8ccb3cb3dbcc6c366304a3536edffc2b.ppt
- Количество слайдов: 77

Methods to analyze real world databases and registries Hilal Maradit Kremers, MD MSc Mayo Clinic, Rochester, MN Clinical Research Methodology Course NYU-Hospital for Joint Diseases December 11, 2008

Disclosure Research funding from • National Institutes of Health (RA) • Amgen (psoriasis) • Pfizer (pulmonary arterial hypertension)

Outline • Terminology • Clinical trials versus observational studies and registries • Types of observational studies in rheumatic diseases – Descriptive epidemiology (incidence, prevalence) – Disease definitions (i. e. classification criteria) – Examining outcomes (including effectiveness of therapy) and risk factors (environmental, genetic) • Tips when interpreting results
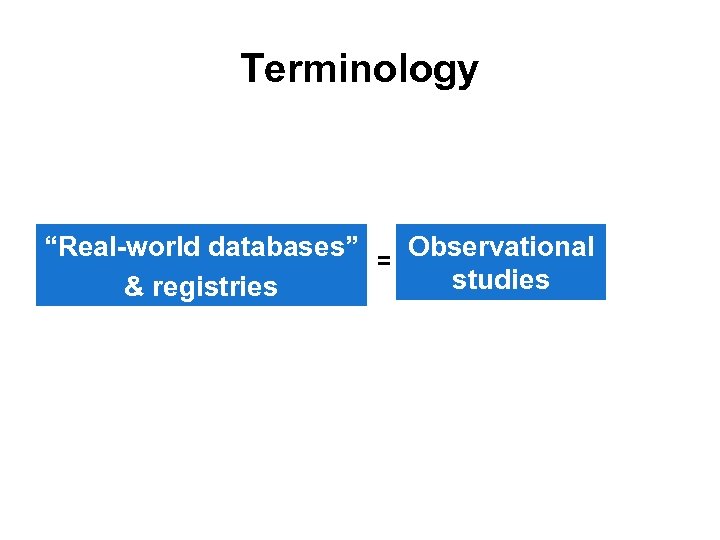
Terminology “Real-world databases” = Observational studies & registries
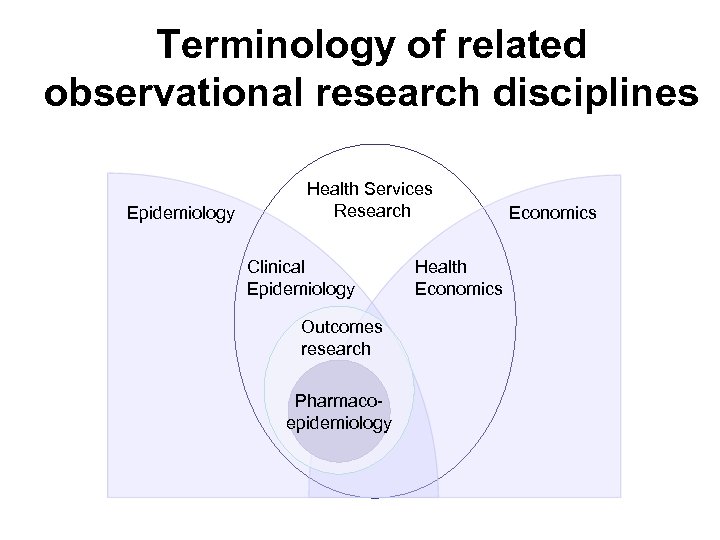
Terminology of related observational research disciplines Epidemiology Health Services Research Clinical Epidemiology Outcomes research Pharmacoepidemiology Health Economics
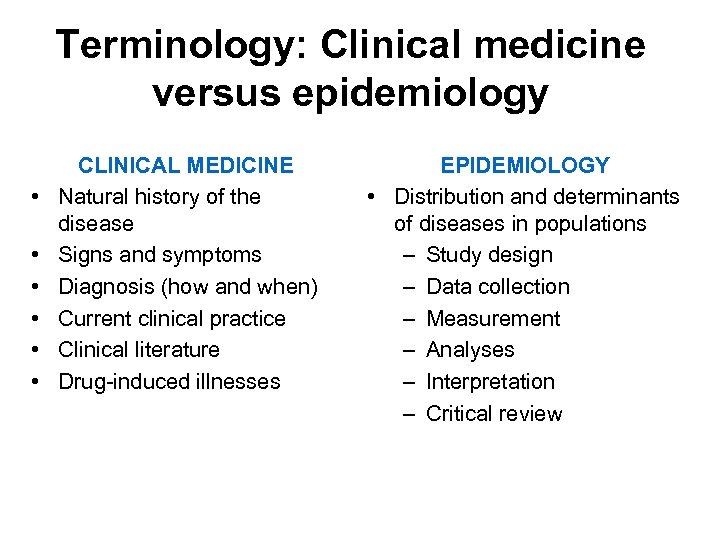
Terminology: Clinical medicine versus epidemiology • • • CLINICAL MEDICINE Natural history of the disease Signs and symptoms Diagnosis (how and when) Current clinical practice Clinical literature Drug-induced illnesses EPIDEMIOLOGY • Distribution and determinants of diseases in populations – Study design – Data collection – Measurement – Analyses – Interpretation – Critical review

Clinical trials versus observational studies and registries
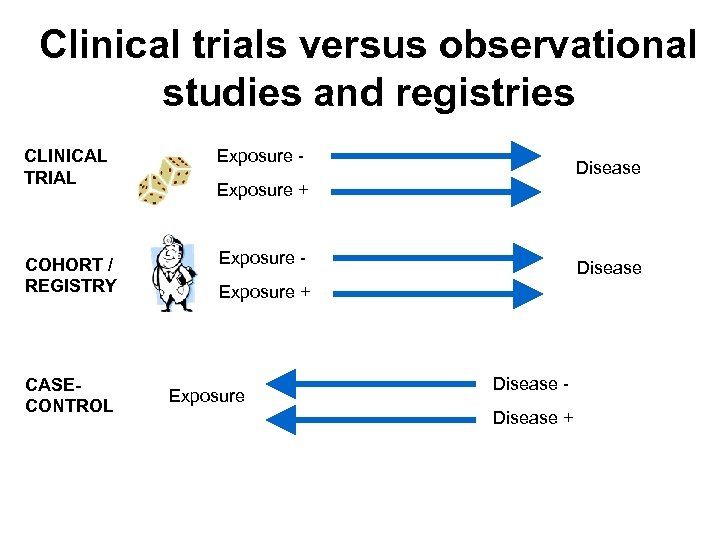
Clinical trials versus observational studies and registries CLINICAL TRIAL COHORT / REGISTRY CASECONTROL Exposure - Disease Exposure + Exposure Disease +

Why do we need registries • Limitations of pre-marketing trials • Unresolved issues from pre-marketing studies • New signals or inconsistent signals from postmarketing surveillance • Evolving concerns about safety • Establishing risk-benefit margins • Learn about use, Rx decisions, compliance and other physician/patient behaviors • To evaluate a risk management program

Clinical trial vs observational studies/registries – four “toos” • Too few • Too brief • Too simple • Too median-aged

Implications of four “toos” • Relative effectiveness unknown – Effectiveness in comparison to alternative therapies • Surrogate vs. clinical endpoints – Bone mineral density, blood pressure, lipid levels, tumor size, joint counts vs radiographic damage • Infrequent adverse events • Long latency adverse events – DES & adenocarcinoma of vagina • Special populations – Women, children, elderly, multiple comorbidities • Drug use in clinical practice

What is a registry? • Definition of a registry – An organized system that uses observational study methods to collect uniform data (clinical and other) to evaluate specified outcomes for a population defined by particular disease, condition or exposure, and that serves a predetermined scientific, clinical, or policy purpose(s). • Different types of registries – Disease registry – Product registry – Health services registry • Pregnancy registries Registries for Evaluating Patient Outcomes. AHRQ Publication No. 07 -EHC 001. May 2007.

Purpose of a registry • Describe the natural history of disease • Determine clinical effectiveness or cost effectiveness of health care products, drugs and services • Measure or monitor safety and harm • Measure quality of care

Registry types • Disease registry – Patients who have the same diagnosis – e. g. all RA or SLE patients or rheumatic diseases • Product registry – Patients who have been exposed to biopharmaceutical products or medical devices • Health services registry – Patients who have had a common procedure, clinical encounter or hospitalization (TKA-THA registries)

Registries useful when: • Outcome is relatively common, well-defined and ascertainable & serious • Extensive drug exposure • Appropriate reference group • Data on relevant covariates ascertainable • Minimal channeling (preferential prescribing of a new drug to patients at a higher risk) • Minimal confounding by indication • Onset latency <2 -3 years • Required drug exposure <2 -3 years • Not an urgent drug safety crisis

Registries may not be useful when: • Outcome: poorly-defined, difficult to validate outcomes (depression, psychosis) • Exposure – Rare drug exposure – Intermittent exposure – OTC drugs, herbals • Significant confounding by indication – Antidepressants and suicides – Inhaled beta-agonists and asthma death • Certain settings – Specialty clinics, in-hospital drug use

Consequences of not doing registries or observational studies • Arguing over case reports • Lack of data on real benefit-risk balance • Less effective and usually biased decisionmaking • Possibly false conclusions • Law suits

Types of observational studies in rheumatology
Observational study designs • Drug exposed patients • Exposed vs. unexposed – Case reports – Cross-sectional – Case series – Prospective cohort – Registries – Case-control • Other – Ecological studies
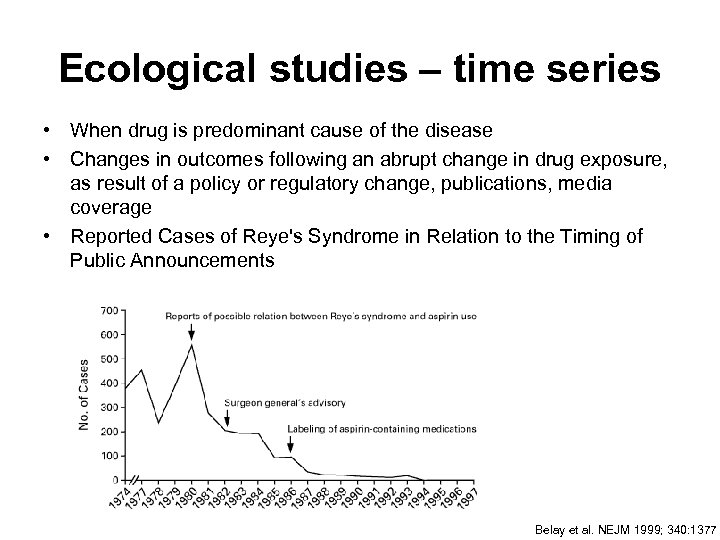
Ecological studies – time series • When drug is predominant cause of the disease • Changes in outcomes following an abrupt change in drug exposure, as result of a policy or regulatory change, publications, media coverage • Reported Cases of Reye's Syndrome in Relation to the Timing of Public Announcements Belay et al. NEJM 1999; 340: 1377
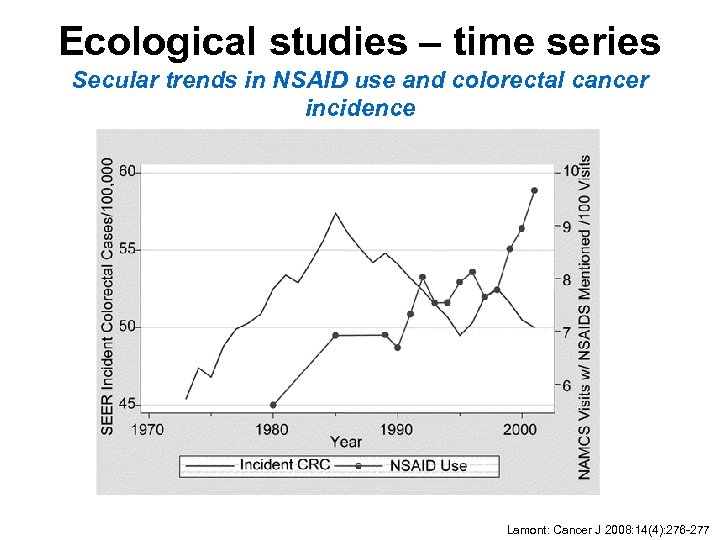
Ecological studies – time series Secular trends in NSAID use and colorectal cancer incidence Lamont: Cancer J 2008: 14(4): 276 -277
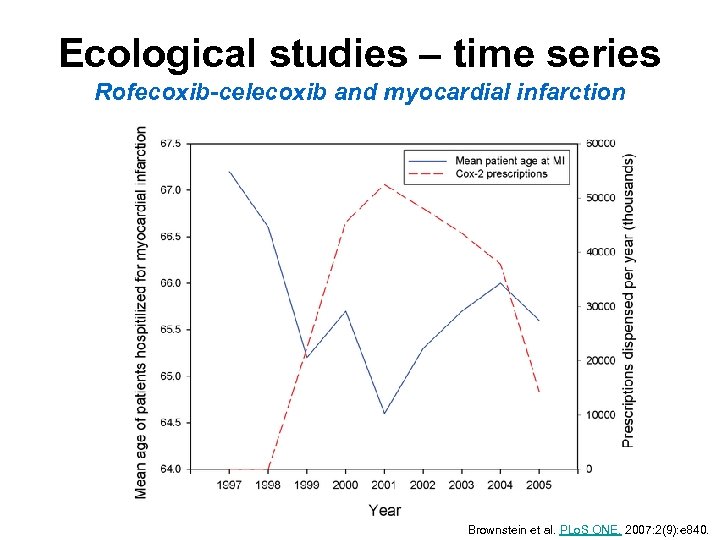
Ecological studies – time series Rofecoxib-celecoxib and myocardial infarction Brownstein et al. PLo. S ONE. 2007: 2(9): e 840.

Summary: ecological studies Limitations • Complexity of disease causation • Confounding by the “ecological fallacy” Advantages • Cost ↓, time ↓, using routinely collected data • New hypotheses about the causes of a disease and new potential risk factors (e. g. air pollution) • Provides estimates of causal effects that are not attenuated by measurement error • Some risk factors for disease operate at the population level (i. e. SES status)

Studies on descriptive epidemiology of rheumatic diseases Incidence Prevalence Mortality
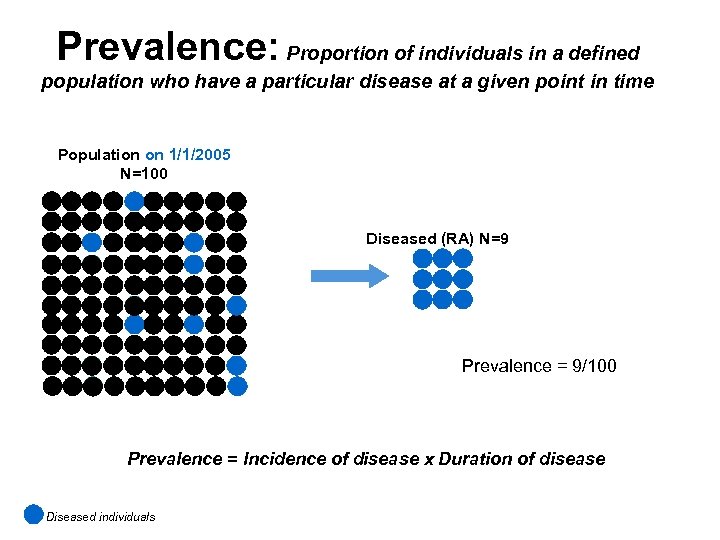
Prevalence: Proportion of individuals in a defined population who have a particular disease at a given point in time Population on 1/1/2005 N=100 Diseased (RA) N=9 Prevalence = 9/100 Prevalence = Incidence of disease x Duration of disease Diseased individuals
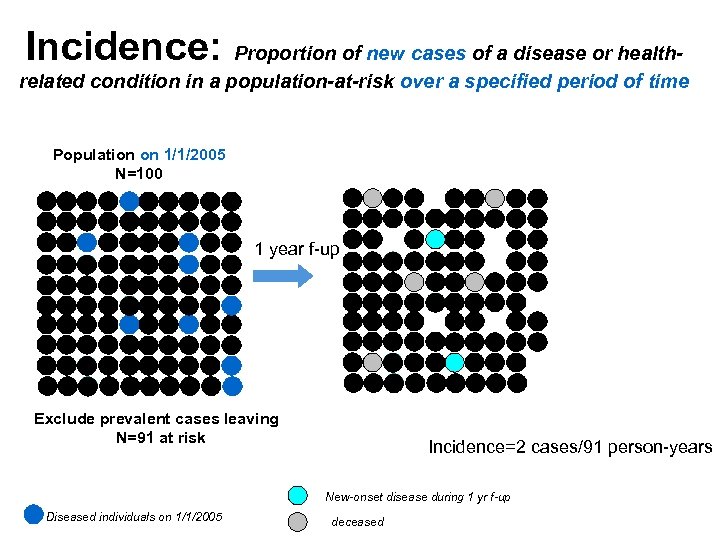
Incidence: Proportion of new cases of a disease or healthrelated condition in a population-at-risk over a specified period of time Population on 1/1/2005 N=100 1 year f-up Exclude prevalent cases leaving N=91 at risk Incidence=2 cases/91 person-years New-onset disease during 1 yr f-up Diseased individuals on 1/1/2005 deceased
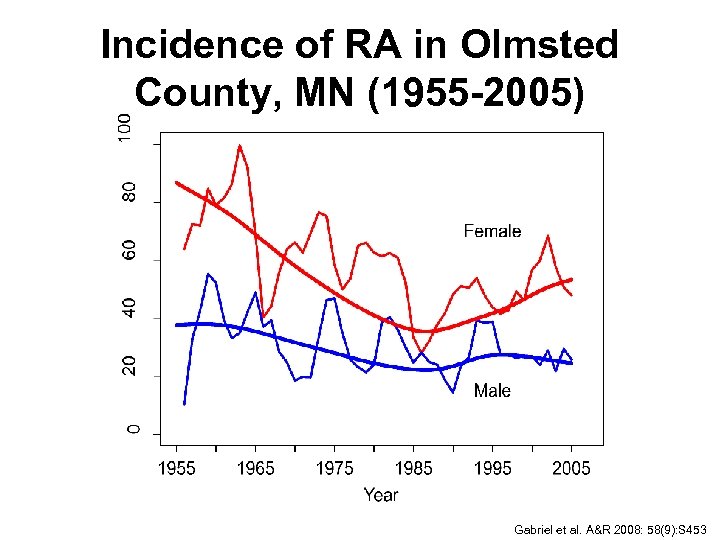
Incidence of RA in Olmsted County, MN (1955 -2005) Gabriel et al. A&R 2008: 58(9): S 453
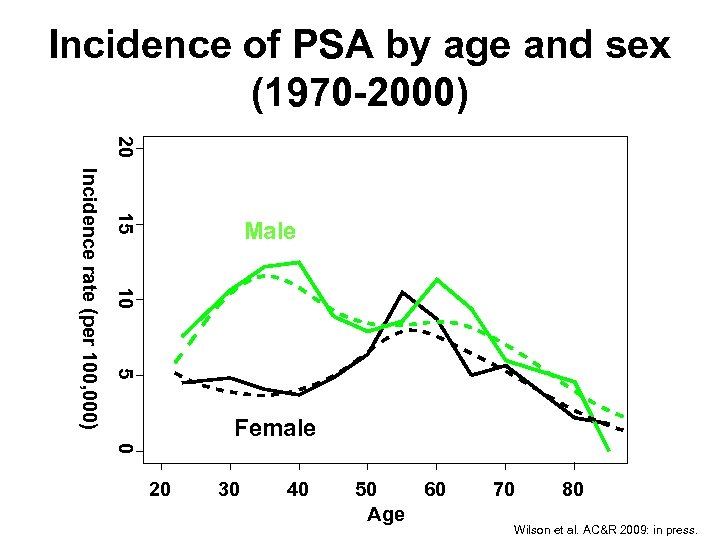
Incidence of PSA by age and sex (1970 -2000) 20 15 10 5 Incidence rate (per 100, 000) Male Female 0 20 30 40 50 60 Age 70 80 Wilson et al. AC&R 2009: in press.
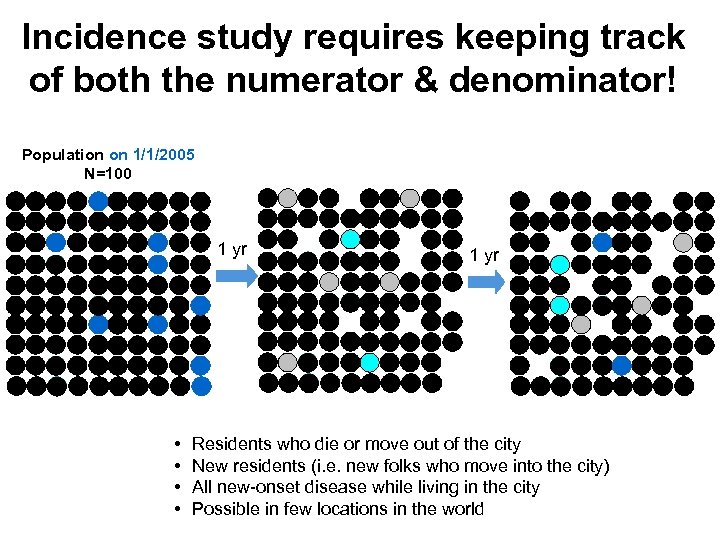
Incidence study requires keeping track of both the numerator & denominator! Population on 1/1/2005 N=100 1 yr • • 1 yr Residents who die or move out of the city New residents (i. e. new folks who move into the city) All new-onset disease while living in the city Possible in few locations in the world

Mortality analyses • RA: 124 studies in 84 unique cohorts 1 • 15 key points in interpretation 1 – Incident vs prevalent cases – Population-based vs clinic-based – SMR • Cause-specific mortality 2 • 3 time dimensions in mortality analyses 3 – Duration of RA – Timing of onset of RA relative to death – Calendar time 1 Sokka et al. Clinical Exp Rheum 2008; 26(Suppl. 51): S 35 -S 61 2 Aviña-Zubieta et al. A&R 2008; 59: 1690 -1697 3 Ward. A&R 2008; 59: 1687 -1689
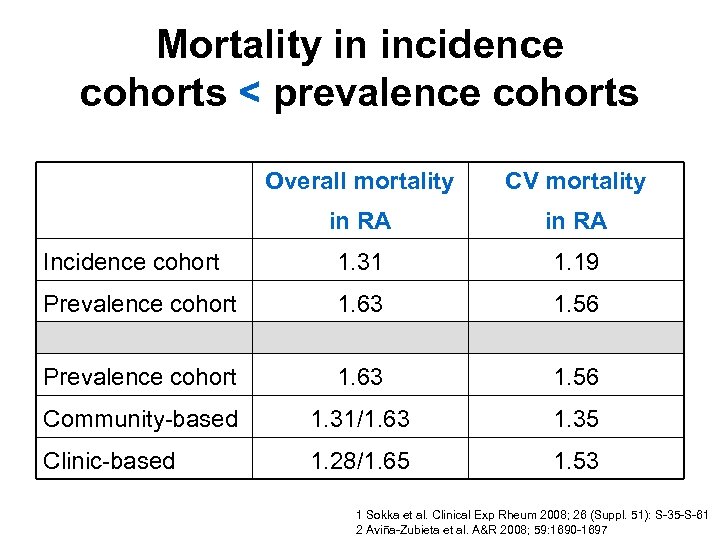
Mortality in incidence cohorts < prevalence cohorts Overall mortality CV mortality in RA Incidence cohort 1. 31 1. 19 Prevalence cohort 1. 63 1. 56 Community-based 1. 31/1. 63 1. 35 Clinic-based 1. 28/1. 65 1. 53 1 Sokka et al. Clinical Exp Rheum 2008; 26 (Suppl. 51): S-35 -S-61 2 Aviña-Zubieta et al. A&R 2008; 59: 1690 -1697
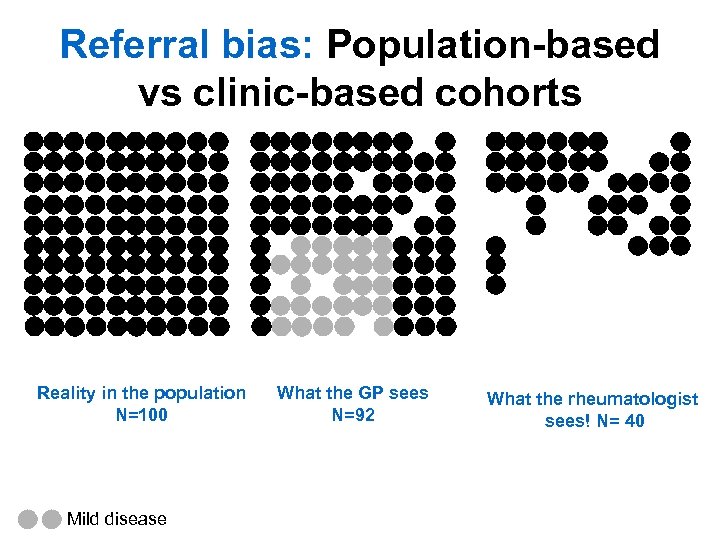
Referral bias: Population-based vs clinic-based cohorts Reality in the population N=100 Mild disease What the GP sees N=92 What the rheumatologist sees! N= 40
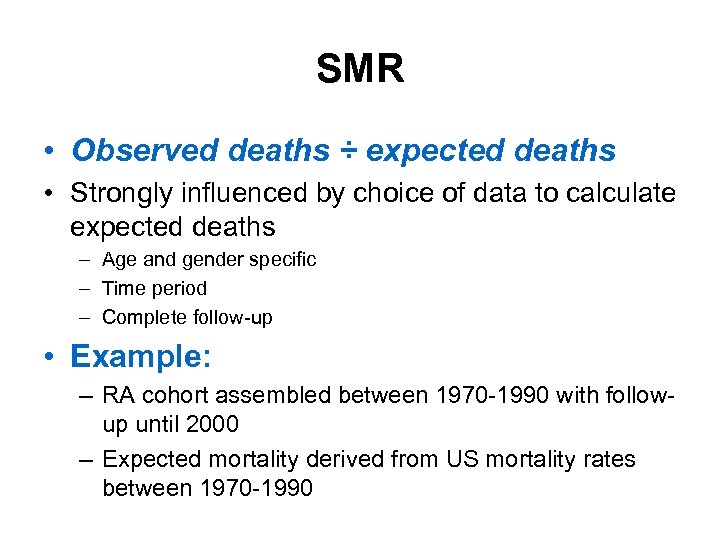
SMR • Observed deaths ÷ expected deaths • Strongly influenced by choice of data to calculate expected deaths – Age and gender specific – Time period – Complete follow-up • Example: – RA cohort assembled between 1970 -1990 with followup until 2000 – Expected mortality derived from US mortality rates between 1970 -1990
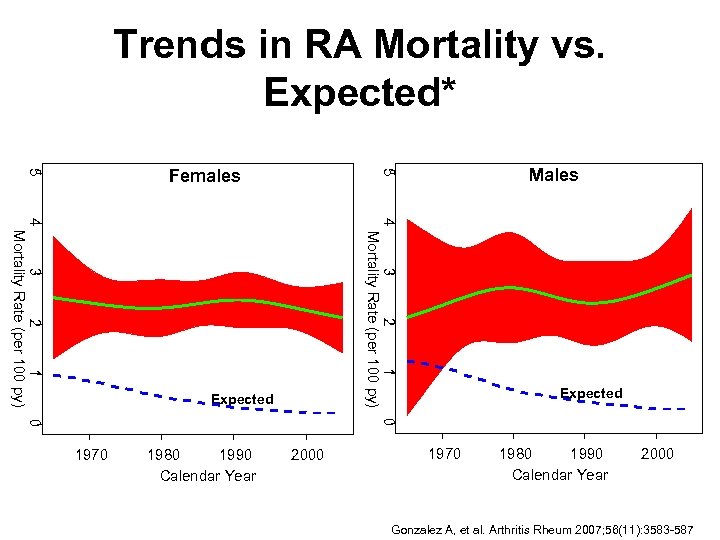
Trends in RA Mortality vs. Expected* 4 3 2 1 0 Mortality Rate (per 100 py) 3 2 1 0 4 Mortality Rate (per 100 py) RA Expected 1970 1980 1990 Calendar Year Males 5 5 Females 2000 RA Expected 1970 1980 1990 Calendar Year 2000 Gonzalez A, et al. Arthritis Rheum 2007; 56(11): 3583 -587
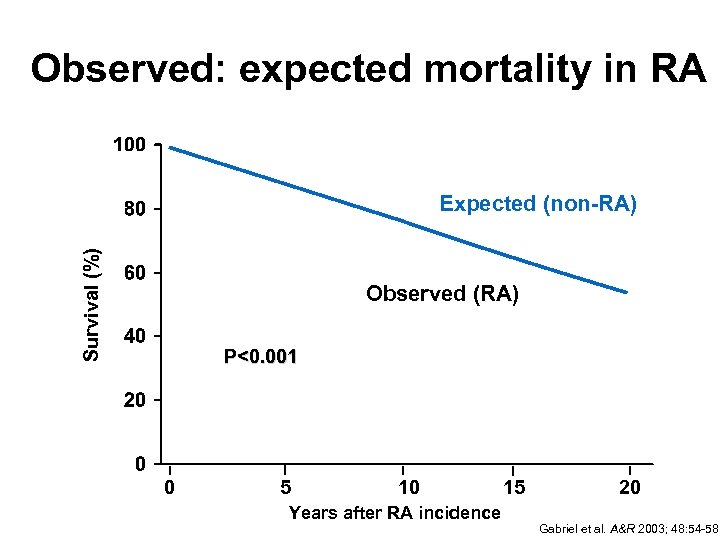
Observed: expected mortality in RA Survival (%) Expected (non-RA) Observed (RA) P<0. 001 0 5 10 Years after RA incidence 15 20 Gabriel et al. A&R 2003; 48: 54 -58
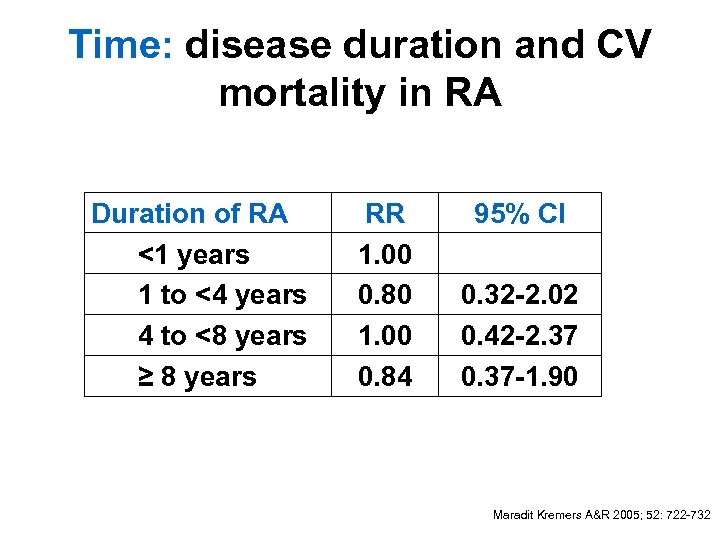
Time: disease duration and CV mortality in RA Duration of RA <1 years 1 to <4 years 4 to <8 years ≥ 8 years RR 1. 00 0. 80 1. 00 0. 84 95% CI 0. 32 -2. 02 0. 42 -2. 37 0. 37 -1. 90 Maradit Kremers A&R 2005; 52: 722 -732
Summary: incidence, prevalence and mortality Consider • Underlying data source – Population-based or not – Incident vs prevalent cases • Methodology – Case ascertainment – Completeness of follow-up • Comparison data!

Disease definitions and classification criteria in rheumatic diseases Developed using observational study methodologies
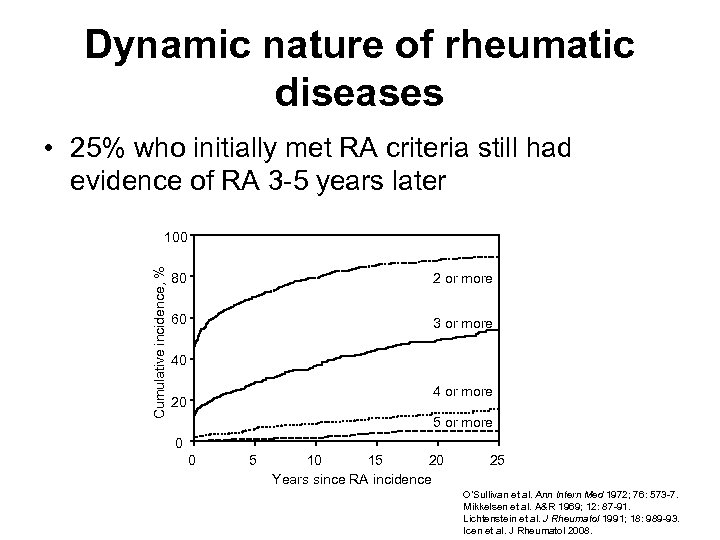
Dynamic nature of rheumatic diseases • 25% who initially met RA criteria still had evidence of RA 3 -5 years later Cumulative incidence, % 100 80 2 or more 60 3 or more 40 4 or more 20 5 or more 0 0 5 10 15 20 Years since RA incidence 25 O’Sullivan et al. Ann Intern Med 1972; 76: 573 -7. Mikkelsen et al. A&R 1969; 12: 87 -91. Lichtenstein et al. J Rheumatol 1991; 18: 989 -93. Icen et al. J Rheumatol 2008.
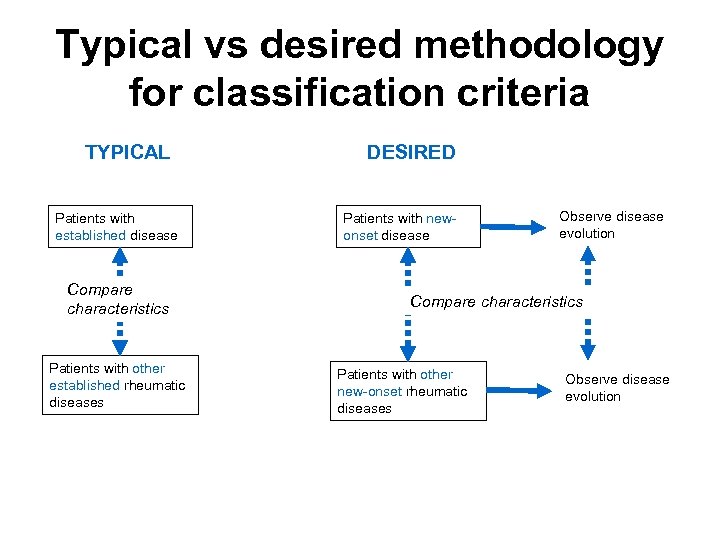
Typical vs desired methodology for classification criteria TYPICAL Patients with established disease Compare characteristics Patients with other established rheumatic diseases DESIRED Patients with newonset disease Observe disease evolution Compare characteristics Patients with other new-onset rheumatic diseases Observe disease evolution

Examining outcomes and risk factors in rheumatic diseases Cohort Studies (outcomes) Registries (outcomes) Case-control studies (risk factors)

Types of Cohort Studies • Designated by the timing of data collection in the investigator’s time: – Prospective – Retrospective (historical) – Mixed • Mayo studies: retrospective • Registries: prospective
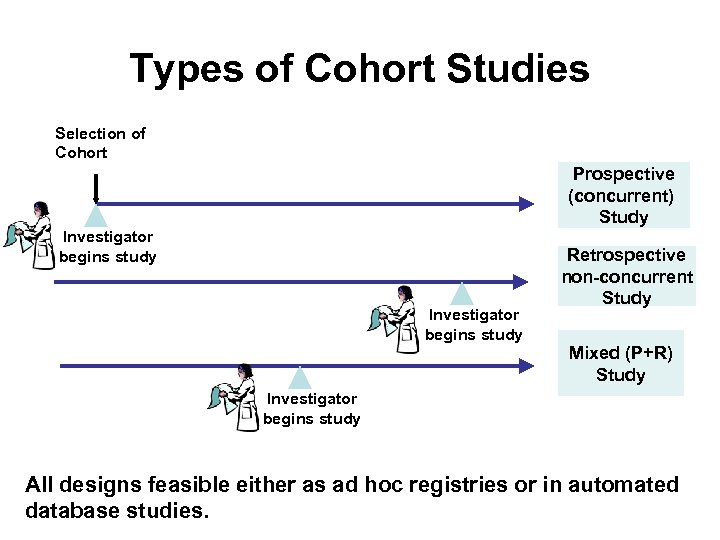
Types of Cohort Studies Selection of Cohort Prospective (concurrent) Study Investigator begins study Retrospective non-concurrent Study Mixed (P+R) Study Investigator begins study All designs feasible either as ad hoc registries or in automated database studies.

Cohort study: design options • • Prospective vs. retrospective Entry into cohort: closed or open Timing of exposure: new users or not Source of un-exposed cohort – Internal – External • drug exposed subjects only, registries

Cohort Study: Steps 1. Cohort identification • Define subjects & follow-up period 2. Risk factor/drug exposure measurement throughout follow-up 3. Outcome (disease) ascertainment 4. Confounder measurements (throughout followup) 5. Analysis

Step 1 - Cohort identification • Trade-off between external and internal validity • Retrospective vs. prospective – Consider feasibility and costs • Follow-up – Tracking of drug changes over time – Losses to follow-up, esp. if likely to be differential (different for drug users and non-users)

Step 2 – Risk factor/Drug exposure measurement • New versus old users – Ability to account confounders before drug started – Ability to quantify outcomes early after starting the drug (compliance, early drop-offs due to intolerance) • Incomplete drug exposure – E. g. One time measurement of DMARD use and mortality • Drug exposure metric – Ever/never, dose (average, cumulative), duration • Reference group – Non-users, past users, users of other drugs • Misclassification of episodic use
Step 2 - Timing: patterns of drug use Antibiotic NSAIDs DMARDs

Step 2 - Drug exposure measurement methods • Interviews • Face-to-face, phone or self-administered • Excellent to capture current use but not for past use or changing drug use over time • Loss of memory – cognitively intact subjects & regularly used drugs • Biological testing • Blood or urine • Excellent to capture current use but not for past use • Non-differential (unless disease affects the assay) • Pharmacy or claims records • Medical records

Step 2 - Pharmacy or claims records for drug exposure • Drugs obtained by prescription • Drug details available • Accurate & complete for both past and current drug exposure • Temporal tracking possible • Limitation compliance – Prescription filled and drug taking • Validation studies are necessary
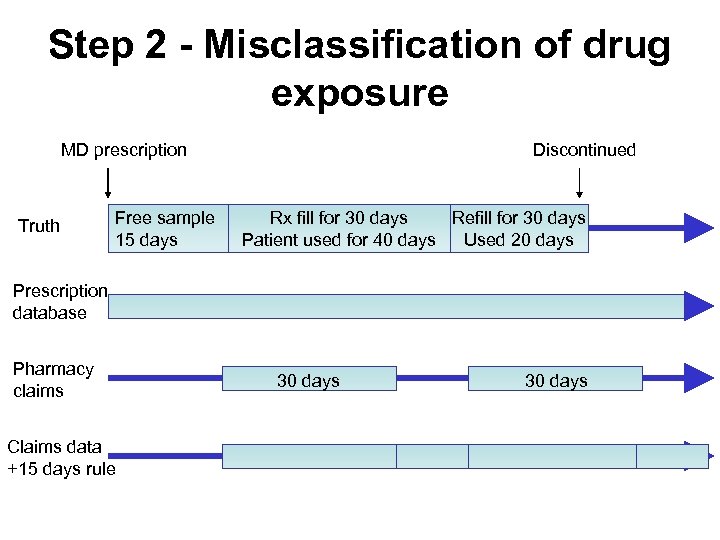
Step 2 - Misclassification of drug exposure MD prescription Truth Free sample 15 days Discontinued Rx fill for 30 days Refill for 30 days Patient used for 40 days Used 20 days Prescription database Pharmacy claims Claims data +15 days rule 30 days

Step 2 summary: Aspects of drug exposure measurement • • Completeness & accuracy Response rate Temporal change over time Special populations Details of the drug Details of utilization Availability & cost (reimbursement) Differential or non-differential
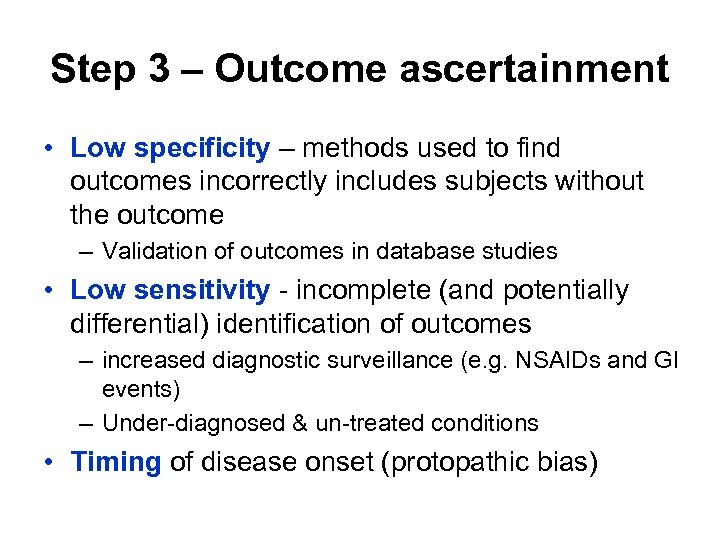
Step 3 – Outcome ascertainment • Low specificity – methods used to find outcomes incorrectly includes subjects without the outcome – Validation of outcomes in database studies • Low sensitivity - incomplete (and potentially differential) identification of outcomes – increased diagnostic surveillance (e. g. NSAIDs and GI events) – Under-diagnosed & un-treated conditions • Timing of disease onset (protopathic bias)
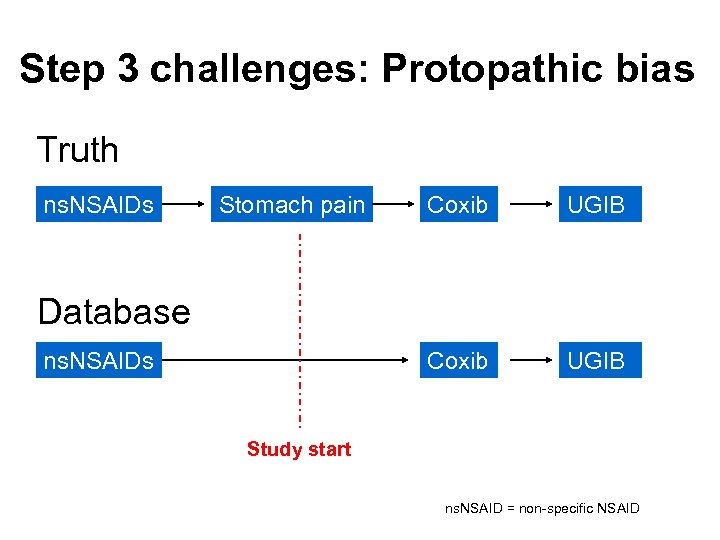
Step 3 challenges: Protopathic bias Truth ns. NSAIDs Stomach pain Coxib UGIB Database ns. NSAIDs Study start ns. NSAID = non-specific NSAID

Step 3 – Outcome of interest in rheumatology • Beneficial effects/effectiveness – Disease progression • Adverse effects – – – – Mortality Cardiovascular morbidity Infections Lymphomas & solid malignancies Autoimmunity GI events (NSAIDs) Pregnancy outcomes
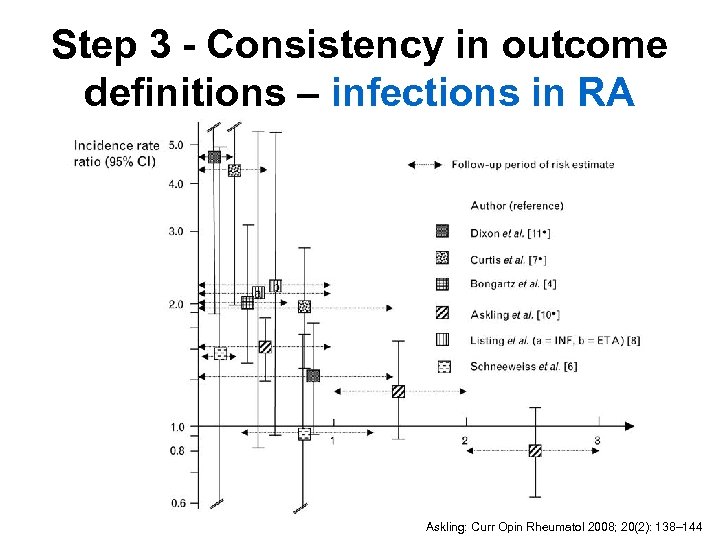
Step 3 - Consistency in outcome definitions – infections in RA Askling: Curr Opin Rheumatol 2008; 20(2): 138– 144

Step 3 challenges: Differential misclassification of outcome • Cohort study: May result from misclassification of outcome/disease free (specificity) or incomplete identification of persons with outcome (sensitivity) in exposed and unexposed subjects – Under-diagnosed conditions – Example: Patients with RA, especially those on biologics are more likely to see their doctors more often and more likely to be examined for labs, or CVD • Using medication-taking as a surrogate of outcome can be problematic
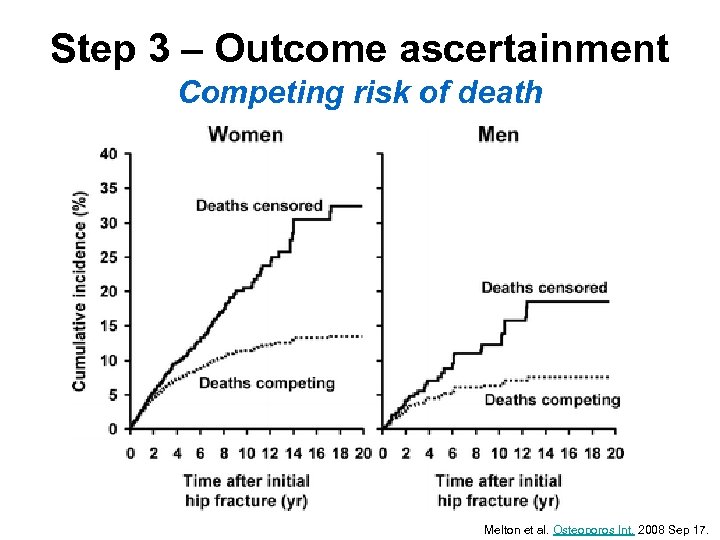
Step 3 – Outcome ascertainment Competing risk of death Melton et al. Osteoporos Int. 2008 Sep 17.
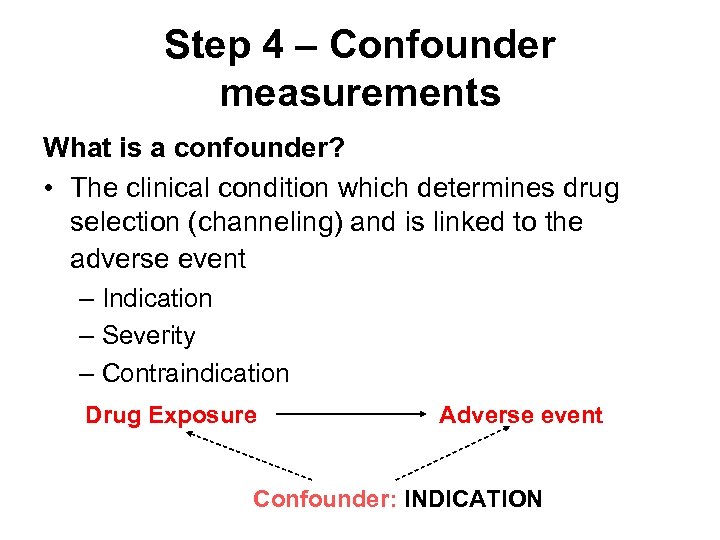
Step 4 – Confounder measurements What is a confounder? • The clinical condition which determines drug selection (channeling) and is linked to the adverse event – Indication – Severity – Contraindication Drug Exposure Adverse event Confounder: INDICATION

Step 4 - Confounding by indication • The indications for drug use, because of their natural association with prognosis, may confound the comparison so that it looks as if the treatment causes the disease “You’d better avoid antihypertensive treatment because treated patients have higher stroke rates”
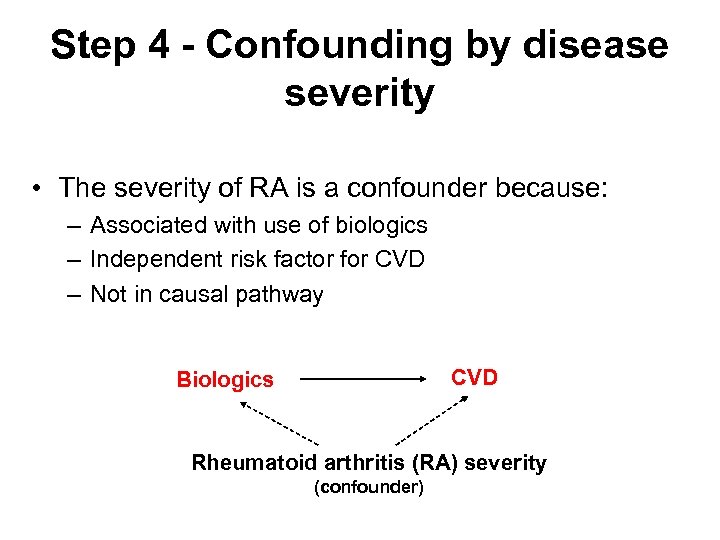
Step 4 - Confounding by disease severity • The severity of RA is a confounder because: – Associated with use of biologics – Independent risk factor for CVD – Not in causal pathway CVD Biologics Rheumatoid arthritis (RA) severity (confounder)
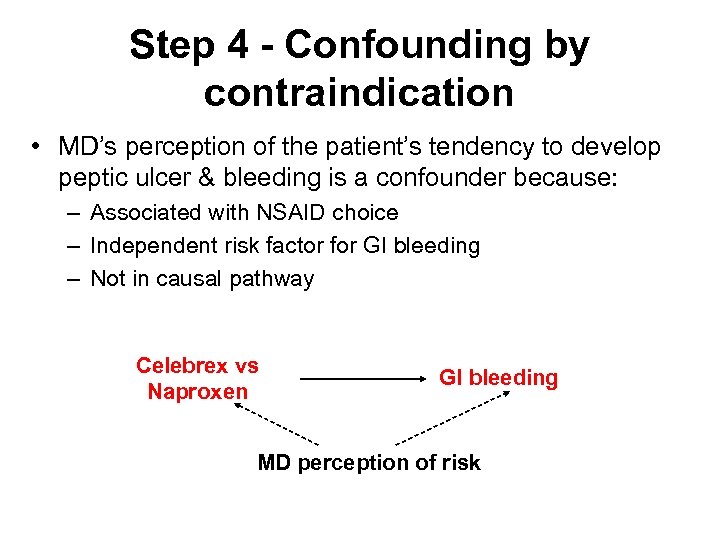
Step 4 - Confounding by contraindication • MD’s perception of the patient’s tendency to develop peptic ulcer & bleeding is a confounder because: – Associated with NSAID choice – Independent risk factor for GI bleeding – Not in causal pathway Celebrex vs Naproxen GI bleeding MD perception of risk
Step 4 - Confounding by indication • Prescription Channeling – New versus older products – Example: Comparison of the risk of upper GI bleeding among coxibs versus traditional NSAIDs • Coxibs preferentially prescribed to patients at high risk for upper GI bleeding Moride et al. Arthritis Res Ther. 2005; 7: R 333 -342.
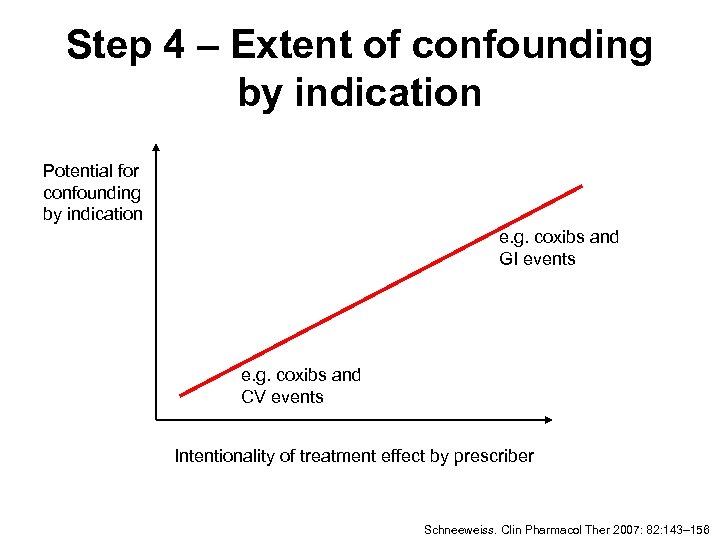
Step 4 – Extent of confounding by indication Potential for confounding by indication e. g. coxibs and GI events e. g. coxibs and CV events Intentionality of treatment effect by prescriber Schneeweiss. Clin Pharmacol Ther 2007: 82: 143– 156

Step 5 - Analysis • Conventional methods to control for confounding – – – • • • Randomization (clinical trials) Restriction - homogeneous study population Matching - select controls comparable to cases re. confounders Stratified analysis Statistical modeling Sensitivity analyses Active-competing comparator designs Propensity scores Marginal structural models Instrumental variable analysis
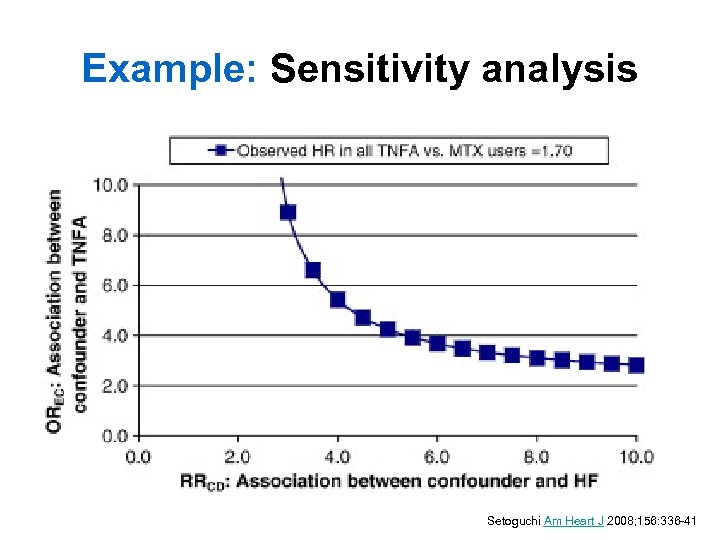
Example: Sensitivity analysis Setoguchi Am Heart J 2008; 156: 336 -41
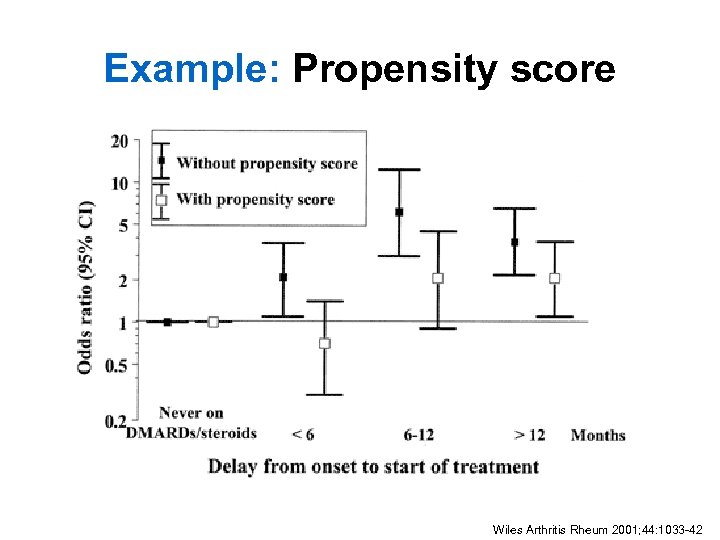
Example: Propensity score Wiles Arthritis Rheum 2001; 44: 1033 -42
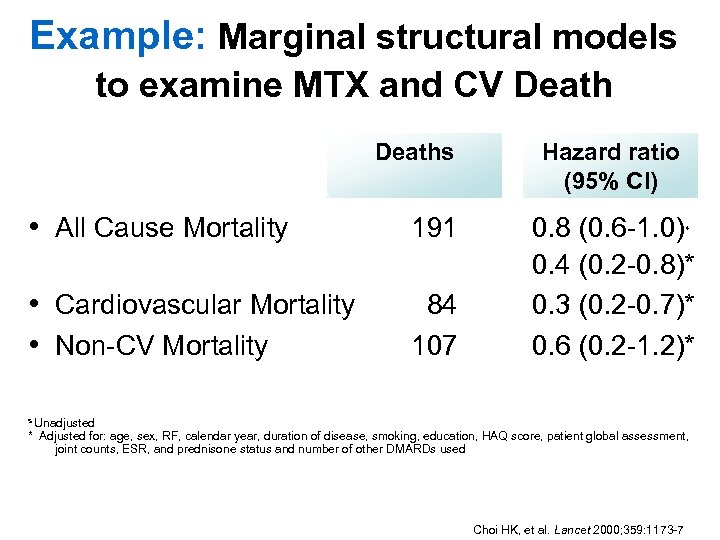
Example: Marginal structural models to examine MTX and CV Deaths • All Cause Mortality 191 • Cardiovascular Mortality • Non-CV Mortality 84 107 Hazard ratio (95% CI) 0. 8 (0. 6 -1. 0) 0. 4 (0. 2 -0. 8)* 0. 3 (0. 2 -0. 7)* 0. 6 (0. 2 -1. 2)* s s Unadjusted * Adjusted for: age, sex, RF, calendar year, duration of disease, smoking, education, HAQ score, patient global assessment, joint counts, ESR, and prednisone status and number of other DMARDs used Choi HK, et al. Lancet 2000; 359: 1173 -7
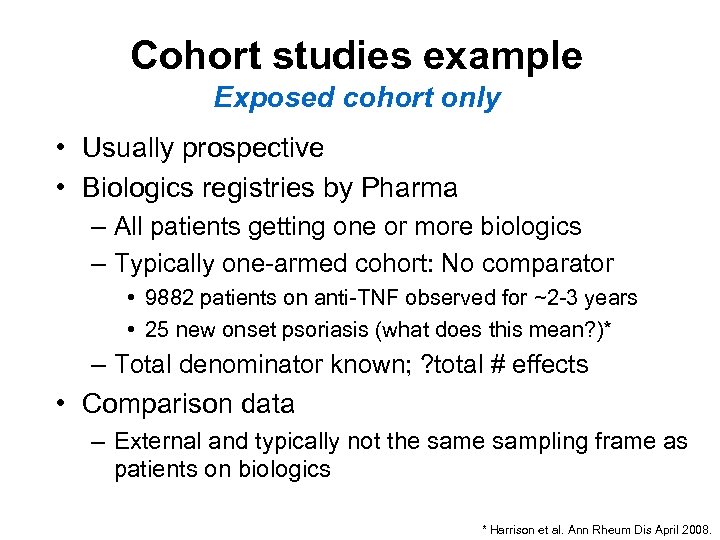
Cohort studies example Exposed cohort only • Usually prospective • Biologics registries by Pharma – All patients getting one or more biologics – Typically one-armed cohort: No comparator • 9882 patients on anti-TNF observed for ~2 -3 years • 25 new onset psoriasis (what does this mean? )* – Total denominator known; ? total # effects • Comparison data – External and typically not the sampling frame as patients on biologics * Harrison et al. Ann Rheum Dis April 2008.
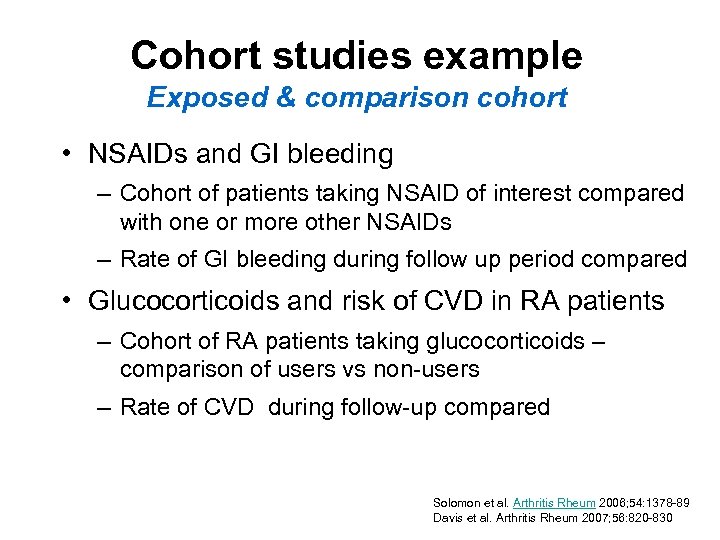
Cohort studies example Exposed & comparison cohort • NSAIDs and GI bleeding – Cohort of patients taking NSAID of interest compared with one or more other NSAIDs – Rate of GI bleeding during follow up period compared • Glucocorticoids and risk of CVD in RA patients – Cohort of RA patients taking glucocorticoids – comparison of users vs non-users – Rate of CVD during follow-up compared Solomon et al. Arthritis Rheum 2006; 54: 1378 -89 Davis et al. Arthritis Rheum 2007; 56: 820 -830
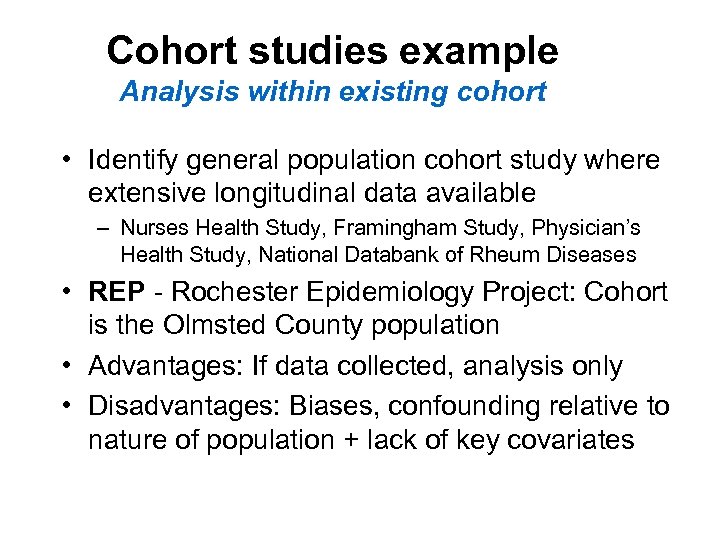
Cohort studies example Analysis within existing cohort • Identify general population cohort study where extensive longitudinal data available – Nurses Health Study, Framingham Study, Physician’s Health Study, National Databank of Rheum Diseases • REP - Rochester Epidemiology Project: Cohort is the Olmsted County population • Advantages: If data collected, analysis only • Disadvantages: Biases, confounding relative to nature of population + lack of key covariates
Cohort studies example Database cohort study • Most common form in pharmacoepidemiology • Usually retrospective, but can be mixed • Many large multi-purpose databases are used – HMO, Managed Care (Puget Sound, United Health Care) – Electronic medical records (GPRD, Medi. Plus) – Provincial health plans (Saskatchewan) • Advantages: large, data already exists, complete for billable services ? • Disadvantages: Claims = diagnoses
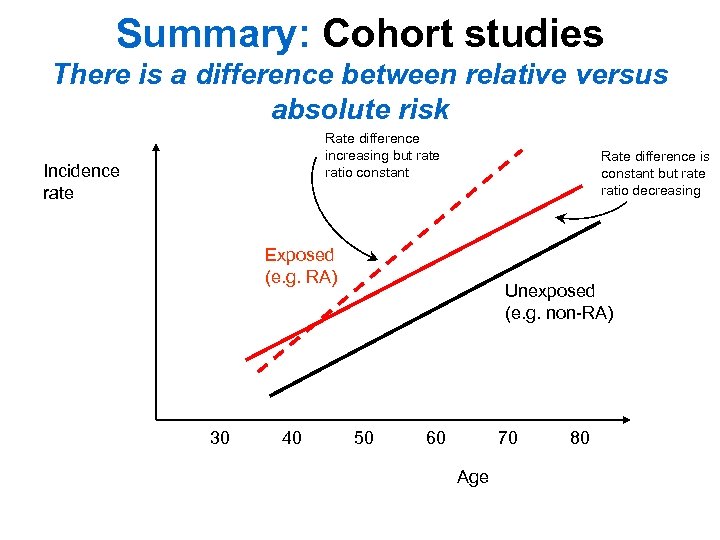
Summary: Cohort studies There is a difference between relative versus absolute risk Rate difference increasing but rate ratio constant Incidence rate Rate difference is constant but rate ratio decreasing Exposed (e. g. RA) 30 40 Unexposed (e. g. non-RA) 50 60 70 Age 80
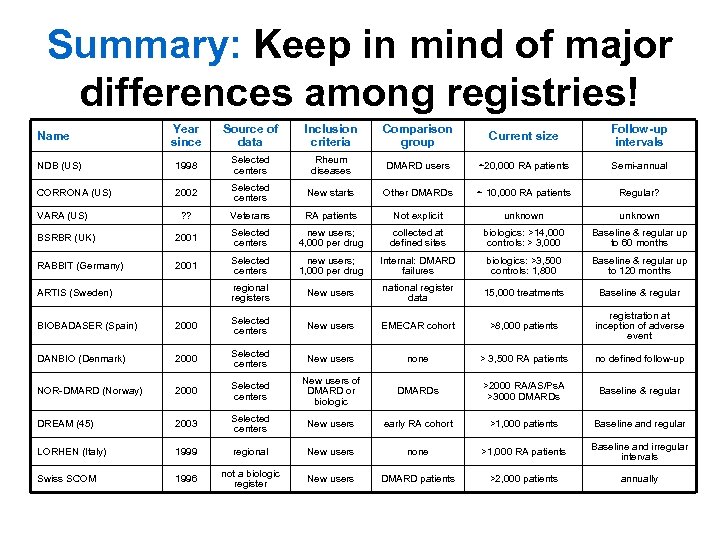
Summary: Keep in mind of major differences among registries! Year since Source of data Inclusion criteria Comparison group Current size Follow-up intervals NDB (US) 1998 Selected centers Rheum diseases DMARD users ~20, 000 RA patients Semi-annual CORRONA (US) 2002 Selected centers New starts Other DMARDs ~ 10, 000 RA patients Regular? ? ? Veterans RA patients Not explicit unknown BSRBR (UK) 2001 Selected centers new users; 4, 000 per drug collected at defined sites biologics: >14, 000 controls: > 3, 000 Baseline & regular up to 60 months RABBIT (Germany) 2001 Selected centers new users; 1, 000 per drug Internal: DMARD failures biologics: >3, 500 controls: 1, 800 Baseline & regular up to 120 months regional registers New users national register data 15, 000 treatments Baseline & regular Name VARA (US) ARTIS (Sweden) BIOBADASER (Spain) 2000 Selected centers New users EMECAR cohort >8, 000 patients registration at inception of adverse event DANBIO (Denmark) 2000 Selected centers New users none > 3, 500 RA patients no defined follow-up NOR-DMARD (Norway) 2000 Selected centers New users of DMARD or biologic DMARDs >2000 RA/AS/Ps. A >3000 DMARDs Baseline & regular DREAM (45) 2003 Selected centers New users early RA cohort >1, 000 patients Baseline and regular LORHEN (Italy) 1999 regional New users none >1, 000 RA patients Baseline and irregular intervals Swiss SCOM 1996 not a biologic register New users DMARD patients >2, 000 patients annually

Tips when interpreting studies

Consider these before you believe the results! • If negative study – Power – Outcome & exposure definition – Comparison group – Non-differential misclassification – Replication

Consider these before you believe the results! • If positive study – Confounding – Channeling – Differential misclassification – Generalizability – Implications – Replication
8ccb3cb3dbcc6c366304a3536edffc2b.ppt