65f21e1bde25cd2c9abbf9287d4b8e5a.ppt
- Количество слайдов: 75
 MHRA GCP Inspection 21 st – 24 th June 2011 Medicines and Healthcare products Regulatory Agency
MHRA GCP Inspection 21 st – 24 th June 2011 Medicines and Healthcare products Regulatory Agency
 n n What do the MHRA inspect? University systems that support conduct of CTIMPs in compliance with regulations and GCP. Areas of interest include: – Approval processes and regulatory submissions – Contract management – Trial file and data management – Quality assurance and monitoring – Training – IT systems – Pharmacovigilance – Archiving – Laboratories – Pharmacy
n n What do the MHRA inspect? University systems that support conduct of CTIMPs in compliance with regulations and GCP. Areas of interest include: – Approval processes and regulatory submissions – Contract management – Trial file and data management – Quality assurance and monitoring – Training – IT systems – Pharmacovigilance – Archiving – Laboratories – Pharmacy
 What do the MHRA inspect? Specific examples of CTIMPs that demonstrate those systems n n Uo. A sponsors or co-sponsors 8 CTIMP studies Uo. A hosts 33 CTIMP studies MHRA have chosen 4 to look at in depth However……. they can change their minds before the visit or decide to look at other studies during the visit…. we must all be prepared! n
What do the MHRA inspect? Specific examples of CTIMPs that demonstrate those systems n n Uo. A sponsors or co-sponsors 8 CTIMP studies Uo. A hosts 33 CTIMP studies MHRA have chosen 4 to look at in depth However……. they can change their minds before the visit or decide to look at other studies during the visit…. we must all be prepared! n
 Aims of this session Brief researchers on what the inspectors will be looking at in your CTIMP study n - Qualifications & training Study files & documentation Pharmacovigilance Serious breaches Informed consent Communication Describe new overarching SOP’s n Prepare researchers for interviews with inspectors n
Aims of this session Brief researchers on what the inspectors will be looking at in your CTIMP study n - Qualifications & training Study files & documentation Pharmacovigilance Serious breaches Informed consent Communication Describe new overarching SOP’s n Prepare researchers for interviews with inspectors n
 Preparation for MHRA Inspection Regulations, Qualifications & Training
Preparation for MHRA Inspection Regulations, Qualifications & Training
 Legislation: Letter from MHRA: n “The main references used for the inspection will be EU Directives 2001/20/EC and 2005/28/EC and supporting guidance documents as incorporated in UK National Legislation, Statutory Instrument 2004, Number 1031, the Medicines for Human Use (Clinical Trials) Regulations 2004 and subsequent amendments. ”
Legislation: Letter from MHRA: n “The main references used for the inspection will be EU Directives 2001/20/EC and 2005/28/EC and supporting guidance documents as incorporated in UK National Legislation, Statutory Instrument 2004, Number 1031, the Medicines for Human Use (Clinical Trials) Regulations 2004 and subsequent amendments. ”
 Legislation: n n 2001 2004 n 2005 2006 n 2008 n 2009 n EU Clinical Trial Directive: Directive 2001/20/EC Medicines for Human Use (Clinical Trials) Regulations 2004 (SI: 1031) EU Directive on Good Clinical Practice 2005/28/EC The Medicines for Human Use (Clinical Trials) Amendment Regulations 2006 (SI: 1928) The Medicines for Human Use (Clinical Trials) Amendment (No. 2) Regulations 2006 The Medicines for Human Use (Clinical Trials) and Blood Safety and Quality (Amendment) Regulations 2008 MHRA GCP guideline - Laboratories
Legislation: n n 2001 2004 n 2005 2006 n 2008 n 2009 n EU Clinical Trial Directive: Directive 2001/20/EC Medicines for Human Use (Clinical Trials) Regulations 2004 (SI: 1031) EU Directive on Good Clinical Practice 2005/28/EC The Medicines for Human Use (Clinical Trials) Amendment Regulations 2006 (SI: 1928) The Medicines for Human Use (Clinical Trials) Amendment (No. 2) Regulations 2006 The Medicines for Human Use (Clinical Trials) and Blood Safety and Quality (Amendment) Regulations 2008 MHRA GCP guideline - Laboratories
 Medicines for Human Use (Clinical Trials) Regulations 2004 (SI: 1031) “Each individual involved in conducting a trial shall be qualified by education, training, and experience to perform his or her respective task(s)”
Medicines for Human Use (Clinical Trials) Regulations 2004 (SI: 1031) “Each individual involved in conducting a trial shall be qualified by education, training, and experience to perform his or her respective task(s)”
 Qualifications and Training: n n Delegation Log Training Record
Qualifications and Training: n n Delegation Log Training Record
 Delegation of Duties: n n Delegation log should be established, documenting which tasks are undertaken by each member of the research team These should be signed by each team member to confirm that they agree to undertake the task they have been delegated
Delegation of Duties: n n Delegation log should be established, documenting which tasks are undertaken by each member of the research team These should be signed by each team member to confirm that they agree to undertake the task they have been delegated
 MHRA Inspection: n Those listed on the delegation log should be qualified to carry out their specific task(s) – CV – GCP Training – Training Record
MHRA Inspection: n Those listed on the delegation log should be qualified to carry out their specific task(s) – CV – GCP Training – Training Record
 SOP: Establishing and Maintaining a Training Record Uo. A-NHSG-SOP-016 n Applies to all staff conducting or supporting clinical research sponsored or co sponsored by Uo. A / NHSG n Responsibility of the individual to create an update their own training record
SOP: Establishing and Maintaining a Training Record Uo. A-NHSG-SOP-016 n Applies to all staff conducting or supporting clinical research sponsored or co sponsored by Uo. A / NHSG n Responsibility of the individual to create an update their own training record
 Contents of the Training Record: n n n Current CV Job Description(s) Certificates of training Training Log: ongoing list of all internal and external training - may include training from previous post (training courses, conferences, seminars, relevant meetings) Keep copies of handouts / agendas If a staff leave – take original training record, but leave a copy with the study file
Contents of the Training Record: n n n Current CV Job Description(s) Certificates of training Training Log: ongoing list of all internal and external training - may include training from previous post (training courses, conferences, seminars, relevant meetings) Keep copies of handouts / agendas If a staff leave – take original training record, but leave a copy with the study file
 Possible Questions Tell me about your qualifications What type of GCP training have you had / who was the provider Have you done any other research training What is your clinical experience / experience on clinical trials How do you assess that your team are competent to complete their delegated tasks – Is this documented
Possible Questions Tell me about your qualifications What type of GCP training have you had / who was the provider Have you done any other research training What is your clinical experience / experience on clinical trials How do you assess that your team are competent to complete their delegated tasks – Is this documented
 Study Files and Documentation: Trial Master Files n Investigator Site Files n
Study Files and Documentation: Trial Master Files n Investigator Site Files n
 Medicines for Human Use (Clinical Trials) Regulations 2004 (SI: 1031) “All Clinical Trial information should be recorded, handled and stored in a way that allows its accurate reporting, interpretation and verification” “The confidentiality of records that could identity subjects shall be protected, respecting the privacy and confidentiality rules in accordance with the requirements of the Data Protection Act 1998 and the law relating to confidentiality”
Medicines for Human Use (Clinical Trials) Regulations 2004 (SI: 1031) “All Clinical Trial information should be recorded, handled and stored in a way that allows its accurate reporting, interpretation and verification” “The confidentiality of records that could identity subjects shall be protected, respecting the privacy and confidentiality rules in accordance with the requirements of the Data Protection Act 1998 and the law relating to confidentiality”
 Study Files and Documentation: n Chief/Principal Investigators are required to keep, and maintain, a CORE set of documents for EACH research project they manage n Should be kept in a designated file called a Investigator Site File (ISF) and/or Trial Master File (TMF)
Study Files and Documentation: n Chief/Principal Investigators are required to keep, and maintain, a CORE set of documents for EACH research project they manage n Should be kept in a designated file called a Investigator Site File (ISF) and/or Trial Master File (TMF)
 SOPs: n Establishing and Maintaining a TMF: Uo. ANHSG-SOP-008 – Uo. A-NHSG-TMP-003 – TMF Checklist n Establishing and Maintaining an ISF: Uo. ANHSG-SOP-009 – Uo. A-NHSG-TMP-002 – ISF Checklist (If single centre: both can be combined to save duplication) n Applies to all staff conducting or supporting CTIMPs sponsored or co sponsored by Uo. A / NHSG
SOPs: n Establishing and Maintaining a TMF: Uo. ANHSG-SOP-008 – Uo. A-NHSG-TMP-003 – TMF Checklist n Establishing and Maintaining an ISF: Uo. ANHSG-SOP-009 – Uo. A-NHSG-TMP-002 – ISF Checklist (If single centre: both can be combined to save duplication) n Applies to all staff conducting or supporting CTIMPs sponsored or co sponsored by Uo. A / NHSG
 TMF / ISF n n n Maintaining TMF / ISF is the responsibility of the CI/PI – can be delegated to research team Use file index / checklist. Alternative version can be used, but must retain all the listed documentation as minimum standard If documents stored elsewhere – add in file note Updates / amendments added to TMF / ISF and reviewed by sponsor. Stored in a secure environment – but remain accessible to trial staff
TMF / ISF n n n Maintaining TMF / ISF is the responsibility of the CI/PI – can be delegated to research team Use file index / checklist. Alternative version can be used, but must retain all the listed documentation as minimum standard If documents stored elsewhere – add in file note Updates / amendments added to TMF / ISF and reviewed by sponsor. Stored in a secure environment – but remain accessible to trial staff
 Possible Questions: Who is managing your TMF / ISF Do you keep electronic versions of documents Who has access to your files How do you ensure the security of your records
Possible Questions: Who is managing your TMF / ISF Do you keep electronic versions of documents Who has access to your files How do you ensure the security of your records
 Archiving: n n What Where How For how long
Archiving: n n What Where How For how long
 SOP: Archiving Clinical Research Data: Uo. A-NHSG-SOP-021 n Not yet finalised Applies to all staff conducting or supporting CTIMPs sponsored or co sponsored by Uo. A / NHSG n Responsibility of the sponsor and CI to ensure essential documents are retained for an appropriate period of time - and made available for monitoring and audit n
SOP: Archiving Clinical Research Data: Uo. A-NHSG-SOP-021 n Not yet finalised Applies to all staff conducting or supporting CTIMPs sponsored or co sponsored by Uo. A / NHSG n Responsibility of the sponsor and CI to ensure essential documents are retained for an appropriate period of time - and made available for monitoring and audit n
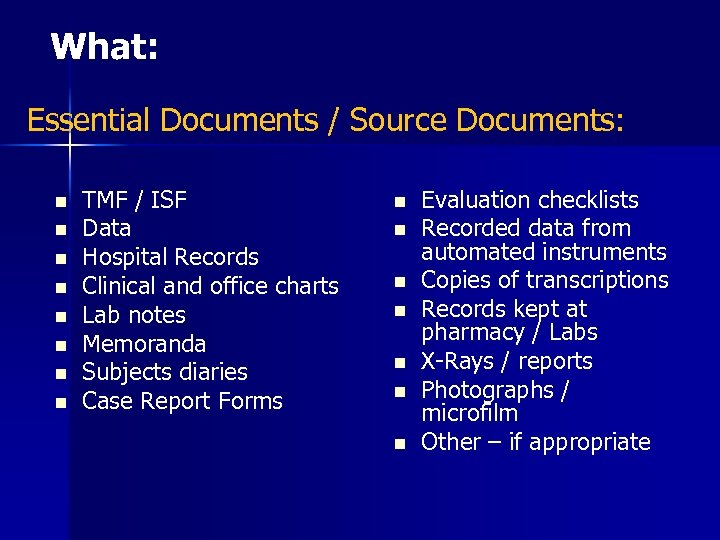 What: Essential Documents / Source Documents: n n n n TMF / ISF Data Hospital Records Clinical and office charts Lab notes Memoranda Subjects diaries Case Report Forms n n n n Evaluation checklists Recorded data from automated instruments Copies of transcriptions Records kept at pharmacy / Labs X-Rays / reports Photographs / microfilm Other – if appropriate
What: Essential Documents / Source Documents: n n n n TMF / ISF Data Hospital Records Clinical and office charts Lab notes Memoranda Subjects diaries Case Report Forms n n n n Evaluation checklists Recorded data from automated instruments Copies of transcriptions Records kept at pharmacy / Labs X-Rays / reports Photographs / microfilm Other – if appropriate
 Hospital Records: Hospital records and source data therein should be retained throughout the archiving period: n Adhere sticker to inside of all medical records documenting: – Study Title – Study ID no – R&D/ Eudra. CT – Name of local CI or PI – Department name / contact number – Date to which notes should be retained
Hospital Records: Hospital records and source data therein should be retained throughout the archiving period: n Adhere sticker to inside of all medical records documenting: – Study Title – Study ID no – R&D/ Eudra. CT – Name of local CI or PI – Department name / contact number – Date to which notes should be retained
 Where: Suitable for type of archived material n Building / room / fireproof safe / locked cabinet n Environmental conditions (avoid extreme fluctuations in temp and humidity) n Risk of fire / flood n Pest control n Secure – accessible only to delegated staff n
Where: Suitable for type of archived material n Building / room / fireproof safe / locked cabinet n Environmental conditions (avoid extreme fluctuations in temp and humidity) n Risk of fire / flood n Pest control n Secure – accessible only to delegated staff n
 Where: Uo. A- sponsored / co-sponsored CTIMPs – Health Sciences Building. n NHSG Sponsored CTIMPs – The Vault Box (Removal Services Scotland Ltd) n Multicentre trials may have site files and relevant records archived at host sites. Should be agreed by sponsor / CI / host site at the beginning of the trial n
Where: Uo. A- sponsored / co-sponsored CTIMPs – Health Sciences Building. n NHSG Sponsored CTIMPs – The Vault Box (Removal Services Scotland Ltd) n Multicentre trials may have site files and relevant records archived at host sites. Should be agreed by sponsor / CI / host site at the beginning of the trial n
 How: After the trial closeout visit: n CTIMPs sponsored / co-sponsored by Uo. A – CI should contact Technical Resource Manager (School of Medicine and Dentistry) n CTIMPs sponsored by NHSG – QA Manager will contact re Archiving arrangements
How: After the trial closeout visit: n CTIMPs sponsored / co-sponsored by Uo. A – CI should contact Technical Resource Manager (School of Medicine and Dentistry) n CTIMPs sponsored by NHSG – QA Manager will contact re Archiving arrangements
 For How Long: n n At least 5 years after the conclusion of the trial (or at least 2 years after the last approval of a marketing application in the EU) Duration of Archiving - agreed by Sponsor / CI at the beginning of the trial Approved by Ethics (require ethical approval if these require to be kept for longer) Do not destroy early or take with you if you leave – must be retained within the Sponsors locality
For How Long: n n At least 5 years after the conclusion of the trial (or at least 2 years after the last approval of a marketing application in the EU) Duration of Archiving - agreed by Sponsor / CI at the beginning of the trial Approved by Ethics (require ethical approval if these require to be kept for longer) Do not destroy early or take with you if you leave – must be retained within the Sponsors locality
 Possible Questions: What happens with the archiving at other sites What will be forwarded to the TMF for archiving What happens to the study material and patient medical notes at the end (archiving arrangements, who, where, how long)
Possible Questions: What happens with the archiving at other sites What will be forwarded to the TMF for archiving What happens to the study material and patient medical notes at the end (archiving arrangements, who, where, how long)
 Preparation for MHRA Inspection Pharmacovigilance
Preparation for MHRA Inspection Pharmacovigilance
 Medicines for Human Use (Clinical Trials) Regulations 2004 (SI 1031) Part 5 Pharmacovigilance Notification of adverse events 32. (1) An investigator shall report any serious adverse event which occurs in a subject at a trial site at which he is responsible for the conduct of a clinical trial immediately to the sponsor. (2) An immediate report under paragraph (1) may be made orally or in writing. (3) Following the immediate report of a serious adverse event, the investigator shall make a detailed written report of the event. (4) Paragraphs (1) to (3) do not apply to serious adverse events specified in the protocol or the investigators' brochure as not requiring immediate reporting.
Medicines for Human Use (Clinical Trials) Regulations 2004 (SI 1031) Part 5 Pharmacovigilance Notification of adverse events 32. (1) An investigator shall report any serious adverse event which occurs in a subject at a trial site at which he is responsible for the conduct of a clinical trial immediately to the sponsor. (2) An immediate report under paragraph (1) may be made orally or in writing. (3) Following the immediate report of a serious adverse event, the investigator shall make a detailed written report of the event. (4) Paragraphs (1) to (3) do not apply to serious adverse events specified in the protocol or the investigators' brochure as not requiring immediate reporting.
 Medicines for Human Use (Clinical Trials) Regulations 2004 (SI 1031) Part 5 Pharmacovigilance Notification of adverse events 32. Key components of the regulations : n Notification of serious adverse events to sponsors n Immediate reporting of SUSARs n Annual reporting of serious adverse reaction
Medicines for Human Use (Clinical Trials) Regulations 2004 (SI 1031) Part 5 Pharmacovigilance Notification of adverse events 32. Key components of the regulations : n Notification of serious adverse events to sponsors n Immediate reporting of SUSARs n Annual reporting of serious adverse reaction
 Medicines for Human Use (Clinical Trials) Regulations 2004 (SI 1031) Amendment 2006 (SI 1928) Condition which applies to all clinical trials: Rights, safety, and well being of trial participants are the most important considerations and shall prevail over interests of science and society
Medicines for Human Use (Clinical Trials) Regulations 2004 (SI 1031) Amendment 2006 (SI 1928) Condition which applies to all clinical trials: Rights, safety, and well being of trial participants are the most important considerations and shall prevail over interests of science and society
 SOP: Procedure for Reporting Serious Adverse Events and Suspected Unexpected Serious Adverse Reactions (Uo. A-NHSG-SOP-014) Not yet finalised “describes the correct procedure for reporting SAEs to the sponsor and expediting reports to ethics and the MHRA when required. ”
SOP: Procedure for Reporting Serious Adverse Events and Suspected Unexpected Serious Adverse Reactions (Uo. A-NHSG-SOP-014) Not yet finalised “describes the correct procedure for reporting SAEs to the sponsor and expediting reports to ethics and the MHRA when required. ”
 CI pharmacovigilance responsibilities n Timely collection of data recording and notification to sponsor n Appropriate assessments undertaken data completeness seriousness relatedness expectedness n Expedited and periodic reporting REC, MHRA, Sponsor (& others as appropriate).
CI pharmacovigilance responsibilities n Timely collection of data recording and notification to sponsor n Appropriate assessments undertaken data completeness seriousness relatedness expectedness n Expedited and periodic reporting REC, MHRA, Sponsor (& others as appropriate).
 Requirements for Pharmacovigilance n All protocols must have a PV section. n Risk to participants is dependent on the clinical trial. Responsibilities and systems to deal with recording, assessment and reporting must be clearly stated. n Time frames for notification, assessment and reporting are critical. n n SOPs are required.
Requirements for Pharmacovigilance n All protocols must have a PV section. n Risk to participants is dependent on the clinical trial. Responsibilities and systems to deal with recording, assessment and reporting must be clearly stated. n Time frames for notification, assessment and reporting are critical. n n SOPs are required.
 Requirements for Pharmacovigilance CI’s need to understand their responsibilities with respect to adverse event recording and notification n −Reports SAEs to the sponsor immediately (in practice 24 – 48 hours). −Report SUSARs to the MHRA within 7 days if fatal/life threatening otherwise within 15 days. −Urgent safety measures implemented, notify MHRA within 3 days. n Assessment of adverse events: −Seriousness −Relatedness/causality −Expectedness
Requirements for Pharmacovigilance CI’s need to understand their responsibilities with respect to adverse event recording and notification n −Reports SAEs to the sponsor immediately (in practice 24 – 48 hours). −Report SUSARs to the MHRA within 7 days if fatal/life threatening otherwise within 15 days. −Urgent safety measures implemented, notify MHRA within 3 days. n Assessment of adverse events: −Seriousness −Relatedness/causality −Expectedness
 Current Procedure for Pharmacovigilance CI/delegate to report serious adverse event to the Research Governance Manager (RGM) (email: g. holland@abdn. ac. uk) −Initial report may be by telephone (Ext: 55076) −Detailed written report by email within 24 hours n CI/delegate to report SAEs/SUSARs to REC and MHRA (as required). n n CI to forward copy of e. SUSAR report to RGM.
Current Procedure for Pharmacovigilance CI/delegate to report serious adverse event to the Research Governance Manager (RGM) (email: g. holland@abdn. ac. uk) −Initial report may be by telephone (Ext: 55076) −Detailed written report by email within 24 hours n CI/delegate to report SAEs/SUSARs to REC and MHRA (as required). n n CI to forward copy of e. SUSAR report to RGM.
 Current Procedure for Pharmacovigilance RGM to provide guidance/support for SUSAR reporting on MHRA electronic reporting site. n n Website for SUSAR reporting: https: //esusar. mhra. gov. uk/? CI/delegate will require registration to the e. SUSAR website. RGM will facilitate. n
Current Procedure for Pharmacovigilance RGM to provide guidance/support for SUSAR reporting on MHRA electronic reporting site. n n Website for SUSAR reporting: https: //esusar. mhra. gov. uk/? CI/delegate will require registration to the e. SUSAR website. RGM will facilitate. n
 Possible questions Would CI report to MHRA if a SUSAR? Who assesses SUSARs? (How) Does the protocol permit for any nonescalated SAES? What is the process for reporting SAEs? Where do you send the annual safety report? What is the process for reporting SUSARs?
Possible questions Would CI report to MHRA if a SUSAR? Who assesses SUSARs? (How) Does the protocol permit for any nonescalated SAES? What is the process for reporting SAEs? Where do you send the annual safety report? What is the process for reporting SUSARs?
 Preparation for MHRA Inspection Serious Breaches
Preparation for MHRA Inspection Serious Breaches
 Medicines for Human Use (Clinical Trials) Regulations 2004 (SI 1031) Amendment 2006 (SI 1928) Notification of serious breaches 29 A (1) The sponsor of a clinical trial shall notify the licensing authority in writing of any serious breach of (a) the conditions and principles of GCP in connection with that trial; or (b) the protocol relating to that trial, as amended from time to time in accordance with regulations 22 to 25, within 7 days of becoming aware of that breach. (2) For the purposes of this regulation, a “serious breach” is a breach which is likely to effect to a significant degree – (a) the safety or physical or mental integrity of the subjects of the trial; or (b) the scientific value of the trial”.
Medicines for Human Use (Clinical Trials) Regulations 2004 (SI 1031) Amendment 2006 (SI 1928) Notification of serious breaches 29 A (1) The sponsor of a clinical trial shall notify the licensing authority in writing of any serious breach of (a) the conditions and principles of GCP in connection with that trial; or (b) the protocol relating to that trial, as amended from time to time in accordance with regulations 22 to 25, within 7 days of becoming aware of that breach. (2) For the purposes of this regulation, a “serious breach” is a breach which is likely to effect to a significant degree – (a) the safety or physical or mental integrity of the subjects of the trial; or (b) the scientific value of the trial”.
 Medicines for Human Use (Clinical Trials) Regulations 2004 (SI 1031) Amendment 2006 (SI 1928) Condition which applies to all clinical trials: Rights, safety, and well being of trial participants are the most important considerations and shall prevail over interests of science and society
Medicines for Human Use (Clinical Trials) Regulations 2004 (SI 1031) Amendment 2006 (SI 1928) Condition which applies to all clinical trials: Rights, safety, and well being of trial participants are the most important considerations and shall prevail over interests of science and society
 SOP: Procedure for Reporting Serious Breaches of the protocol or GCP (Uo. A-NHSG-SOP-015) Not yet finalised “describes the correct procedure for reporting serious breaches to the sponsor, ethics and to the MHRA. ”
SOP: Procedure for Reporting Serious Breaches of the protocol or GCP (Uo. A-NHSG-SOP-015) Not yet finalised “describes the correct procedure for reporting serious breaches to the sponsor, ethics and to the MHRA. ”
 Examples of serious breaches Principal Investigator unable to provide training log. Study protocol not peer-reviewed. It started with a simple case of peer review
Examples of serious breaches Principal Investigator unable to provide training log. Study protocol not peer-reviewed. It started with a simple case of peer review
 Examples of serious breaches No trial specific SOPs. Investigator unaware of the Declaration of Helsinki.
Examples of serious breaches No trial specific SOPs. Investigator unaware of the Declaration of Helsinki.
 Examples of serious breaches Protocol does not contain a section on the exclusion criteria for study participants. Failure to report an SAE to study sponsor.
Examples of serious breaches Protocol does not contain a section on the exclusion criteria for study participants. Failure to report an SAE to study sponsor.
 Examples of serious breaches CRFs contain patient identifiers. After trial commences new data concerning IMP safety not taken into account.
Examples of serious breaches CRFs contain patient identifiers. After trial commences new data concerning IMP safety not taken into account.
 Examples of serious breaches No statement of patient eligibility signed by medically qualified individual No CTA in place before study start.
Examples of serious breaches No statement of patient eligibility signed by medically qualified individual No CTA in place before study start.
 Examples of serious breaches Patient identifiable data on laptop stolen from investigator’s car. Inadequate insurance cover in place.
Examples of serious breaches Patient identifiable data on laptop stolen from investigator’s car. Inadequate insurance cover in place.
 Current Procedure for Serious Breaches CI/delegate to report serious breaches to the Research Governance Manager (RGM) (email: g. holland@abdn. ac. uk) −If unsure a breach has occurred contact the RGM for advise within 24 hours of event. −Initial report may be by telephone (Ext: 55076) −Detailed written report by email within 7 days n CI/delegate to report serious breaches to REC and MHRA within 7 days n CI to forward copy of report & email to MHRA to RGM. n
Current Procedure for Serious Breaches CI/delegate to report serious breaches to the Research Governance Manager (RGM) (email: g. holland@abdn. ac. uk) −If unsure a breach has occurred contact the RGM for advise within 24 hours of event. −Initial report may be by telephone (Ext: 55076) −Detailed written report by email within 7 days n CI/delegate to report serious breaches to REC and MHRA within 7 days n CI to forward copy of report & email to MHRA to RGM. n
 Current Procedure for Reporting Serious Breaches to the MHRA. RGM to provide guidance/support for serious breach reporting to REC and MHRA. n n MHRA notification of serious breach form available at: http: //www. mhra. gov. uk/Howweregulate/Medicines/I nspectionandstandards/Good. Clinical. Practice/News/CO N 084915 n Notification form to be sent to: GCP. Serious. Breaches@mhra. gsi. gov. uk
Current Procedure for Reporting Serious Breaches to the MHRA. RGM to provide guidance/support for serious breach reporting to REC and MHRA. n n MHRA notification of serious breach form available at: http: //www. mhra. gov. uk/Howweregulate/Medicines/I nspectionandstandards/Good. Clinical. Practice/News/CO N 084915 n Notification form to be sent to: GCP. Serious. Breaches@mhra. gsi. gov. uk
 Current Procedure for Reporting Serious Breaches to the REC. RGM to provide guidance/support for serious breach reporting to REC and MHRA. n n No specific REC notification of serious breach form. RECs will accept the MHRA notification of serious breach form. n n Forward letter/email to REC to the RGM.
Current Procedure for Reporting Serious Breaches to the REC. RGM to provide guidance/support for serious breach reporting to REC and MHRA. n n No specific REC notification of serious breach form. RECs will accept the MHRA notification of serious breach form. n n Forward letter/email to REC to the RGM.
 Possible questions What do you class as a deviation? Have there been any breaches of GCP? Have there been any deviations from the protocol? Have there been any persistent deviations of GCP or the protocol?
Possible questions What do you class as a deviation? Have there been any breaches of GCP? Have there been any deviations from the protocol? Have there been any persistent deviations of GCP or the protocol?
 Preparation for MHRA Inspection Informed Consent
Preparation for MHRA Inspection Informed Consent
 Medicines for Human Use (Clinical Trials) Regulations 2004 (SI 1031) n For the purposes of this Schedule, a person gives informed consent to take part, or that a subject is to take part, in a clinical trial only if his decision— (a) is given freely after that person is informed of the nature, significance, implications and risks of the trial; and (b) either — n (i) is evidenced in writing, dated and signed, or otherwise marked, by that person so as to indicate his consent; or n (ii) if the person is unable to sign or to mark a document so as to indiacte his consent, is given orally in the presence of a at least one witness and recorded in writing.
Medicines for Human Use (Clinical Trials) Regulations 2004 (SI 1031) n For the purposes of this Schedule, a person gives informed consent to take part, or that a subject is to take part, in a clinical trial only if his decision— (a) is given freely after that person is informed of the nature, significance, implications and risks of the trial; and (b) either — n (i) is evidenced in writing, dated and signed, or otherwise marked, by that person so as to indicate his consent; or n (ii) if the person is unable to sign or to mark a document so as to indiacte his consent, is given orally in the presence of a at least one witness and recorded in writing.
 SOP: Obtaining Informed Consent from Competent Adults for Research Studies (Uo. A-NHSG-SOP-010) “describes the correct procedure for obtaining written informed consent for clinical research studies”
SOP: Obtaining Informed Consent from Competent Adults for Research Studies (Uo. A-NHSG-SOP-010) “describes the correct procedure for obtaining written informed consent for clinical research studies”
 SOP: Responsibilities of PI n Ethical approval for : consent form PIS adverts Remember all changes need ethical approval! n n n Delegation log Training of staff in informed consent No tests, procedures, data collection before consent
SOP: Responsibilities of PI n Ethical approval for : consent form PIS adverts Remember all changes need ethical approval! n n n Delegation log Training of staff in informed consent No tests, procedures, data collection before consent
 SOP: Procedure - providing information RCT: Pink or Blue Pill for Chocolate Addiction? 2010 -012345 -67 Version 3, 25 June 2010
SOP: Procedure - providing information RCT: Pink or Blue Pill for Chocolate Addiction? 2010 -012345 -67 Version 3, 25 June 2010
 Who can obtain informed consent? 'Thanks for telling me your entire medical history but I'm the hospital barber. ' Investigators/Co-investigators & staff named on delegation log
Who can obtain informed consent? 'Thanks for telling me your entire medical history but I'm the hospital barber. ' Investigators/Co-investigators & staff named on delegation log
 Checks prior to obtaining signature n the participant’s identity and eligibility (there is no new or undisclosed information that would exclude them from the study) the participant’s understanding of the study is adequate and they are happy to continue with entering the study n the participant knows that they can withdraw at any time without giving a reason n the participant has had sufficient time to consider taking part in the study n
Checks prior to obtaining signature n the participant’s identity and eligibility (there is no new or undisclosed information that would exclude them from the study) the participant’s understanding of the study is adequate and they are happy to continue with entering the study n the participant knows that they can withdraw at any time without giving a reason n the participant has had sufficient time to consider taking part in the study n
 Consent Form Must be signed and personally dated by participant and the person taking consent Must be obtained prior to initiation of any screening procedures and before any changes are made to patient’s medication Filing Original -> investigator study file Copy -> to participant/legal representative (Copy -> patient’s notes along with PIS)
Consent Form Must be signed and personally dated by participant and the person taking consent Must be obtained prior to initiation of any screening procedures and before any changes are made to patient’s medication Filing Original -> investigator study file Copy -> to participant/legal representative (Copy -> patient’s notes along with PIS)
 Headed Paper Unit & dept conducting the trial Participant must initial not tick boxes Signed & personally dated by participant Eudract no Person taking consent must sign also Version no
Headed Paper Unit & dept conducting the trial Participant must initial not tick boxes Signed & personally dated by participant Eudract no Person taking consent must sign also Version no
 Vulnerable Participants 1. Difficulty reading/writing - Impartial witness - Read PIS to participant - signature of witness 2. Minor – child under 16 - consent of parent required 3. Adult – unable to give informed consent due to physical or mental incapacity - Adults with Incapacity (Scotland) Act 2000 - consent by a legal representative
Vulnerable Participants 1. Difficulty reading/writing - Impartial witness - Read PIS to participant - signature of witness 2. Minor – child under 16 - consent of parent required 3. Adult – unable to give informed consent due to physical or mental incapacity - Adults with Incapacity (Scotland) Act 2000 - consent by a legal representative
 Common MHRA findings They will check source data from medical notes! – No record of study visit in medical notes – No records of consent being taken – medical notes or ISF – Poor version control – Inconsistencies with protocol – Missing elements e. g. signature – Unclear process
Common MHRA findings They will check source data from medical notes! – No record of study visit in medical notes – No records of consent being taken – medical notes or ISF – Poor version control – Inconsistencies with protocol – Missing elements e. g. signature – Unclear process
 Possible questions How do you approach patients? Who tells participants about the trial Talk me though the consent procedure How have other clinicians been told about the trial? Where do you store PIS & Consent form Can all participants consent on their own?
Possible questions How do you approach patients? Who tells participants about the trial Talk me though the consent procedure How have other clinicians been told about the trial? Where do you store PIS & Consent form Can all participants consent on their own?
 Preparation for MHRA Inspection Communication
Preparation for MHRA Inspection Communication
 Communication Inspectors will look for evidence that a study team communicates well Site File Ind “if it isn’t written down, it didn’t happen” ex
Communication Inspectors will look for evidence that a study team communicates well Site File Ind “if it isn’t written down, it didn’t happen” ex
 Communication – with who? Research team n Clinical team (e. g. ward nurses/doctors) n Pharmacy n Labs – internal & external n Sponsor n Ethics/R&D n
Communication – with who? Research team n Clinical team (e. g. ward nurses/doctors) n Pharmacy n Labs – internal & external n Sponsor n Ethics/R&D n
 Communication – how? Internally: Research team Regular meetings – dates, agenda, minutes n Email updates n Written correspondence n All must be filed appropriately in the TMF/ISF
Communication – how? Internally: Research team Regular meetings – dates, agenda, minutes n Email updates n Written correspondence n All must be filed appropriately in the TMF/ISF
 Communication – how? Externally: Clinical team n n n Ward staff: presentations/posters New staff/rotational staff – documented procedure of how the are informed of study External clinicians – e. g. labels on notes Keep a record of everything & file in TMF/ISF
Communication – how? Externally: Clinical team n n n Ward staff: presentations/posters New staff/rotational staff – documented procedure of how the are informed of study External clinicians – e. g. labels on notes Keep a record of everything & file in TMF/ISF
 Communication – how? Externally: pharmacy, sponsor, ethics, R&D, MHRA etc Email updates n Written correspondence n Amendments – inform correct people n Keep a record of everything & file in TMF/ISF
Communication – how? Externally: pharmacy, sponsor, ethics, R&D, MHRA etc Email updates n Written correspondence n Amendments – inform correct people n Keep a record of everything & file in TMF/ISF
 Possible questions Do you have regular team meetings? What do you cover in these meetings – are they minuted? How do clinicians know this patient is part of a study? How is communication maintained? How do staff on call (not part of core team) know what to do? How have other clinicians been told about the trial?
Possible questions Do you have regular team meetings? What do you cover in these meetings – are they minuted? How do clinicians know this patient is part of a study? How is communication maintained? How do staff on call (not part of core team) know what to do? How have other clinicians been told about the trial?
 Summary ■ Review your trial documentation and training files for staff. ■ Have evidence of training (GCP certificate, CV) ■ Ensure you can explain your role in the trial ■ Review the typical questions and answers provided ■ Familiarise yourself with new SOPs ■ Be confident of your trial and processes.
Summary ■ Review your trial documentation and training files for staff. ■ Have evidence of training (GCP certificate, CV) ■ Ensure you can explain your role in the trial ■ Review the typical questions and answers provided ■ Familiarise yourself with new SOPs ■ Be confident of your trial and processes.
 Main Contacts: Prof Phil Hannaford – p. hannaford@abdn. ac. uk n Prof Alison Mac. Leod – mmd 175@abdn. ac. uk n Dr Gail Holland – g. holland@abdn. ac. uk Tel: 01224 - 555076 n Lynda Sime – lynda. sime@nhs. net Tel: 01224 -554656 n
Main Contacts: Prof Phil Hannaford – p. hannaford@abdn. ac. uk n Prof Alison Mac. Leod – mmd 175@abdn. ac. uk n Dr Gail Holland – g. holland@abdn. ac. uk Tel: 01224 - 555076 n Lynda Sime – lynda. sime@nhs. net Tel: 01224 -554656 n


