b0055320acfe890abb8539fcaaf80cfc.ppt
- Количество слайдов: 42

Methods of determination of protein structure by Ivo Frébort
Architecture of Proteins • Shape - globular or fibrous • The levels of protein structure - Primary - sequence - Secondary - local structures - H-bonds - Tertiary - overall 3 -dimensional shape - Quaternary - subunit organization

What forces determine the structure? • Primary structure - determined by covalent bonds • Secondary, Tertiary, Quaternary structures all determined by weak forces • Weak forces - H-bonds, ionic interactions, van der Waals interactions, hydrophobic interactions
Other Chemical Groups in Proteins may be "conjugated" with other chemical groups • If the non-amino acid part of the protein is important to its function, it is called a prosthetic group. • Glycoprotein, lipoprotein, nucleoprotein, phosphoprotein, metalloprotein, hemoprotein, flavoprotein.
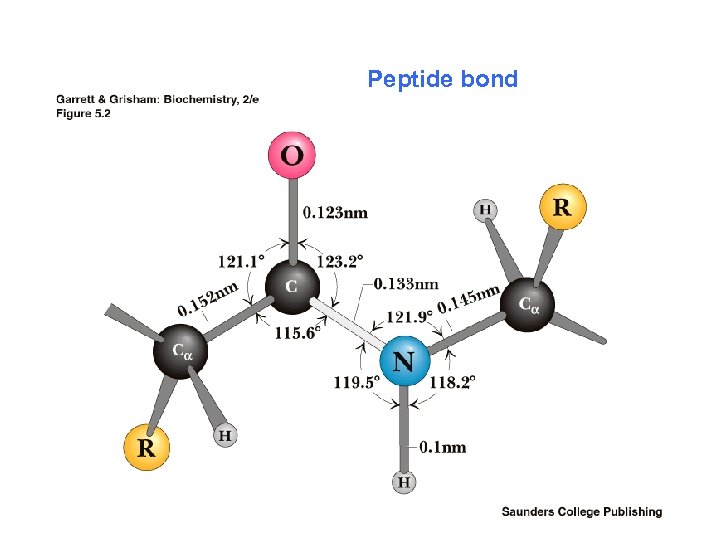
Peptide bond
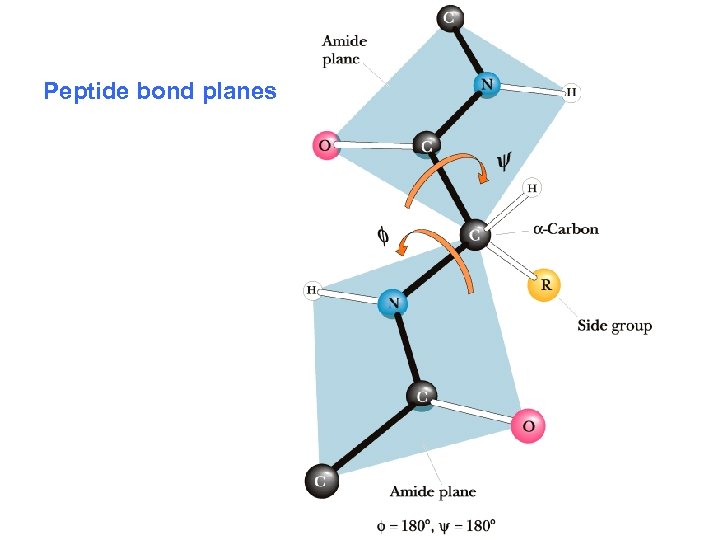
Peptide bond planes
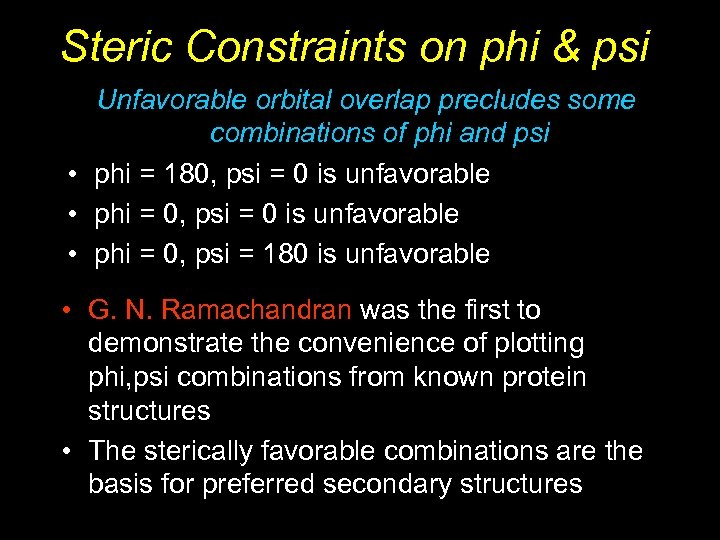
Steric Constraints on phi & psi Unfavorable orbital overlap precludes some combinations of phi and psi • phi = 180, psi = 0 is unfavorable • phi = 0, psi = 180 is unfavorable • G. N. Ramachandran was the first to demonstrate the convenience of plotting phi, psi combinations from known protein structures • The sterically favorable combinations are the basis for preferred secondary structures
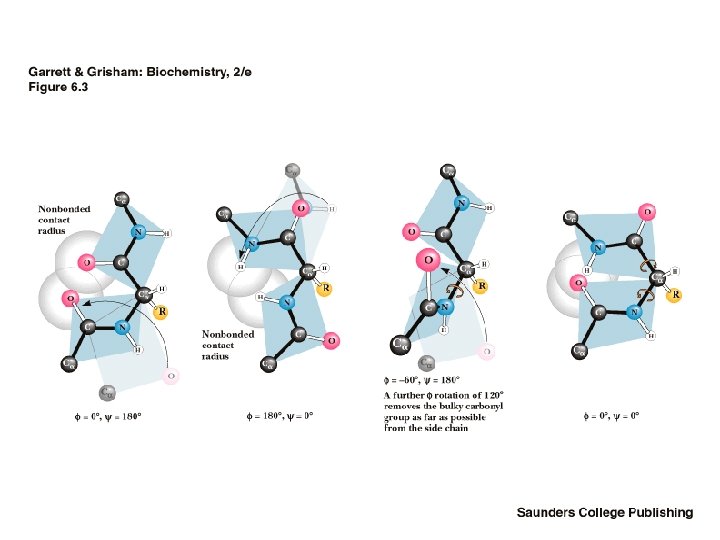
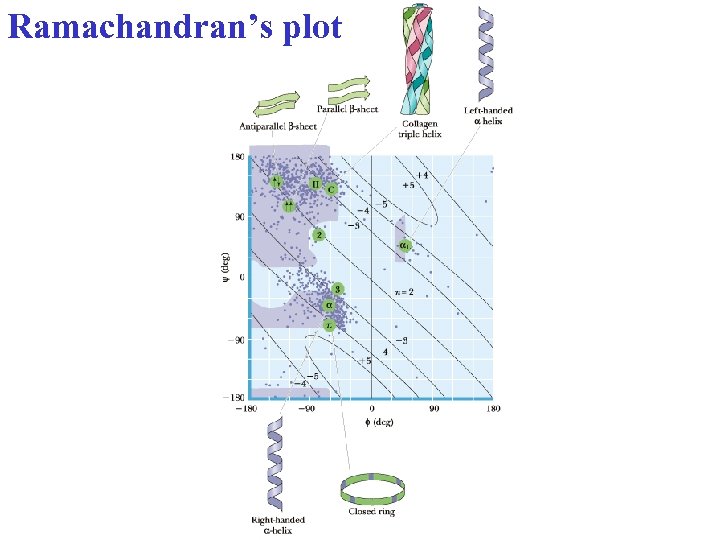
Ramachandran’s plot
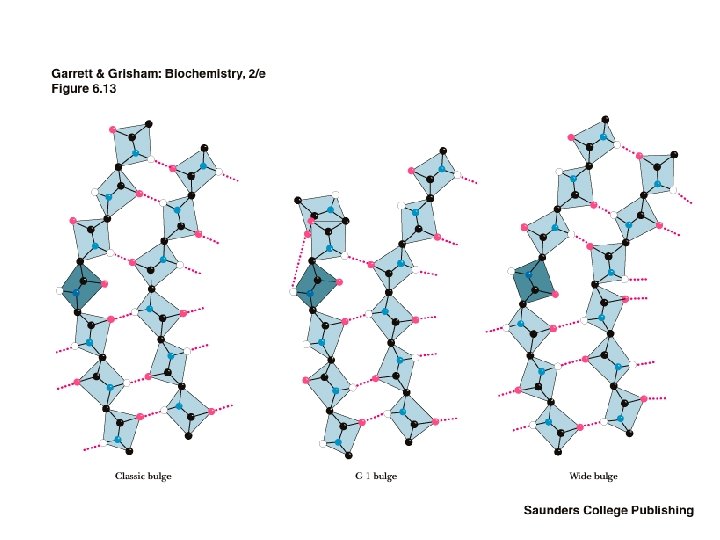
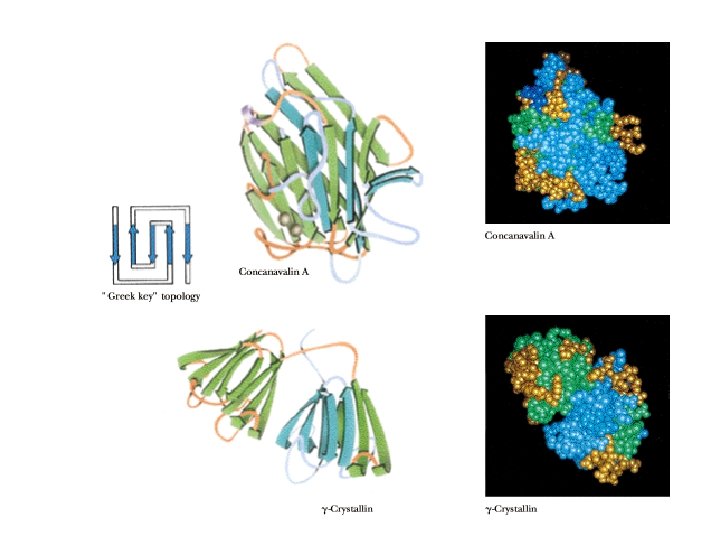
Classes of Secondary Structure • • • All these are local structures that are stabilized by hydrogen bonds Alpha helix Other helices Beta sheet (composed of "beta strands") Tight turns Beta bulge

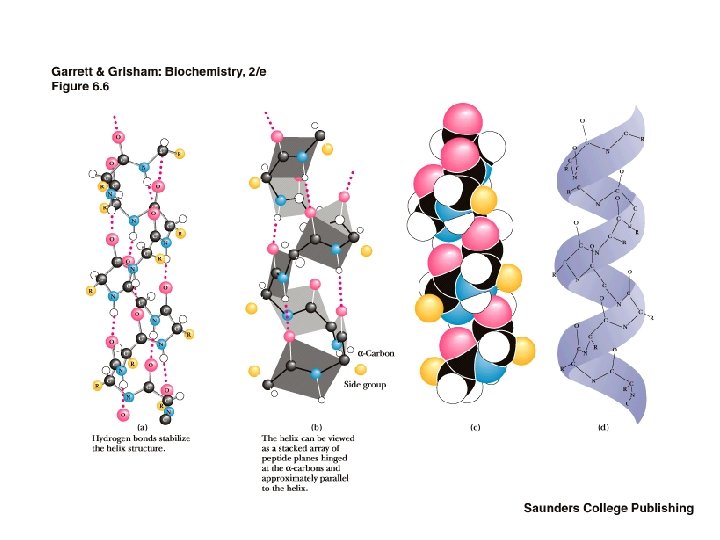
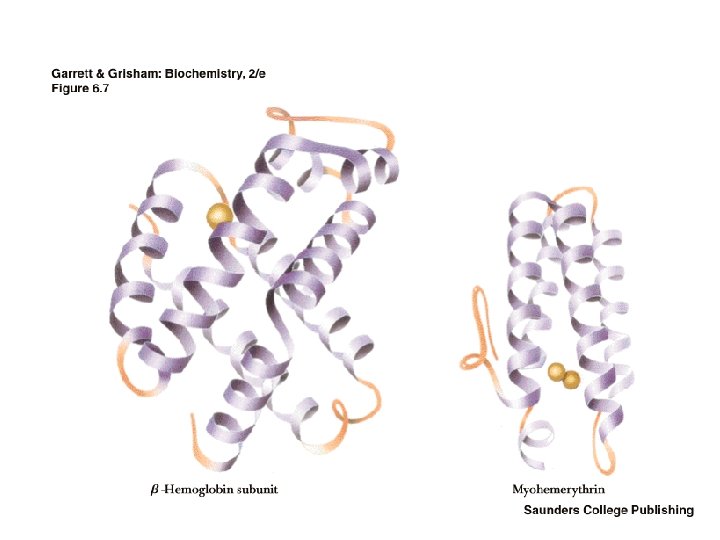
The Alpha Helix • First proposed by Linus Pauling and Robert Corey in 1951 • Identified in keratin by Max Perutz • A ubiquitous component of proteins • Stabilized by H-bonds
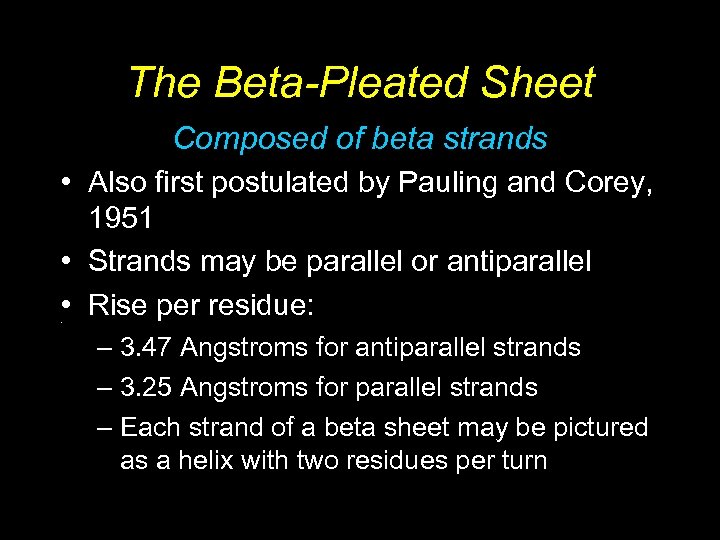
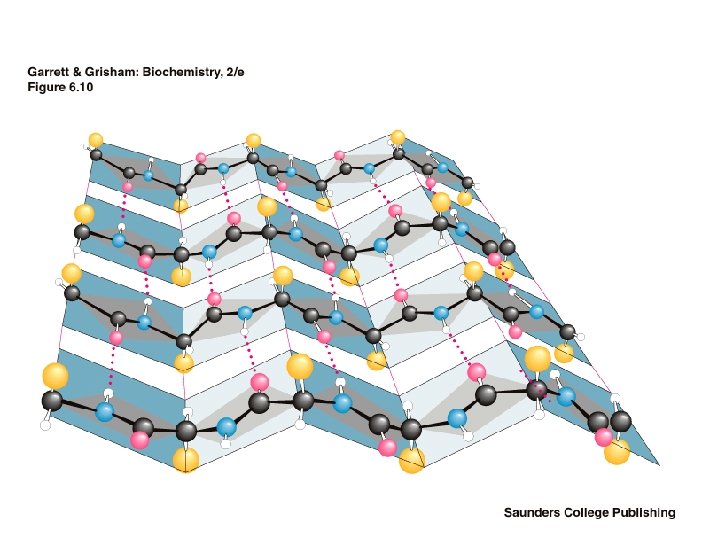
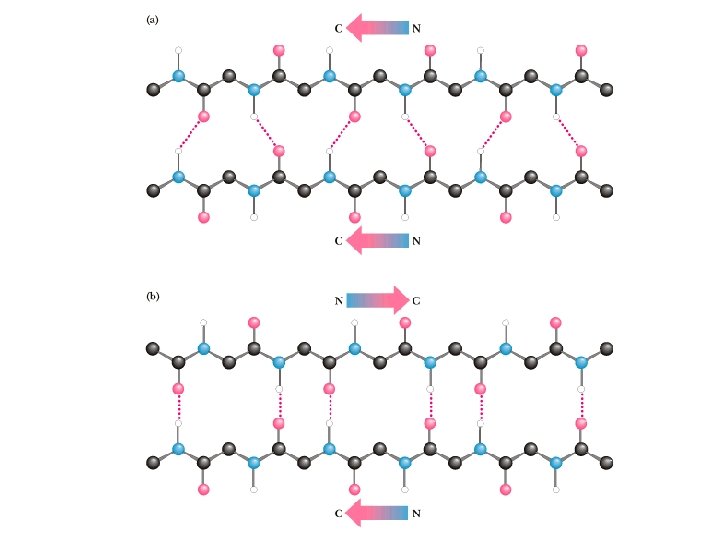
The Beta-Pleated Sheet Composed of beta strands • Also first postulated by Pauling and Corey, 1951 • Strands may be parallel or antiparallel • Rise per residue: • – 3. 47 Angstroms for antiparallel strands – 3. 25 Angstroms for parallel strands – Each strand of a beta sheet may be pictured as a helix with two residues per turn

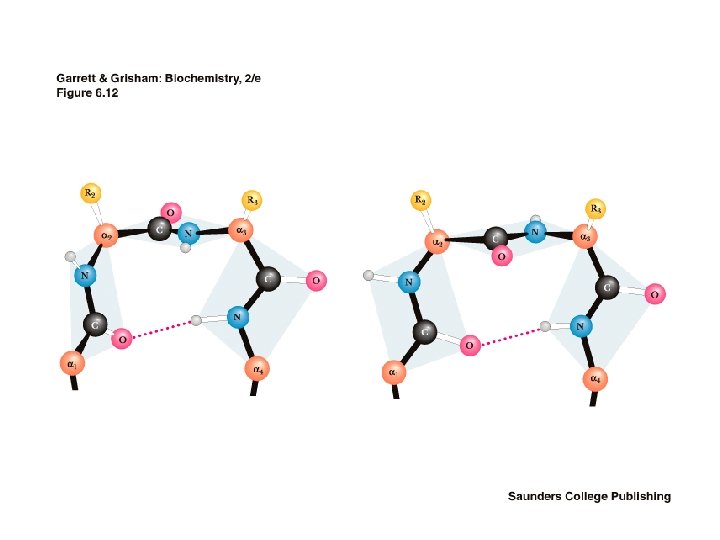
The Beta Turn • allows the peptide chain to reverse direction • carbonyl C of one residue is H-bonded to the amide proton of a residue three residues away • proline and glycine are prevalent in beta turns

What are the structural and functional advantages driving quaternary association? • • Stability: reduction of surface to volume ratio Genetic economy and efficiency Bringing catalytic sites together Cooperativity
MAX FERDINAND PERUTZ 1962 Nobel Laureate in Chemistry for their studies of the structures of globular proteins. Born: 1914 Place of Birth: Vienna, Austria Residence: Great Britain Affiliation: Laboratory of Molecular Biology, Cambridge Died: February 6, 2002
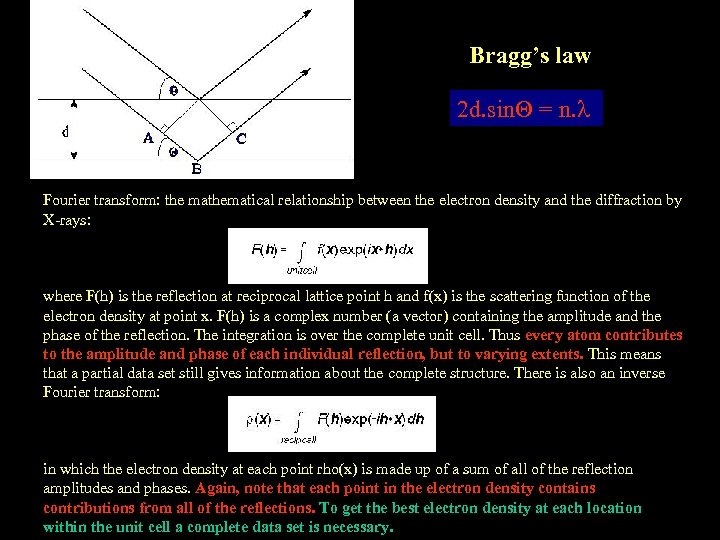
Bragg’s law 2 d. sin = n. Fourier transform: the mathematical relationship between the electron density and the diffraction by X-rays: where F(h) is the reflection at reciprocal lattice point h and f(x) is the scattering function of the electron density at point x. F(h) is a complex number (a vector) containing the amplitude and the phase of the reflection. The integration is over the complete unit cell. Thus every atom contributes to the amplitude and phase of each individual reflection, but to varying extents. This means that a partial data set still gives information about the complete structure. There is also an inverse Fourier transform: in which the electron density at each point rho(x) is made up of a sum of all of the reflection amplitudes and phases. Again, note that each point in the electron density contains contributions from all of the reflections. To get the best electron density at each location within the unit cell a complete data set is necessary.
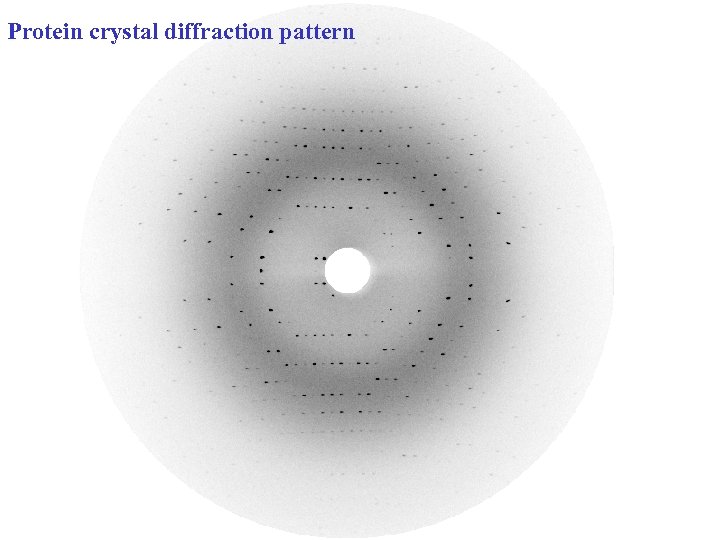
Protein crystal diffraction pattern
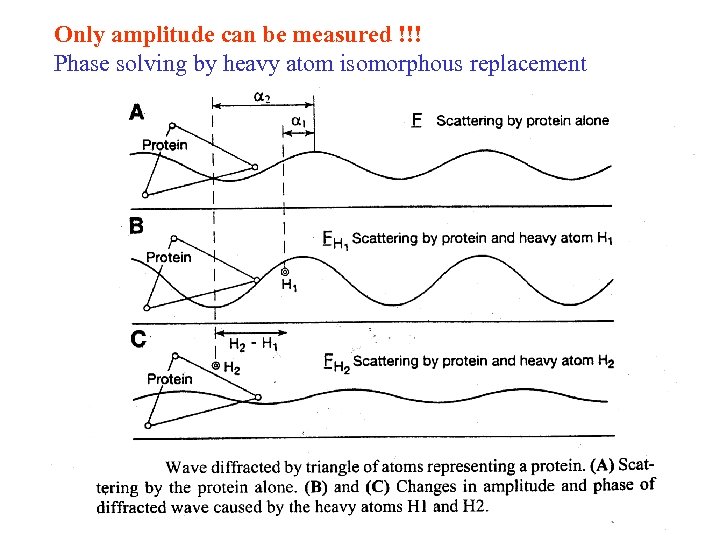
Only amplitude can be measured !!! Phase solving by heavy atom isomorphous replacement
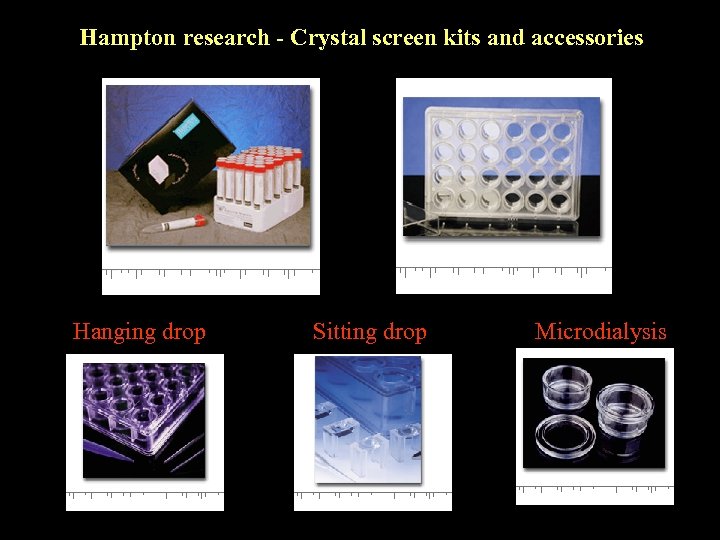
Hampton research - Crystal screen kits and accessories Hanging drop Sitting drop Microdialysis

Crystal screen kit reagents
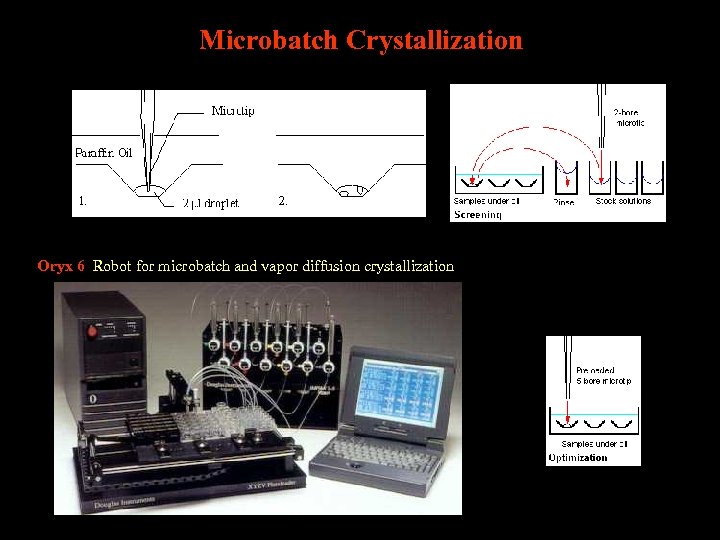
Microbatch Crystallization Oryx 6 Robot for microbatch and vapor diffusion crystallization
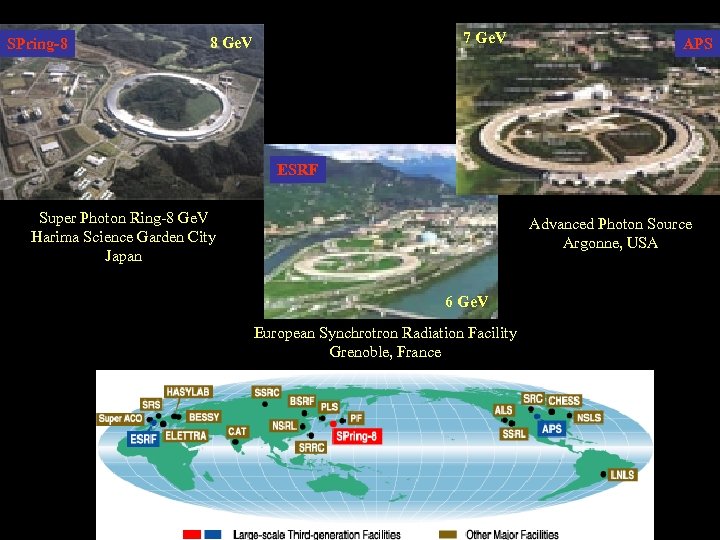
SPring-8 7 Ge. V 8 Ge. V APS ESRF Super Photon Ring-8 Ge. V Harima Science Garden City Japan Advanced Photon Source Argonne, USA 6 Ge. V European Synchrotron Radiation Facility Grenoble, France
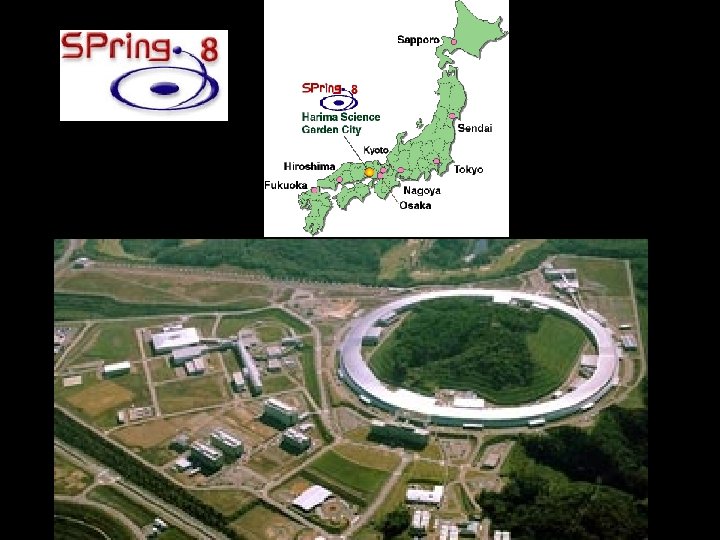
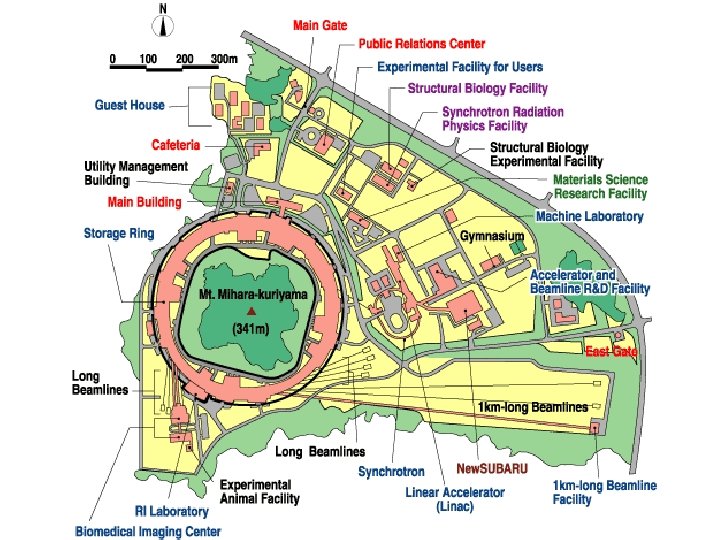

SPring-8 The linac accelerates electrons generated by an electron gun to 1 Ge. V using high frequency electric fields. The synchrotron is a circular accelerator that accelerates electrons injected from the linac to an energy of 8 Ge. V and transfers them to the storage ring. Electrons with an energy of 8 Ge. V are stored and synchrotron radiation is produced in the storage ring. The beamlines guide synchrotron radiation to the experimental hutch, where scientific research is performed. This facility is located at the end of 1 km-long beamline. It is used for research on advanced coherent X-ray optics. The observation of the gravitational effect on light is an example of the experiments carried out there.
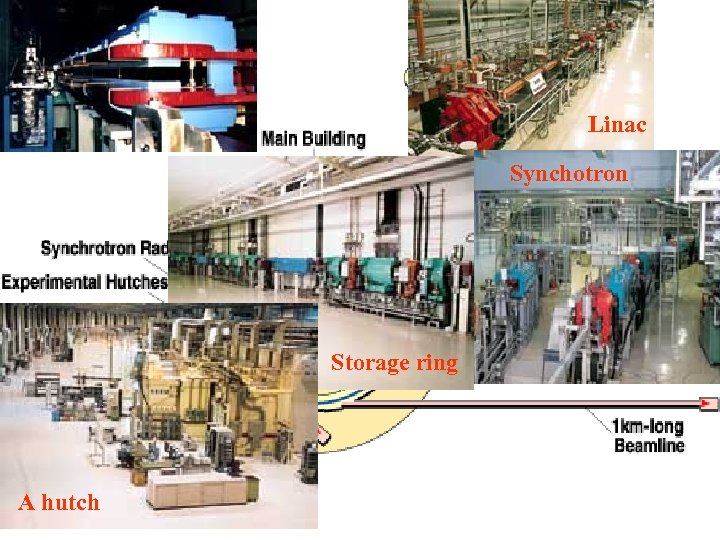
Linac Synchotron Storage ring A hutch
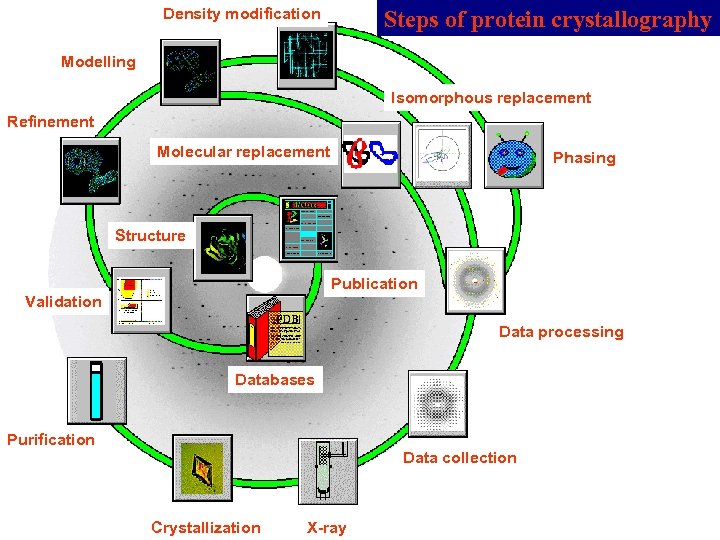
Density modification Steps of protein crystallography Modelling Isomorphous replacement Refinement Molecular replacement Phasing Structure Publication Validation Data processing Databases Purification Data collection Crystallization X-ray
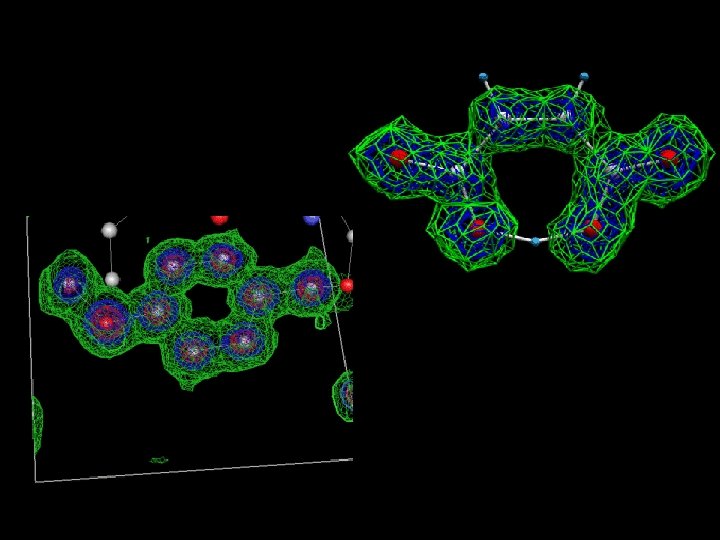
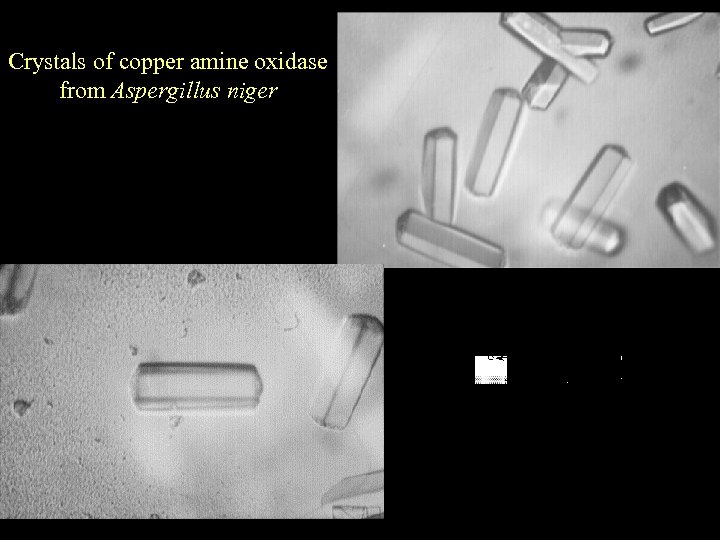
Crystals of copper amine oxidase from Aspergillus niger
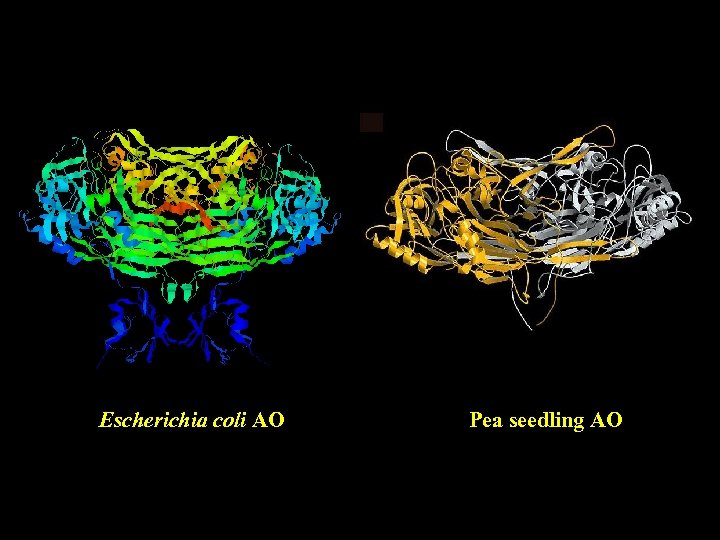
Escherichia coli AO (Parsons et al. , 1995) Pea seedling AO
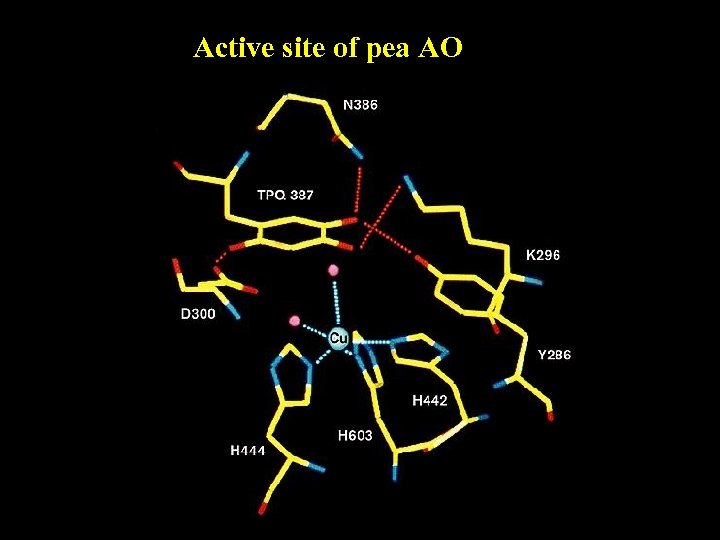
Active site of pea AO
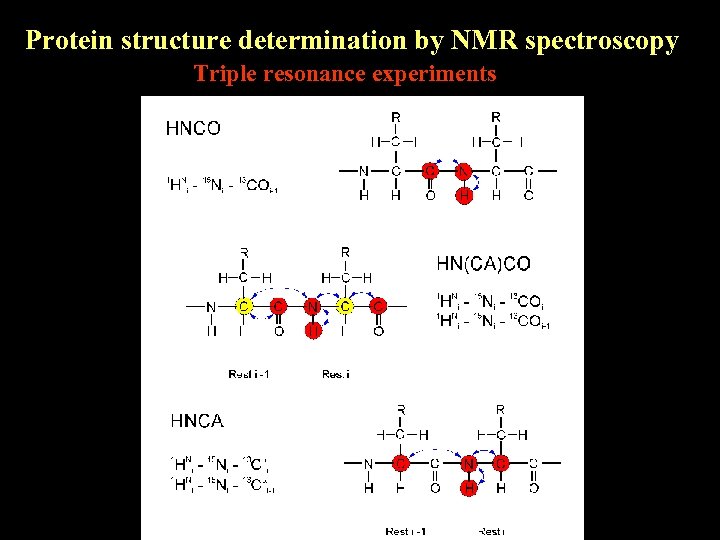
Protein structure determination by NMR spectroscopy Triple resonance experiments
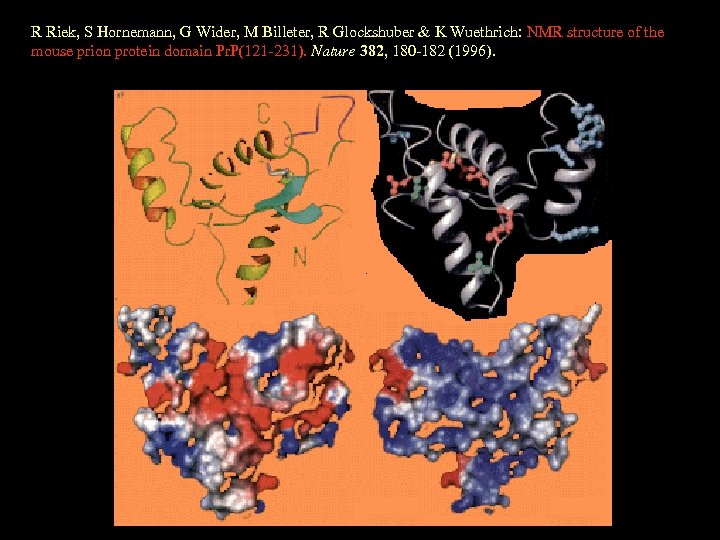
R Riek, S Hornemann, G Wider, M Billeter, R Glockshuber & K Wuethrich: NMR structure of the mouse prion protein domain Pr. P(121 -231). Nature 382, 180 -182 (1996).

Literature Garett, R. and Grisham, C. : Biochemistry 2 nd ed. , Harcourt Brace & Company, Orlando, FL, USA 1999. Jones, C. , Mulloy, B. , and Sanderson, M. R. : Crystallographic methods and protocols, Methods in Molecular Biology Vol. 56, Humana Press, Totowa, NJ, USA 1996. Spring-8 web page (www. spring 8. jp) and other Internet resources
b0055320acfe890abb8539fcaaf80cfc.ppt