0b1aea3063700c81419b79daaeed8c6a.ppt
- Количество слайдов: 55
 Metal Finishing (Electroplating) P 2 Workshop Part 1 Ohio EPA Division of Hazardous Waste Management Office of Pollution Prevention
Metal Finishing (Electroplating) P 2 Workshop Part 1 Ohio EPA Division of Hazardous Waste Management Office of Pollution Prevention
 What’s in it for Me? • Understanding of Metal Finishing Unit Processes • Skills for Identifying Process Optimization (P 2!) Opportunities • Knowledge of Tools and Resources for Providing Technical Assistance
What’s in it for Me? • Understanding of Metal Finishing Unit Processes • Skills for Identifying Process Optimization (P 2!) Opportunities • Knowledge of Tools and Resources for Providing Technical Assistance
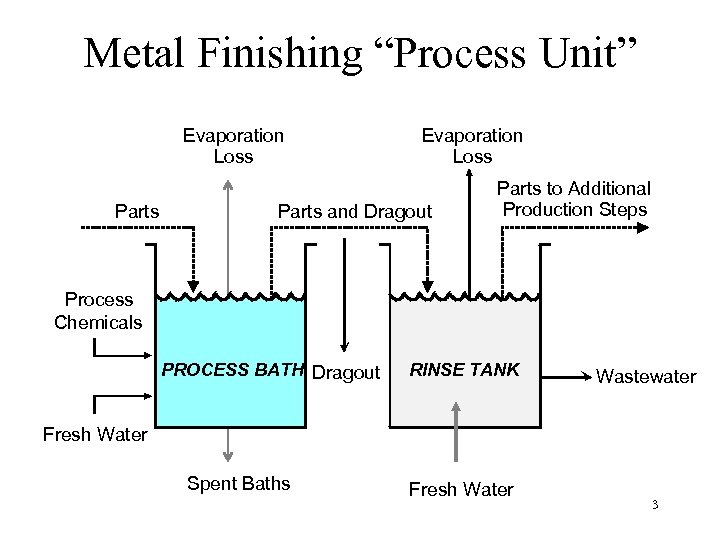 Metal Finishing “Process Unit” Evaporation Loss Parts and Dragout Parts to Additional Production Steps Process Chemicals PROCESS BATH Dragout RINSE TANK Wastewater Fresh Water Spent Baths Fresh Water 3
Metal Finishing “Process Unit” Evaporation Loss Parts and Dragout Parts to Additional Production Steps Process Chemicals PROCESS BATH Dragout RINSE TANK Wastewater Fresh Water Spent Baths Fresh Water 3
 Metal Finishing Processes 1. Surface Preparation and Cleaning: Ø alkaline cleaning Ø electropolishing Ø oxide removal 2. Metal Plating: Ø electroplating Ø electroless plating 3. Protection and Finishing Treatments: Ø anodizing Ø chromate conversion Ø phosphating
Metal Finishing Processes 1. Surface Preparation and Cleaning: Ø alkaline cleaning Ø electropolishing Ø oxide removal 2. Metal Plating: Ø electroplating Ø electroless plating 3. Protection and Finishing Treatments: Ø anodizing Ø chromate conversion Ø phosphating
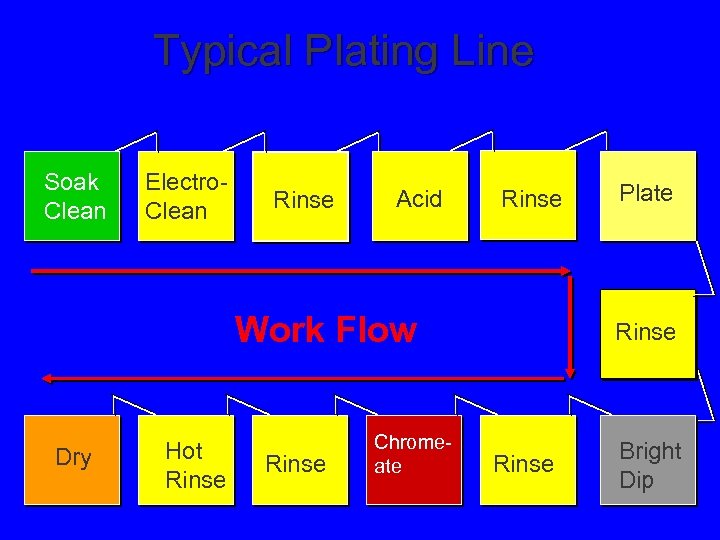 Typical Plating Line Soak Clean Electro. Clean Rinse Acid Rinse Work Flow Dry Hot Rinse Chromeate Plate Rinse Bright Dip
Typical Plating Line Soak Clean Electro. Clean Rinse Acid Rinse Work Flow Dry Hot Rinse Chromeate Plate Rinse Bright Dip
 Common Metal Finishing Wastes • • Rinse water effluent Spent plating baths Spent alkaline and acidic etchants and cleaners Spent strippers Solvent degreasers Waste and process bath treatment sludges Miscellaneous wastes (filters, empty containers, floor grates, off-spec chemicals) * Some of these may be Persistent Bioaccumulative Toxic substances such as Cadmium, Chromium, Copper, Lead, Nickel, Zinc & Cyanide
Common Metal Finishing Wastes • • Rinse water effluent Spent plating baths Spent alkaline and acidic etchants and cleaners Spent strippers Solvent degreasers Waste and process bath treatment sludges Miscellaneous wastes (filters, empty containers, floor grates, off-spec chemicals) * Some of these may be Persistent Bioaccumulative Toxic substances such as Cadmium, Chromium, Copper, Lead, Nickel, Zinc & Cyanide
 P 2 Principles for Metal Finishing ü Use the least toxic/easiest to manage chemicals ü Extract the most life (use) out of process chemistries ü Keep process chemistry solutions where they belong: in the tanks ü Return as much escaping solution (dragout) as possible to the tanks ü Use the least amount of rinse water required for good rinsing 7
P 2 Principles for Metal Finishing ü Use the least toxic/easiest to manage chemicals ü Extract the most life (use) out of process chemistries ü Keep process chemistry solutions where they belong: in the tanks ü Return as much escaping solution (dragout) as possible to the tanks ü Use the least amount of rinse water required for good rinsing 7
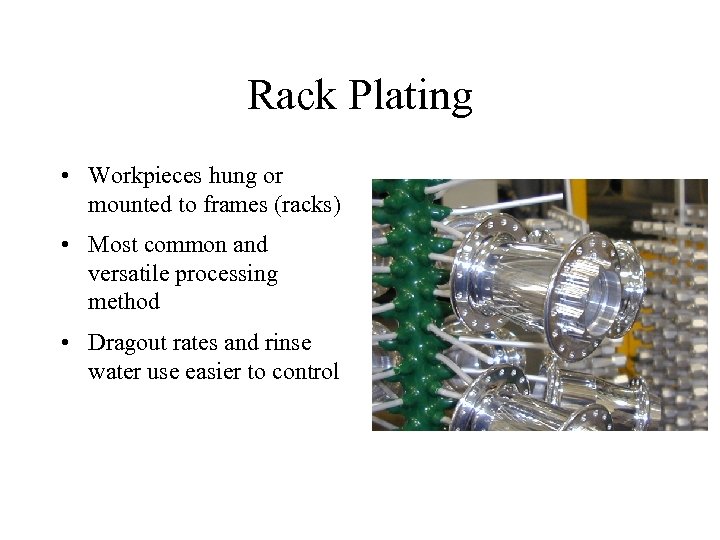 Rack Plating • Workpieces hung or mounted to frames (racks) • Most common and versatile processing method • Dragout rates and rinse water use easier to control
Rack Plating • Workpieces hung or mounted to frames (racks) • Most common and versatile processing method • Dragout rates and rinse water use easier to control
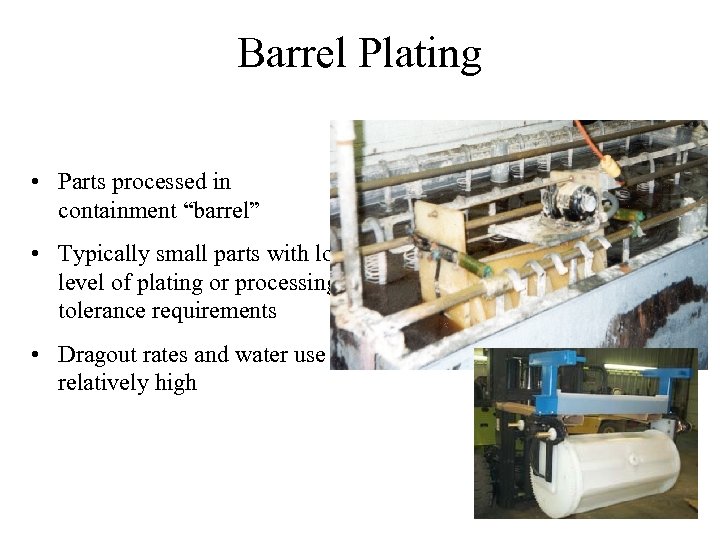 Barrel Plating • Parts processed in containment “barrel” • Typically small parts with low level of plating or processing tolerance requirements • Dragout rates and water use relatively high
Barrel Plating • Parts processed in containment “barrel” • Typically small parts with low level of plating or processing tolerance requirements • Dragout rates and water use relatively high
 Manual Plating • Process steps performed by hand • Smaller size parts, lower production
Manual Plating • Process steps performed by hand • Smaller size parts, lower production
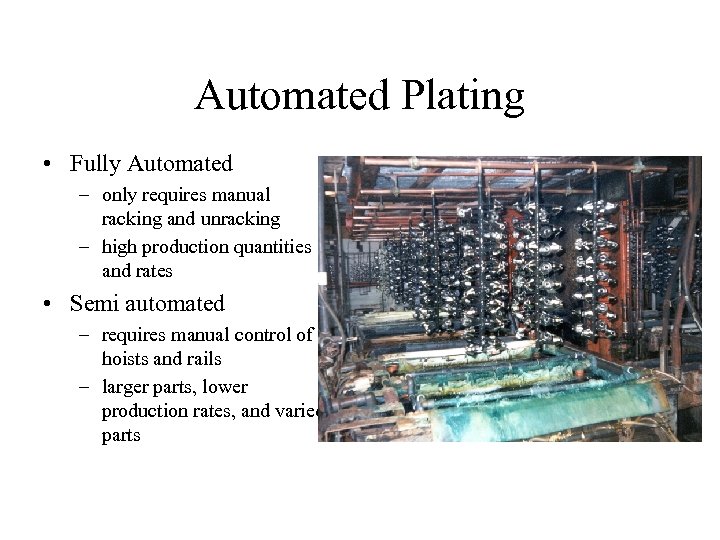 Automated Plating • Fully Automated – only requires manual racking and unracking – high production quantities and rates • Semi automated – requires manual control of hoists and rails – larger parts, lower production rates, and varied parts
Automated Plating • Fully Automated – only requires manual racking and unracking – high production quantities and rates • Semi automated – requires manual control of hoists and rails – larger parts, lower production rates, and varied parts
 Keys to Identifying P 2 Opportunities at Metal Finishing Facilities • Basics of Metal Finishing – does the facility monitor and measure their plating or coating solutions, how are additions made – does the facility have specifications for each part they finish, mil thickness, quality standards? – does the facility have procedures and training in place for coating time, withdrawal rate, drain time, rinsing, part orientation? – does the facility know addition and flow rates for water in their cleaning and plating solutions and rinses?
Keys to Identifying P 2 Opportunities at Metal Finishing Facilities • Basics of Metal Finishing – does the facility monitor and measure their plating or coating solutions, how are additions made – does the facility have specifications for each part they finish, mil thickness, quality standards? – does the facility have procedures and training in place for coating time, withdrawal rate, drain time, rinsing, part orientation? – does the facility know addition and flow rates for water in their cleaning and plating solutions and rinses?
 Measuring and Understanding Dragout 13
Measuring and Understanding Dragout 13
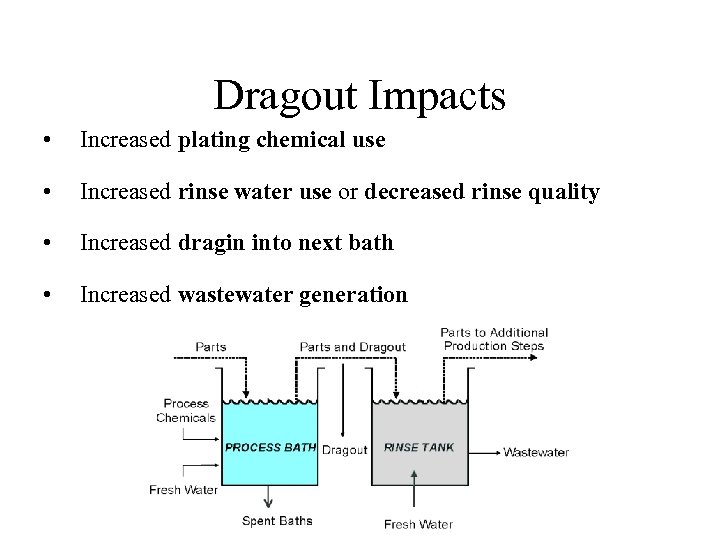 Dragout Impacts • Increased plating chemical use • Increased rinse water use or decreased rinse quality • Increased dragin into next bath • Increased wastewater generation
Dragout Impacts • Increased plating chemical use • Increased rinse water use or decreased rinse quality • Increased dragin into next bath • Increased wastewater generation
 Dragout Impacts (continued) • Increased WWTS treatment chemicals • Increased WWTS filter cake • Increased WWTS effluent metal concentration
Dragout Impacts (continued) • Increased WWTS treatment chemicals • Increased WWTS filter cake • Increased WWTS effluent metal concentration
 Dragout Measurement • Direct volume measurement (dragout volume drained from parts) • Metal concentration/conductivity in rinse tanks • Wastewater contaminant concentration 16
Dragout Measurement • Direct volume measurement (dragout volume drained from parts) • Metal concentration/conductivity in rinse tanks • Wastewater contaminant concentration 16
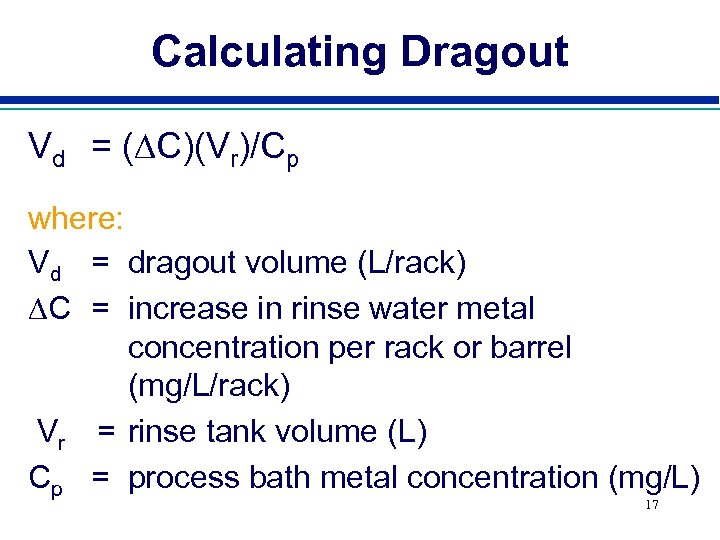 Calculating Dragout Vd = (DC)(Vr)/Cp where: Vd = dragout volume (L/rack) DC = increase in rinse water metal concentration per rack or barrel (mg/L/rack) Vr = rinse tank volume (L) Cp = process bath metal concentration (mg/L) 17
Calculating Dragout Vd = (DC)(Vr)/Cp where: Vd = dragout volume (L/rack) DC = increase in rinse water metal concentration per rack or barrel (mg/L/rack) Vr = rinse tank volume (L) Cp = process bath metal concentration (mg/L) 17
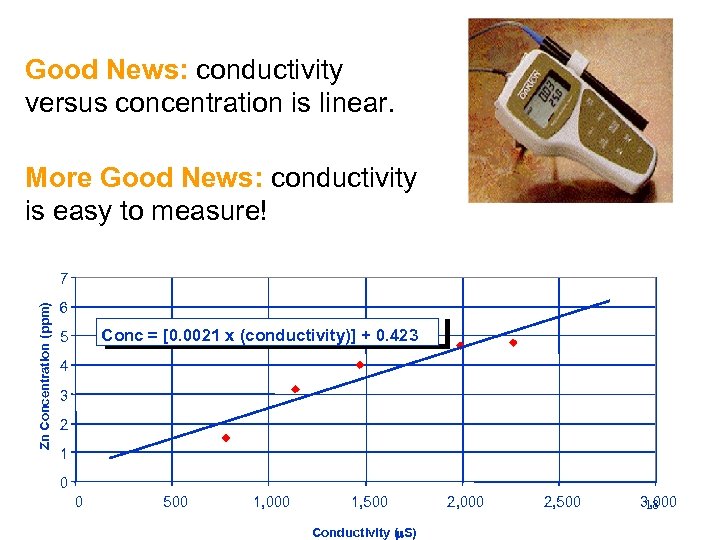 Good News: conductivity versus concentration is linear. More Good News: conductivity is easy to measure! Zn Concentration (ppm) 7 6 Conc = [0. 0021 x (conductivity)] + 0. 423 5 4 3 2 1 0 0 500 1, 000 1, 500 Conductivity (m. S) 2, 000 2, 500 3, 000 18
Good News: conductivity versus concentration is linear. More Good News: conductivity is easy to measure! Zn Concentration (ppm) 7 6 Conc = [0. 0021 x (conductivity)] + 0. 423 5 4 3 2 1 0 0 500 1, 000 1, 500 Conductivity (m. S) 2, 000 2, 500 3, 000 18
 Using Dragout Measurements • Estimate costs of dragout for particular parts • Make cost/benefit decisions – – – Lower dragout vs. slower withdrawal rates Lower dragout vs. longer hang time Worker training Incentive programs WWTS Recovery technologies • Benchmarking 19
Using Dragout Measurements • Estimate costs of dragout for particular parts • Make cost/benefit decisions – – – Lower dragout vs. slower withdrawal rates Lower dragout vs. longer hang time Worker training Incentive programs WWTS Recovery technologies • Benchmarking 19
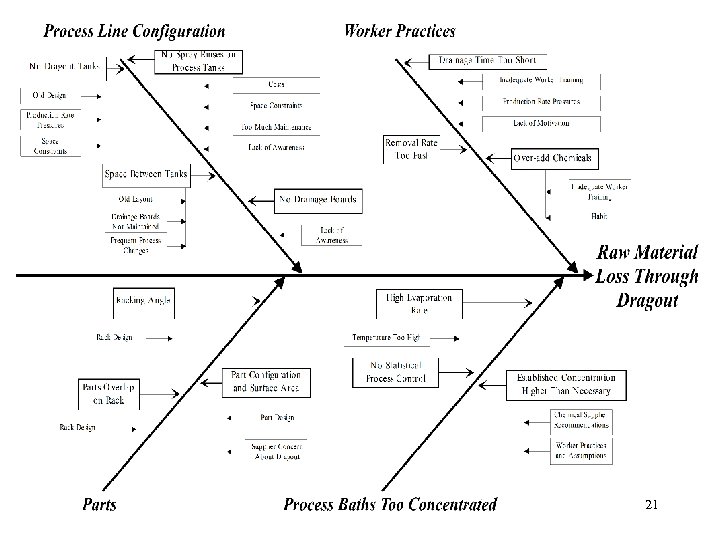
 Dragout Reduction Techniques 20
Dragout Reduction Techniques 20
 21
21
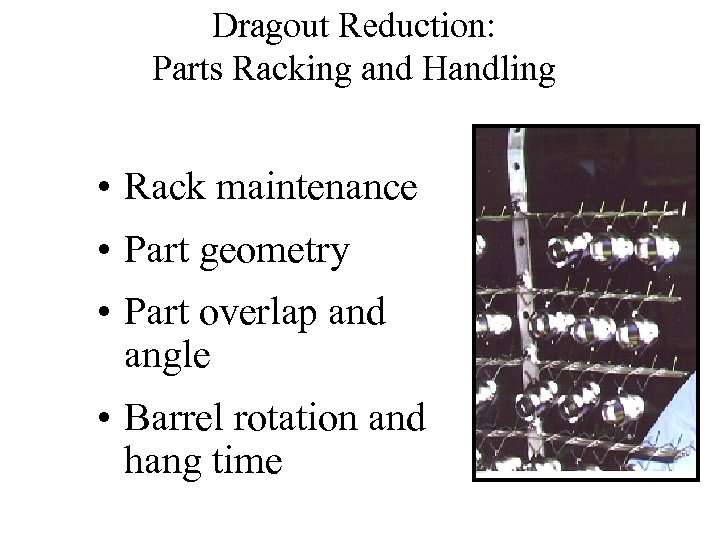 Dragout Reduction: Parts Racking and Handling • Rack maintenance • Part geometry • Part overlap and angle • Barrel rotation and hang time
Dragout Reduction: Parts Racking and Handling • Rack maintenance • Part geometry • Part overlap and angle • Barrel rotation and hang time
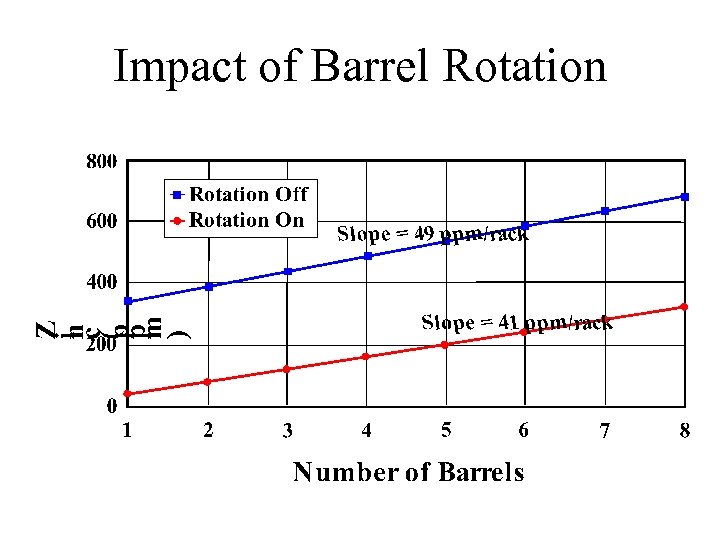 Impact of Barrel Rotation
Impact of Barrel Rotation
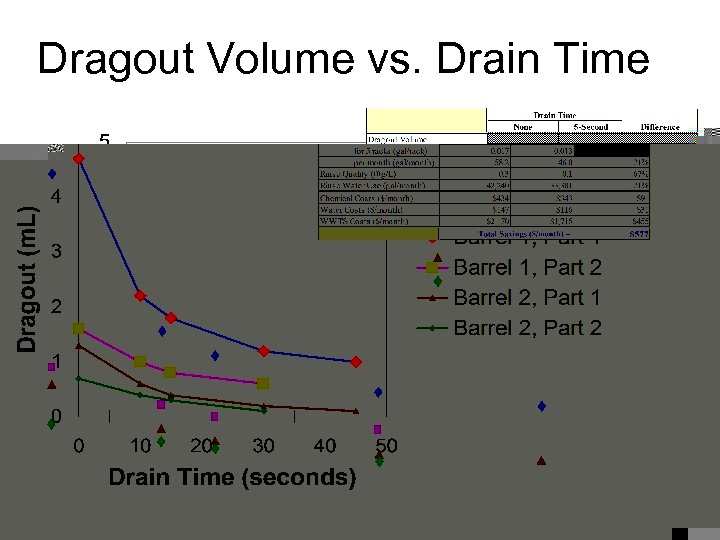 Dragout Volume vs. Drain Time
Dragout Volume vs. Drain Time
 Dragout Reduction: Worker Practices • Withdrawal rate • Drainage time • Tilting • Use hang bars and other devices
Dragout Reduction: Worker Practices • Withdrawal rate • Drainage time • Tilting • Use hang bars and other devices
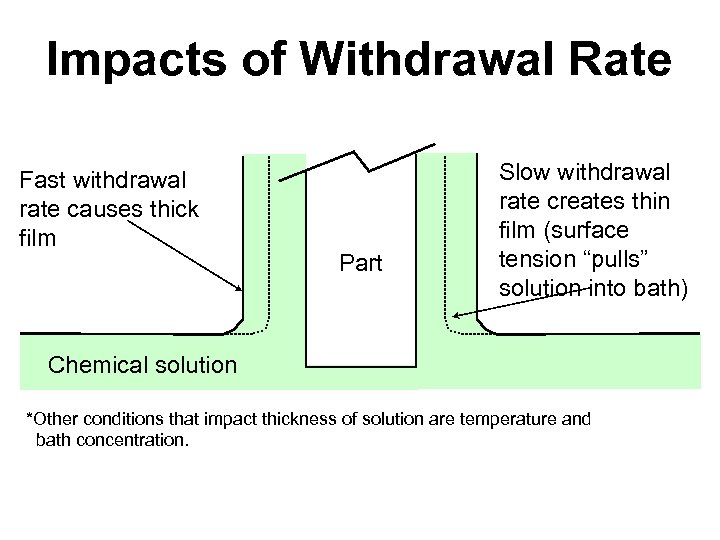 Impacts of Withdrawal Rate Fast withdrawal rate causes thick film Part Slow withdrawal rate creates thin film (surface tension “pulls” solution into bath) Chemical solution *Other conditions that impact thickness of solution are temperature and bath concentration.
Impacts of Withdrawal Rate Fast withdrawal rate causes thick film Part Slow withdrawal rate creates thin film (surface tension “pulls” solution into bath) Chemical solution *Other conditions that impact thickness of solution are temperature and bath concentration.
 Dragout Reduction: Process Layout, Maintenance • Tank spacing and drain boards • Tank sequence • Dragout tanks (with or without sprays) • Spray rinses
Dragout Reduction: Process Layout, Maintenance • Tank spacing and drain boards • Tank sequence • Dragout tanks (with or without sprays) • Spray rinses
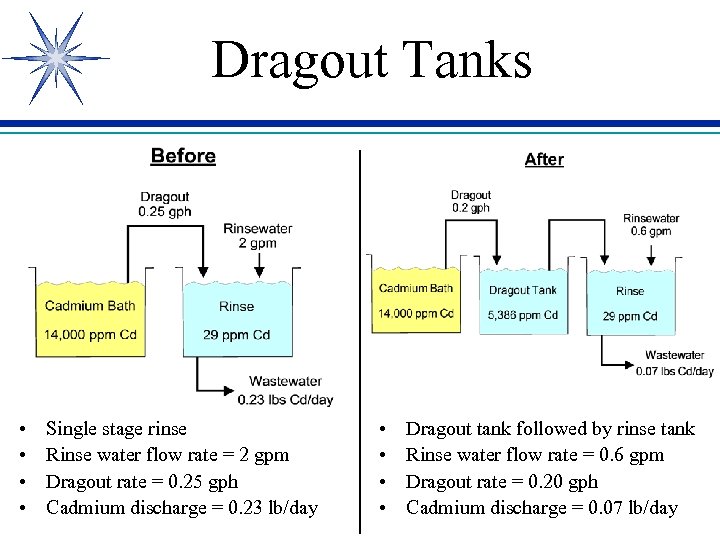 Dragout Tanks • • Single stage rinse Rinse water flow rate = 2 gpm Dragout rate = 0. 25 gph Cadmium discharge = 0. 23 lb/day • • Dragout tank followed by rinse tank Rinse water flow rate = 0. 6 gpm Dragout rate = 0. 20 gph Cadmium discharge = 0. 07 lb/day
Dragout Tanks • • Single stage rinse Rinse water flow rate = 2 gpm Dragout rate = 0. 25 gph Cadmium discharge = 0. 23 lb/day • • Dragout tank followed by rinse tank Rinse water flow rate = 0. 6 gpm Dragout rate = 0. 20 gph Cadmium discharge = 0. 07 lb/day
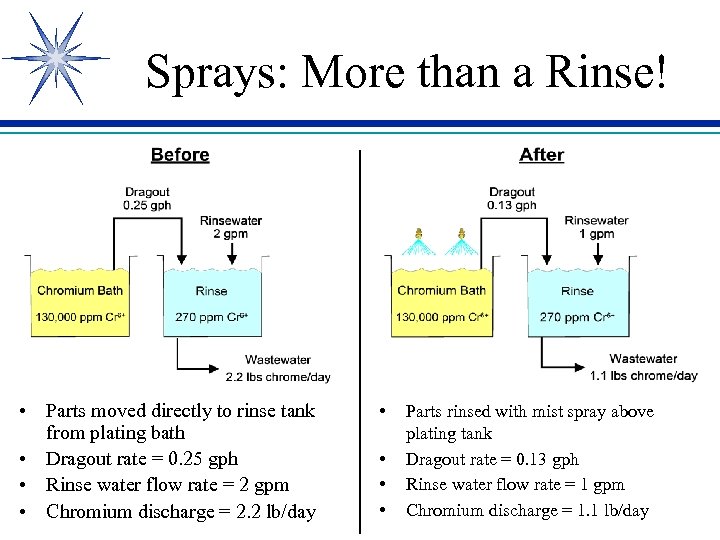 Sprays: More than a Rinse! • Parts moved directly to rinse tank from plating bath • Dragout rate = 0. 25 gph • Rinse water flow rate = 2 gpm • Chromium discharge = 2. 2 lb/day • • Parts rinsed with mist spray above plating tank Dragout rate = 0. 13 gph Rinse water flow rate = 1 gpm Chromium discharge = 1. 1 lb/day
Sprays: More than a Rinse! • Parts moved directly to rinse tank from plating bath • Dragout rate = 0. 25 gph • Rinse water flow rate = 2 gpm • Chromium discharge = 2. 2 lb/day • • Parts rinsed with mist spray above plating tank Dragout rate = 0. 13 gph Rinse water flow rate = 1 gpm Chromium discharge = 1. 1 lb/day
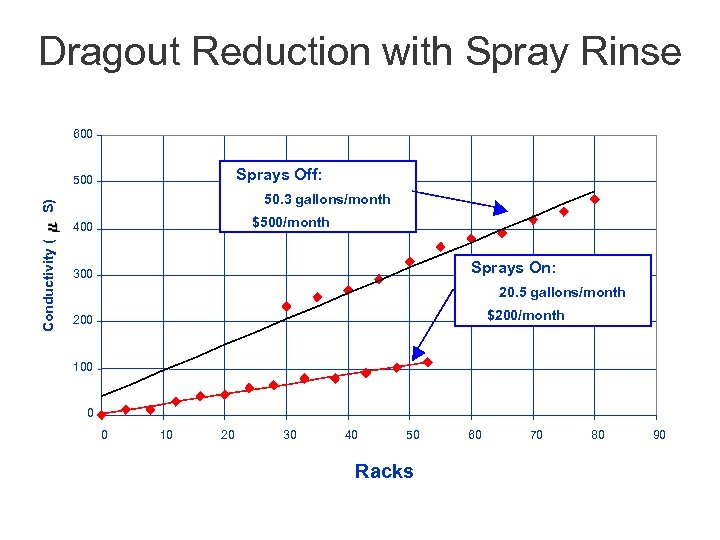 Dragout Reduction with Spray Rinse 600 Sprays Off: 500 S) 50. 3 gallons/month $500/month Conductivity ( 400 Sprays On: 300 20. 5 gallons/month $200/month 200 100 0 0 10 20 30 40 50 Racks 60 70 80 90
Dragout Reduction with Spray Rinse 600 Sprays Off: 500 S) 50. 3 gallons/month $500/month Conductivity ( 400 Sprays On: 300 20. 5 gallons/month $200/month 200 100 0 0 10 20 30 40 50 Racks 60 70 80 90
 Conductivity Control Systems 31
Conductivity Control Systems 31
 Keys to Identifying P 2 Opportunities at Metal Finishing Facilities • Conductivity Control – does the facility use conductivity measurement to manage their rinse system(s)? – if they have a system, what type is it, how do they operate it, what are their results? – if they do not have system do they know the relative costs and benefits of installing one?
Keys to Identifying P 2 Opportunities at Metal Finishing Facilities • Conductivity Control – does the facility use conductivity measurement to manage their rinse system(s)? – if they have a system, what type is it, how do they operate it, what are their results? – if they do not have system do they know the relative costs and benefits of installing one?
 Analyzer • The system “brain” • Receives input from sensor • Displays conductivity reading – Digital, analog, or none • Sends output signal to solenoid valve • Key features: – Programmable set point – Programmable deadband 33
Analyzer • The system “brain” • Receives input from sensor • Displays conductivity reading – Digital, analog, or none • Sends output signal to solenoid valve • Key features: – Programmable set point – Programmable deadband 33
 Conventional Sensor • The sensor has an anode and cathode which is placed in a solution • Electrical potential is measured between these electrodes • Prone to fouling • $150 -250 34
Conventional Sensor • The sensor has an anode and cathode which is placed in a solution • Electrical potential is measured between these electrodes • Prone to fouling • $150 -250 34
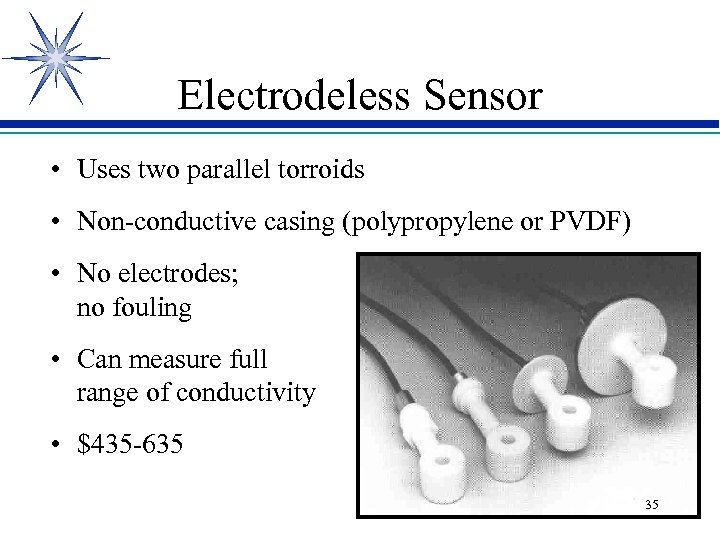 Electrodeless Sensor • Uses two parallel torroids • Non-conductive casing (polypropylene or PVDF) • No electrodes; no fouling • Can measure full range of conductivity • $435 -635 35
Electrodeless Sensor • Uses two parallel torroids • Non-conductive casing (polypropylene or PVDF) • No electrodes; no fouling • Can measure full range of conductivity • $435 -635 35
 Sensor Installation • Sensor placement – Halfway down from top of water level – Away from stagnant areas – Away from clean water inlet – In final stage of multistage counterflow rinse • Good circulation of rinse water – Mechanical mixing – Double dipping parts 36
Sensor Installation • Sensor placement – Halfway down from top of water level – Away from stagnant areas – Away from clean water inlet – In final stage of multistage counterflow rinse • Good circulation of rinse water – Mechanical mixing – Double dipping parts 36
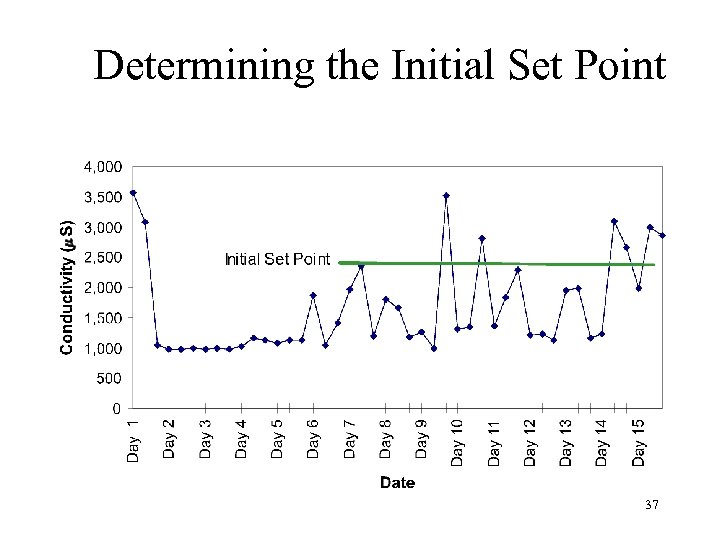 Determining the Initial Set Point 37
Determining the Initial Set Point 37
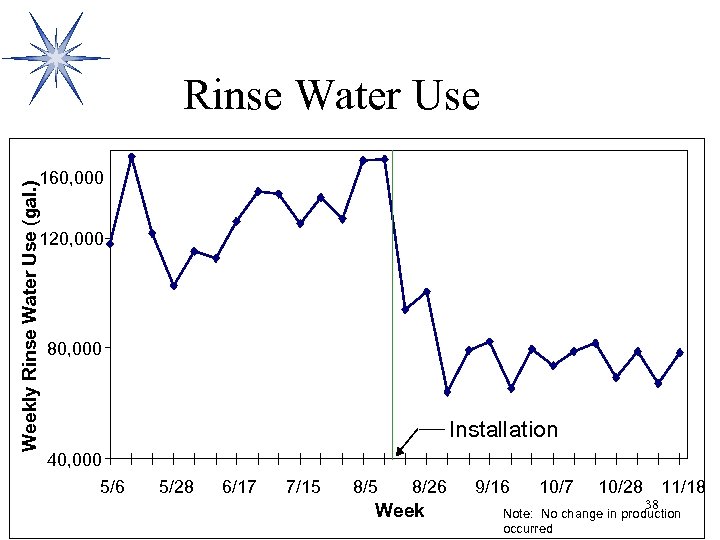 Rinse Water Use Weekly Rinse Water Use (gal. ) 160, 000 120, 000 80, 000 Installation 40, 000 5/6 5/28 6/17 7/15 8/26 Week 9/16 10/7 10/28 38 11/18 Note: No change in production occurred
Rinse Water Use Weekly Rinse Water Use (gal. ) 160, 000 120, 000 80, 000 Installation 40, 000 5/6 5/28 6/17 7/15 8/26 Week 9/16 10/7 10/28 38 11/18 Note: No change in production occurred
 Spray Rinse Systems and Design 39
Spray Rinse Systems and Design 39
 Benefits of Reducing Rinse Water • Lower water bills and sewer fees • Wastewater treatment impacts – Lower treatment chemical costs – Higher retention time – Less O&M requirements • Decreased sludge generation
Benefits of Reducing Rinse Water • Lower water bills and sewer fees • Wastewater treatment impacts – Lower treatment chemical costs – Higher retention time – Less O&M requirements • Decreased sludge generation
 Techniques that Improve Rinse Efficiency • Agitation – – Rack motion Forced air and/or forced water Sprays Double dipping • Flow Controls and Water Quality – Flow restrictors – Conductivity control systems – Tap water vs. deionized water
Techniques that Improve Rinse Efficiency • Agitation – – Rack motion Forced air and/or forced water Sprays Double dipping • Flow Controls and Water Quality – Flow restrictors – Conductivity control systems – Tap water vs. deionized water
 Techniques that Improve Rinse Efficiency • Tank Design – Size (not bigger than necessary) – Eliminate short-circuiting • Tank Layout – Multiple tanks – Countercurrent rinses are extremely efficient • 90% reduction compared to a single rinse • Most old shops can not accommodate the larger “footprint”
Techniques that Improve Rinse Efficiency • Tank Design – Size (not bigger than necessary) – Eliminate short-circuiting • Tank Layout – Multiple tanks – Countercurrent rinses are extremely efficient • 90% reduction compared to a single rinse • Most old shops can not accommodate the larger “footprint”
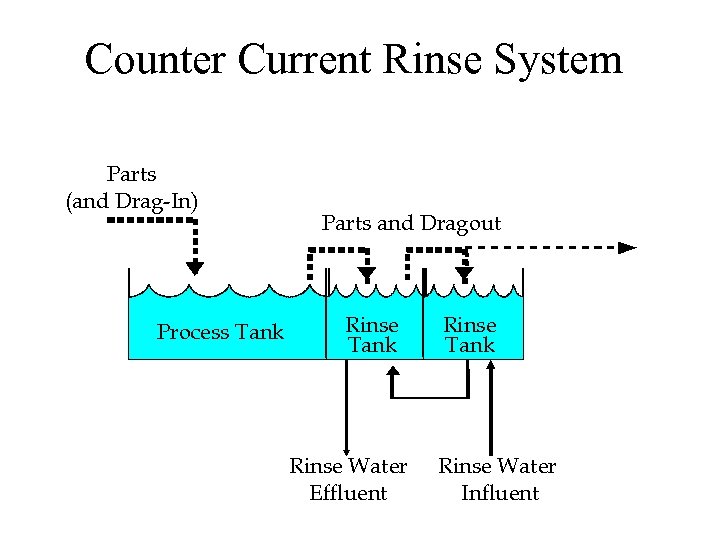 Counter Current Rinse System Parts (and Drag-In) Process Tank Parts and Dragout Rinse Tank Rinse Water Effluent Rinse Tank Rinse Water Influent
Counter Current Rinse System Parts (and Drag-In) Process Tank Parts and Dragout Rinse Tank Rinse Water Effluent Rinse Tank Rinse Water Influent
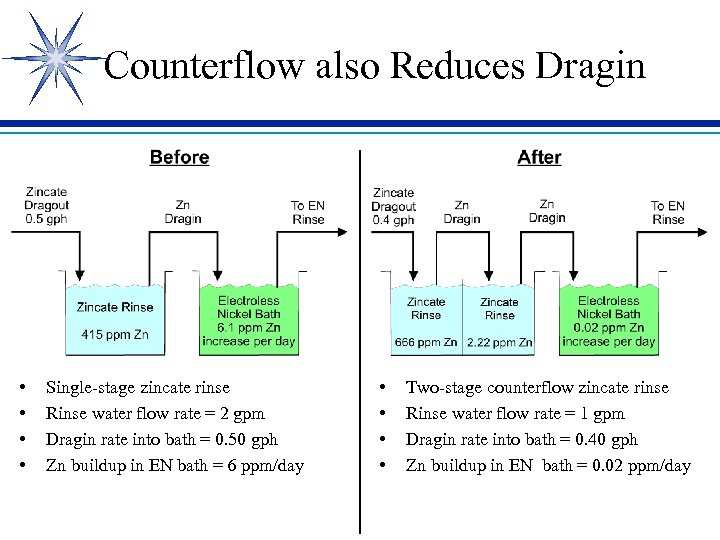 Counterflow also Reduces Dragin • • Single-stage zincate rinse Rinse water flow rate = 2 gpm Dragin rate into bath = 0. 50 gph Zn buildup in EN bath = 6 ppm/day • • Two-stage counterflow zincate rinse Rinse water flow rate = 1 gpm Dragin rate into bath = 0. 40 gph Zn buildup in EN bath = 0. 02 ppm/day
Counterflow also Reduces Dragin • • Single-stage zincate rinse Rinse water flow rate = 2 gpm Dragin rate into bath = 0. 50 gph Zn buildup in EN bath = 6 ppm/day • • Two-stage counterflow zincate rinse Rinse water flow rate = 1 gpm Dragin rate into bath = 0. 40 gph Zn buildup in EN bath = 0. 02 ppm/day
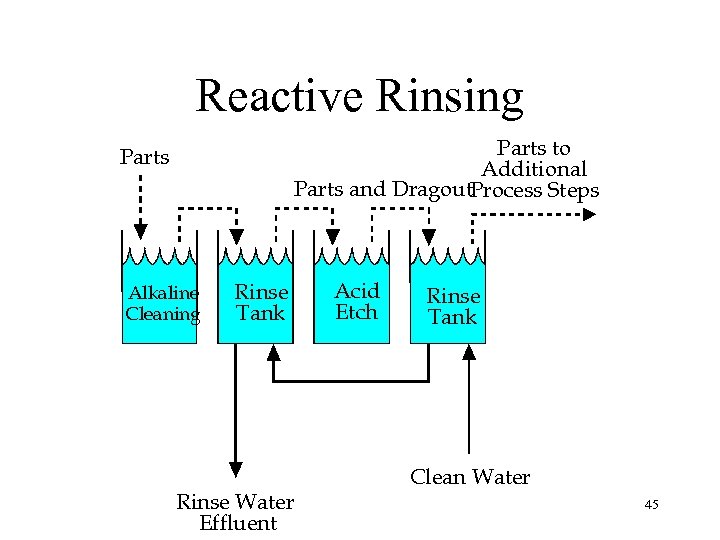 Reactive Rinsing Parts to Additional Parts and Dragout. Process Steps Parts Alkaline Cleaning Rinse Tank Rinse Water Effluent Acid Etch Rinse Tank Clean Water 45
Reactive Rinsing Parts to Additional Parts and Dragout. Process Steps Parts Alkaline Cleaning Rinse Tank Rinse Water Effluent Acid Etch Rinse Tank Clean Water 45
 Reactive Rinsing Example • 172 Acid Bath Dumps/Year – 200 gallon bath – $23 k/year for bath makeup • In-Shop Measurements: – Alkaline Rinse p. H: 12. 15 – Acid Rinse p. H: 1. 32 – Combination: 1. 77 • Estimated 50% Reduction in Acid Use/Dumps – Capital: – Payback: $1, 250 <1 month
Reactive Rinsing Example • 172 Acid Bath Dumps/Year – 200 gallon bath – $23 k/year for bath makeup • In-Shop Measurements: – Alkaline Rinse p. H: 12. 15 – Acid Rinse p. H: 1. 32 – Combination: 1. 77 • Estimated 50% Reduction in Acid Use/Dumps – Capital: – Payback: $1, 250 <1 month
 Spray Rinse Systems and Design 47
Spray Rinse Systems and Design 47
 Keys to Identifying P 2 Opportunities at Metal Finishing Facilities • Rinse Tank Optimization & Spray Rinsing – are any measures in place to extend the life of the rinse baths, skimmers, agitation, sludge removal, water treatment? – are spray rinses utilized, if so, where are they located, how are they operated and why? – are rinse tanks utilizing counter current flow, are there flow restrictors or controls? – is the quality of the rinse water monitored or measured? – has the facility experimented with different rinse configurations, flows, or sprays?
Keys to Identifying P 2 Opportunities at Metal Finishing Facilities • Rinse Tank Optimization & Spray Rinsing – are any measures in place to extend the life of the rinse baths, skimmers, agitation, sludge removal, water treatment? – are spray rinses utilized, if so, where are they located, how are they operated and why? – are rinse tanks utilizing counter current flow, are there flow restrictors or controls? – is the quality of the rinse water monitored or measured? – has the facility experimented with different rinse configurations, flows, or sprays?
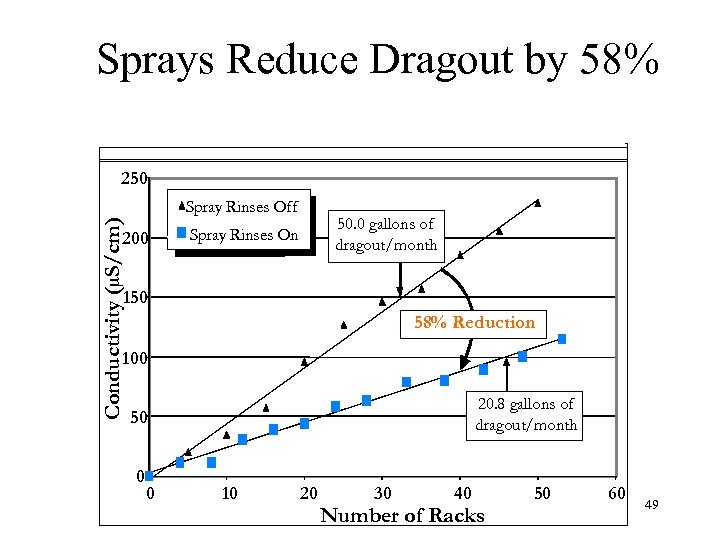 Sprays Reduce Dragout by 58% 250 Conductivity (µS/cm) Spray Rinses Off 200 50. 0 gallons of dragout/month Spray Rinses On 150 58% Reduction 100 20. 8 gallons of dragout/month 50 0 0 10 20 30 40 Number of Racks 50 60 49
Sprays Reduce Dragout by 58% 250 Conductivity (µS/cm) Spray Rinses Off 200 50. 0 gallons of dragout/month Spray Rinses On 150 58% Reduction 100 20. 8 gallons of dragout/month 50 0 0 10 20 30 40 Number of Racks 50 60 49
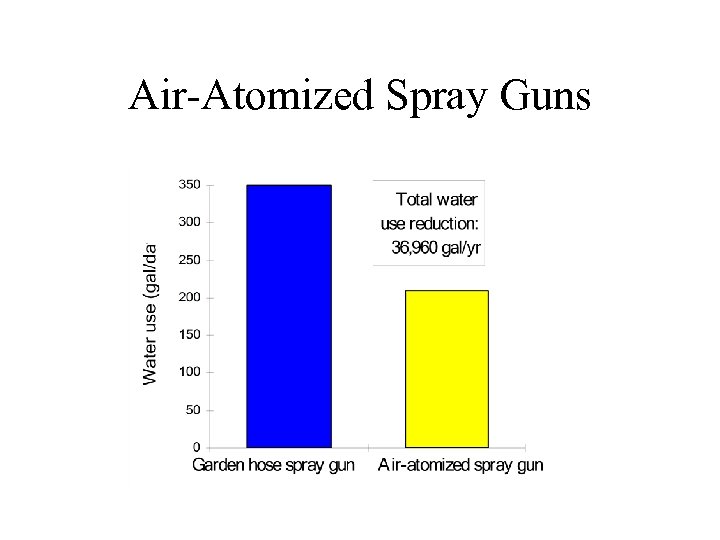 Air-Atomized Spray Guns
Air-Atomized Spray Guns
 Air-Atomized Spray Guns
Air-Atomized Spray Guns
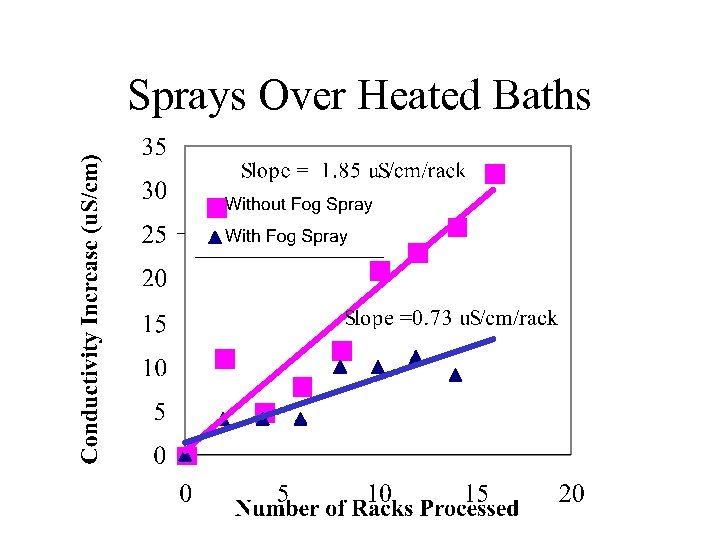 Sprays Over Heated Baths
Sprays Over Heated Baths
 Sprays Over Heated Baths
Sprays Over Heated Baths
 Tools and Resources for Assistance 54
Tools and Resources for Assistance 54
 Available Tools • Metal Finishing P 2 Videos 1. DHWM Preserving the Legacy Series: “The Metal Plating and Finishing Industries” 2. USEPA “Pollution Prevention for Metal Platers: Drag-out Reduction” • Advanced P 2 Technical Assistance - TECHSOLVE P 2 IRIS “Metal Finishing” http: //www. techsolve. org/iamsorg/p 2 iris/metalfinish/default. html U. S. EPA/NAMF/AESF - Strategic Goals Program http: //www. strategicgoals. org/
Available Tools • Metal Finishing P 2 Videos 1. DHWM Preserving the Legacy Series: “The Metal Plating and Finishing Industries” 2. USEPA “Pollution Prevention for Metal Platers: Drag-out Reduction” • Advanced P 2 Technical Assistance - TECHSOLVE P 2 IRIS “Metal Finishing” http: //www. techsolve. org/iamsorg/p 2 iris/metalfinish/default. html U. S. EPA/NAMF/AESF - Strategic Goals Program http: //www. strategicgoals. org/


