6d286d0b344e0c7b629e71d35a1761ab.ppt
- Количество слайдов: 59
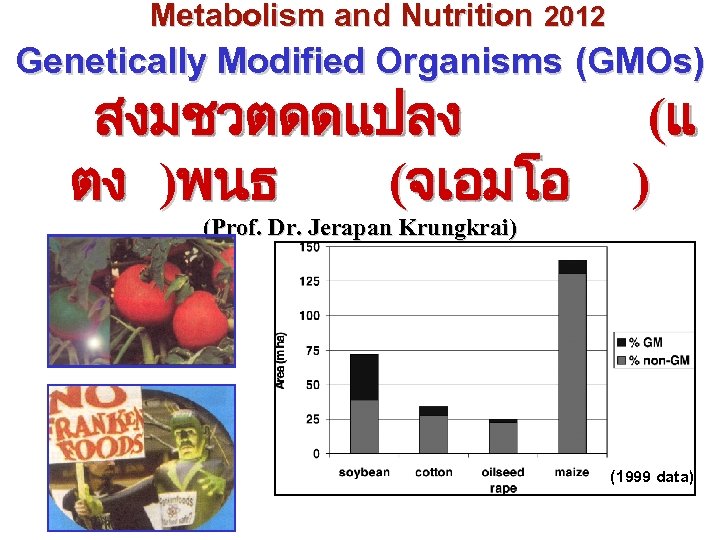
Metabolism and Nutrition 2012 Genetically Modified Organisms (GMOs) สงมชวตดดแปลง ตง )พนธ (จเอมโอ (แ ) (Prof. Dr. Jerapan Krungkrai) (1999 data)
Genetically Modified Organisms (GMOs) (จเอมโอ ) Objectives: After this topic, the students should be able to: 1. Describe GMOs and examples of GMOs in our daily life. 2. Describe construction of GMOs using genetic engineering techniques. . 3 Discuss safety and regulation of GMOs (environment and public health. (. 4 Describe detection and identification of GMO products. Contents: 1. Definition of GMOs and examples of GMOs. 2. Construction of GMOs using genectic engineering. . 3 Safety of GMOs in view of environment and public health aspects. . 4 Regulation of GMOs by labeling and/or percentage of GMOs in food products. . 5 Detection techniques and identification of GMO products. . 6 Future trends of GMOs. Teaching methods: 1 -1. 5 h lecture with power-points and hand-out sheets provided with genetic engineering introduction. Evaluations: 6 MCQs with 5 choices (including genetic engineering introduction)ใ References: lists in the provided hand-out power-point sheets.
1. What are GMOs? - Genetically Modified Organisms (GMOs) are living organisms created through genetic engineering. This has been described earlier. - Scientists transplant the genes of one species into another species to try to transfer "desirable" characteristics.
1. 1. Here, we concern only plant GMOs or GM crops - Plants containing stably-integrated exogenous DNA encoding: - Herbicide resistance Insect resistance Reporter Others - Created using modern molecular biology techniques rather than traditional selective breeding - Biolistic particle bombardment (gene gun) - Agrobacterium tumefaciens transformation

1. 2. Modification Objectives Agronomic trait Pest resistance - Drought resistance - Bacterial resistance - Herbicide or salt tolerance - Nitrate reduction - Temperature resistance - Fungal resistance - Insect resistance - Nematode resistance - Viral resistance
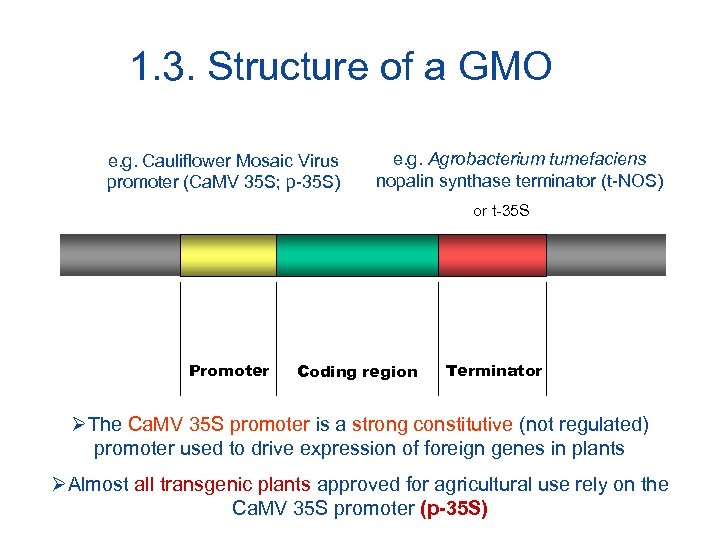
1. 3. Structure of a GMO e. g. Cauliflower Mosaic Virus promoter (Ca. MV 35 S; p-35 S) e. g. Agrobacterium tumefaciens nopalin synthase terminator (t-NOS) or t-35 S Promoter Coding region Terminator ØThe Ca. MV 35 S promoter is a strong constitutive (not regulated) promoter used to drive expression of foreign genes in plants ØAlmost all transgenic plants approved for agricultural use rely on the Ca. MV 35 S promoter (p-35 S)
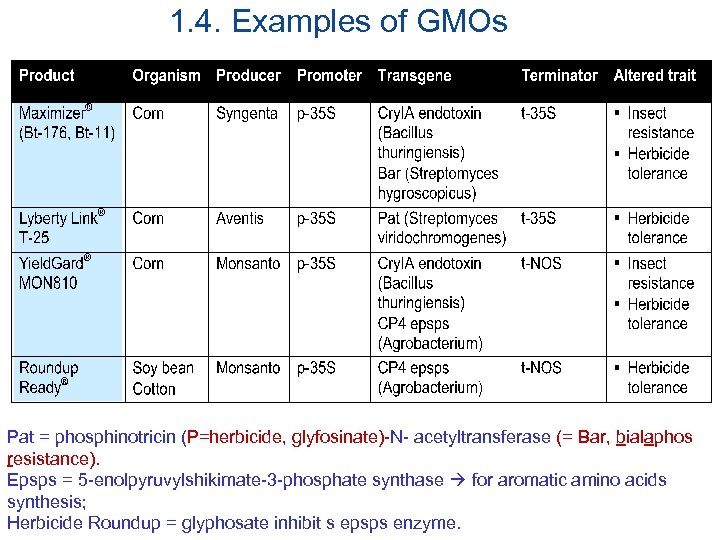
1. 4. Examples of GMOs Pat = phosphinotricin (P=herbicide, glyfosinate)-N- acetyltransferase (= Bar, bialaphos resistance). Epsps = 5 -enolpyruvylshikimate-3 -phosphate synthase for aromatic amino acids synthesis; Herbicide Roundup = glyphosate inhibit s epsps enzyme.

1. 4. Examples of GMOs (cont’) More GMOs: • • tomato (ripening slower), potato(insect resistance), corn (male sterility), squash (virus resistance), melon (virus resistance), papaya (virus resistance), rape seed or canola (high level of lauric acid), tobacco (herbicide tolerance), ……………. . .

1. 5. GM crops: History - The first genetically improved crop was the Flavr Savr™ tomato (Calgene company) which was approved in 1994, USA. - 1983 Plant Genetic Engineering Technology - 1990 Recombinant chymosin (from - fungus Aspergillus niger of bovine gene) replaces rennet (from bovine intestine) enzyme used in cheese production, US FDA approved. Polygalacturonase << (reverse 3’ 5’) - 1994 Flavr Savr tomato approved in USA - 1995 Genetically modified soybeans introduced on the market - 1997 20 GM crops approved in US - 1999 Golden Rice developed Golden rice adds 3 enzymes for beta carotene synthesis from geranyl diphosphate = phytoene synthase, phytoene desaturase, lycopene beta-cyclase.
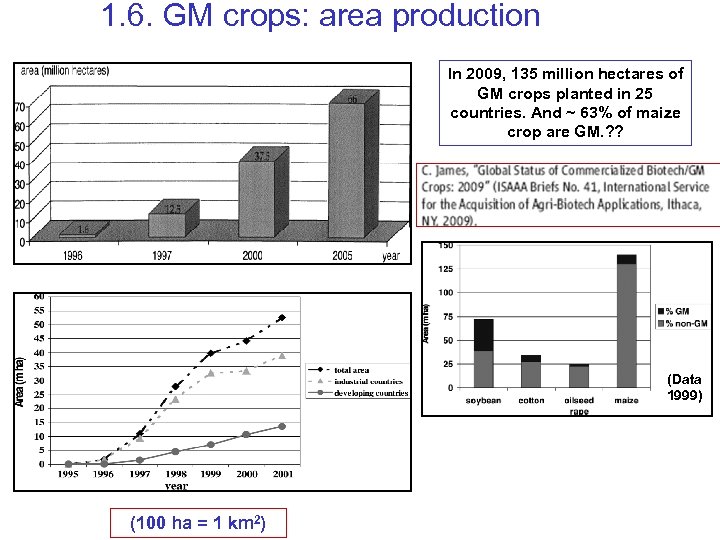
1. 6. GM crops: area production In 2009, 135 million hectares of GM crops planted in 25 countries. And ~ 63% of maize crop are GM. ? ? (Data 1999) (100 ha = 1 km 2)
2. GMO construction 2. 1. Plant Transformation Systems: ØMonocot plants are usually transformed using a biolistic particle bombardment or “gene gun” ØDicot plants are usually transformed using Agrobacterium tumefaciens ØBoth systems require a mechanism for selection of transformants and a plant promoter to drive heterologous gene expression
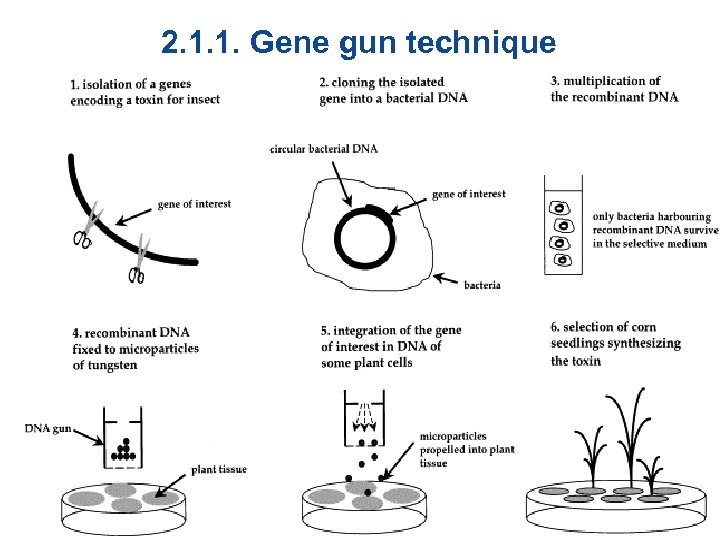
2. 1. 1. Gene gun technique
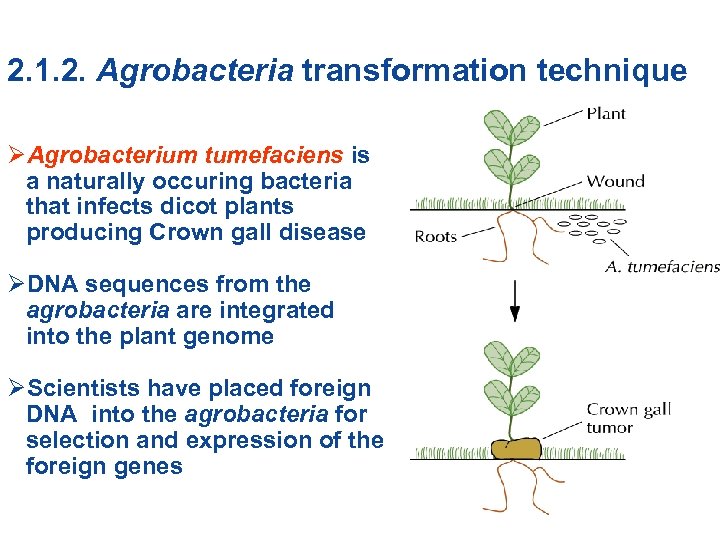
2. 1. 2. Agrobacteria transformation technique ØAgrobacterium tumefaciens is a naturally occuring bacteria that infects dicot plants producing Crown gall disease ØDNA sequences from the agrobacteria are integrated into the plant genome ØScientists have placed foreign DNA into the agrobacteria for selection and expression of the foreign genes
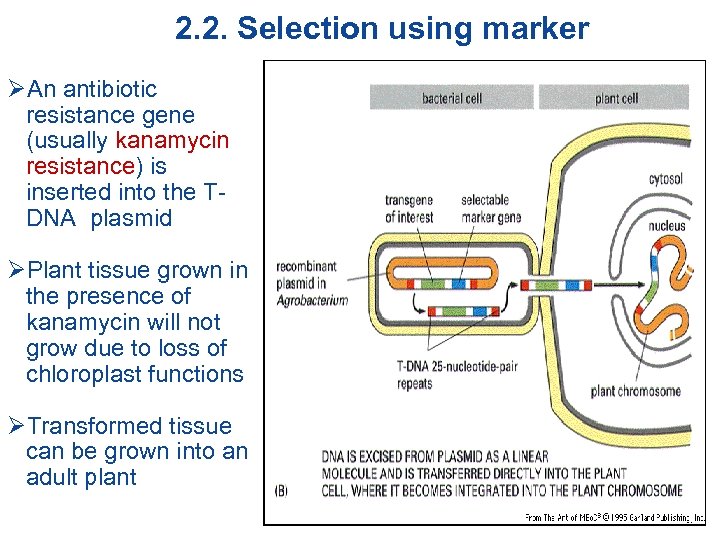
2. 2. Selection using marker ØAn antibiotic resistance gene (usually kanamycin resistance) is inserted into the TDNA plasmid ØPlant tissue grown in the presence of kanamycin will not grow due to loss of chloroplast functions ØTransformed tissue can be grown into an adult plant

3. Genetically Modified Crops: Example Ø Numerous crop plants have been genetically engineered for tolerance to the herbicide Roundup ØAn insecticidal protein from Bacillus thuringiensis has been cloned and expressed in corn, soybean, and cotton plants, namely Bt crops ØB. thuringiensis is a bacteria that naturally produces proteins toxic to certain insects (B. t. toxin= Cryl. A endotoxin)) ØB. t. toxin produces channels in the membranes of the gut in insects ØB. t. toxin is not harmful to animals or humans
B. t. Toxin comments & B. t. cotton ØAre B. t. crops protected from insect pests? ØDo B. t. crops increase production yields? ØDo farmers use less insecticides with B. t. crops? ØAre B. t. crops harmful to insects not considered pests (butterfly)? ØDo insects become resistant to B. t. toxin? ØAre B. t. crops harmful to the environment and biodiversity?

4. Environmental and public health safety issues of the introduced genes in GM crops - 1. Potential gene flow to other organisms: bacteria, plants, mammals - 2. Destruction of agricultural diversity - 3. Allergenicity & toxicity - 4. Antibiotic resistance - 5. Gastrointestinal problems/nutritional changes/anti-nutrient effect

5. Questions About GM Foods ØHow are plants genetically engineered? ØWhat foreign DNA sequences are used in genetic engineering and why? ØHow are crop plants modified and why? ØWhat are the safety, social, ethical, and environmental issues of GM foods? ØHow are GM foods regulated (US and worldwide)?

6. Labeling of GM Food Products ØShould products derived from GM crops be labeled as such? ØWhat tests are currently used to detect GM ingredients in food products? ØWhat is an acceptable level of GM ingredients in food products? ØWhat is the current labeling policy (US and worldwide)?

Labeling Regulations - USA - GMOs must be labeled. - Switzerland - Establishes 1% threshold for labeling effective July 1, 1999 - European Union - EC/1139/1998 requires labeling of foods containing GM ingredients, especially soy and maize - EC/49/2000 establishes 1% labeling thresholds - EC/1829/2003 establishes 0. 9% labeling thresholds for authorized GMOs and 0. 5% for non-authorized GMOs in EC - EC/1830/2003 requires traceability and labeling of GMOs throughout the entire food and feed chain - Japan - Establishes 5% labeling threshold on April 1, 2000 based upon major ingredients containing GMO and enforcement effective April 1, 2001
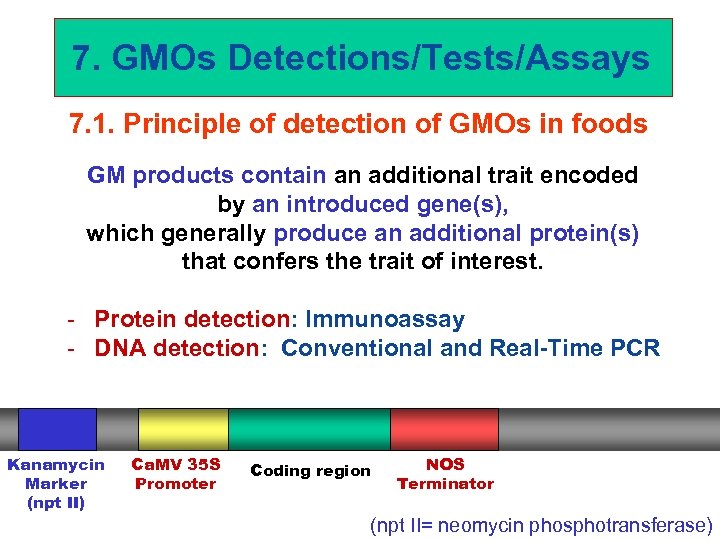
7. GMOs Detections/Tests/Assays 7. 1. Principle of detection of GMOs in foods GM products contain an additional trait encoded by an introduced gene(s), which generally produce an additional protein(s) that confers the trait of interest. - Protein detection: Immunoassay - DNA detection: Conventional and Real-Time PCR Kanamycin Marker (npt II) Ca. MV 35 S Promoter Coding region NOS Terminator (npt II= neomycin phosphotransferase)
7. 2. GMOs Immunoassay - Fast and inexpensive (dipsticks) Antibodies detect expressed protein Qualitative, not quantitative Only detects gene products expressed in test material (tissue-specific expression) - Sensitivity and specificity dependent upon antibody - Protein degradation during sample extraction can limit detection - Negative result does not indicate absence of transgene (false negative result)
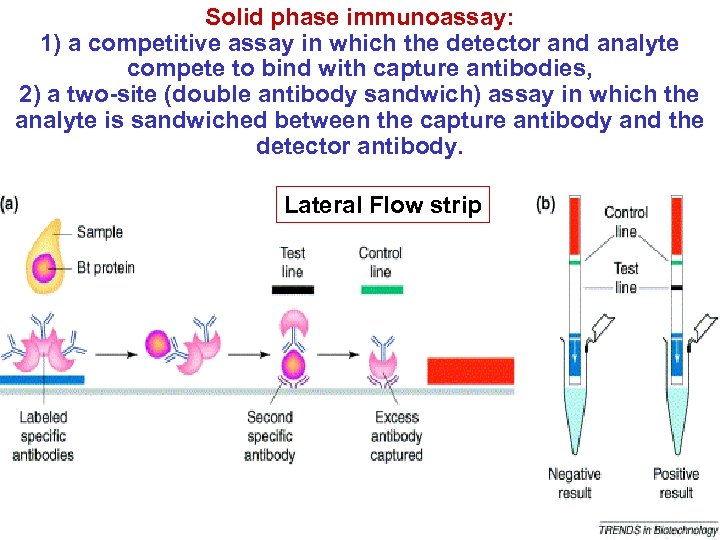
Solid phase immunoassay: 1) a competitive assay in which the detector and analyte compete to bind with capture antibodies, 2) a two-site (double antibody sandwich) assay in which the analyte is sandwiched between the capture antibody and the detector antibody. Lateral Flow strip
7. 3. GMOs conventional PCR - Semi-quantitative endpoint analysis Target present in all tissues Potential for carry-over contamination Sensitivity dependent upon detection method Gel electrophoresis-based analysis Labor-intensive, especially competitive PCR Low throughput Negative result does not indicate absence of transgene (false negative result) PCR Primers: p-35 S or t-NOS
7. 4. GMOs Real-Time PCR using Taqman Probes - Quantitative results Greater specificity provided by Taq. Man probe No carry-over contamination Integrated and automated amplification, detection, data collection and analysis - Gel-free analysis - High throughput, 96 - and 384 -well formats - Prevention of false negative result
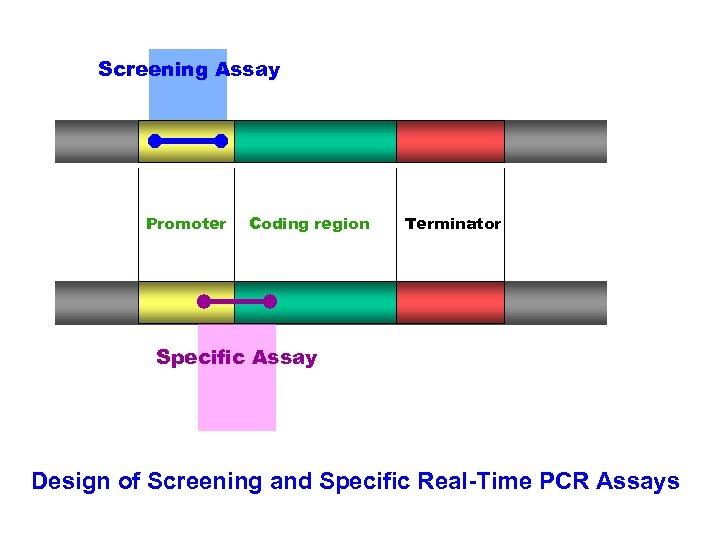
Screening Assay Promoter Coding region Terminator Specific Assay Design of Screening and Specific Real-Time PCR Assays
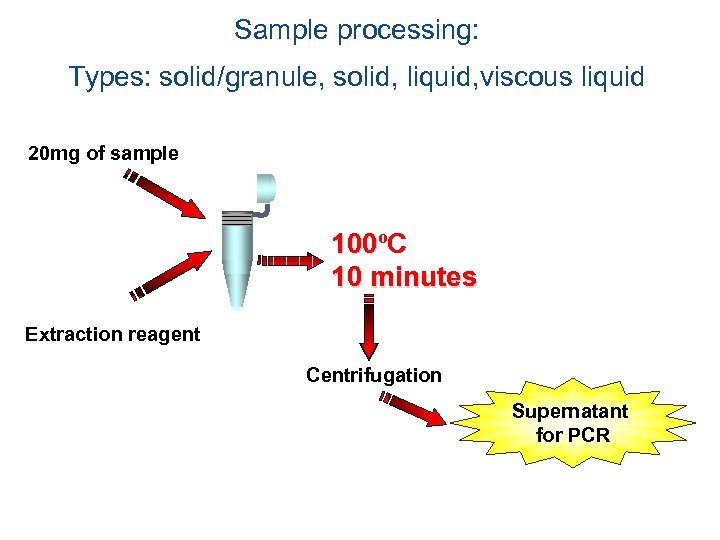
Sample processing: Types: solid/granule, solid, liquid, viscous liquid 20 mg of sample 100ºC 100 10 minutes Extraction reagent Centrifugation Supernatant for PCR
7. 5. GMOs Reference Standards - Manufactured by the Institute of Reference Materials and Measurements, IRMM (Geel, Belgium) - GMO flour blended with non-GMO flour to obtain specific % GMO content - Soy and Maize reference standards available - Provide quantitation standard and DNA template preparation control
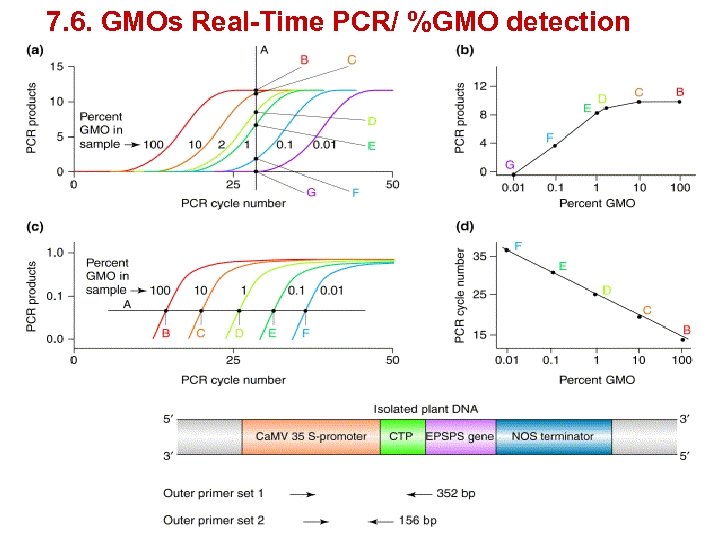
7. 6. GMOs Real-Time PCR/ %GMO detection
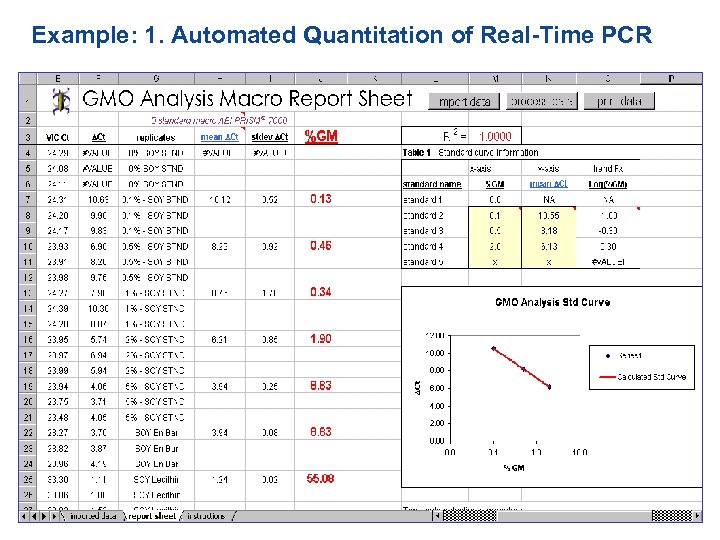
Example: 1. Automated Quantitation of Real-Time PCR
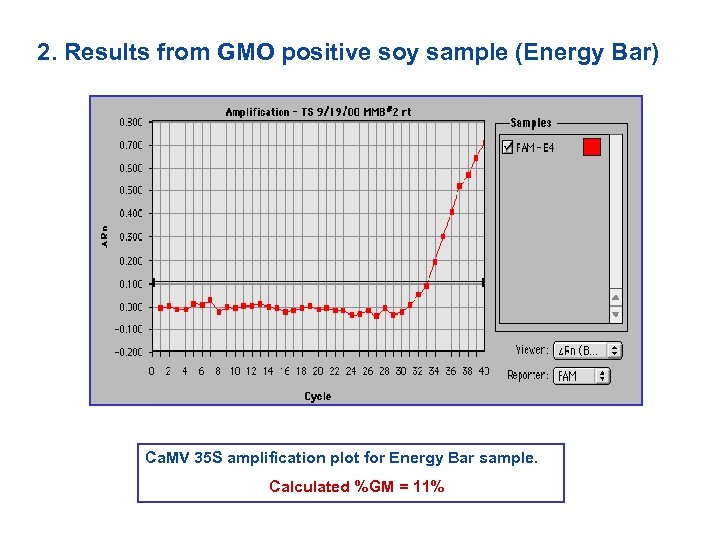
2. Results from GMO positive soy sample (Energy Bar) Ca. MV 35 S amplification plot for Energy Bar sample. Calculated %GM = 11%
8. GM crop safety evaluation strategies - Protein safety evaluation: In addition to acute toxicity, the other main adverse effects associated with proteins are antinutrient effects (e. g. soybean trypsin inhibitors), effects on the immune system (e. g. lectins) and allergenicity (e. g. soybean) - Requirement for animal studies: If the characterization of the food indicates that the available data are insufficient for a thorough safety assessment, animal testing may be deemed necessary.

9. Thailand GM crops • 1. Chili • 2. Tomato • 3. Rice (Stunt virus) • 4. Papaya (Ringspot virus, not from Cornell university) • 5. Cotton (Thailand native lines, not from Monsanto company) • 6. Pineapple In Thailand, we concern GM crops and foods since 1993, however, in 1999 there are regulations for the GMOs. “ GM crops productions for commercialization are prohibited, only research in laboratory /controlled area can be studied. ”

10. Future Trends of GMOs Up to 2010, more than 48 GM crops will be developed !!! 1. Agronomical quality: slower ripening (banana), herbicide tolerance (wheat, sunflower…. ), virus and fungus resistance (rice, melon, cucumber…)…… 2. Nutritional quality: richer in starch (potato), high level of amino acids (soy bean), lower level of saturated fats in oil (corn)…… 3. Pharmaceutical quality: higher level of a natural anticancer agent (strawberry), producing a vaccine against hepatitis B virus (banana), producing antioxidant agents (Broccoli), synthesizing hemoglobin (tobacco)……. 4. Industrial quality: colored fibers (cotton)……. . 5. Transgenic animals (pigs) expressing plant genes coding for PUFAs.

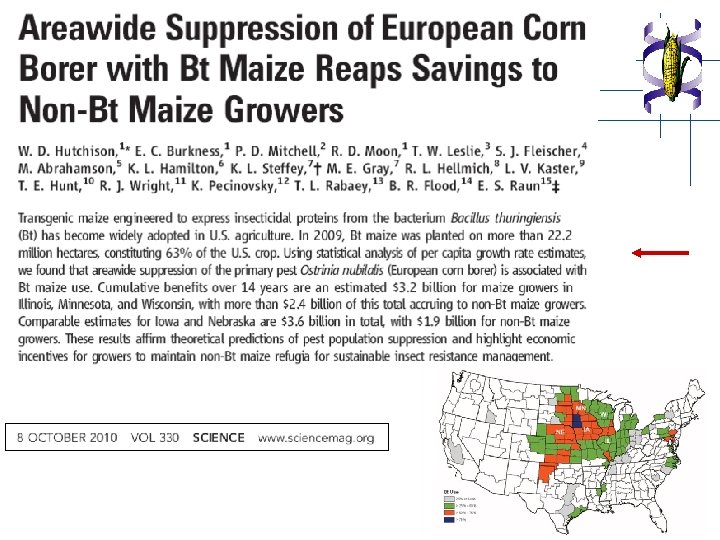
References 1. Gachet, E. et al. (1998) Trends in Food Science & Technology 9, 380 -388. 2. Beachy, R. E. (1999) Science 285, 335. 3. Doerfler, W. et al. (2001) Annals of the New York Academy of Sciences 945, 276 -288. 4. Ahmed, F. A. (2002) Trends in Biotechnology 20, 215 -223. 5. Nap, J. P. et al. (2003) Plant Journal 33, 1 -18. 6. Corner, A. J. et al. (2003) Plant Journal 33, 19 -46. 7. Taverniers, I. et al. (2004) Biotech International 16, 20 -23. 8. Niemann, H. (2004) Proceedings of the National Academy of Sciences 101, 7211 -7212. 9. Rosi-Marshall, E. et al. (2007) Proceedings of the National Academy of Sciences 104, 16204 -16208. 10. Waltz E. (2009) Nature 461, 27 -32. 11. Hutchison, W. D. et al. (2010) Science 330, 222 -225. 12. Tabashnik, B. (2010) Science 330, 189 -190.



Evaluation: MCQs, 6 questions/5 choices Good Luck and Have a Good Examination

Metabolism and Nutrition 2012 Genetic Engineering (Recombinant DNA Technology) - Prof. Dr. Jerapan Krungkrai Techniques used in GMOs • Cloning of gene • PCR Tobacco histone tagged with green fluorescent protein al. (2000 ( Reference: Alberts, B. et(GFP( Molecular Biology of the Cell
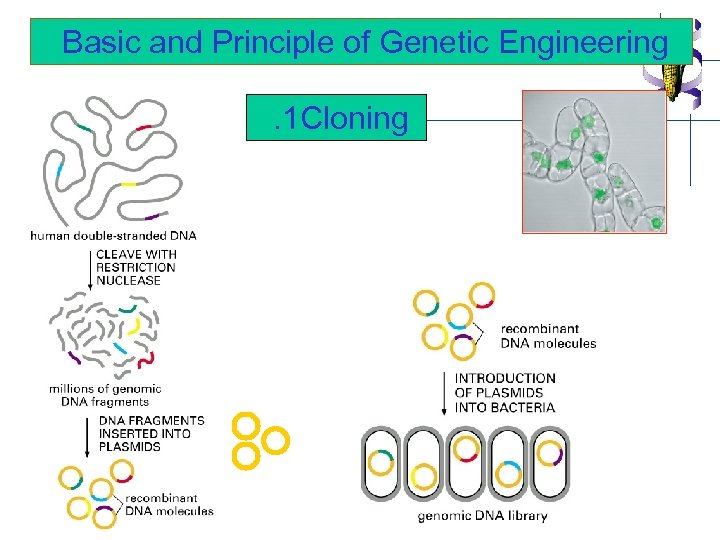
Basic and Principle of Genetic Engineering. 1 Cloning
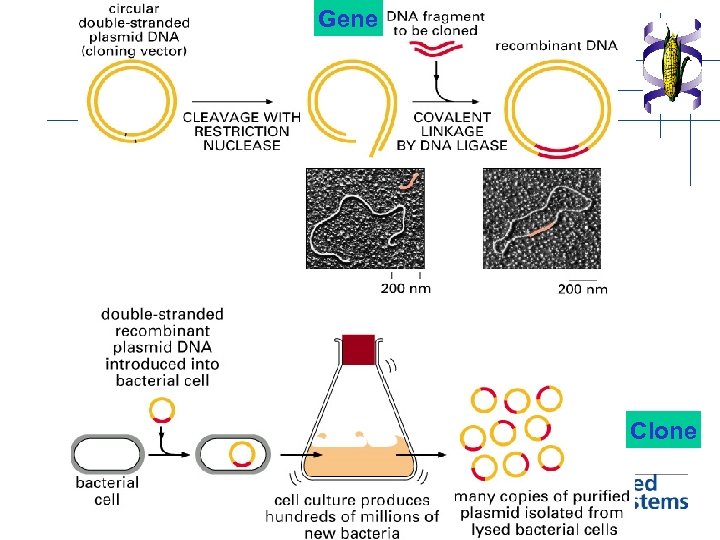
Gene Clone
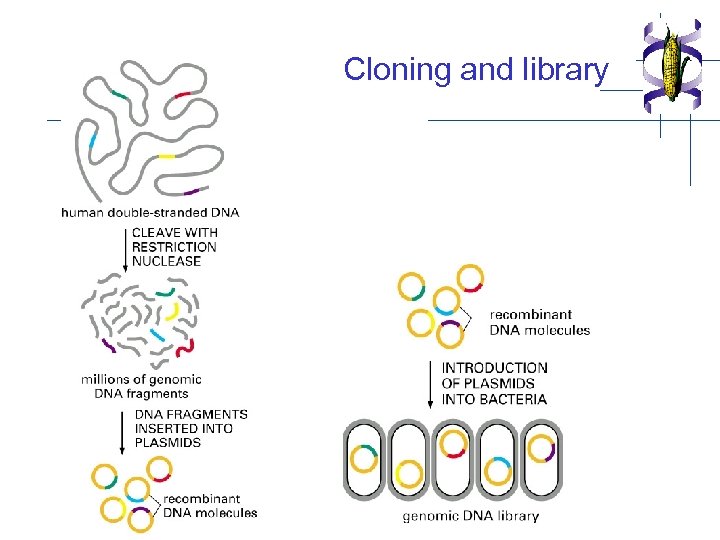
Cloning and library
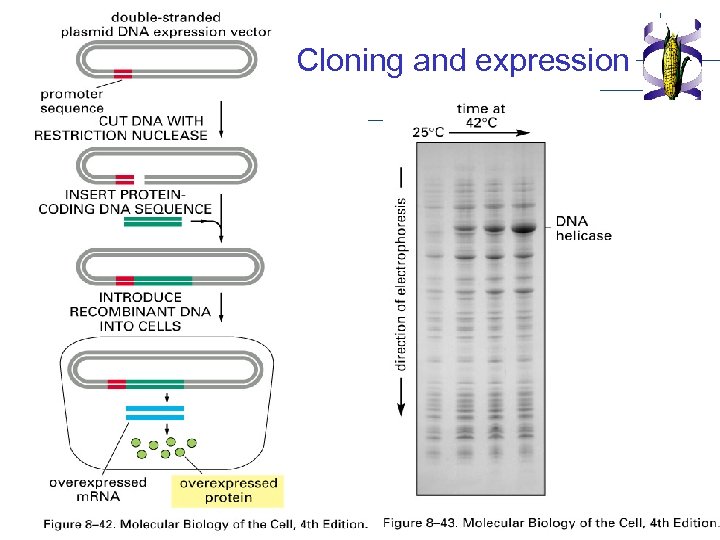
Cloning and expression
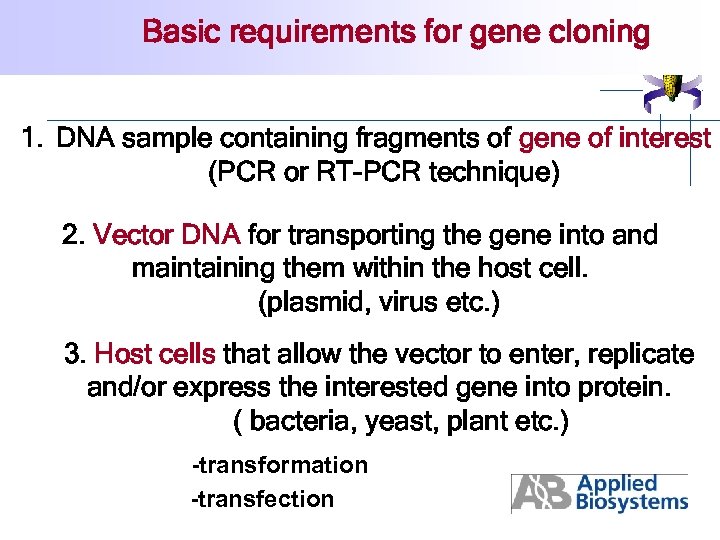
Basic requirements for gene cloning 1. DNA sample containing fragments of gene of interest (PCR or RT-PCR technique) 2. Vector DNA for transporting the gene into and maintaining them within the host cell. (plasmid, virus etc. ) 3. Host cells that allow the vector to enter, replicate and/or express the interested gene into protein. ( bacteria, yeast, plant etc. ) -transformation -transfection
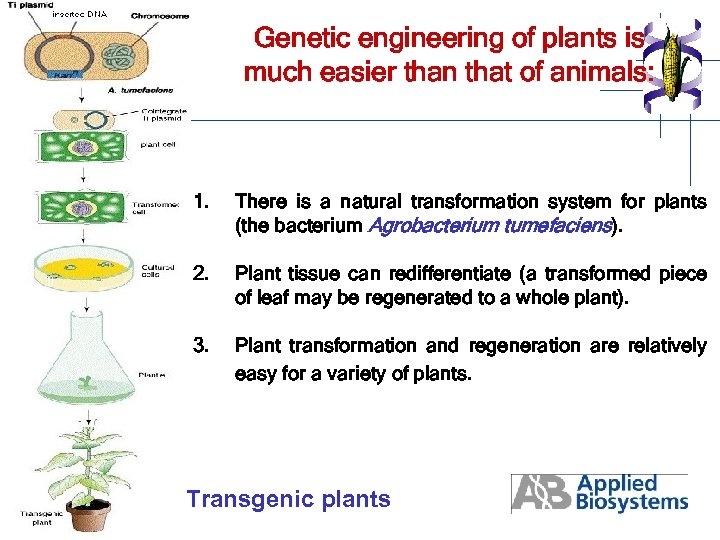
Genetic engineering of plants is much easier than that of animals: 1. There is a natural transformation system for plants (the bacterium Agrobacterium tumefaciens). 2. Plant tissue can redifferentiate (a transformed piece of leaf may be regenerated to a whole plant). 3. Plant transformation and regeneration are relatively easy for a variety of plants. Transgenic plants
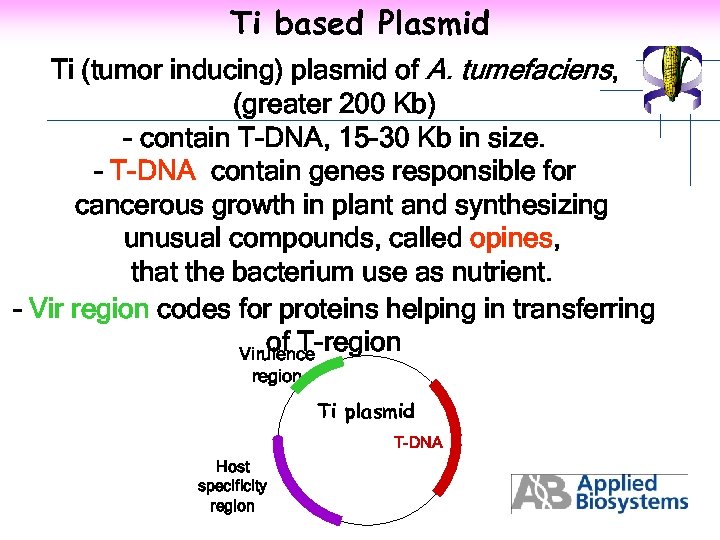
Ti based Plasmid Ti (tumor inducing) plasmid of A. tumefaciens, (greater 200 Kb) - contain T-DNA, 15 -30 Kb in size. - T-DNA contain genes responsible for cancerous growth in plant and synthesizing unusual compounds, called opines, that the bacterium use as nutrient. - Vir region codes for proteins helping in transferring of T-region Virulence region Ti plasmid T-DNA Host specificity region
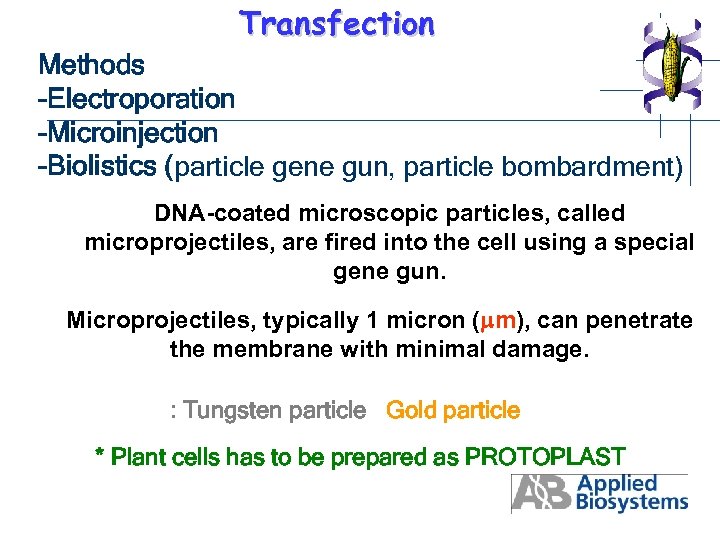
Transfection Methods -Electroporation -Microinjection -Biolistics (particle gene gun, particle bombardment) DNA-coated microscopic particles, called microprojectiles, are fired into the cell using a special gene gun. Microprojectiles, typically 1 micron ( m), can penetrate the membrane with minimal damage. : Tungsten particle Gold particle * Plant cells has to be prepared as PROTOPLAST
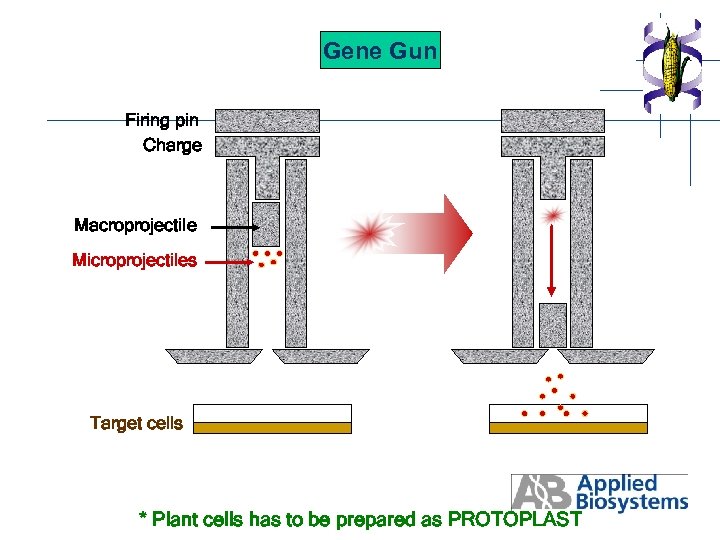
Gene Gun Firing pin Charge Macroprojectile Microprojectiles Target cells * Plant cells has to be prepared as PROTOPLAST
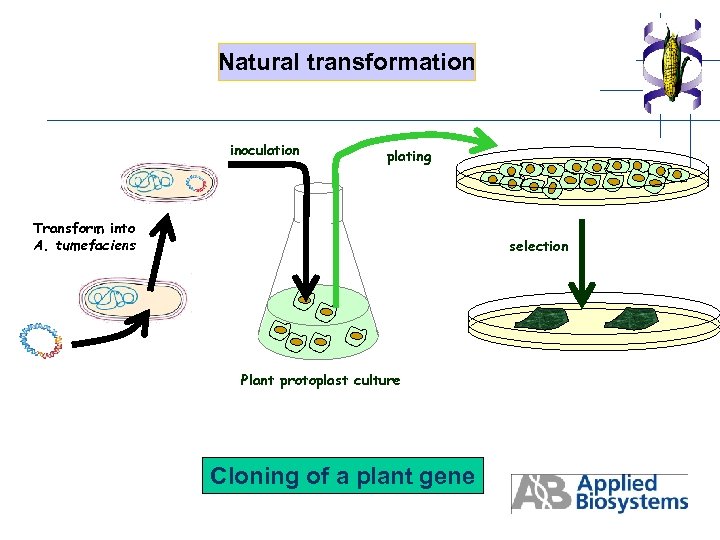
Natural transformation inoculation plating Transform into A. tumefaciens selection Plant protoplast culture Cloning of a plant gene
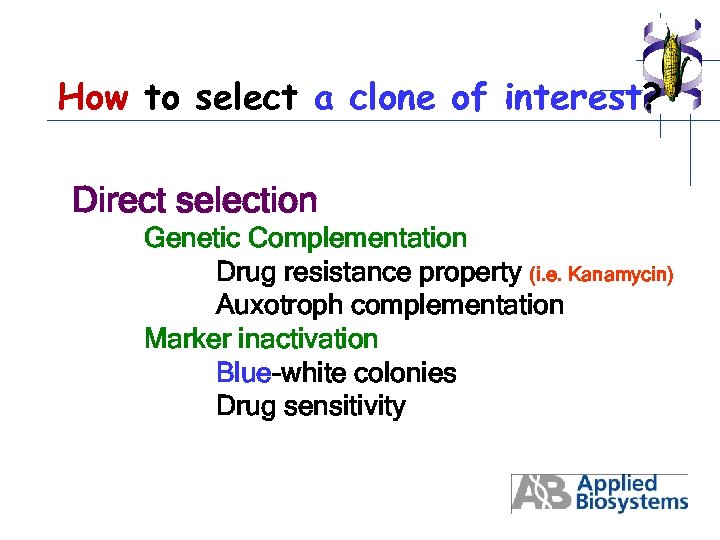
How to select a clone of interest? Direct selection Genetic Complementation Drug resistance property (i. e. Kanamycin) Auxotroph complementation Marker inactivation Blue-white colonies Drug sensitivity
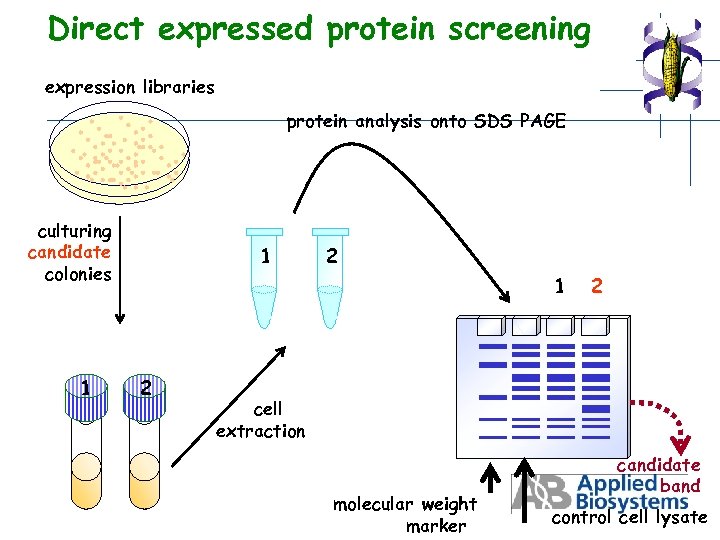
Direct expressed protein screening expression libraries protein analysis onto SDS PAGE culturing candidate colonies 1 1 2 2 cell extraction molecular weight marker candidate band control cell lysate

Human Cloning statement
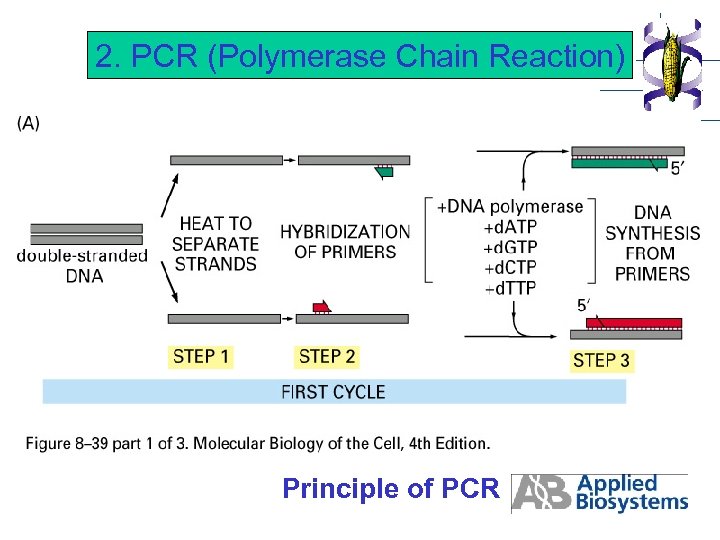
2. PCR (Polymerase Chain Reaction) Principle of PCR
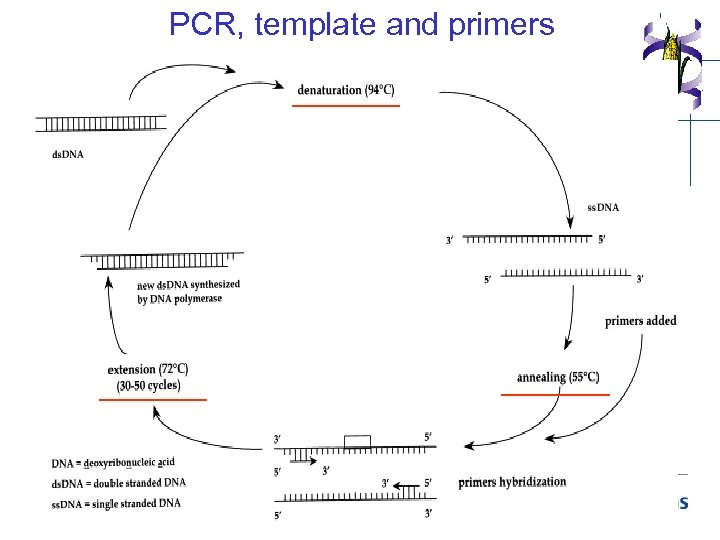
PCR, template and primers
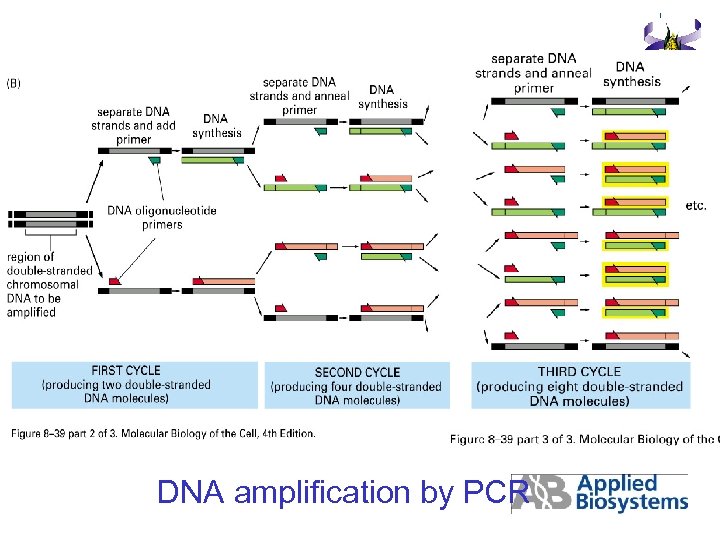
DNA amplification by PCR
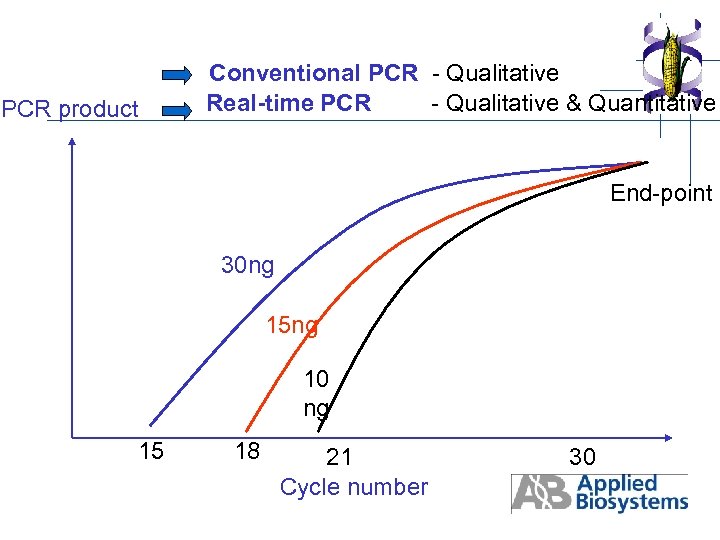
PCR product Conventional PCR - Qualitative Real-time PCR - Qualitative & Quantitative End-point 30 ng 15 ng 10 ng 15 18 21 Cycle number 30
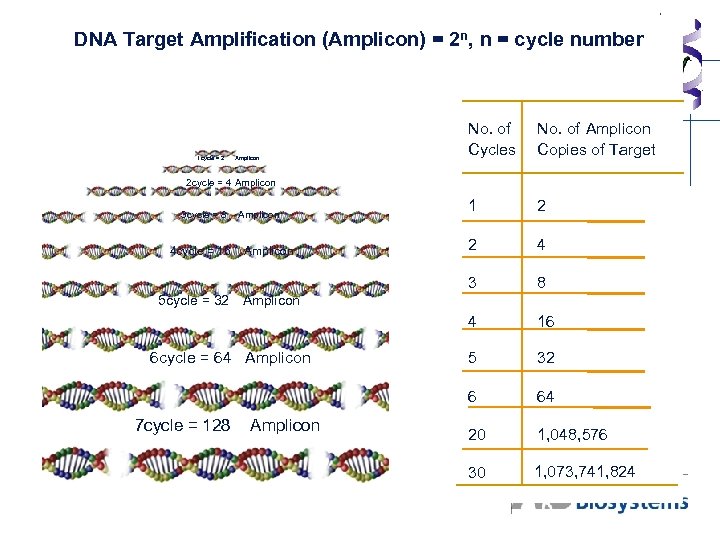
DNA Target Amplification (Amplicon) = 2 n, n = cycle number 1 2 2 4 8 4 16 5 32 6 Amplicon No. of Amplicon Copies of Target 3 1 cycle = 2 No. of Cycles 64 20 1, 048, 576 30 1, 073, 741, 824 2 cycle = 4 Amplicon 3 cycle = 8 4 cycle = 16 Amplicon 5 cycle = 32 Amplicon 6 cycle = 64 Amplicon 7 cycle = 128 Amplicon
6d286d0b344e0c7b629e71d35a1761ab.ppt