1e39b8437a3a4a5c59eeb173d5d11506.ppt
- Количество слайдов: 40
 MFDS drug approval system 2013 We are dedicated to professional, individualized and market-specific solutions to make our clients’ expansions into Korea and success in the global market
MFDS drug approval system 2013 We are dedicated to professional, individualized and market-specific solutions to make our clients’ expansions into Korea and success in the global market
 § Contents üMFDS Organization Overview ü Regulatory Infrastructure ü Review and Approval Process MFDS : Ministry of Food & Drug Safety, it was changed from KFDA since April, 2013 www. mfds. go. kr KFDA : Korea FDA MFDS > KFDA
§ Contents üMFDS Organization Overview ü Regulatory Infrastructure ü Review and Approval Process MFDS : Ministry of Food & Drug Safety, it was changed from KFDA since April, 2013 www. mfds. go. kr KFDA : Korea FDA MFDS > KFDA
 § Contents üMFDS(KFDA) Organization Overview ü Regulatory Infrastructure ü Review and Approval Process
§ Contents üMFDS(KFDA) Organization Overview ü Regulatory Infrastructure ü Review and Approval Process
 MFDS Organization Ministry of Food and Drug Safety
MFDS Organization Ministry of Food and Drug Safety
 MFDS Organization National Institute of Food and Drug Sadety Evalustion
MFDS Organization National Institute of Food and Drug Sadety Evalustion
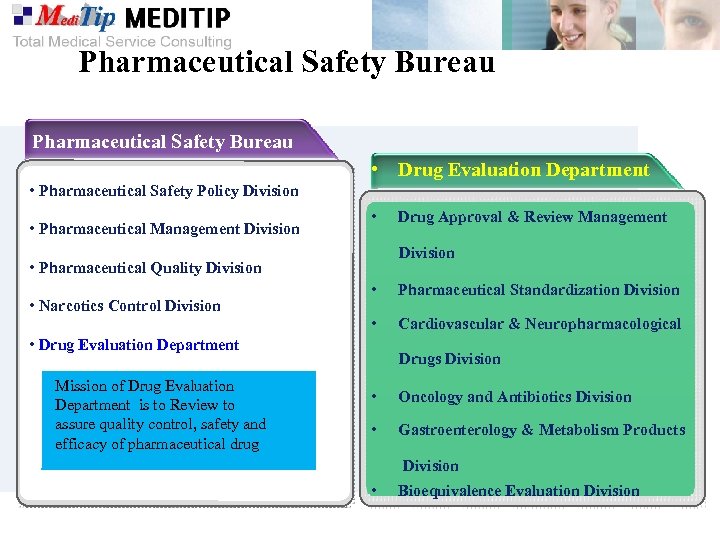 Pharmaceutical Safety Bureau • Drug Evaluation Department • Pharmaceutical Safety Policy Division • Pharmaceutical Management Division • Pharmaceutical Quality Division • Narcotics Control Division • Pharmaceutical Standardization Division • Cardiovascular & Neuropharmacological • Drug Evaluation Department Mission of Drug Evaluation Department is to Review to assure quality control, safety and efficacy of pharmaceutical drug Drug Approval & Review Management Drugs Division • Oncology and Antibiotics Division • Gastroenterology & Metabolism Products Division • Bioequivalence Evaluation Division
Pharmaceutical Safety Bureau • Drug Evaluation Department • Pharmaceutical Safety Policy Division • Pharmaceutical Management Division • Pharmaceutical Quality Division • Narcotics Control Division • Pharmaceutical Standardization Division • Cardiovascular & Neuropharmacological • Drug Evaluation Department Mission of Drug Evaluation Department is to Review to assure quality control, safety and efficacy of pharmaceutical drug Drug Approval & Review Management Drugs Division • Oncology and Antibiotics Division • Gastroenterology & Metabolism Products Division • Bioequivalence Evaluation Division
 Pharmaceutical Safety Bureau ü Marketing Authorization ü Clinical Trial Review ü Advertisement Regulation ü Post-Marketing Surveillance ü Quality Control
Pharmaceutical Safety Bureau ü Marketing Authorization ü Clinical Trial Review ü Advertisement Regulation ü Post-Marketing Surveillance ü Quality Control
 § Contents üMFDS(KFDA) Organization Overview ü Regulatory Infrastructure ü Review and Approval Process
§ Contents üMFDS(KFDA) Organization Overview ü Regulatory Infrastructure ü Review and Approval Process
 Regulatory Infrastructure § Regulatory hierarchy on Clinical Trials & Drug Approval • • • Pharmaceutical Affairs Law Enforcement Rule of Pharmaceutical Affairs Law Guideline • Guideline on GCP • Guideline on IND • Guideline for Accredited Clinical Institutes • Regulation for Review and Approval of Drugs • Regulation for Review and Approval for Biological Products § Continuous legalization efforts to support Regulatory Harmonization in order to achieve globalization
Regulatory Infrastructure § Regulatory hierarchy on Clinical Trials & Drug Approval • • • Pharmaceutical Affairs Law Enforcement Rule of Pharmaceutical Affairs Law Guideline • Guideline on GCP • Guideline on IND • Guideline for Accredited Clinical Institutes • Regulation for Review and Approval of Drugs • Regulation for Review and Approval for Biological Products § Continuous legalization efforts to support Regulatory Harmonization in order to achieve globalization
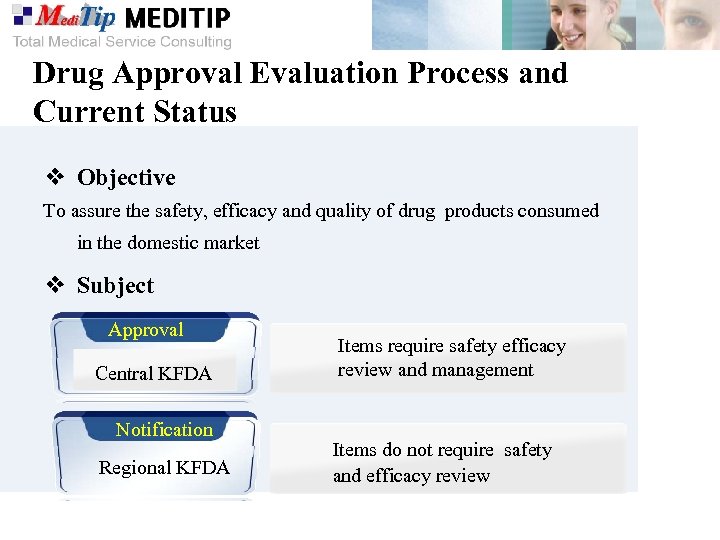 Drug Approval Evaluation Process and Current Status ❖ Objective To assure the safety, efficacy and quality of drug products consumed in the domestic market ❖ Subject Approval Central KFDA Notification Regional KFDA Items require safety efficacy review and management Items do not require safety and efficacy review
Drug Approval Evaluation Process and Current Status ❖ Objective To assure the safety, efficacy and quality of drug products consumed in the domestic market ❖ Subject Approval Central KFDA Notification Regional KFDA Items require safety efficacy review and management Items do not require safety and efficacy review
 § Contents üMFDS(KFDA) Organization Overview ü Regulatory Infrastructure ü Review and Approval Process
§ Contents üMFDS(KFDA) Organization Overview ü Regulatory Infrastructure ü Review and Approval Process
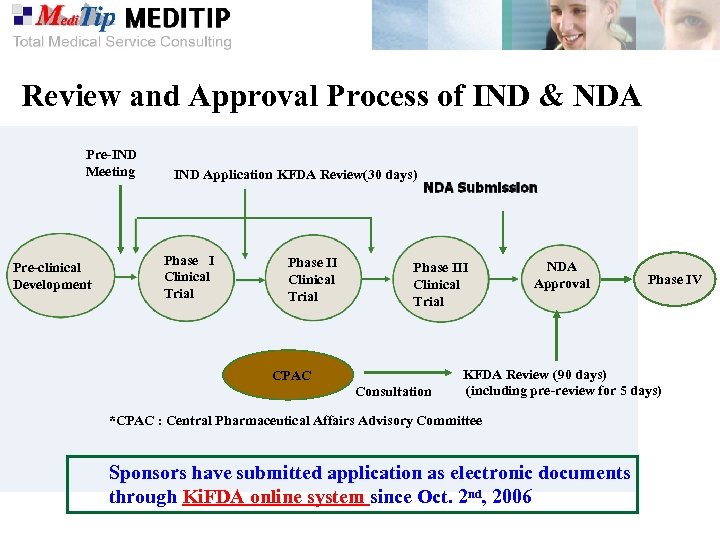 Review and Approval Process of IND & NDA Pre-IND Meeting Pre-clinical Development IND Application KFDA Review(30 days) Phase I Clinical Trial Phase III Clinical Trial CPAC Consultation NDA Approval Phase IV KFDA Review (90 days) (including pre-review for 5 days) *CPAC : Central Pharmaceutical Affairs Advisory Committee Sponsors have submitted application as electronic documents through Ki. FDA online system since Oct. 2 nd, 2006
Review and Approval Process of IND & NDA Pre-IND Meeting Pre-clinical Development IND Application KFDA Review(30 days) Phase I Clinical Trial Phase III Clinical Trial CPAC Consultation NDA Approval Phase IV KFDA Review (90 days) (including pre-review for 5 days) *CPAC : Central Pharmaceutical Affairs Advisory Committee Sponsors have submitted application as electronic documents through Ki. FDA online system since Oct. 2 nd, 2006
 Central Pharmaceutical Affairs Council (CPAC) u CPAC deliberates on the following; ¿ Provide advice and experts opinion on scientific issues ¿ CPAC are outside experts such as physician, researchers, pharmacologists, chemists, and statisticians ¿ Other important subcommittee
Central Pharmaceutical Affairs Council (CPAC) u CPAC deliberates on the following; ¿ Provide advice and experts opinion on scientific issues ¿ CPAC are outside experts such as physician, researchers, pharmacologists, chemists, and statisticians ¿ Other important subcommittee
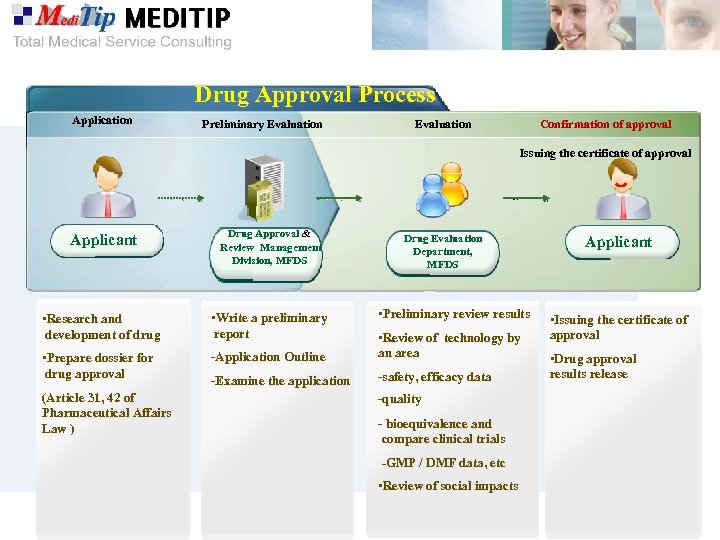 Drug Approval Process Application Preliminary Evaluation Confirmation of approval Issuing the certificate of approval Applicant Drug Approval & Review Management Division, MFDS • Research and development of drug • Write a preliminary report • Prepare dossier for drug approval -Application Outline • Review of technology by an area -Examine the application -safety, efficacy data (Article 31, 42 of Pharmaceutical Affairs Law ) Drug Evaluation Department, MFDS • Preliminary review results -quality - bioequivalence and compare clinical trials -GMP / DMF data, etc • Review of social impacts Applicant • Issuing the certificate of approval • Drug approval results release
Drug Approval Process Application Preliminary Evaluation Confirmation of approval Issuing the certificate of approval Applicant Drug Approval & Review Management Division, MFDS • Research and development of drug • Write a preliminary report • Prepare dossier for drug approval -Application Outline • Review of technology by an area -Examine the application -safety, efficacy data (Article 31, 42 of Pharmaceutical Affairs Law ) Drug Evaluation Department, MFDS • Preliminary review results -quality - bioequivalence and compare clinical trials -GMP / DMF data, etc • Review of social impacts Applicant • Issuing the certificate of approval • Drug approval results release
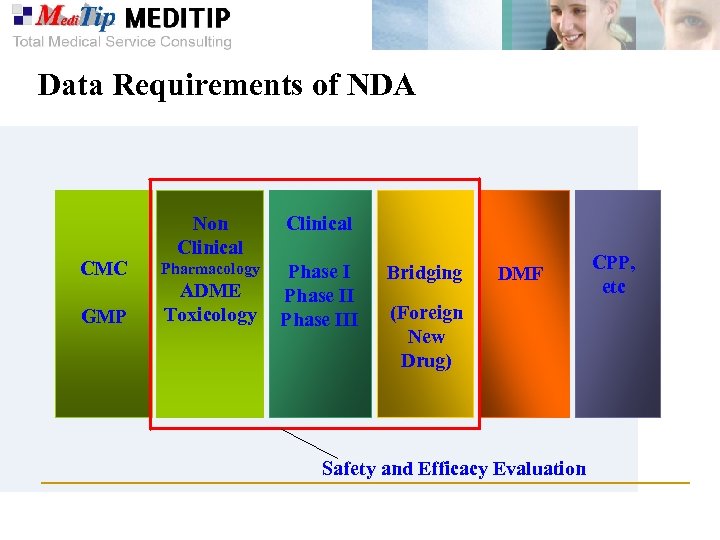 Data Requirements of NDA Clinical CMC Non Clinical Pharmacology GMP ADME Toxicology Phase III Bridging DMF (Foreign New Drug) Safety and Efficacy Evaluation CPP, etc
Data Requirements of NDA Clinical CMC Non Clinical Pharmacology GMP ADME Toxicology Phase III Bridging DMF (Foreign New Drug) Safety and Efficacy Evaluation CPP, etc
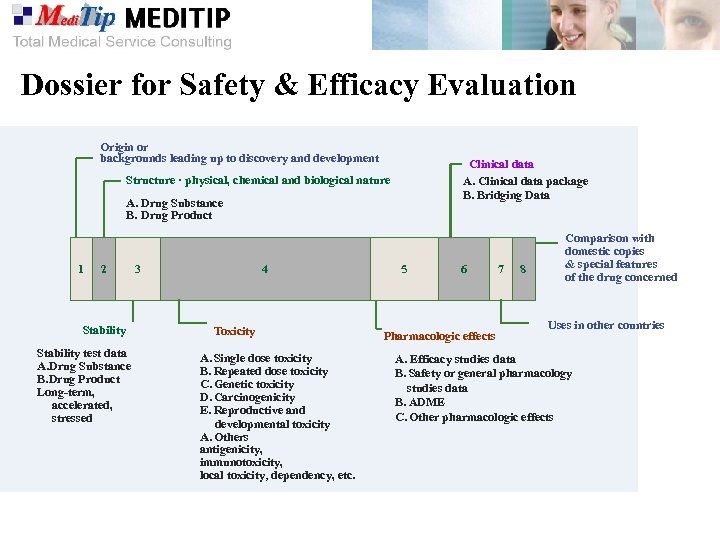 Dossier for Safety & Efficacy Evaluation Origin or backgrounds leading up to discovery and development Clinical data A. Clinical data package B. Bridging Data Structure · physical, chemical and biological nature A. Drug Substance B. Drug Product 1 2 Stability test data A. Drug Substance B. Drug Product Long-term, accelerated, stressed 3 4 Toxicity A. Single dose toxicity B. Repeated dose toxicity C. Genetic toxicity D. Carcinogenicity E. Reproductive and developmental toxicity A. Others antigenicity, immunotoxicity, local toxicity, dependency, etc. 5 6 Pharmacologic effects 7 8 Comparison with domestic copies & special features of the drug concerned Uses in other countries A. Efficacy studies data B. Safety or general pharmacology studies data B. ADME C. Other pharmacologic effects
Dossier for Safety & Efficacy Evaluation Origin or backgrounds leading up to discovery and development Clinical data A. Clinical data package B. Bridging Data Structure · physical, chemical and biological nature A. Drug Substance B. Drug Product 1 2 Stability test data A. Drug Substance B. Drug Product Long-term, accelerated, stressed 3 4 Toxicity A. Single dose toxicity B. Repeated dose toxicity C. Genetic toxicity D. Carcinogenicity E. Reproductive and developmental toxicity A. Others antigenicity, immunotoxicity, local toxicity, dependency, etc. 5 6 Pharmacologic effects 7 8 Comparison with domestic copies & special features of the drug concerned Uses in other countries A. Efficacy studies data B. Safety or general pharmacology studies data B. ADME C. Other pharmacologic effects
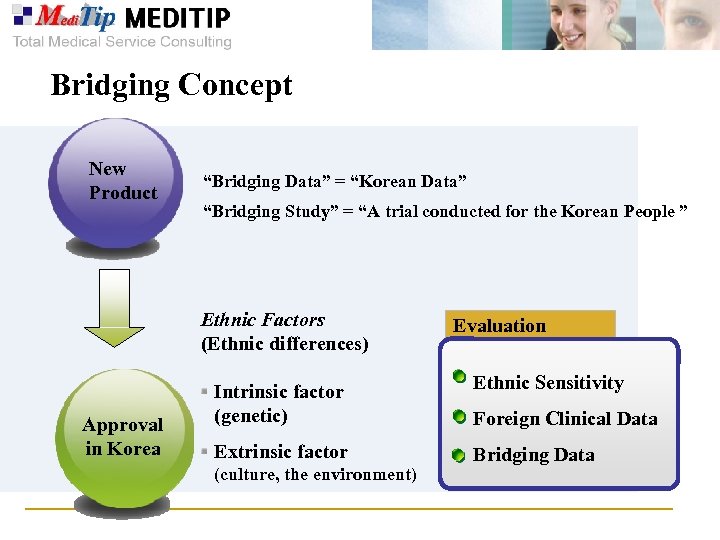 Bridging Concept New Product “Bridging Data” = “Korean Data” “Bridging Study” = “A trial conducted for the Korean People ” Ethnic Factors (Ethnic differences) Approval in Korea Evaluation Intrinsic factor (genetic) Ethnic Sensitivity Extrinsic factor Bridging Data (culture, the environment) Foreign Clinical Data
Bridging Concept New Product “Bridging Data” = “Korean Data” “Bridging Study” = “A trial conducted for the Korean People ” Ethnic Factors (Ethnic differences) Approval in Korea Evaluation Intrinsic factor (genetic) Ethnic Sensitivity Extrinsic factor Bridging Data (culture, the environment) Foreign Clinical Data
 DMF Introduction Ø KDMF : API registration system (effective as of July 1 st, 2002) Background • Concerns about using low quality of drug substances • Quality control of drug substances Scope • New chemical entities used as APIs • Phase-in of other APIs registration (designated by KFDA) # Only drug substances registered can be used.
DMF Introduction Ø KDMF : API registration system (effective as of July 1 st, 2002) Background • Concerns about using low quality of drug substances • Quality control of drug substances Scope • New chemical entities used as APIs • Phase-in of other APIs registration (designated by KFDA) # Only drug substances registered can be used.
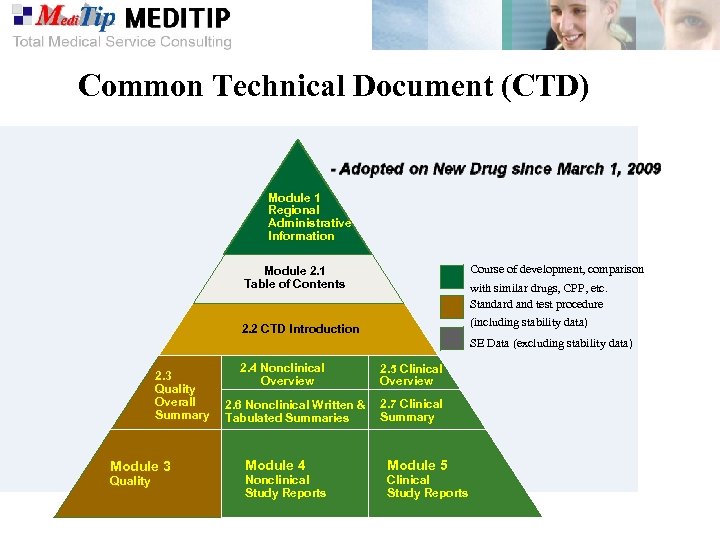 Common Technical Document (CTD) Module 1 Regional Administrative Information Course of development, comparison Module 2. 1 Table of Contents with similar drugs, CPP, etc. Standard and test procedure (including stability data) 2. 2 CTD Introduction SE Data (excluding stability data) 2. 3 Quality Overall Summary Module 3 Quality 2. 4 Nonclinical Overview 2. 6 Nonclinical Written & Tabulated Summaries Module 4 Nonclinical Study Reports 2. 5 Clinical Overview 2. 7 Clinical Summary Module 5 Clinical Study Reports
Common Technical Document (CTD) Module 1 Regional Administrative Information Course of development, comparison Module 2. 1 Table of Contents with similar drugs, CPP, etc. Standard and test procedure (including stability data) 2. 2 CTD Introduction SE Data (excluding stability data) 2. 3 Quality Overall Summary Module 3 Quality 2. 4 Nonclinical Overview 2. 6 Nonclinical Written & Tabulated Summaries Module 4 Nonclinical Study Reports 2. 5 Clinical Overview 2. 7 Clinical Summary Module 5 Clinical Study Reports
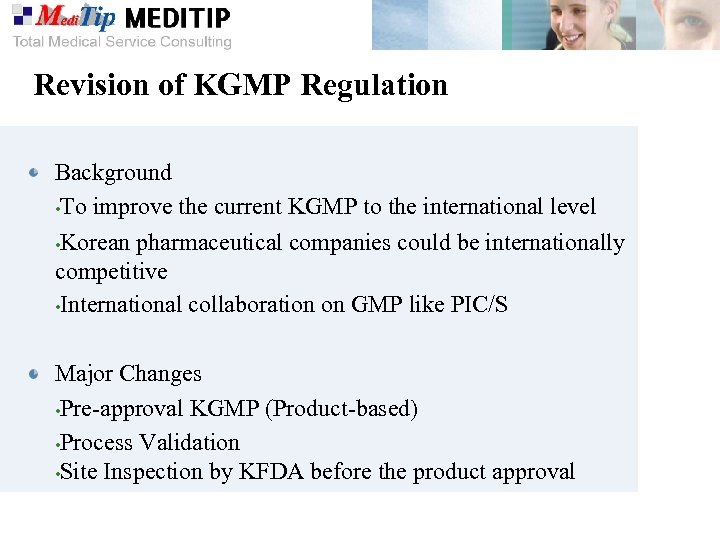 Revision of KGMP Regulation Background • To improve the current KGMP to the international level • Korean pharmaceutical companies could be internationally competitive • International collaboration on GMP like PIC/S Major Changes • Pre-approval KGMP (Product-based) • Process Validation • Site Inspection by KFDA before the product approval
Revision of KGMP Regulation Background • To improve the current KGMP to the international level • Korean pharmaceutical companies could be internationally competitive • International collaboration on GMP like PIC/S Major Changes • Pre-approval KGMP (Product-based) • Process Validation • Site Inspection by KFDA before the product approval
 Definition u New drug ¿ Is a drug containing a new medicinal substance with entirely different chemical structure or material composition from those of drugs which have already been approved in Korea. ¿ Is combined preparations containing new medicinal substances as active ingredient
Definition u New drug ¿ Is a drug containing a new medicinal substance with entirely different chemical structure or material composition from those of drugs which have already been approved in Korea. ¿ Is combined preparations containing new medicinal substances as active ingredient
 Definition u Non-new drug requiring data submission Is not new drug but is subject to evaluation of safety and efficacy ¿ Consist of as follows; u New efficacy u New composition u New formulation u New dosage form u New administration route ¿
Definition u Non-new drug requiring data submission Is not new drug but is subject to evaluation of safety and efficacy ¿ Consist of as follows; u New efficacy u New composition u New formulation u New dosage form u New administration route ¿
 Exception of Safety and Efficacy Data u Drugs may be excluded evaluation for safety and efficacy. ¿ Drugs listed in the followings; ¿ Pharmacopia (KP, USP, JP, BP, EP, Deutsches Arzneibuch and Pharmacipee Francaise) ¿ Pharmaceutical compendia published in the past 3 yrs (US PDR, Drugs in Japan, ABPI Data Sheet Compendium, Rote Liste, VIDAL, L'iniformatore Farmaceutico, Arzneimittel Kompendium der Schweiz, Compendium of Pharmaceuticals and Specialties )
Exception of Safety and Efficacy Data u Drugs may be excluded evaluation for safety and efficacy. ¿ Drugs listed in the followings; ¿ Pharmacopia (KP, USP, JP, BP, EP, Deutsches Arzneibuch and Pharmacipee Francaise) ¿ Pharmaceutical compendia published in the past 3 yrs (US PDR, Drugs in Japan, ABPI Data Sheet Compendium, Rote Liste, VIDAL, L'iniformatore Farmaceutico, Arzneimittel Kompendium der Schweiz, Compendium of Pharmaceuticals and Specialties )
 NDA Filing 1. Origin, background of discovery and development 2. Structure, physicochemical properties, and biological properties 3. Stability a. Long-term storage or accelerated testing b. Stress testing 4. Toxicology a. Acute toxicity b. Subacute and chronic toxicity c. Reproductive and development toxicity d. Inhaling toxicity, e. Genetic toxicity, f. Immunological toxicity g. Carcinogenetic toxicity h. Local toxicity 5. Pharmacology a. Efficacy b. General/Safety Pharmacology c. Pharmacokinetics 6. Clinical data a. Safety and Effectiveness b. Bridging data 7. Usage in foreign countries 8. Comparison with similar drugs that are currently available in Korea
NDA Filing 1. Origin, background of discovery and development 2. Structure, physicochemical properties, and biological properties 3. Stability a. Long-term storage or accelerated testing b. Stress testing 4. Toxicology a. Acute toxicity b. Subacute and chronic toxicity c. Reproductive and development toxicity d. Inhaling toxicity, e. Genetic toxicity, f. Immunological toxicity g. Carcinogenetic toxicity h. Local toxicity 5. Pharmacology a. Efficacy b. General/Safety Pharmacology c. Pharmacokinetics 6. Clinical data a. Safety and Effectiveness b. Bridging data 7. Usage in foreign countries 8. Comparison with similar drugs that are currently available in Korea
 Stability • Data that are prepared from test in according to Guidelines for Stability of drugs (MFDS Notification 2000) or ICH guidelines a. Long Term Storage testing is ; – Study under the recommended storage condition, for the retest period or shelf life proposed (or approved) for labeling – 25℃ / 60% RH b. Accelerated testing – 40℃ / 75% RH c. Stress testing (photostability) – 50 ℃, -20 ℃, light, acid/base solution
Stability • Data that are prepared from test in according to Guidelines for Stability of drugs (MFDS Notification 2000) or ICH guidelines a. Long Term Storage testing is ; – Study under the recommended storage condition, for the retest period or shelf life proposed (or approved) for labeling – 25℃ / 60% RH b. Accelerated testing – 40℃ / 75% RH c. Stress testing (photostability) – 50 ℃, -20 ℃, light, acid/base solution
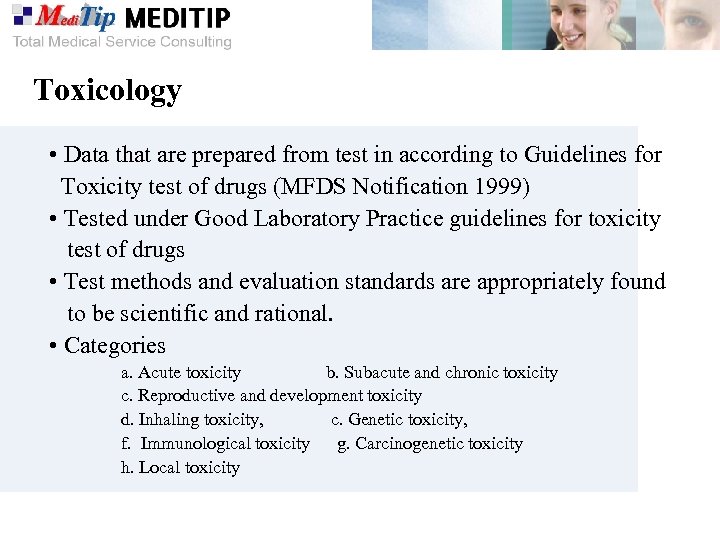 Toxicology • Data that are prepared from test in according to Guidelines for Toxicity test of drugs (MFDS Notification 1999) • Tested under Good Laboratory Practice guidelines for toxicity test of drugs • Test methods and evaluation standards are appropriately found to be scientific and rational. • Categories a. Acute toxicity b. Subacute and chronic toxicity c. Reproductive and development toxicity d. Inhaling toxicity, c. Genetic toxicity, f. Immunological toxicity g. Carcinogenetic toxicity h. Local toxicity
Toxicology • Data that are prepared from test in according to Guidelines for Toxicity test of drugs (MFDS Notification 1999) • Tested under Good Laboratory Practice guidelines for toxicity test of drugs • Test methods and evaluation standards are appropriately found to be scientific and rational. • Categories a. Acute toxicity b. Subacute and chronic toxicity c. Reproductive and development toxicity d. Inhaling toxicity, c. Genetic toxicity, f. Immunological toxicity g. Carcinogenetic toxicity h. Local toxicity
 Pharmacology Data shows pharmacological action and mechanisms of drugs Categories a. Efficacy b. General Pharmacology or Safety Pharmacology c. Pharmacokinetics (Absorption, Distribution, Metabolism, and Excretion)
Pharmacology Data shows pharmacological action and mechanisms of drugs Categories a. Efficacy b. General Pharmacology or Safety Pharmacology c. Pharmacokinetics (Absorption, Distribution, Metabolism, and Excretion)
 Clinical Trials Data are prepared in according to Korea GCP or ICH GCP Clinical data was therapeutic confirmatory study that is to ascertain the safety and efficacy of the test drug in a large number of selected patients in order to determine the indication, dosage & administration and precaution in human use
Clinical Trials Data are prepared in according to Korea GCP or ICH GCP Clinical data was therapeutic confirmatory study that is to ascertain the safety and efficacy of the test drug in a large number of selected patients in order to determine the indication, dosage & administration and precaution in human use
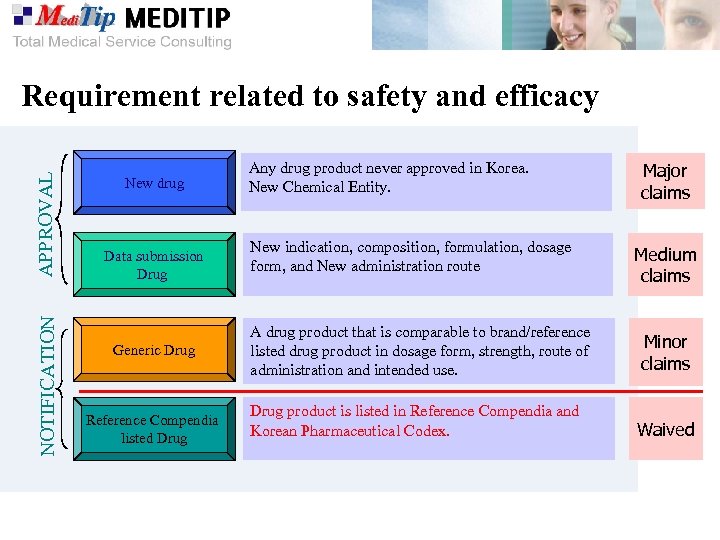 NOTIFICATION APPROVAL Requirement related to safety and efficacy New drug Data submission Drug Generic Drug Reference Compendia listed Drug Any drug product never approved in Korea. New Chemical Entity. New indication, composition, formulation, dosage form, and New administration route Major claims Medium claims A drug product that is comparable to brand/reference listed drug product in dosage form, strength, route of administration and intended use. Minor claims Drug product is listed in Reference Compendia and Korean Pharmaceutical Codex. Waived
NOTIFICATION APPROVAL Requirement related to safety and efficacy New drug Data submission Drug Generic Drug Reference Compendia listed Drug Any drug product never approved in Korea. New Chemical Entity. New indication, composition, formulation, dosage form, and New administration route Major claims Medium claims A drug product that is comparable to brand/reference listed drug product in dosage form, strength, route of administration and intended use. Minor claims Drug product is listed in Reference Compendia and Korean Pharmaceutical Codex. Waived
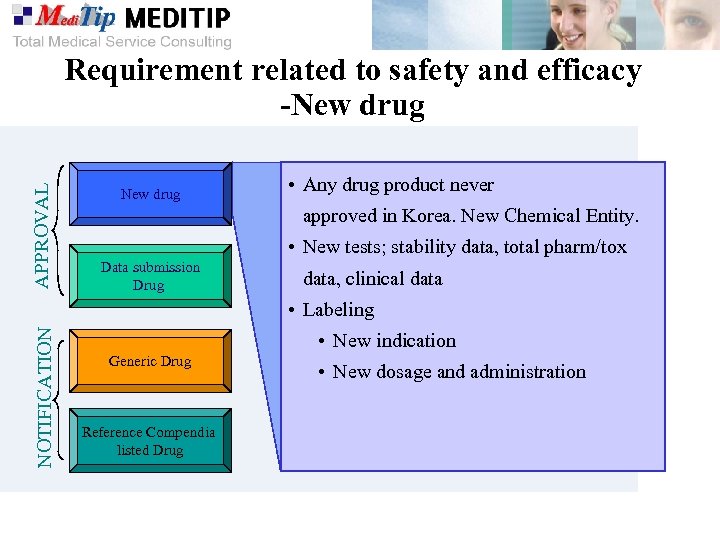 APPROVAL Requirement related to safety and efficacy -New drug • Any drug product never approved in Korea. New Chemical Entity. • New tests; stability data, total pharm/tox Data submission Drug data, clinical data NOTIFICATION • Labeling • New indication Generic Drug Reference Compendia listed Drug • New dosage and administration
APPROVAL Requirement related to safety and efficacy -New drug • Any drug product never approved in Korea. New Chemical Entity. • New tests; stability data, total pharm/tox Data submission Drug data, clinical data NOTIFICATION • Labeling • New indication Generic Drug Reference Compendia listed Drug • New dosage and administration
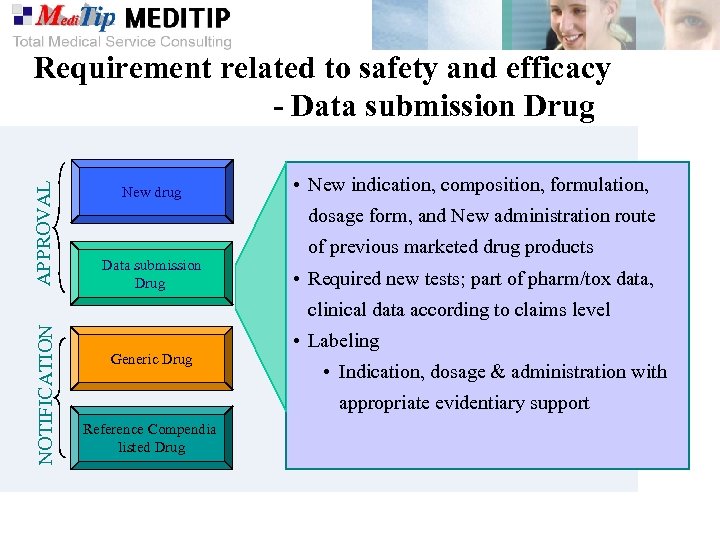 APPROVAL Requirement related to safety and efficacy - Data submission Drug New drug • New indication, composition, formulation, dosage form, and New administration route Data submission Drug of previous marketed drug products • Required new tests; part of pharm/tox data, NOTIFICATION clinical data according to claims level Generic Drug • Labeling • Indication, dosage & administration with appropriate evidentiary support Reference Compendia listed Drug
APPROVAL Requirement related to safety and efficacy - Data submission Drug New drug • New indication, composition, formulation, dosage form, and New administration route Data submission Drug of previous marketed drug products • Required new tests; part of pharm/tox data, NOTIFICATION clinical data according to claims level Generic Drug • Labeling • Indication, dosage & administration with appropriate evidentiary support Reference Compendia listed Drug
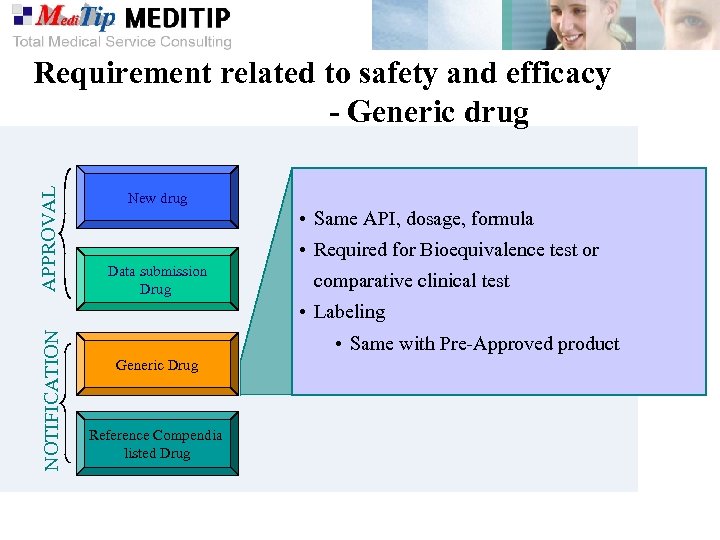 APPROVAL Requirement related to safety and efficacy - Generic drug New drug • Same API, dosage, formula • Required for Bioequivalence test or Data submission Drug comparative clinical test NOTIFICATION • Labeling • Same with Pre-Approved product Generic Drug Reference Compendia listed Drug
APPROVAL Requirement related to safety and efficacy - Generic drug New drug • Same API, dosage, formula • Required for Bioequivalence test or Data submission Drug comparative clinical test NOTIFICATION • Labeling • Same with Pre-Approved product Generic Drug Reference Compendia listed Drug
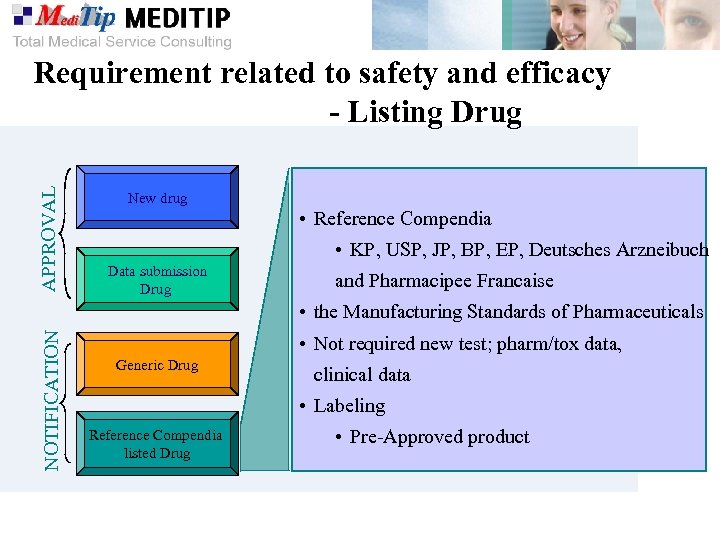 APPROVAL Requirement related to safety and efficacy - Listing Drug New drug • Reference Compendia • KP, USP, JP, BP, EP, Deutsches Arzneibuch Data submission Drug and Pharmacipee Francaise NOTIFICATION • the Manufacturing Standards of Pharmaceuticals • Not required new test; pharm/tox data, Generic Drug clinical data • Labeling Reference Compendia listed Drug • Pre-Approved product
APPROVAL Requirement related to safety and efficacy - Listing Drug New drug • Reference Compendia • KP, USP, JP, BP, EP, Deutsches Arzneibuch Data submission Drug and Pharmacipee Francaise NOTIFICATION • the Manufacturing Standards of Pharmaceuticals • Not required new test; pharm/tox data, Generic Drug clinical data • Labeling Reference Compendia listed Drug • Pre-Approved product
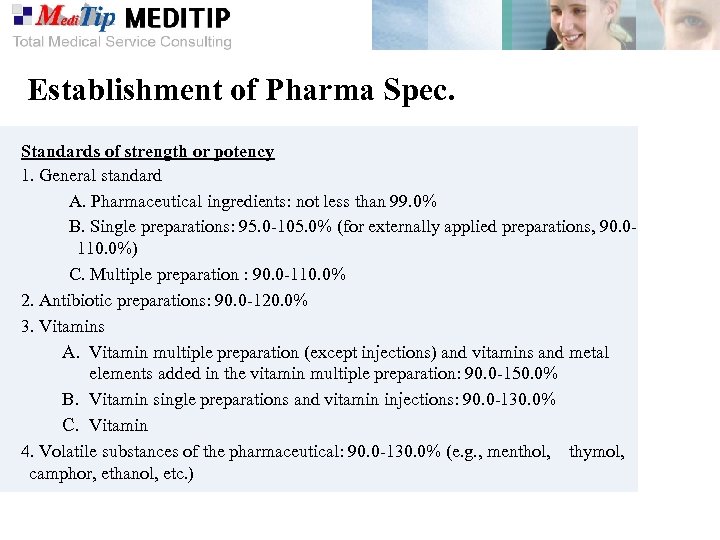 Establishment of Pharma Spec. Standards of strength or potency 1. General standard A. Pharmaceutical ingredients: not less than 99. 0% B. Single preparations: 95. 0 -105. 0% (for externally applied preparations, 90. 0110. 0%) C. Multiple preparation : 90. 0 -110. 0% 2. Antibiotic preparations: 90. 0 -120. 0% 3. Vitamins A. Vitamin multiple preparation (except injections) and vitamins and metal elements added in the vitamin multiple preparation: 90. 0 -150. 0% B. Vitamin single preparations and vitamin injections: 90. 0 -130. 0% C. Vitamin 4. Volatile substances of the pharmaceutical: 90. 0 -130. 0% (e. g. , menthol, thymol, camphor, ethanol, etc. )
Establishment of Pharma Spec. Standards of strength or potency 1. General standard A. Pharmaceutical ingredients: not less than 99. 0% B. Single preparations: 95. 0 -105. 0% (for externally applied preparations, 90. 0110. 0%) C. Multiple preparation : 90. 0 -110. 0% 2. Antibiotic preparations: 90. 0 -120. 0% 3. Vitamins A. Vitamin multiple preparation (except injections) and vitamins and metal elements added in the vitamin multiple preparation: 90. 0 -150. 0% B. Vitamin single preparations and vitamin injections: 90. 0 -130. 0% C. Vitamin 4. Volatile substances of the pharmaceutical: 90. 0 -130. 0% (e. g. , menthol, thymol, camphor, ethanol, etc. )
 Establishment of Pharma Spec. 5. Single and multi nucleic acids: 90. 0 -130. 0% derivatives preparations: 90. 0 -130. 0% 6. Enzyme preparations A. Pharmaceutical ingredients: not less than 100. 0% B. Digestive enzyme preparations: not less than 90. 0% C. Anti-inflammatory enzyme preparations: 90. 0 -130. 0% 7. Single or multi amino acids and their derivatives preparations: 90. 0 -130. 0% 8. Lactic acid bacteria preparations and other probiotics: not less than 90. 0% 9. Disinfectants: 90. 0 -110. 0% 10. Protein∙organ extracts (hydrolysis) preparations A. Pharmaceutical ingredients: not less than 100. 0% B. Drug products: not less than 90. 0%
Establishment of Pharma Spec. 5. Single and multi nucleic acids: 90. 0 -130. 0% derivatives preparations: 90. 0 -130. 0% 6. Enzyme preparations A. Pharmaceutical ingredients: not less than 100. 0% B. Digestive enzyme preparations: not less than 90. 0% C. Anti-inflammatory enzyme preparations: 90. 0 -130. 0% 7. Single or multi amino acids and their derivatives preparations: 90. 0 -130. 0% 8. Lactic acid bacteria preparations and other probiotics: not less than 90. 0% 9. Disinfectants: 90. 0 -110. 0% 10. Protein∙organ extracts (hydrolysis) preparations A. Pharmaceutical ingredients: not less than 100. 0% B. Drug products: not less than 90. 0%
 Establishment of Pharma Spec. 11. If it is impossible or unnecessary to determine the content of active pharmaceutical ingredients and so, the specification of content is not established, the efficacy test, performance test, or pharmaceutical test may be conducted instead (e. g. , medicinal carbon: absorption capacity test).
Establishment of Pharma Spec. 11. If it is impossible or unnecessary to determine the content of active pharmaceutical ingredients and so, the specification of content is not established, the efficacy test, performance test, or pharmaceutical test may be conducted instead (e. g. , medicinal carbon: absorption capacity test).
 Establishment of Pharma Spec. Test standards for other quality control 1. p. H: ± 1. 0 as for actual statistical values 2. Specific gravity: ± 0. 05 as for actual statistical values 3. Residue on evaporation: Upper limit shall be established on the basis of actual statistical values. 4. Alcohol number: This test shall be established for oral preparations containing not less than 4% of ethanol and the limit shall be not less than 90. 0% of t 5. Preservatives test: This test shall be established for drug products containing preservatives. In this case, the preservative shall be identified and the content shall be not more than the labeled value. As for all preparations except oral solutions, the amount of the content shall be 80. 0 -120. 0% of the labeled value. If necessary, other appropriate ranges may be established separately. he labeled value.
Establishment of Pharma Spec. Test standards for other quality control 1. p. H: ± 1. 0 as for actual statistical values 2. Specific gravity: ± 0. 05 as for actual statistical values 3. Residue on evaporation: Upper limit shall be established on the basis of actual statistical values. 4. Alcohol number: This test shall be established for oral preparations containing not less than 4% of ethanol and the limit shall be not less than 90. 0% of t 5. Preservatives test: This test shall be established for drug products containing preservatives. In this case, the preservative shall be identified and the content shall be not more than the labeled value. As for all preparations except oral solutions, the amount of the content shall be 80. 0 -120. 0% of the labeled value. If necessary, other appropriate ranges may be established separately. he labeled value.
 Establishment of Pharma Spec. Test standards for other quality control 6. Uniformity of dosage units: Korean Pharmacopoeia and Specification & Test Methods for Mass (Volume) Variation of Pharmaceuticals, etc. (KFDA's Notification) shall be applied. 7. Microbial limit test: Specification & Test Methods for Microbial Limit Test of Pharmaceuticals, etc. (KFDA's Notification) shall be applied. 8. Adhesive strength: When tested according to the Adhesive strength method described in the adhesive plaster of the Korean Pharmacopoeia, plasters and pressure sensitive adhesives, and cataplasmas shall have the adhesive strength of not less than 42 g per 12 mm wide. 9. Texture (length and width): When tested according to the Texture described in the adhesive plaster of the Korean Pharmacopoeia, the length and width shall be not less than 98. 0% of the labeled values.
Establishment of Pharma Spec. Test standards for other quality control 6. Uniformity of dosage units: Korean Pharmacopoeia and Specification & Test Methods for Mass (Volume) Variation of Pharmaceuticals, etc. (KFDA's Notification) shall be applied. 7. Microbial limit test: Specification & Test Methods for Microbial Limit Test of Pharmaceuticals, etc. (KFDA's Notification) shall be applied. 8. Adhesive strength: When tested according to the Adhesive strength method described in the adhesive plaster of the Korean Pharmacopoeia, plasters and pressure sensitive adhesives, and cataplasmas shall have the adhesive strength of not less than 42 g per 12 mm wide. 9. Texture (length and width): When tested according to the Texture described in the adhesive plaster of the Korean Pharmacopoeia, the length and width shall be not less than 98. 0% of the labeled values.
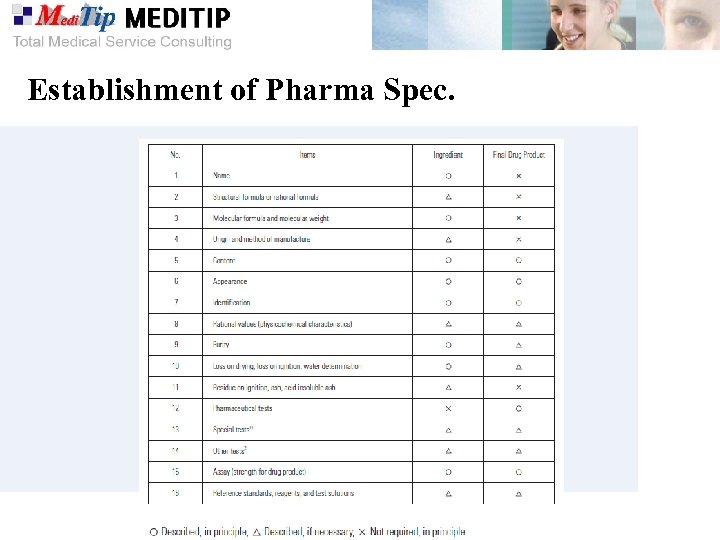 Establishment of Pharma Spec.
Establishment of Pharma Spec.
 Thank you !
Thank you !


