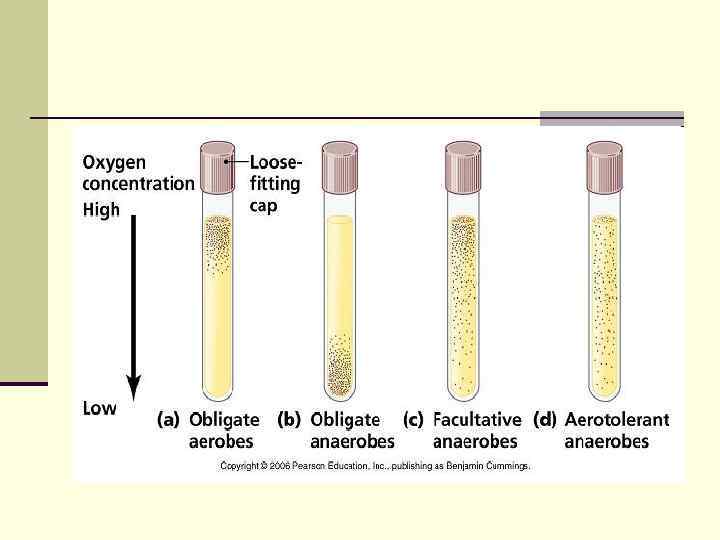
Depending on the O 2 requirement Strict (Obligate) Aerobes – O 2 present, require O 2 for growth n e. g. Pseudomonas aeruginosa § § Obligate aerobe – 20% O 2: only grows with O 2 Microaerophile – 4% O 2: best growth with small amount O 2 n Strict (Obligate) Anaerobes – O 2 depleted, grow in the absence of O 2 & may even die on exposure to O 2 e. g. Bacteroides fragilis n n n Obligate anaerobe: only grows in absence of O 2 Aerotolerant anaerobe: anaerobes that “tolerate” +/or survive in O 2, but do NOT utilize O 2 during E* metabolism n n e. g. Campylobacter spp, Helicobacter spp e. g. Clostridium perfringens Facultative Anaerobe – grows both in presence & absence of O 2; but grows BEST under Aerobic conditions; considered to be aerobic organism; O 2 present – aerobic respiration for E*; O 2 absent – anaerobic pathways (fermentation) n n e. g. Staphylococcus spps Capnophilic organism – requires high CO 2 levels eg Neisseria spps
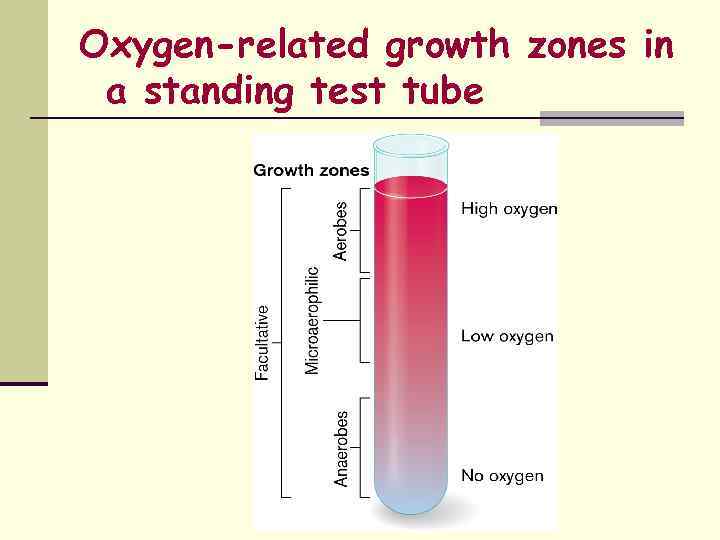
Oxygen-related growth zones in a standing test tube

Oxygen is toxic n Oxygen is readily converted into radicals (singlet oxygen, superoxide, hydrogen peroxide, hydroxyl radical) n Most important detoxifying enzymes are superoxide dismutase and catalase n Cells differ in their content of detoxifying enzymes and hence, ability to grow in the presence of oxygen 90
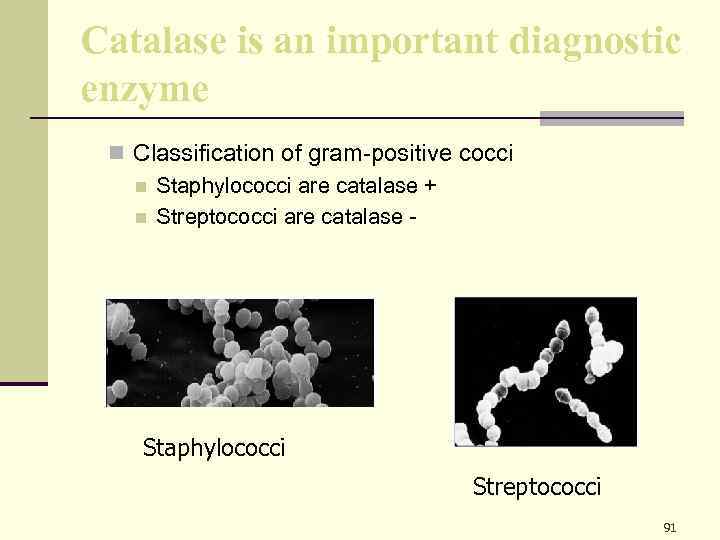
Catalase is an important diagnostic enzyme n Classification of gram-positive cocci n Staphylococci are catalase + n Streptococci are catalase - Staphylococci Streptococci 91

p. H Majority of bacteria grow BEST at neutral or slightly alkaline p. H Ø p. H 7. 0 – 7. 4 => this is near most normal body fluids • Ø Acidophiles: grow BEST at low p. H (acid: p. H 0 – 1. 0) Ø Ø T. B. - p. H 6. 5 -6. 8 Alkalophiles: grow BEST at high p. H (alkaline: p. H 10. 0) Ø V. cholerae - p. H 8. 4 -9. 2
Microbial physiology. Microbial metabolism. Enzymes. Nutrition. Bioenergetics. Bacterial growth and multiplication.
Enzymes n Biological catalysts n Highly specific n Extremely efficient n Increase reaction rates 108 -1010 times n High turnover numbers n Proteins or RNA (ribozymes)
Uptake of nutrients by bacteria Passive diffusion simple diffusion Facilitated diffusion Active transport
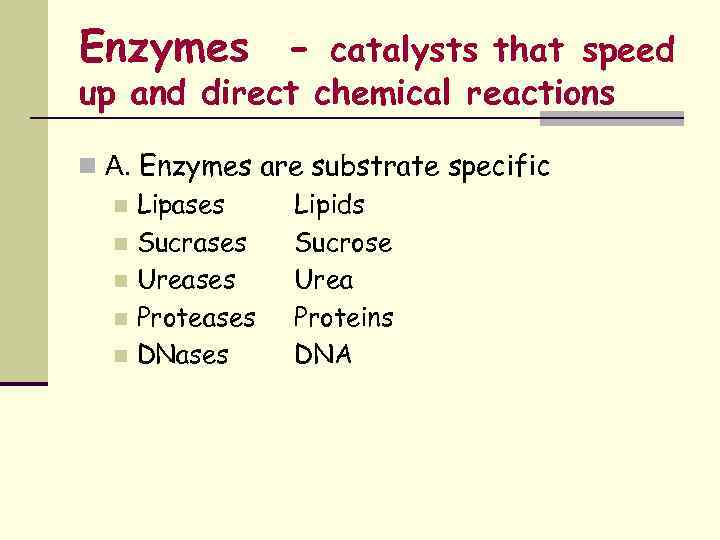
Enzymes - catalysts that speed up and direct chemical reactions n A. Enzymes are substrate specific n Lipases Lipids n Sucrases Sucrose n Ureases Urea n Proteases Proteins n DNases DNA
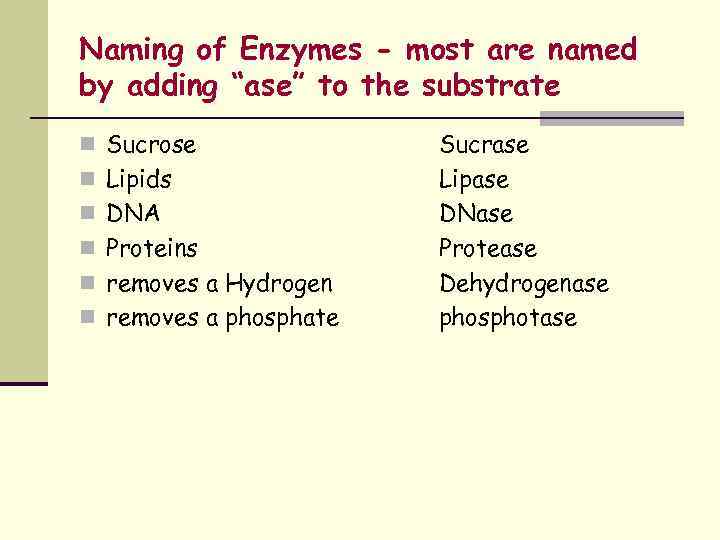
Naming of Enzymes - most are named by adding “ase” to the substrate n Sucrose n Lipids n DNA n Proteins n removes a Hydrogen n removes a phosphate Sucrase Lipase DNase Protease Dehydrogenase phosphotase
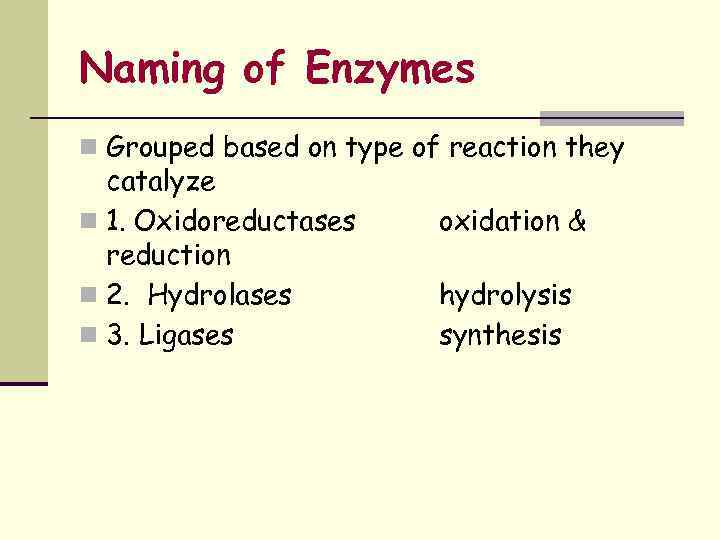
Naming of Enzymes n Grouped based on type of reaction they catalyze n 1. Oxidoreductases reduction n 2. Hydrolases n 3. Ligases oxidation & hydrolysis synthesis
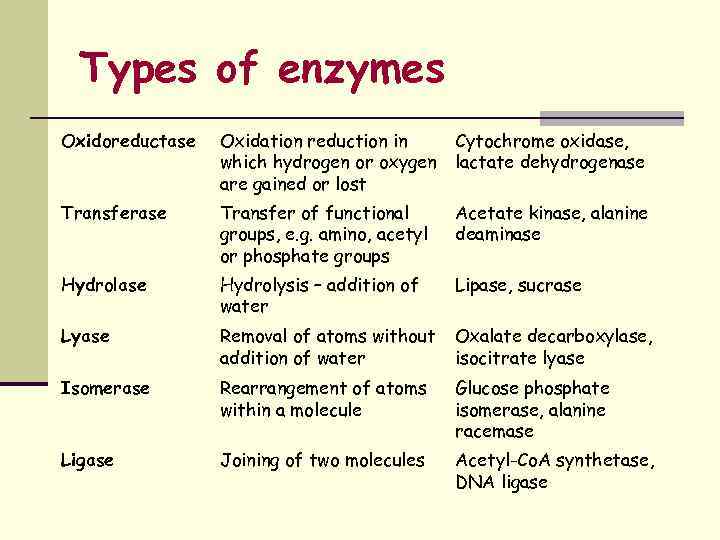
Types of enzymes Oxidoreductase Oxidation reduction in which hydrogen or oxygen are gained or lost Cytochrome oxidase, lactate dehydrogenase Transfer of functional groups, e. g. amino, acetyl or phosphate groups Acetate kinase, alanine deaminase Hydrolysis – addition of water Lipase, sucrase Lyase Removal of atoms without addition of water Oxalate decarboxylase, isocitrate lyase Isomerase Rearrangement of atoms within a molecule Glucose phosphate isomerase, alanine racemase Ligase Joining of two molecules Acetyl-Co. A synthetase, DNA ligase
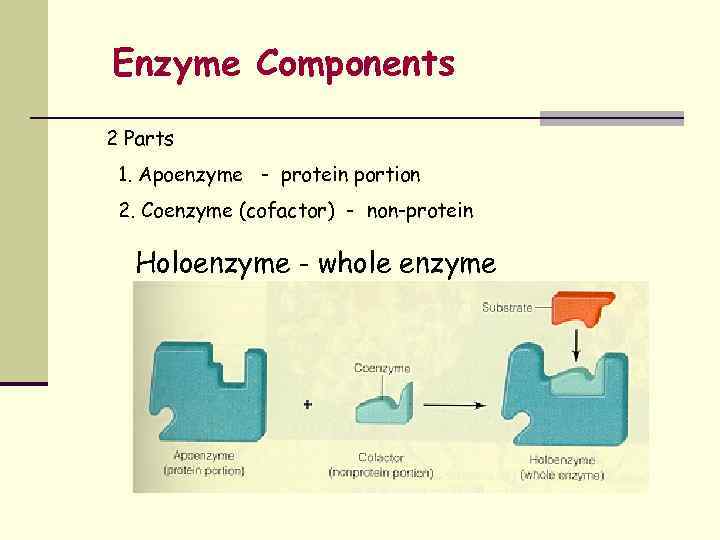
Enzyme Components 2 Parts 1. Apoenzyme - protein portion 2. Coenzyme (cofactor) - non-protein Holoenzyme - whole enzyme

Coenzymes n Many are derived from vitamins n 1. Niacin n NAD (Nicotinamide adenine dinucleotide) n 2. Riboflavin n FAD (Flavin adenine dinucleotide) n 3. Pantothenic Acid n Co. Enzyme A
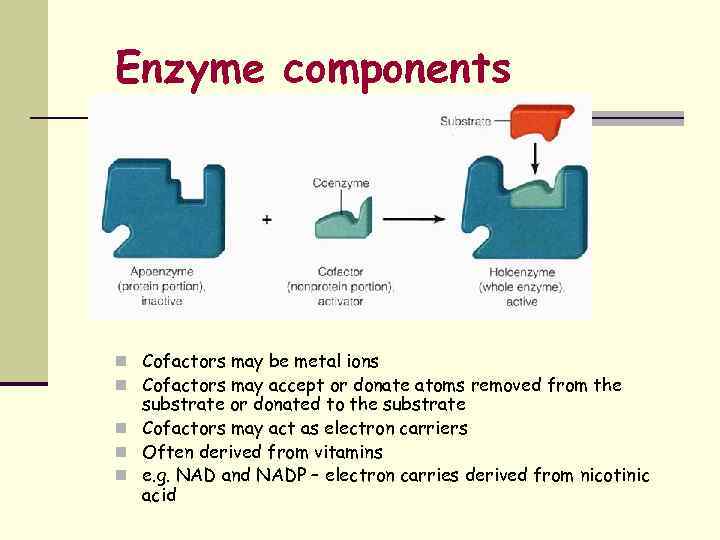
Enzyme components n Cofactors may be metal ions n Cofactors may accept or donate atoms removed from the substrate or donated to the substrate n Cofactors may act as electron carriers n Often derived from vitamins n e. g. NAD and NADP – electron carries derived from nicotinic acid
Microbial metabolism, enzimes and nutrition.ppt