Холдеева.pptx
- Количество слайдов: 42
 Металл-органические каркасы: перспективы использования в гетерогенном окислительном катализе О. А. Холдеева Институт катализа СО РАН Новосибирский государственный университет
Металл-органические каркасы: перспективы использования в гетерогенном окислительном катализе О. А. Холдеева Институт катализа СО РАН Новосибирский государственный университет
 Outline • Introduction Why metal-organic frameworks (MOFs)? Which MOFs? • Selective oxidation catalysis using MOFs and benign oxidants 1. MOFs as heterogeneous catalysts Oxidation of hydrocarbons (alkanes, alkenes, and aromatics) with O 2 and TBHP over Cr- and Fe-MIL-101(100) 2. MOFs as supports for immobilization of active complexes Oxidation of phenols with TBHP over Fe. Pc. S/Cr-MIL-101 Oxidation of alkenes with H 2 O 2 over POM/Cr-MIL-101 3. MOFs as precursors of catalysts Oxidation of alkylphenols to benzoquinones with H 2 O 2 over Ti-MIL-125 Key issues: activity, selectivity, productivity, stability, reusability, and mechanistic features of MOFs in comparison with other types of catalysts • Summary and outlook • Acknowledgements 2
Outline • Introduction Why metal-organic frameworks (MOFs)? Which MOFs? • Selective oxidation catalysis using MOFs and benign oxidants 1. MOFs as heterogeneous catalysts Oxidation of hydrocarbons (alkanes, alkenes, and aromatics) with O 2 and TBHP over Cr- and Fe-MIL-101(100) 2. MOFs as supports for immobilization of active complexes Oxidation of phenols with TBHP over Fe. Pc. S/Cr-MIL-101 Oxidation of alkenes with H 2 O 2 over POM/Cr-MIL-101 3. MOFs as precursors of catalysts Oxidation of alkylphenols to benzoquinones with H 2 O 2 over Ti-MIL-125 Key issues: activity, selectivity, productivity, stability, reusability, and mechanistic features of MOFs in comparison with other types of catalysts • Summary and outlook • Acknowledgements 2
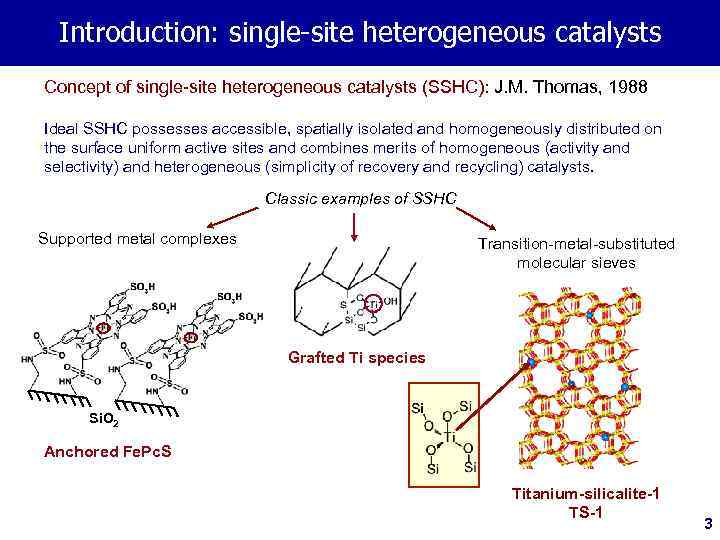 Introduction: single-site heterogeneous catalysts Concept of single-site heterogeneous catalysts (SSHC): J. M. Thomas, 1988 Ideal SSHC possesses accessible, spatially isolated and homogeneously distributed on the surface uniform active sites and combines merits of homogeneous (activity and selectivity) and heterogeneous (simplicity of recovery and recycling) catalysts. Classic examples of SSHC Supported metal complexes Transition-metal-substituted molecular sieves Grafted Ti species Si. O 2 Anchored Fe. Pc. S Titanium-silicalite-1 TS-1 3
Introduction: single-site heterogeneous catalysts Concept of single-site heterogeneous catalysts (SSHC): J. M. Thomas, 1988 Ideal SSHC possesses accessible, spatially isolated and homogeneously distributed on the surface uniform active sites and combines merits of homogeneous (activity and selectivity) and heterogeneous (simplicity of recovery and recycling) catalysts. Classic examples of SSHC Supported metal complexes Transition-metal-substituted molecular sieves Grafted Ti species Si. O 2 Anchored Fe. Pc. S Titanium-silicalite-1 TS-1 3
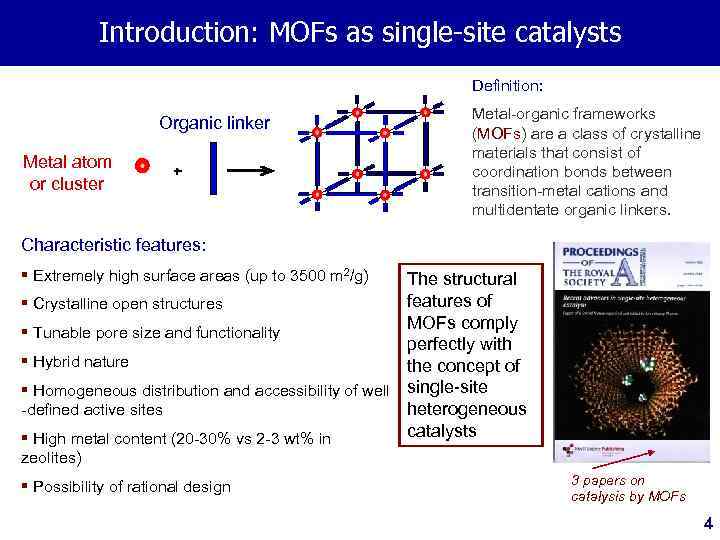 Introduction: MOFs as single-site catalysts Definition: Organic linker Metal atom or cluster + Metal-organic frameworks (MOFs) are a class of crystalline materials that consist of coordination bonds between transition-metal cations and multidentate organic linkers. Characteristic features: § Extremely high surface areas (up to 3500 m 2/g) The structural features of § Crystalline open structures MOFs comply § Tunable pore size and functionality perfectly with § Hybrid nature the concept of § Homogeneous distribution and accessibility of well single-site -defined active sites heterogeneous catalysts § High metal content (20 -30% vs 2 -3 wt% in zeolites) § Possibility of rational design 3 papers on catalysis by MOFs 4
Introduction: MOFs as single-site catalysts Definition: Organic linker Metal atom or cluster + Metal-organic frameworks (MOFs) are a class of crystalline materials that consist of coordination bonds between transition-metal cations and multidentate organic linkers. Characteristic features: § Extremely high surface areas (up to 3500 m 2/g) The structural features of § Crystalline open structures MOFs comply § Tunable pore size and functionality perfectly with § Hybrid nature the concept of § Homogeneous distribution and accessibility of well single-site -defined active sites heterogeneous catalysts § High metal content (20 -30% vs 2 -3 wt% in zeolites) § Possibility of rational design 3 papers on catalysis by MOFs 4
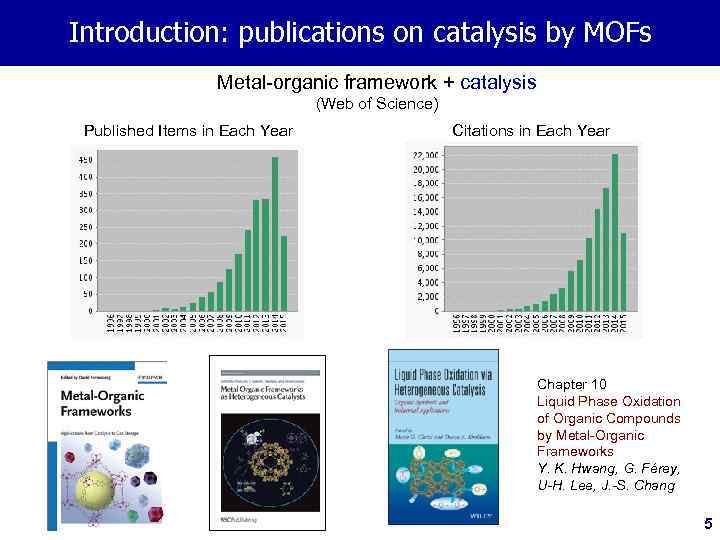 Introduction: publications on catalysis by MOFs Metal-organic framework + catalysis (Web of Science) Published Items in Each Year Citations in Each Year Chapter 10 Liquid Phase Oxidation of Organic Compounds by Metal-Organic Frameworks Y. K. Hwang, G. Férey, U-H. Lee, J. -S. Chang 5
Introduction: publications on catalysis by MOFs Metal-organic framework + catalysis (Web of Science) Published Items in Each Year Citations in Each Year Chapter 10 Liquid Phase Oxidation of Organic Compounds by Metal-Organic Frameworks Y. K. Hwang, G. Férey, U-H. Lee, J. -S. Chang 5
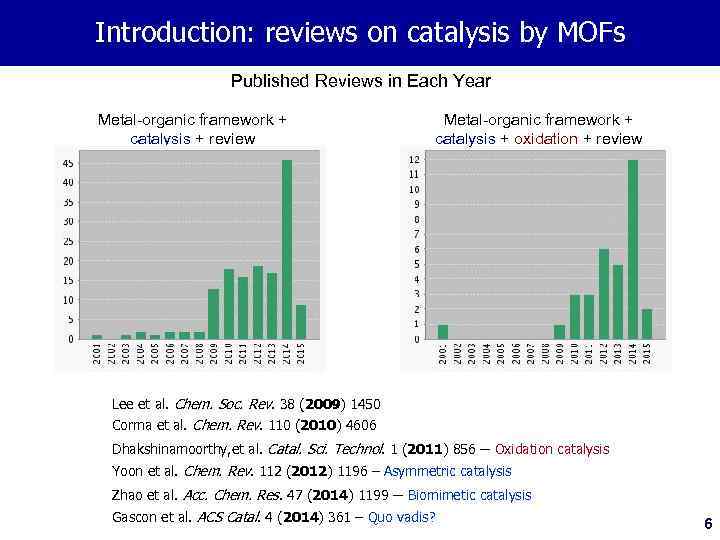 Introduction: reviews on catalysis by MOFs Published Reviews in Each Year Metal-organic framework + catalysis + review Metal-organic framework + catalysis + oxidation + review Lee et al. Chem. Soc. Rev. 38 (2009) 1450 Corma et al. Chem. Rev. 110 (2010) 4606 Dhakshinamoorthy, et al. Catal. Sci. Technol. 1 (2011) 856 – Oxidation catalysis Yoon et al. Chem. Rev. 112 (2012) 1196 – Asymmetric catalysis Zhao et al. Acc. Chem. Res. 47 (2014) 1199 – Biomimetic catalysis Gascon et al. ACS Catal. 4 (2014) 361 – Quo vadis? 6
Introduction: reviews on catalysis by MOFs Published Reviews in Each Year Metal-organic framework + catalysis + review Metal-organic framework + catalysis + oxidation + review Lee et al. Chem. Soc. Rev. 38 (2009) 1450 Corma et al. Chem. Rev. 110 (2010) 4606 Dhakshinamoorthy, et al. Catal. Sci. Technol. 1 (2011) 856 – Oxidation catalysis Yoon et al. Chem. Rev. 112 (2012) 1196 – Asymmetric catalysis Zhao et al. Acc. Chem. Res. 47 (2014) 1199 – Biomimetic catalysis Gascon et al. ACS Catal. 4 (2014) 361 – Quo vadis? 6
 Rien n'est parfait en ce bas monde (Le Petit Prince)
Rien n'est parfait en ce bas monde (Le Petit Prince)
 Introduction: thermal stability of MOFs Thermal stability of MOFs is generally limited to 350– 400 C May be OK for LPSO MOFs with outstanding thermal stability Al-MIL-53: 500 C (air) Loiseau et al. Chem. Eur. J. 2004, 10, 1373 Ui. O-66 and -67 (Zr. IV): 540 C (air) Cavka et al. J. Am. Chem. Soc. 2008, 130, 13850 ZIF-8 and -11 (Zn. II): 550 C (N 2) Park et al. PNAS, 2006, 103, 10186 [Zn 4 O(dmcapz)3]: 450 C (N 2) Montoro et al. J. Am. Chem. Soc. 2011, 133, 11888 Very few examples of application of MOFs in gas-phase oxidation catalysis: Zou et al. Angew. Chem. Int. Ed. , 2006, 45, 2542 Zou et al. J. Am. Chem. Soc. 2007, 129, 8402 CO to CO 2 (200– 300 C) 7
Introduction: thermal stability of MOFs Thermal stability of MOFs is generally limited to 350– 400 C May be OK for LPSO MOFs with outstanding thermal stability Al-MIL-53: 500 C (air) Loiseau et al. Chem. Eur. J. 2004, 10, 1373 Ui. O-66 and -67 (Zr. IV): 540 C (air) Cavka et al. J. Am. Chem. Soc. 2008, 130, 13850 ZIF-8 and -11 (Zn. II): 550 C (N 2) Park et al. PNAS, 2006, 103, 10186 [Zn 4 O(dmcapz)3]: 450 C (N 2) Montoro et al. J. Am. Chem. Soc. 2011, 133, 11888 Very few examples of application of MOFs in gas-phase oxidation catalysis: Zou et al. Angew. Chem. Int. Ed. , 2006, 45, 2542 Zou et al. J. Am. Chem. Soc. 2007, 129, 8402 CO to CO 2 (200– 300 C) 7
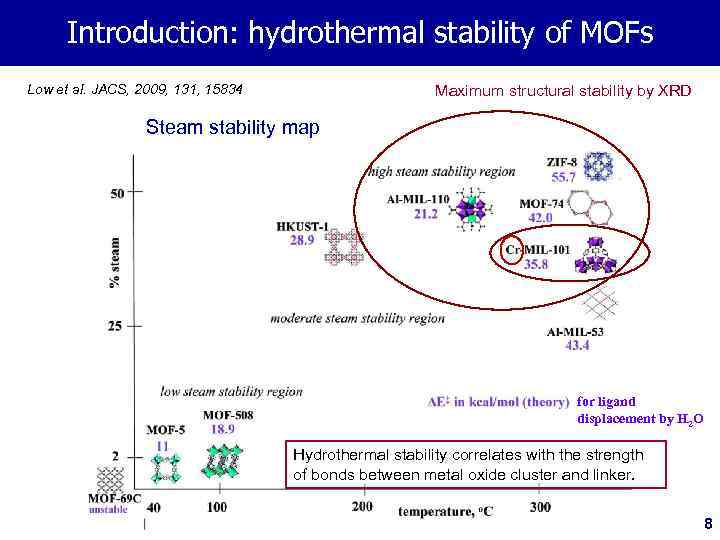 Introduction: hydrothermal stability of MOFs Low et al. JACS, 2009, 131, 15834 Maximum structural stability by XRD Steam stability map for ligand displacement by H 2 O Hydrothermal stability correlates with the strength of bonds between metal oxide cluster and linker. 8
Introduction: hydrothermal stability of MOFs Low et al. JACS, 2009, 131, 15834 Maximum structural stability by XRD Steam stability map for ligand displacement by H 2 O Hydrothermal stability correlates with the strength of bonds between metal oxide cluster and linker. 8
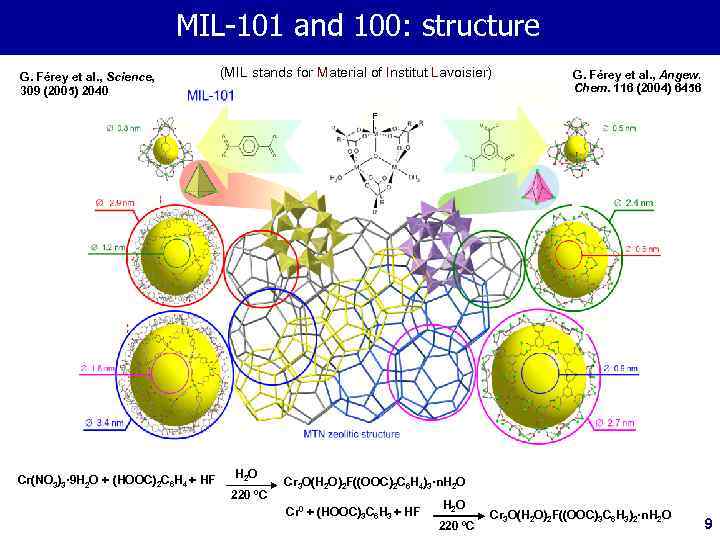 MIL-101 and 100: structure G. Férey et al. , Science, 309 (2005) 2040 (MIL stands for Material of Institut Lavoisier) G. Férey et al. , Angew. Chem. 116 (2004) 6456 F Cr(NO 3)3· 9 H 2 O + (HOOC)2 C 6 H 4 + HF H 2 O 220 ºC Cr 3 O(H 2 O)2 F((OOC)2 C 6 H 4)3·n. H 2 O Cr 0 + (HOOC)3 C 6 H 3 + HF H 2 O 220 ºC Cr 3 O(H 2 O)2 F((OOC)3 C 6 H 3)2·n. H 2 O 9
MIL-101 and 100: structure G. Férey et al. , Science, 309 (2005) 2040 (MIL stands for Material of Institut Lavoisier) G. Férey et al. , Angew. Chem. 116 (2004) 6456 F Cr(NO 3)3· 9 H 2 O + (HOOC)2 C 6 H 4 + HF H 2 O 220 ºC Cr 3 O(H 2 O)2 F((OOC)2 C 6 H 4)3·n. H 2 O Cr 0 + (HOOC)3 C 6 H 3 + HF H 2 O 220 ºC Cr 3 O(H 2 O)2 F((OOC)3 C 6 H 3)2·n. H 2 O 9
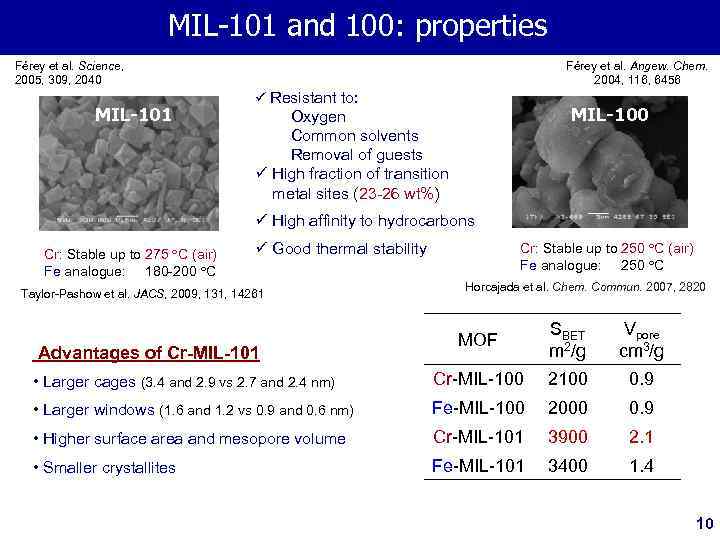 MIL-100 MIL-101 and 100: properties and 101: structure and properties Férey et al. Science, 2005, 309, 2040 MIL-101 Férey et al. Angew. Chem. 2004, 116, 6456 ü Resistant to: MIL-100 Oxygen Common solvents Removal of guests ü High fraction of transition metal sites (23 -26 wt%) ü High affinity to hydrocarbons Cr: Stable up to 275 C (air) Fe analogue: 180 -200 C Cr: Stable up to 250 C (air) Fe analogue: 250 C ü Good thermal stability Taylor-Pashow et al. JACS, 2009, 131, 14261 Horcajada et al. Chem. Commun. 2007, 2820 MOF SBET m 2/g Vpore cm 3/g • Larger cages (3. 4 and 2. 9 vs 2. 7 and 2. 4 nm) Cr-MIL-100 2100 0. 9 • Larger windows (1. 6 and 1. 2 vs 0. 9 and 0. 6 nm) Fe-MIL-100 2000 0. 9 • Higher surface area and mesopore volume Cr-MIL-101 3900 2. 1 • Smaller crystallites Fe-MIL-101 3400 1. 4 Advantages of Cr-MIL-101 10
MIL-100 MIL-101 and 100: properties and 101: structure and properties Férey et al. Science, 2005, 309, 2040 MIL-101 Férey et al. Angew. Chem. 2004, 116, 6456 ü Resistant to: MIL-100 Oxygen Common solvents Removal of guests ü High fraction of transition metal sites (23 -26 wt%) ü High affinity to hydrocarbons Cr: Stable up to 275 C (air) Fe analogue: 180 -200 C Cr: Stable up to 250 C (air) Fe analogue: 250 C ü Good thermal stability Taylor-Pashow et al. JACS, 2009, 131, 14261 Horcajada et al. Chem. Commun. 2007, 2820 MOF SBET m 2/g Vpore cm 3/g • Larger cages (3. 4 and 2. 9 vs 2. 7 and 2. 4 nm) Cr-MIL-100 2100 0. 9 • Larger windows (1. 6 and 1. 2 vs 0. 9 and 0. 6 nm) Fe-MIL-100 2000 0. 9 • Higher surface area and mesopore volume Cr-MIL-101 3900 2. 1 • Smaller crystallites Fe-MIL-101 3400 1. 4 Advantages of Cr-MIL-101 10
 1. MOFs as heterogeneous catalysts: Oxidation of hydrocarbons with O 2 and TBHP over Cr- and Fe-MIL-101(100) In collaboration with Prof. Fedin and Dr. Kovalenko (NIIC)
1. MOFs as heterogeneous catalysts: Oxidation of hydrocarbons with O 2 and TBHP over Cr- and Fe-MIL-101(100) In collaboration with Prof. Fedin and Dr. Kovalenko (NIIC)
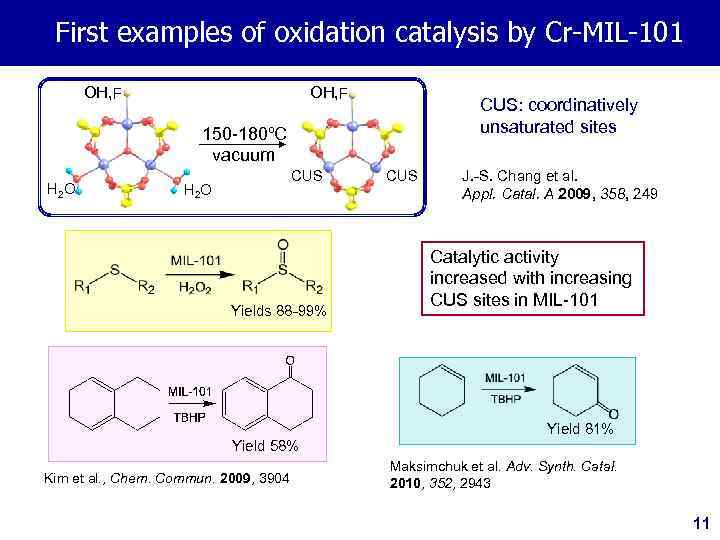 First examples of oxidation catalysis by Cr-MIL-101 OH, F CUS: coordinatively unsaturated sites 150 -180ºC vacuum H 2 O CUS H 2 O Yields 88 -99% CUS J. -S. Chang et al. Appl. Catal. A 2009, 358, 249 Catalytic activity increased with increasing CUS sites in MIL-101 Yield 81% Yield 58% Kim et al. , Chem. Commun. 2009, 3904 Maksimchuk et al. Adv. Synth. Catal. 2010, 352, 2943 11
First examples of oxidation catalysis by Cr-MIL-101 OH, F CUS: coordinatively unsaturated sites 150 -180ºC vacuum H 2 O CUS H 2 O Yields 88 -99% CUS J. -S. Chang et al. Appl. Catal. A 2009, 358, 249 Catalytic activity increased with increasing CUS sites in MIL-101 Yield 81% Yield 58% Kim et al. , Chem. Commun. 2009, 3904 Maksimchuk et al. Adv. Synth. Catal. 2010, 352, 2943 11
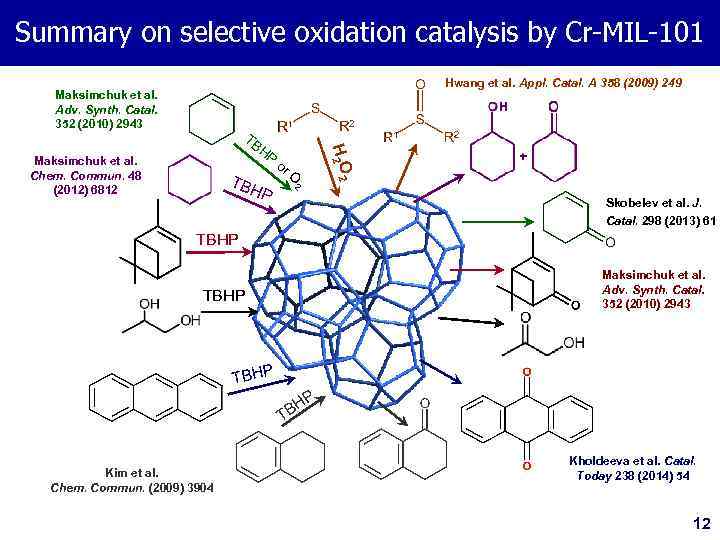 Summary on selective oxidation catalysis by Cr-MIL-101 Hwang et al. Appl. Catal. A 358 (2009) 249 Maksimchuk et al. Adv. Synth. Catal. 352 (2010) 2943 HP Maksimchuk et al. Chem. Commun. 48 (2012) 6812 TB HP or O 2 H 2 O 2 TB Skobelev et al. J. Catal. 298 (2013) 61 TBHP Maksimchuk et al. Adv. Synth. Catal. 352 (2010) 2943 TBHP HP B T Kim et al. Chem. Commun. (2009) 3904 Kholdeeva et al. Catal. Today 238 (2014) 54 12
Summary on selective oxidation catalysis by Cr-MIL-101 Hwang et al. Appl. Catal. A 358 (2009) 249 Maksimchuk et al. Adv. Synth. Catal. 352 (2010) 2943 HP Maksimchuk et al. Chem. Commun. 48 (2012) 6812 TB HP or O 2 H 2 O 2 TB Skobelev et al. J. Catal. 298 (2013) 61 TBHP Maksimchuk et al. Adv. Synth. Catal. 352 (2010) 2943 TBHP HP B T Kim et al. Chem. Commun. (2009) 3904 Kholdeeva et al. Catal. Today 238 (2014) 54 12
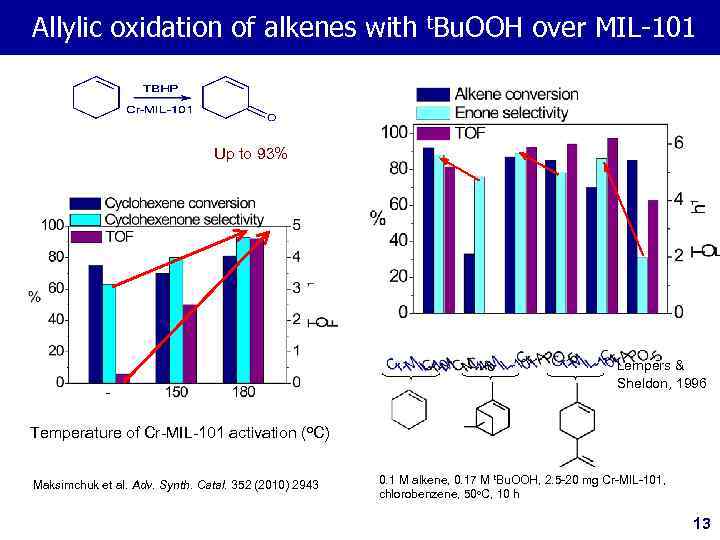 Allylic oxidation of alkenes with t. Bu. OOH over MIL-101 Up to 93% Lempers & Sheldon, 1996 Temperature of Cr-MIL-101 activation (o. C) Maksimchuk et al. Adv. Synth. Catal. 352 (2010) 2943 0. 1 M alkene, 0. 17 M t. Bu. OOH, 2. 5 -20 mg Cr-MIL-101, chlorobenzene, 50 o. C, 10 h 13
Allylic oxidation of alkenes with t. Bu. OOH over MIL-101 Up to 93% Lempers & Sheldon, 1996 Temperature of Cr-MIL-101 activation (o. C) Maksimchuk et al. Adv. Synth. Catal. 352 (2010) 2943 0. 1 M alkene, 0. 17 M t. Bu. OOH, 2. 5 -20 mg Cr-MIL-101, chlorobenzene, 50 o. C, 10 h 13
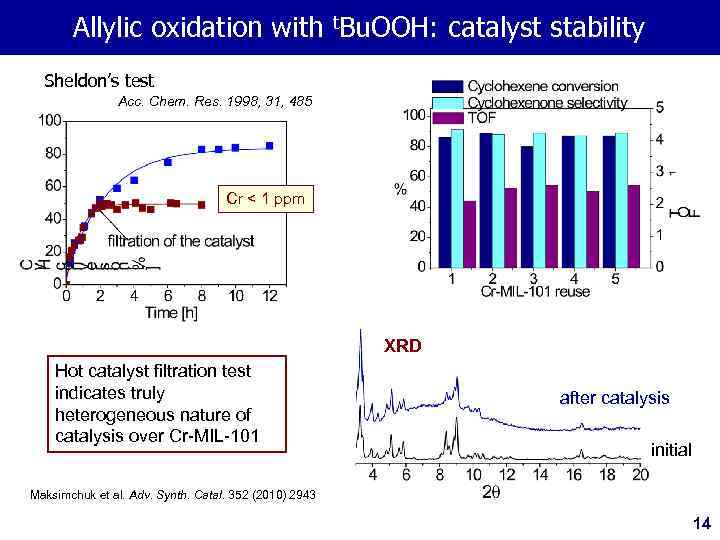 Allylic oxidation with t. Bu. OOH: catalyst stability Sheldon’s test Acc. Chem. Res. 1998, 31, 485 Cr < 1 ppm XRD Hot catalyst filtration test indicates truly heterogeneous nature of catalysis over Cr-MIL-101 after catalysis initial Maksimchuk et al. Adv. Synth. Catal. 352 (2010) 2943 14
Allylic oxidation with t. Bu. OOH: catalyst stability Sheldon’s test Acc. Chem. Res. 1998, 31, 485 Cr < 1 ppm XRD Hot catalyst filtration test indicates truly heterogeneous nature of catalysis over Cr-MIL-101 after catalysis initial Maksimchuk et al. Adv. Synth. Catal. 352 (2010) 2943 14
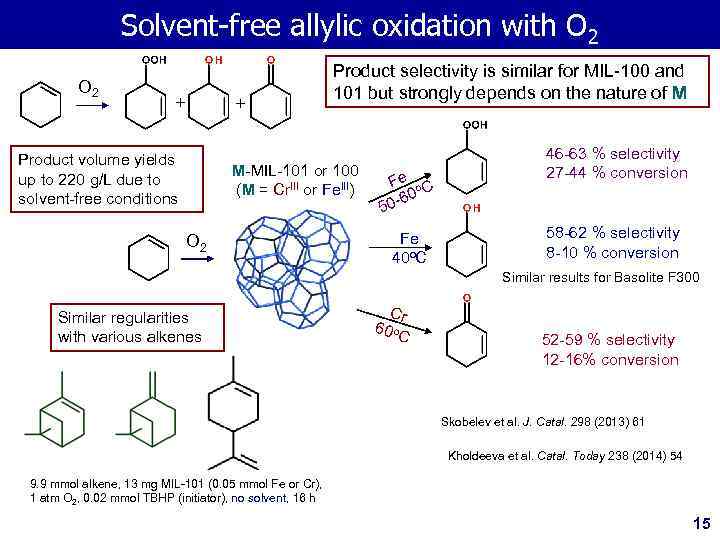 Solvent-free allylic oxidation with O 2 + + Product volume yields up to 220 g/L due to solvent-free conditions Product selectivity is similar for MIL-100 and 101 but strongly depends on the nature of M M-MIL-101 or 100 (M = Cr. III or Fe. III) O 2 Fe o C 60 50 - Fe 40 o. C 46 -63 % selectivity 27 -44 % conversion 58 -62 % selectivity 8 -10 % conversion Similar results for Basolite F 300 Similar regularities with various alkenes Cr 60 o. C 52 -59 % selectivity 12 -16% conversion Skobelev et al. J. Catal. 298 (2013) 61 Kholdeeva et al. Catal. Today 238 (2014) 54 9. 9 mmol alkene, 13 mg MIL-101 (0. 05 mmol Fe or Cr), 1 atm O 2, 0. 02 mmol TBHP (initiator), no solvent, 16 h 15
Solvent-free allylic oxidation with O 2 + + Product volume yields up to 220 g/L due to solvent-free conditions Product selectivity is similar for MIL-100 and 101 but strongly depends on the nature of M M-MIL-101 or 100 (M = Cr. III or Fe. III) O 2 Fe o C 60 50 - Fe 40 o. C 46 -63 % selectivity 27 -44 % conversion 58 -62 % selectivity 8 -10 % conversion Similar results for Basolite F 300 Similar regularities with various alkenes Cr 60 o. C 52 -59 % selectivity 12 -16% conversion Skobelev et al. J. Catal. 298 (2013) 61 Kholdeeva et al. Catal. Today 238 (2014) 54 9. 9 mmol alkene, 13 mg MIL-101 (0. 05 mmol Fe or Cr), 1 atm O 2, 0. 02 mmol TBHP (initiator), no solvent, 16 h 15
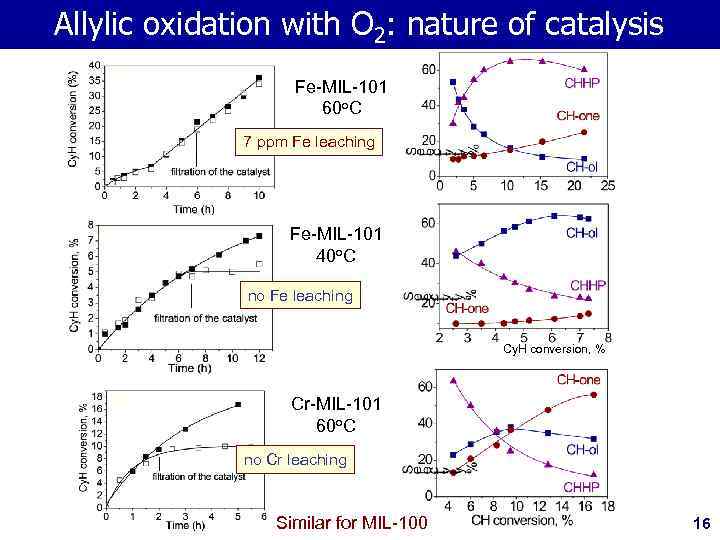 Allylic oxidation with O 2: nature of catalysis Fe-MIL-101 60 o. C 7 ppm Fe leaching Fe-MIL-101 40 o. C no Fe leaching Cy. H conversion, % Cr-MIL-101 60 o. C no Cr leaching Similar for MIL-100 16
Allylic oxidation with O 2: nature of catalysis Fe-MIL-101 60 o. C 7 ppm Fe leaching Fe-MIL-101 40 o. C no Fe leaching Cy. H conversion, % Cr-MIL-101 60 o. C no Cr leaching Similar for MIL-100 16
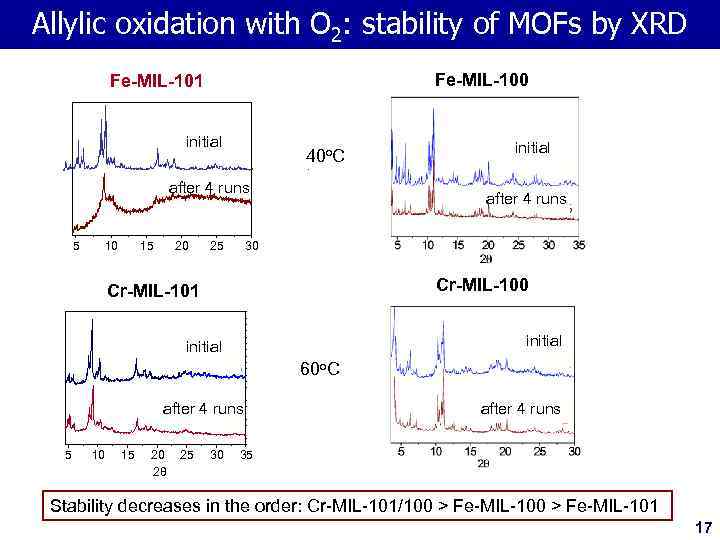 Allylic oxidation with O 2: stability of MOFs by XRD Fe-MIL-100 Fe-MIL-101 initial 40 o. C after 4 runs 5 10 15 20 25 after 4 runs 30 Cr-MIL-101 initial A after 4 runs Б 5 10 15 20 2θ 25 initial 30 60 o. C after 4 runs 35 Stability decreases in the order: Cr-MIL-101/100 > Fe-MIL-101 17
Allylic oxidation with O 2: stability of MOFs by XRD Fe-MIL-100 Fe-MIL-101 initial 40 o. C after 4 runs 5 10 15 20 25 after 4 runs 30 Cr-MIL-101 initial A after 4 runs Б 5 10 15 20 2θ 25 initial 30 60 o. C after 4 runs 35 Stability decreases in the order: Cr-MIL-101/100 > Fe-MIL-101 17
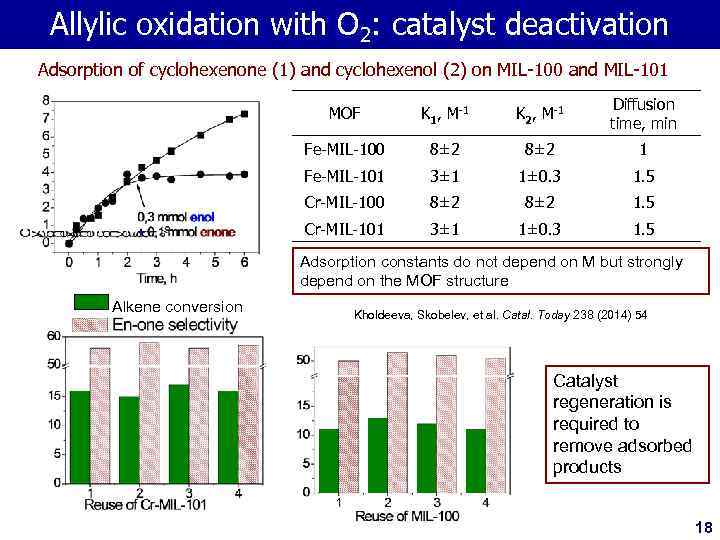 Allylic oxidation with O 2: catalyst deactivation Adsorption of cyclohexenone (1) and cyclohexenol (2) on MIL-100 and MIL-101 MOF K 1, M-1 K 2, M-1 Diffusion time, min Fe-MIL-100 8± 2 1 Fe-MIL-101 3± 1 1± 0. 3 1. 5 Cr-MIL-100 8± 2 1. 5 Cr-MIL-101 3± 1 1± 0. 3 1. 5 Adsorption constants do not depend on M but strongly depend on the MOF structure Alkene conversion Kholdeeva, Skobelev, et al. Catal. Today 238 (2014) 54 Catalyst regeneration is required to remove adsorbed products 18
Allylic oxidation with O 2: catalyst deactivation Adsorption of cyclohexenone (1) and cyclohexenol (2) on MIL-100 and MIL-101 MOF K 1, M-1 K 2, M-1 Diffusion time, min Fe-MIL-100 8± 2 1 Fe-MIL-101 3± 1 1± 0. 3 1. 5 Cr-MIL-100 8± 2 1. 5 Cr-MIL-101 3± 1 1± 0. 3 1. 5 Adsorption constants do not depend on M but strongly depend on the MOF structure Alkene conversion Kholdeeva, Skobelev, et al. Catal. Today 238 (2014) 54 Catalyst regeneration is required to remove adsorbed products 18
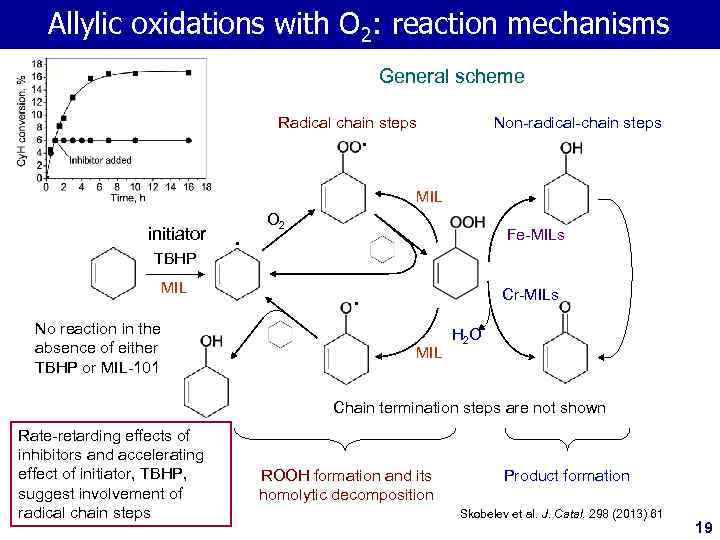 Allylic oxidations with O 2: reaction mechanisms General scheme Radical chain steps Non-radical-chain steps • MIL initiator TBHP O 2 Fe-MILs • MIL Cr-MILs • No reaction in the absence of either TBHP or MIL-101 MIL H 2 O Chain termination steps are not shown Rate-retarding effects of inhibitors and accelerating effect of initiator, TBHP, suggest involvement of radical chain steps ROOH formation and its homolytic decomposition Product formation Skobelev et al. J. Catal. 298 (2013) 61 19
Allylic oxidations with O 2: reaction mechanisms General scheme Radical chain steps Non-radical-chain steps • MIL initiator TBHP O 2 Fe-MILs • MIL Cr-MILs • No reaction in the absence of either TBHP or MIL-101 MIL H 2 O Chain termination steps are not shown Rate-retarding effects of inhibitors and accelerating effect of initiator, TBHP, suggest involvement of radical chain steps ROOH formation and its homolytic decomposition Product formation Skobelev et al. J. Catal. 298 (2013) 61 19
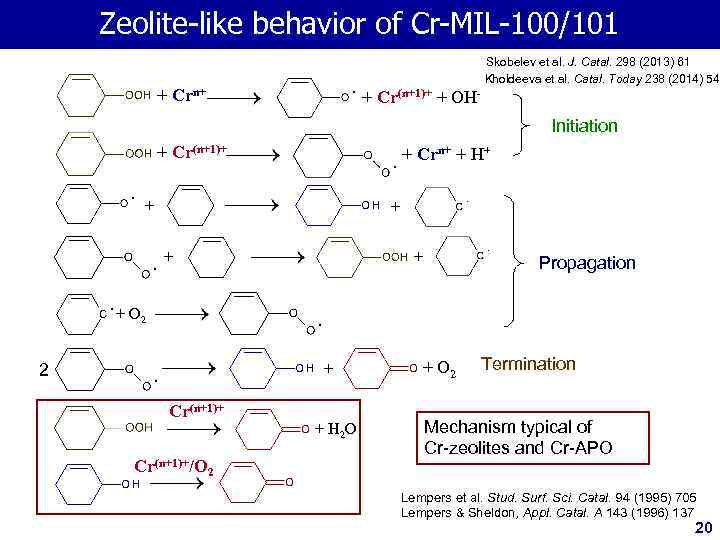 Zeolite-like behavior of Cr-MIL-100/101 Skobelev et al. J. Catal. 298 (2013) 61 Kholdeeva et al. Catal. Today 238 (2014) 54 + Crn+ + Cr(n+1)+ + OHInitiation + Cr(n+1)+ + Crn+ + H+ + + Propagation + O 2 + 2 Cr(n+1)+/О 2 + H 2 O + O 2 Termination Mechanism typical of Cr-zeolites and Cr-APO Lempers et al. Stud. Surf. Sci. Catal. 94 (1995) 705 Lempers & Sheldon, Appl. Catal. A 143 (1996) 137 20
Zeolite-like behavior of Cr-MIL-100/101 Skobelev et al. J. Catal. 298 (2013) 61 Kholdeeva et al. Catal. Today 238 (2014) 54 + Crn+ + Cr(n+1)+ + OHInitiation + Cr(n+1)+ + Crn+ + H+ + + Propagation + O 2 + 2 Cr(n+1)+/О 2 + H 2 O + O 2 Termination Mechanism typical of Cr-zeolites and Cr-APO Lempers et al. Stud. Surf. Sci. Catal. 94 (1995) 705 Lempers & Sheldon, Appl. Catal. A 143 (1996) 137 20
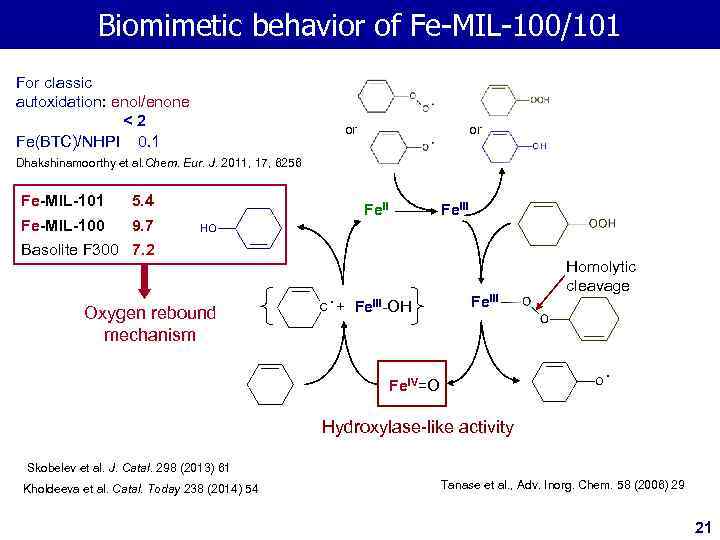 Biomimetic behavior of Fe-MIL-100/101 For classic autoxidation: enol/enone <2 Fe(BTC)/NHPI 0. 1 or or Dhakshinamoorthy et al. Chem. Eur. J. 2011, 17, 6256 Fe-MIL-101 5. 4 Fe-MIL-100 9. 7 Fe. III Basolite F 300 7. 2 Oxygen rebound mechanism + Fe. III-ОH Fe. III Homolytic cleavage Fe. IV=О Hydroxylase-like activity Skobelev et al. J. Catal. 298 (2013) 61 Kholdeeva et al. Catal. Today 238 (2014) 54 Tanase et al. , Adv. Inorg. Chem. 58 (2006) 29 21
Biomimetic behavior of Fe-MIL-100/101 For classic autoxidation: enol/enone <2 Fe(BTC)/NHPI 0. 1 or or Dhakshinamoorthy et al. Chem. Eur. J. 2011, 17, 6256 Fe-MIL-101 5. 4 Fe-MIL-100 9. 7 Fe. III Basolite F 300 7. 2 Oxygen rebound mechanism + Fe. III-ОH Fe. III Homolytic cleavage Fe. IV=О Hydroxylase-like activity Skobelev et al. J. Catal. 298 (2013) 61 Kholdeeva et al. Catal. Today 238 (2014) 54 Tanase et al. , Adv. Inorg. Chem. 58 (2006) 29 21
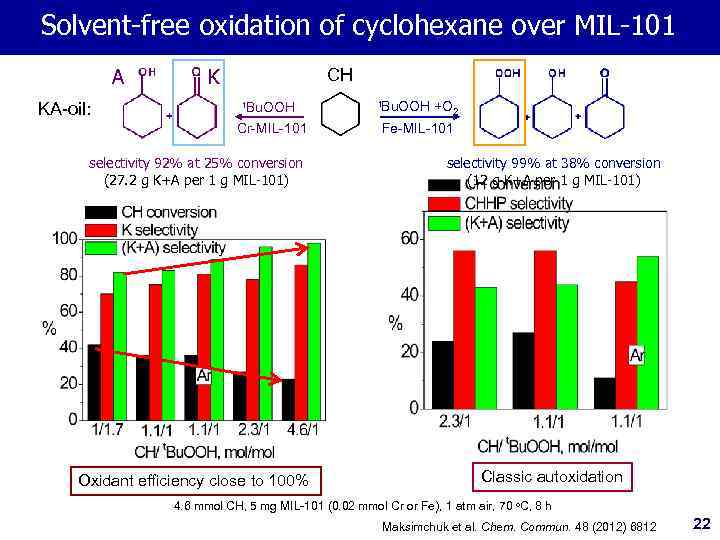 Solvent-free oxidation of cyclohexane over MIL-101 A KA-oil: K CH t. Bu. OOH Cr-MIL-101 t. Bu. OOH +O 2 Fe-MIL-101 selectivity 92% at 25% conversion (27. 2 g K+A per 1 g MIL-101) selectivity 99% at 38% conversion (12 g К+А per 1 g MIL-101) Oxidant efficiency close to 100% Classic autoxidation 4. 6 mmol CH, 5 mg MIL-101 (0. 02 mmol Cr or Fe), 1 atm air, 70 o. C, 8 h Maksimchuk et al. Chem. Commun. 48 (2012) 6812 22
Solvent-free oxidation of cyclohexane over MIL-101 A KA-oil: K CH t. Bu. OOH Cr-MIL-101 t. Bu. OOH +O 2 Fe-MIL-101 selectivity 92% at 25% conversion (27. 2 g K+A per 1 g MIL-101) selectivity 99% at 38% conversion (12 g К+А per 1 g MIL-101) Oxidant efficiency close to 100% Classic autoxidation 4. 6 mmol CH, 5 mg MIL-101 (0. 02 mmol Cr or Fe), 1 atm air, 70 o. C, 8 h Maksimchuk et al. Chem. Commun. 48 (2012) 6812 22
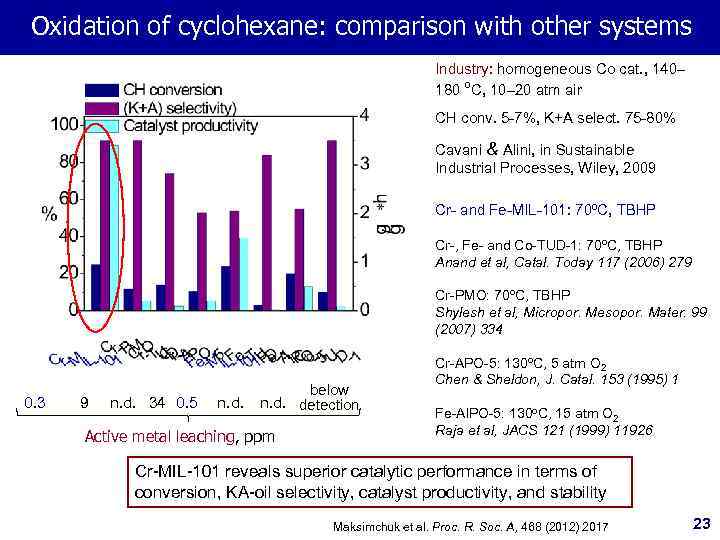 Oxidation of cyclohexane: comparison with other systems Industry: homogeneous Co cat. , 140– 180 ºC, 10– 20 atm air CH conv. 5 -7%, K+A select. 75 -80% Cavani & Alini, in Sustainable Industrial Processes, Wiley, 2009 Cr- and Fe-MIL-101: 70ºC, TBHP Cr-, Fe- and Co-TUD-1: 70ºC, TBHP Anand et al, Catal. Today 117 (2006) 279 Cr-PMO: 70ºC, TBHP Shylesh et al, Micropor. Mesopor. Mater. 99 (2007) 334 0. 3 9 n. d. 34 0. 5 n. d. below n. d. detection Active metal leaching, ppm Cr-APO-5: 130 o. C, 5 atm O 2 Chen & Sheldon, J. Catal. 153 (1995) 1 Fe-Al. PO-5: 130 o. C, 15 atm O 2 Raja et al, JACS 121 (1999) 11926 Cr-MIL-101 reveals superior catalytic performance in terms of conversion, KA-oil selectivity, catalyst productivity, and stability Maksimchuk et al. Proc. R. Soc. A, 468 (2012) 2017 23
Oxidation of cyclohexane: comparison with other systems Industry: homogeneous Co cat. , 140– 180 ºC, 10– 20 atm air CH conv. 5 -7%, K+A select. 75 -80% Cavani & Alini, in Sustainable Industrial Processes, Wiley, 2009 Cr- and Fe-MIL-101: 70ºC, TBHP Cr-, Fe- and Co-TUD-1: 70ºC, TBHP Anand et al, Catal. Today 117 (2006) 279 Cr-PMO: 70ºC, TBHP Shylesh et al, Micropor. Mesopor. Mater. 99 (2007) 334 0. 3 9 n. d. 34 0. 5 n. d. below n. d. detection Active metal leaching, ppm Cr-APO-5: 130 o. C, 5 atm O 2 Chen & Sheldon, J. Catal. 153 (1995) 1 Fe-Al. PO-5: 130 o. C, 15 atm O 2 Raja et al, JACS 121 (1999) 11926 Cr-MIL-101 reveals superior catalytic performance in terms of conversion, KA-oil selectivity, catalyst productivity, and stability Maksimchuk et al. Proc. R. Soc. A, 468 (2012) 2017 23
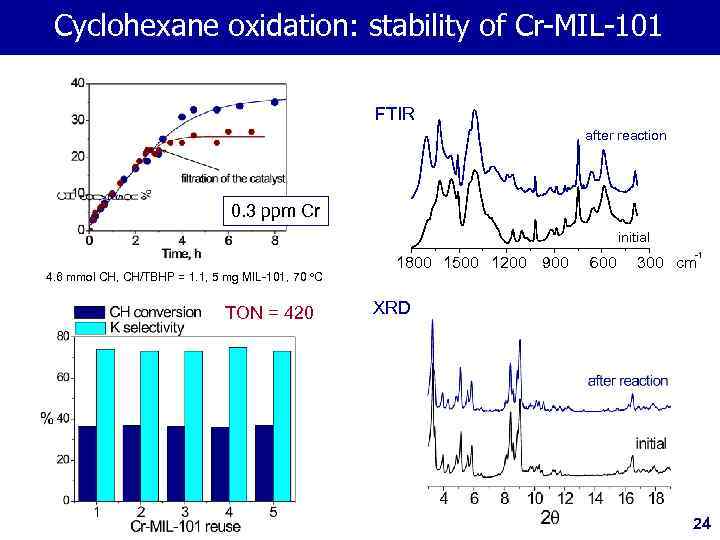 Cyclohexane oxidation: stability of Cr-MIL-101 FTIR after reaction 0. 3 ppm Cr initial 4. 6 mmol CH, CH/TBHP = 1. 1, 5 mg MIL-101, 70 o. C TON = 420 1800 1500 1200 900 600 -1 300 cm XRD 24
Cyclohexane oxidation: stability of Cr-MIL-101 FTIR after reaction 0. 3 ppm Cr initial 4. 6 mmol CH, CH/TBHP = 1. 1, 5 mg MIL-101, 70 o. C TON = 420 1800 1500 1200 900 600 -1 300 cm XRD 24
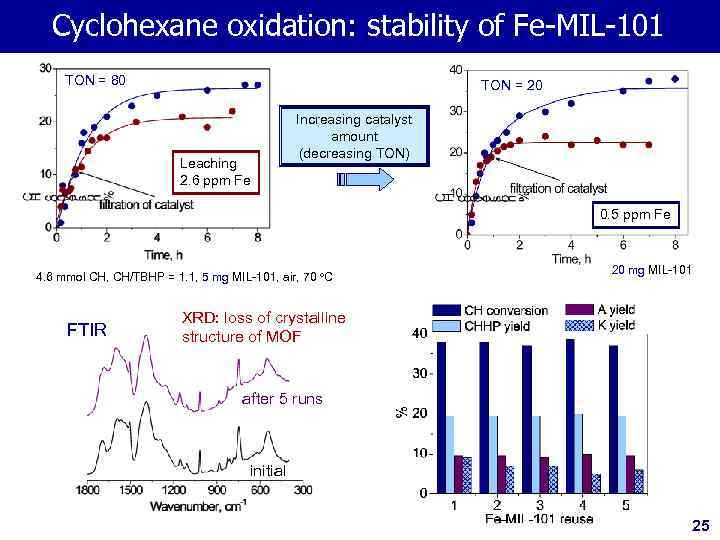 Cyclohexane oxidation: stability of Fe-MIL-101 TON = 80 TON = 20 Leaching 2. 6 ppm Fe Increasing catalyst amount (decreasing TON) 0. 5 ppm Fe 4. 6 mmol CH, CH/TBHP = 1. 1, 5 mg MIL-101, air, 70 o. C FTIR 20 mg MIL-101 XRD: loss of crystalline structure of MOF after 5 runs initial 25
Cyclohexane oxidation: stability of Fe-MIL-101 TON = 80 TON = 20 Leaching 2. 6 ppm Fe Increasing catalyst amount (decreasing TON) 0. 5 ppm Fe 4. 6 mmol CH, CH/TBHP = 1. 1, 5 mg MIL-101, air, 70 o. C FTIR 20 mg MIL-101 XRD: loss of crystalline structure of MOF after 5 runs initial 25
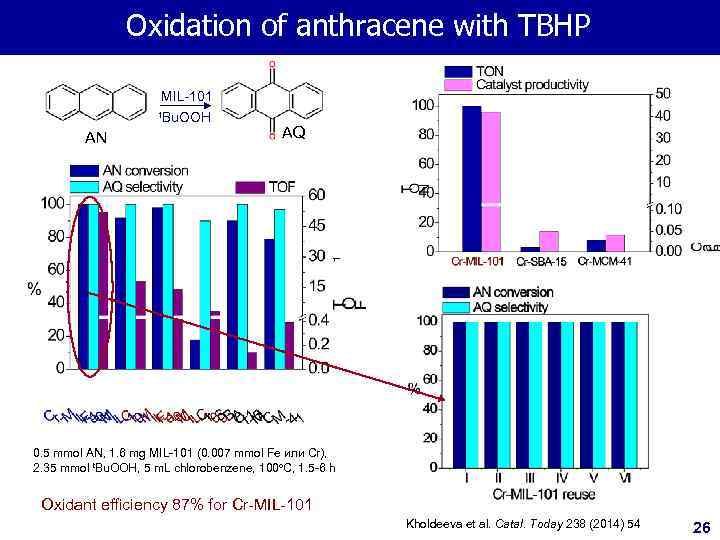 Oxidation of anthracene with TBHP MIL-101 t. Bu. OOH AN AQ 0. 5 mmol AN, 1. 6 mg MIL-101 (0. 007 mmol Fe или Cr), 2. 35 mmol t. Bu. OOH, 5 m. L chlorobenzene, 100 o. C, 1. 5 -6 h Oxidant efficiency 87% for Cr-MIL-101 Kholdeeva et al. Catal. Today 238 (2014) 54 26
Oxidation of anthracene with TBHP MIL-101 t. Bu. OOH AN AQ 0. 5 mmol AN, 1. 6 mg MIL-101 (0. 007 mmol Fe или Cr), 2. 35 mmol t. Bu. OOH, 5 m. L chlorobenzene, 100 o. C, 1. 5 -6 h Oxidant efficiency 87% for Cr-MIL-101 Kholdeeva et al. Catal. Today 238 (2014) 54 26
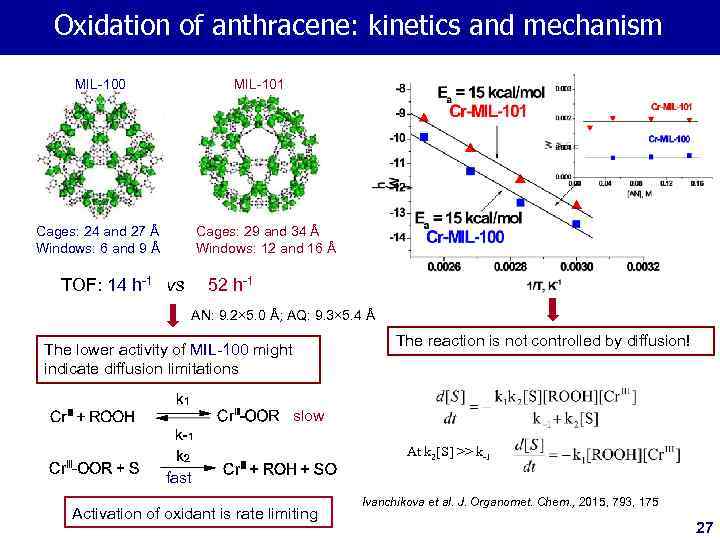 Oxidation of anthracene: kinetics and mechanism MIL-100 MIL-101 Cages: 24 and 27 Å Windows: 6 and 9 Å Cages: 29 and 34 Å Windows: 12 and 16 Å TOF: 14 h-1 vs 52 h-1 AN: 9. 2× 5. 0 Å; AQ: 9. 3× 5. 4 Å The reaction is not controlled by diffusion! The lower activity of MIL-100 might indicate diffusion limitations slow At k 2[S] >> k-1 fast Activation of oxidant is rate limiting Ivanchikova et al. J. Organomet. Chem. , 2015, 793, 175 27
Oxidation of anthracene: kinetics and mechanism MIL-100 MIL-101 Cages: 24 and 27 Å Windows: 6 and 9 Å Cages: 29 and 34 Å Windows: 12 and 16 Å TOF: 14 h-1 vs 52 h-1 AN: 9. 2× 5. 0 Å; AQ: 9. 3× 5. 4 Å The reaction is not controlled by diffusion! The lower activity of MIL-100 might indicate diffusion limitations slow At k 2[S] >> k-1 fast Activation of oxidant is rate limiting Ivanchikova et al. J. Organomet. Chem. , 2015, 793, 175 27
 Catalysis by MOF-supported metal nanoparticles: Moon et al. Chem Soc. Rev. 42 (2013)1807 Ishida et al. Chem. Eur. J. 14 (2008) 8456 Liu et al. J. Phys. Chem. C 114 (2010) 13362 Juan-Alcañiz et al. J. Catal. 307 (2013) 295 Selective oxidation of alcohols 2. MOFs as supports for active complexes Oxidation of phenols with TBHP over Fe. Pc. S/Cr-MIL-101 In collaboration with Dr. A. Sorokin (IRCELYON) Oxidation of alkenes with H 2 O 2 over POM/Cr-MIL-101 In collaboration with Prof. Fedin, Dr. Dybtsev, and Dr. Kovalenko (NIIC)
Catalysis by MOF-supported metal nanoparticles: Moon et al. Chem Soc. Rev. 42 (2013)1807 Ishida et al. Chem. Eur. J. 14 (2008) 8456 Liu et al. J. Phys. Chem. C 114 (2010) 13362 Juan-Alcañiz et al. J. Catal. 307 (2013) 295 Selective oxidation of alcohols 2. MOFs as supports for active complexes Oxidation of phenols with TBHP over Fe. Pc. S/Cr-MIL-101 In collaboration with Dr. A. Sorokin (IRCELYON) Oxidation of alkenes with H 2 O 2 over POM/Cr-MIL-101 In collaboration with Prof. Fedin, Dr. Dybtsev, and Dr. Kovalenko (NIIC)
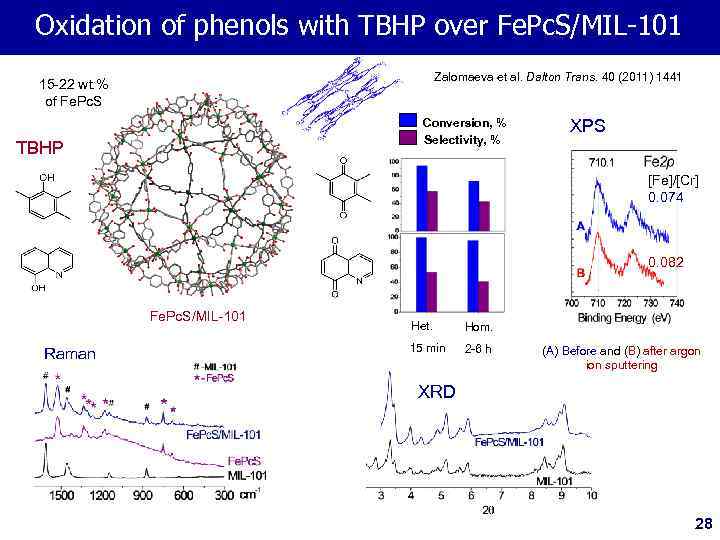 Oxidation of phenols with TBHP over Fe. Pc. S/MIL-101 Zalomaeva et al. Dalton Trans. 40 (2011) 1441 15 -22 wt. % of Fe. Pc. S Conversion, % Selectivity, % TBHP XPS [Fe]/[Cr] 0. 074 0. 082 Fe. Pc. S/MIL-101 Raman Het. Hom. 15 min 2 -6 h (A) Before and (B) after argon ion sputtering XRD 28
Oxidation of phenols with TBHP over Fe. Pc. S/MIL-101 Zalomaeva et al. Dalton Trans. 40 (2011) 1441 15 -22 wt. % of Fe. Pc. S Conversion, % Selectivity, % TBHP XPS [Fe]/[Cr] 0. 074 0. 082 Fe. Pc. S/MIL-101 Raman Het. Hom. 15 min 2 -6 h (A) Before and (B) after argon ion sputtering XRD 28
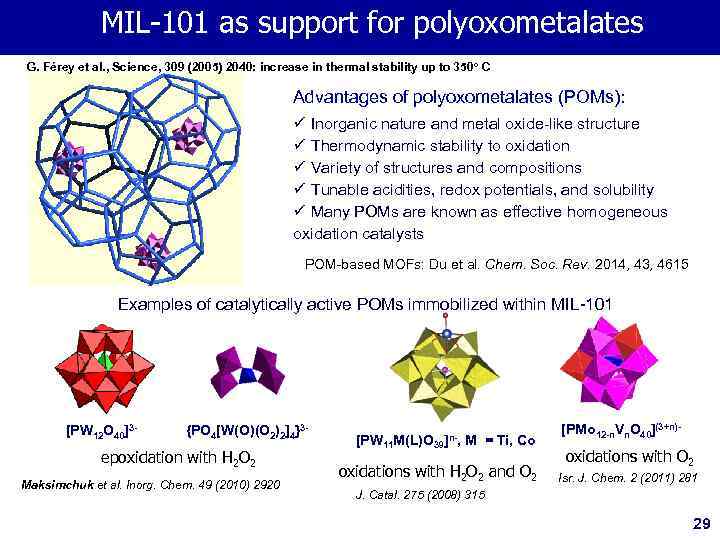 MIL-101 as support for polyoxometalates G. Férey et al. , Science, 309 (2005) 2040: increase in thermal stability up to 350 o C Advantages of polyoxometalates (POMs): ü Inorganic nature and metal oxide-like structure ü Thermodynamic stability to oxidation ü Variety of structures and compositions ü Tunable acidities, redox potentials, and solubility ü Many POMs are known as effective homogeneous oxidation catalysts POM-based MOFs: Du et al. Chem. Soc. Rev. 2014, 43, 4615 Examples of catalytically active POMs immobilized within MIL-101 [PW 12 O 40]3 - {PO 4[W(O)(O 2)2]4}3 - epoxidation with Н 2 О 2 Maksimchuk et al. Inorg. Chem. 49 (2010) 2920 [PW 11 M(L)O 39]n-, M = Ti, Co oxidations with Н 2 О 2 and О 2 [PMo 12 -n. Vn. O 40](3+n)- oxidations with О 2 Isr. J. Chem. 2 (2011) 281 J. Catal. 275 (2008) 315 29
MIL-101 as support for polyoxometalates G. Férey et al. , Science, 309 (2005) 2040: increase in thermal stability up to 350 o C Advantages of polyoxometalates (POMs): ü Inorganic nature and metal oxide-like structure ü Thermodynamic stability to oxidation ü Variety of structures and compositions ü Tunable acidities, redox potentials, and solubility ü Many POMs are known as effective homogeneous oxidation catalysts POM-based MOFs: Du et al. Chem. Soc. Rev. 2014, 43, 4615 Examples of catalytically active POMs immobilized within MIL-101 [PW 12 O 40]3 - {PO 4[W(O)(O 2)2]4}3 - epoxidation with Н 2 О 2 Maksimchuk et al. Inorg. Chem. 49 (2010) 2920 [PW 11 M(L)O 39]n-, M = Ti, Co oxidations with Н 2 О 2 and О 2 [PMo 12 -n. Vn. O 40](3+n)- oxidations with О 2 Isr. J. Chem. 2 (2011) 281 J. Catal. 275 (2008) 315 29
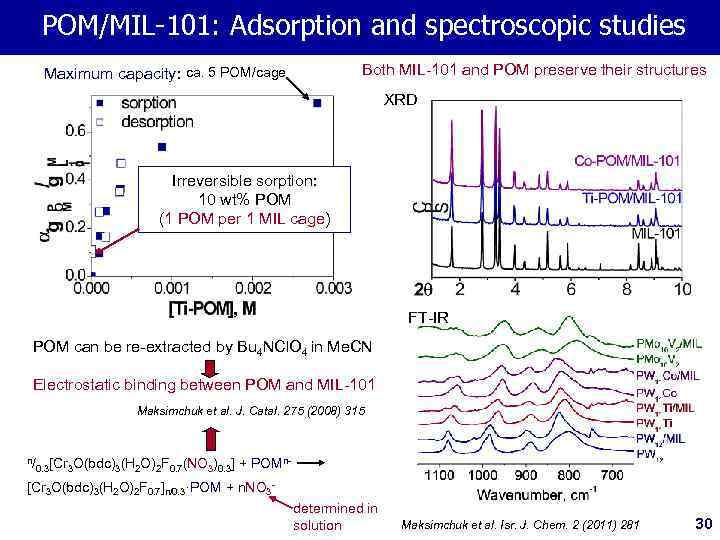 POM/MIL-101: Adsorption and spectroscopic studies Both MIL-101 and POM preserve their structures Maximum capacity: ca. 5 POM/cage XRD Irreversible sorption: 10 wt% POM (1 POM per 1 MIL cage) FT-IR POM can be re-extracted by Bu 4 NCl. O 4 in Me. CN Electrostatic binding between POM and MIL-101 Maksimchuk et al. J. Catal. 275 (2008) 315 n/ 0. 3[Cr 3 O(bdc)3(H 2 O)2 F 0. 7(NO 3)0. 3] + POMn- [Cr 3 O(bdc)3(H 2 O)2 F 0. 7]n/0. 3·POM + n. NO 3 determined in solution Maksimchuk et al. Isr. J. Chem. 2 (2011) 281 30
POM/MIL-101: Adsorption and spectroscopic studies Both MIL-101 and POM preserve their structures Maximum capacity: ca. 5 POM/cage XRD Irreversible sorption: 10 wt% POM (1 POM per 1 MIL cage) FT-IR POM can be re-extracted by Bu 4 NCl. O 4 in Me. CN Electrostatic binding between POM and MIL-101 Maksimchuk et al. J. Catal. 275 (2008) 315 n/ 0. 3[Cr 3 O(bdc)3(H 2 O)2 F 0. 7(NO 3)0. 3] + POMn- [Cr 3 O(bdc)3(H 2 O)2 F 0. 7]n/0. 3·POM + n. NO 3 determined in solution Maksimchuk et al. Isr. J. Chem. 2 (2011) 281 30
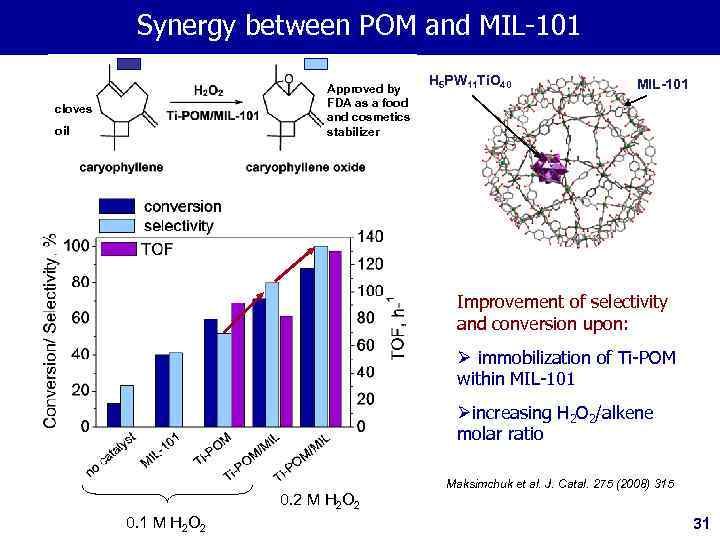 Synergy between PОМ and MIL-101 Approved by FDA as a food and cosmetics stabilizer cloves oil H 5 PW 11 Ti. O 40 MIL-101 Improvement of selectivity and conversion upon: Ø immobilization of Ti-POM within MIL-101 Øincreasing H 2 O 2/alkene molar ratio Maksimchuk et al. J. Catal. 275 (2008) 315 0. 2 M H 2 O 2 0. 1 M H 2 O 2 31
Synergy between PОМ and MIL-101 Approved by FDA as a food and cosmetics stabilizer cloves oil H 5 PW 11 Ti. O 40 MIL-101 Improvement of selectivity and conversion upon: Ø immobilization of Ti-POM within MIL-101 Øincreasing H 2 O 2/alkene molar ratio Maksimchuk et al. J. Catal. 275 (2008) 315 0. 2 M H 2 O 2 0. 1 M H 2 O 2 31
![Alkene epoxidation with H 2 O 2 over PW 12/MIL-101 [PW 12 O 40]3 Alkene epoxidation with H 2 O 2 over PW 12/MIL-101 [PW 12 O 40]3](https://present5.com/presentation/-104014181_425111232/image-35.jpg) Alkene epoxidation with H 2 O 2 over PW 12/MIL-101 [PW 12 O 40]3 - PW 12/MIL H 2 O 2, M Increasing selectivity and conversion with increasing H 2 O 2/alkene molar ratio 0. 1 M alkene, 0. 2 M Н 2 О 2, 5. 8· 10 -3 M of W, Me. CN, 50 о. С, 3 h Maksimchuk et al. Inorg. Chem. 49 (2010) 2920 Immobilization increases stability of POM TON: 770 PW 12/MIL vs 155 PW 12 32
Alkene epoxidation with H 2 O 2 over PW 12/MIL-101 [PW 12 O 40]3 - PW 12/MIL H 2 O 2, M Increasing selectivity and conversion with increasing H 2 O 2/alkene molar ratio 0. 1 M alkene, 0. 2 M Н 2 О 2, 5. 8· 10 -3 M of W, Me. CN, 50 о. С, 3 h Maksimchuk et al. Inorg. Chem. 49 (2010) 2920 Immobilization increases stability of POM TON: 770 PW 12/MIL vs 155 PW 12 32
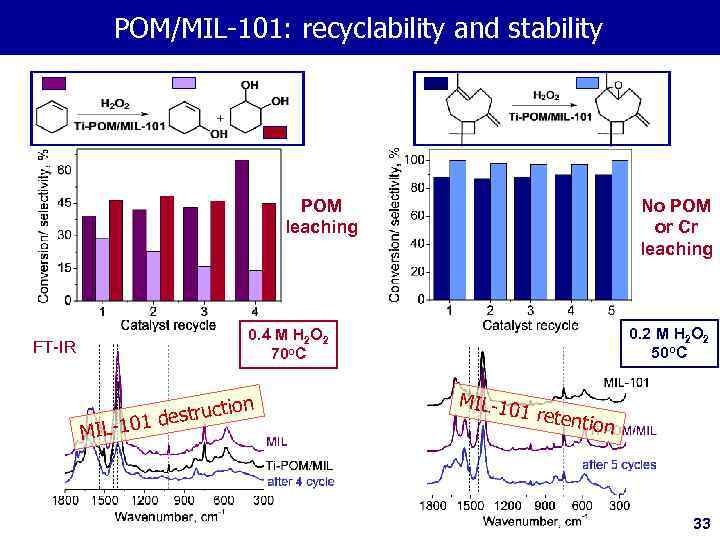 PОМ/MIL-101: recyclability and stability IR-spectra No POM or Cr leaching POM leaching FT-IR 0. 2 М Н 2 О 2 50 o. C 0. 4 М Н 2 О 2 70 o. C n structio 1 de MIL-10 1 retentio n 33
PОМ/MIL-101: recyclability and stability IR-spectra No POM or Cr leaching POM leaching FT-IR 0. 2 М Н 2 О 2 50 o. C 0. 4 М Н 2 О 2 70 o. C n structio 1 de MIL-10 1 retentio n 33
 3. MOFs as precursors of catalysts Oxidation of alkylphenols to benzoquinones with H 2 O 2 over Ti-MIL-125 In collaboration with group of Prof. J. -S. Chang (KRIST)
3. MOFs as precursors of catalysts Oxidation of alkylphenols to benzoquinones with H 2 O 2 over Ti-MIL-125 In collaboration with group of Prof. J. -S. Chang (KRIST)
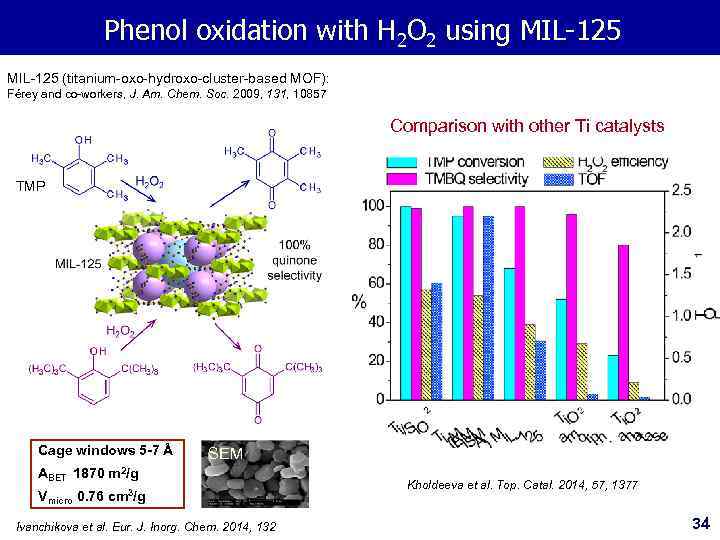 Phenol oxidation with H 2 O 2 using MIL-125 (titanium-oxo-hydroxo-cluster-based MOF): Férey and co-workers, J. Am. Chem. Soc. 2009, 131, 10857 Comparison with other Ti catalysts TMP Cage windows 5 -7 Å SEM ABET 1870 m 2/g Vmicro 0. 76 cm 3/g Ivanchikova et al. Eur. J. Inorg. Chem. 2014, 132 Kholdeeva et al. Top. Catal. 2014, 57, 1377 34
Phenol oxidation with H 2 O 2 using MIL-125 (titanium-oxo-hydroxo-cluster-based MOF): Férey and co-workers, J. Am. Chem. Soc. 2009, 131, 10857 Comparison with other Ti catalysts TMP Cage windows 5 -7 Å SEM ABET 1870 m 2/g Vmicro 0. 76 cm 3/g Ivanchikova et al. Eur. J. Inorg. Chem. 2014, 132 Kholdeeva et al. Top. Catal. 2014, 57, 1377 34
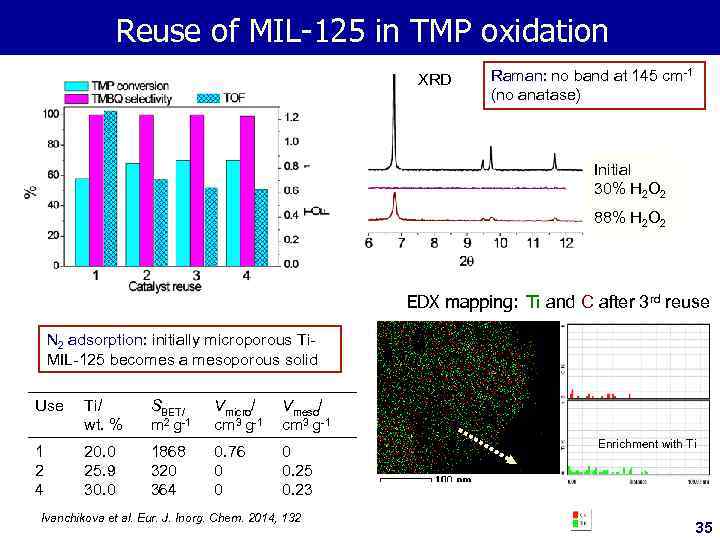 Reuse of MIL-125 in TMP oxidation XRD Raman: no band at 145 cm-1 (no anatase) Initial 30% H 2 O 2 88% H 2 O 2 EDX mapping: Ti and C after 3 rd reuse N 2 adsorption: initially microporous Ti. MIL-125 becomes a mesoporous solid Use Ti/ wt. % SBET/ m 2 g-1 Vmicro/ cm 3 g-1 Vmeso/ cm 3 g-1 1 2 4 20. 0 25. 9 30. 0 1868 320 364 0. 76 0 0. 25 0. 23 Ivanchikova et al. Eur. J. Inorg. Chem. 2014, 132 Enrichment with Ti 35
Reuse of MIL-125 in TMP oxidation XRD Raman: no band at 145 cm-1 (no anatase) Initial 30% H 2 O 2 88% H 2 O 2 EDX mapping: Ti and C after 3 rd reuse N 2 adsorption: initially microporous Ti. MIL-125 becomes a mesoporous solid Use Ti/ wt. % SBET/ m 2 g-1 Vmicro/ cm 3 g-1 Vmeso/ cm 3 g-1 1 2 4 20. 0 25. 9 30. 0 1868 320 364 0. 76 0 0. 25 0. 23 Ivanchikova et al. Eur. J. Inorg. Chem. 2014, 132 Enrichment with Ti 35
 Summary Cr- and Fe-MIL-101(100) can act as active and selective catalysts for liquidphase oxidations with environmentally benign oxidants – O 2 and TBHP. The nature of metal (Cr or Fe) has a strong impact on the product selectivity. Fe-MIL-101(100) reveal biomimetic behavior in aerobic alkene oxidation. Drastic change of selectivity into autoxidation indicates MOF destruction. Stability under turnover conditions follows similar trend as thermal stability: Cr. MIL-101, Cr-MIL-100 > Fe-MIL-101. Within stability limits, product adsorption is the main reason of MIL-101/100 deactivation. Stable recycling performance is possible after MOF regeneration by washing and evacuation. Cr-MIL-101 combines high activity, selectivity and stability, which enables product yields and productivities superior to other SSHC (Cr-APO, Cr-Si, Cr. PMO). Combination of Cr-MIL-101 and POM enables use of H 2 O 2 as oxidant and leads to improvement of both catalytic performance and stability. Unstable MOF (MIL-125) can serve as precursors for active, selective and recyclable catalysts. 36
Summary Cr- and Fe-MIL-101(100) can act as active and selective catalysts for liquidphase oxidations with environmentally benign oxidants – O 2 and TBHP. The nature of metal (Cr or Fe) has a strong impact on the product selectivity. Fe-MIL-101(100) reveal biomimetic behavior in aerobic alkene oxidation. Drastic change of selectivity into autoxidation indicates MOF destruction. Stability under turnover conditions follows similar trend as thermal stability: Cr. MIL-101, Cr-MIL-100 > Fe-MIL-101. Within stability limits, product adsorption is the main reason of MIL-101/100 deactivation. Stable recycling performance is possible after MOF regeneration by washing and evacuation. Cr-MIL-101 combines high activity, selectivity and stability, which enables product yields and productivities superior to other SSHC (Cr-APO, Cr-Si, Cr. PMO). Combination of Cr-MIL-101 and POM enables use of H 2 O 2 as oxidant and leads to improvement of both catalytic performance and stability. Unstable MOF (MIL-125) can serve as precursors for active, selective and recyclable catalysts. 36
 Outlook The main area of application of MOFs as oxidation catalysts: Synthesis of fine and specialty chemicals high-added-value oxygenated products liquid-phase mild conditions Challenging MOFs are those that combine Ø metals capable of activation of O 2 and H 2 O 2 (heterolytically) Ø mesoporosity Ø hydrophobicity Ø chirality (for enantioselective reactions) Ø thermal, hydrothermal and chemical stability Ø mechanical stability Ø low cost (relatively to the cost of products) 37
Outlook The main area of application of MOFs as oxidation catalysts: Synthesis of fine and specialty chemicals high-added-value oxygenated products liquid-phase mild conditions Challenging MOFs are those that combine Ø metals capable of activation of O 2 and H 2 O 2 (heterolytically) Ø mesoporosity Ø hydrophobicity Ø chirality (for enantioselective reactions) Ø thermal, hydrothermal and chemical stability Ø mechanical stability Ø low cost (relatively to the cost of products) 37
 Acknowledgements Co-workers from BIC Catalytic studies Dr. Maxim Melgunov (N 2 adsorption) Dr. Alexander Shmakov (XRD) Dr. Yurii Chesalov (IR and Raman) Dr. Vladimir Zaikovskii (TEM) Dr. Vasiliy Kaichev (XPS) Mr. Viktor Utkin (GC-MS) Dr. Natalia Maksimchuk Dr. Irina Ivanchikova Collaborators from NIIC Prof. Vladimir P. Fedin Dr. Danyl Dybtsev Dr. Konstantin Kovalenko (syntheses of MIL-101/100) KRIST Prof. Jong-San Chang Ji Sun Lee (synthesis of MIL-125) Dr. Igor Skobelev Dr. Olga Zalomaeva IRCELYON Dr. Alexander Sorokin, (Fe. Pc. S) Financial support: Russian Foundation for Basic Research (grant No. 13 -03 -00413) 38
Acknowledgements Co-workers from BIC Catalytic studies Dr. Maxim Melgunov (N 2 adsorption) Dr. Alexander Shmakov (XRD) Dr. Yurii Chesalov (IR and Raman) Dr. Vladimir Zaikovskii (TEM) Dr. Vasiliy Kaichev (XPS) Mr. Viktor Utkin (GC-MS) Dr. Natalia Maksimchuk Dr. Irina Ivanchikova Collaborators from NIIC Prof. Vladimir P. Fedin Dr. Danyl Dybtsev Dr. Konstantin Kovalenko (syntheses of MIL-101/100) KRIST Prof. Jong-San Chang Ji Sun Lee (synthesis of MIL-125) Dr. Igor Skobelev Dr. Olga Zalomaeva IRCELYON Dr. Alexander Sorokin, (Fe. Pc. S) Financial support: Russian Foundation for Basic Research (grant No. 13 -03 -00413) 38


