76aacfb021748451f3412436f14851fd.ppt
- Количество слайдов: 116

Metabolic regulation 2310611 Advanced Biochemistry Piamsook Pongsawasdi Oct 2017
PART I n n OVERVIEW OF METABOLISM REGULATION OF METABOLIC PATHWAYS 2
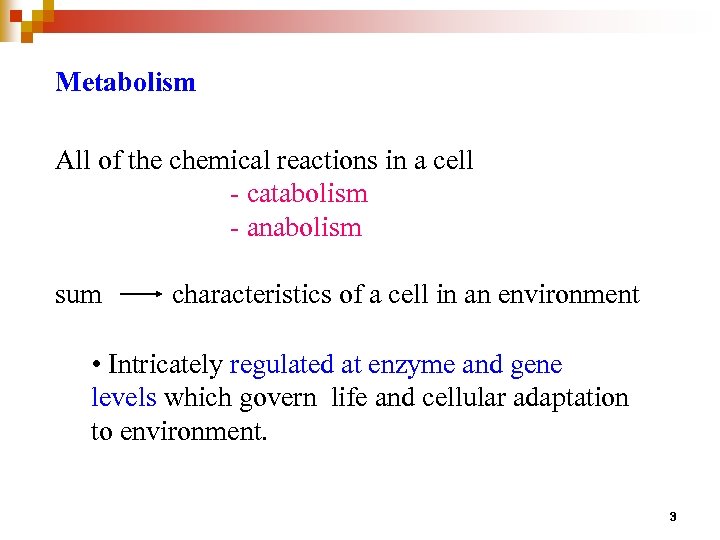
Metabolism All of the chemical reactions in a cell - catabolism - anabolism sum characteristics of a cell in an environment • Intricately regulated at enzyme and gene levels which govern life and cellular adaptation to environment. 3

Metabolism involves: Transformation of nutrients n Energy transformations n Synthesis and degradation processes n Excretion of waste products n Glycolysis and TCA cycle are central core of metabolism n All reactions are enzyme catalyzed. 4
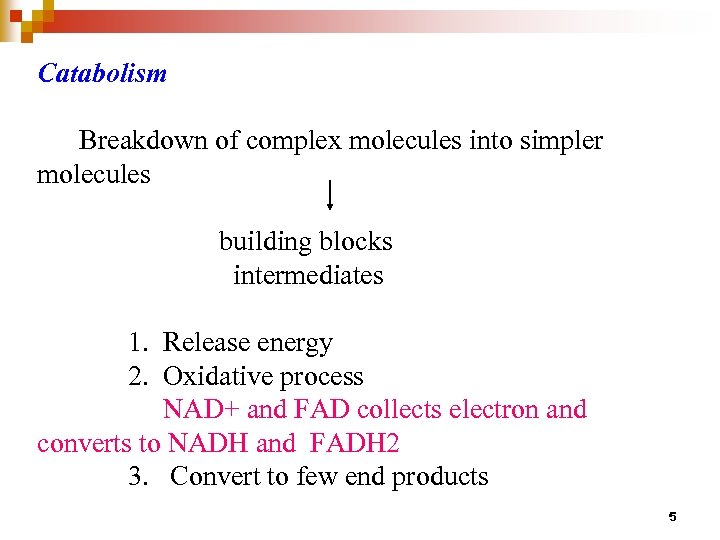
Catabolism Breakdown of complex molecules into simpler molecules building blocks intermediates 1. Release energy 2. Oxidative process NAD+ and FAD collects electron and converts to NADH and FADH 2 3. Convert to few end products 5
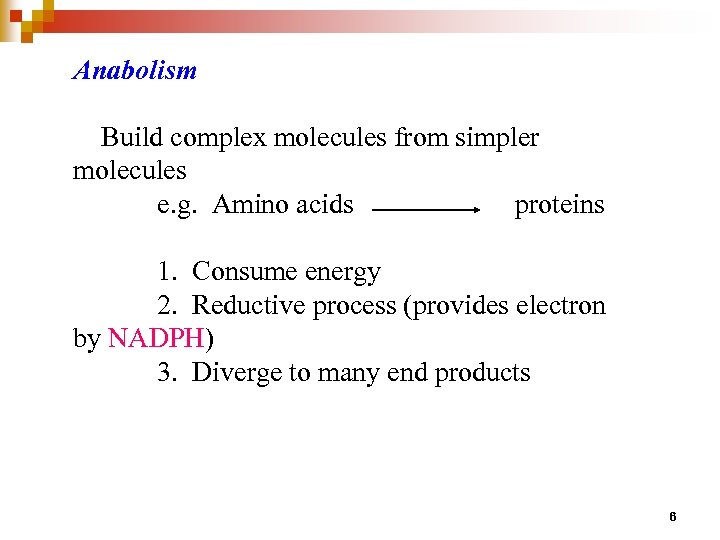
Anabolism Build complex molecules from simpler molecules e. g. Amino acids proteins 1. Consume energy 2. Reductive process (provides electron by NADPH) 3. Diverge to many end products 6
Amphibolic pathway • Intermediary pathway that serves both in catabolism and anabolism Anaplerotic reactions. • Reactions that replenish depleted intermediates, e. g. Pyr Carboxylase replenishes OAA. Replenished reaction can be by breakdown of amino acids (transamination/deamination) or fatty acids. 7
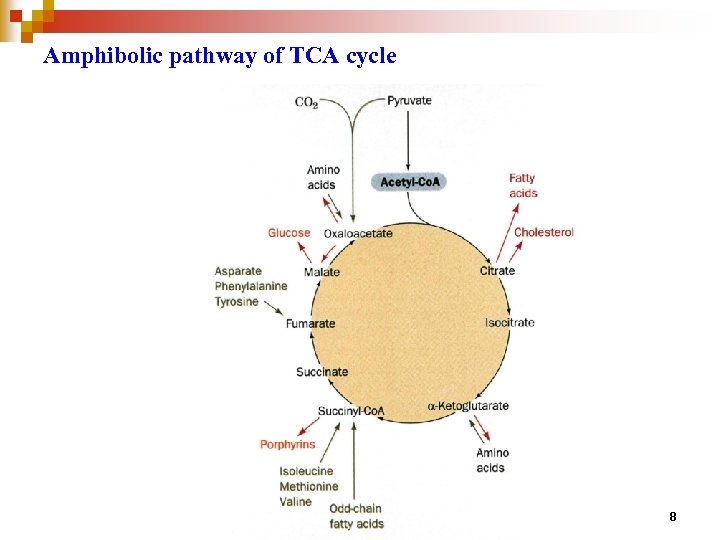
Amphibolic pathway of TCA cycle 8
Summary of metabolism n n n Metabolism is composed of interconnecting reactions and pathways Thermodynamics determine the direction of the pathways ATP is the universal currency Oxidation of carbon fuel is major source of cellular energy Metabolic pathways are tightly regulated 9
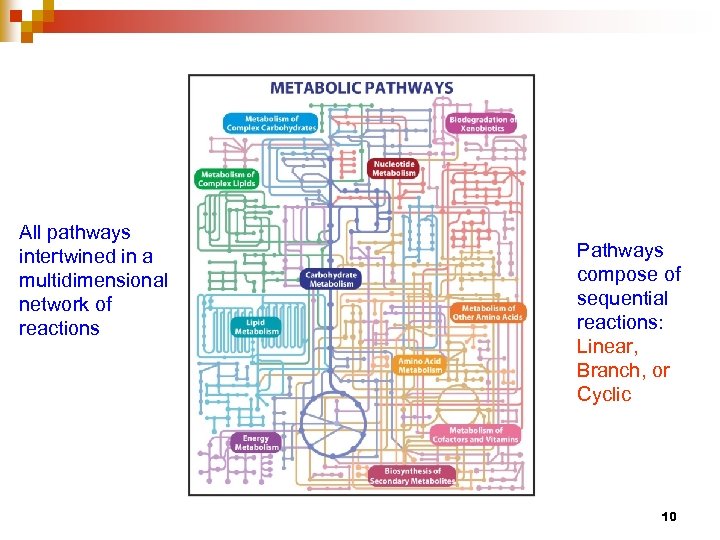
All pathways intertwined in a multidimensional network of reactions Pathways compose of sequential reactions: Linear, Branch, or Cyclic 10
Summary of metabolism (continued) Metabolic pathways are irreversible at some steps (to be spontaneous - overall ΔG of the pathway is negative) n Every pathway has a committed step (usually the first irreversible step unique to the pathway) n Synthetic and degradative pathways differ, they are distinct by compartmentation or bypass reaction 11 n

Synthetic and degradative pathways don’t happen at the same time They can share some common steps but they are never simply the reverse of one another provides separate control of the processes (at irreversible step) n Synthetic pathways always use more ATP than a degradative pathway will produce. n If both synthetic and degradative pathways occurred at the same time, “wasteful” hydrolysis of ATP would result. This is termed a “futile cycle. ” 12 n
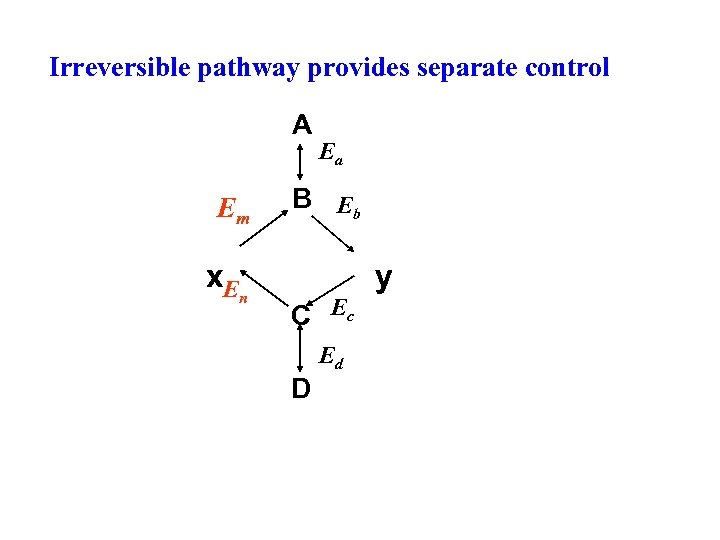
Irreversible pathway provides separate control A Em x. E n Ea B Eb C Ec Ed D y
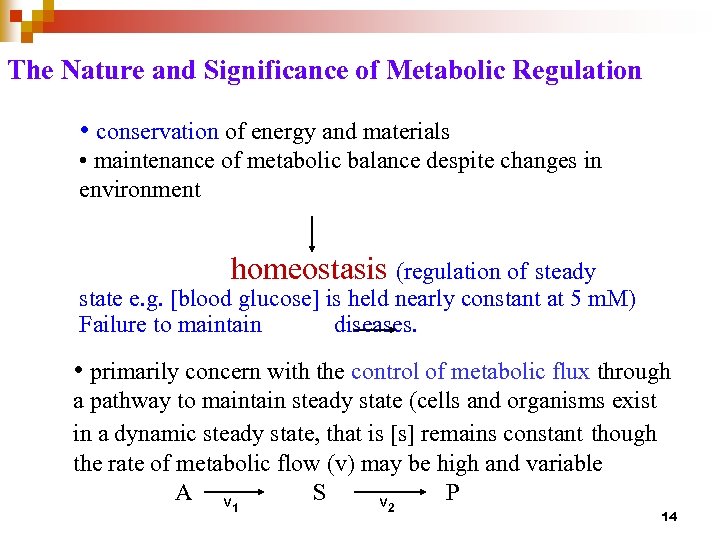
The Nature and Significance of Metabolic Regulation • conservation of energy and materials • maintenance of metabolic balance despite changes in environment homeostasis (regulation of steady state e. g. [blood glucose] is held nearly constant at 5 m. M) Failure to maintain diseases. • primarily concern with the control of metabolic flux through a pathway to maintain steady state (cells and organisms exist in a dynamic steady state, that is [s] remains constant though the rate of metabolic flow (v) may be high and variable A v S P v 1 2 14
Metabolic flux The rate of material processing through a metabolic pathway n Flux = flow of metabolites through a pathway - quantitatively predict system behaviour as a response to any external signal(s) 15
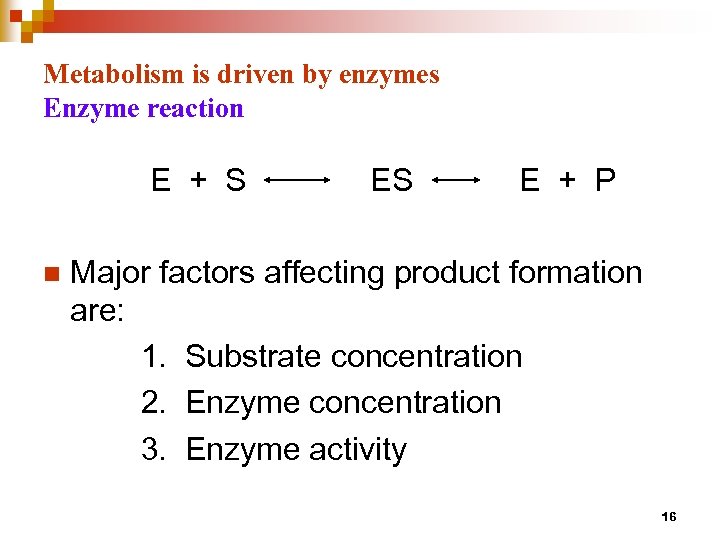
Metabolism is driven by enzymes Enzyme reaction E + S n ES E + P Major factors affecting product formation are: 1. Substrate concentration 2. Enzyme concentration 3. Enzyme activity 16
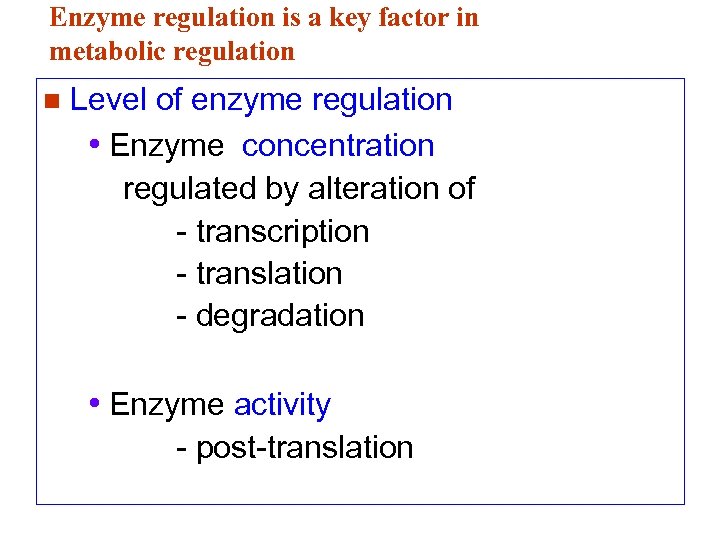
Enzyme regulation is a key factor in metabolic regulation n Level of enzyme regulation • Enzyme concentration regulated by alteration of - transcription - translation - degradation • Enzyme activity - post-translation
![Enzyme Activity is affected by many factors Step 1 -5 change in [E] Step Enzyme Activity is affected by many factors Step 1 -5 change in [E] Step](https://present5.com/presentation/76aacfb021748451f3412436f14851fd/image-18.jpg)
Enzyme Activity is affected by many factors Step 1 -5 change in [E] Step 6 -10 change in activity 18
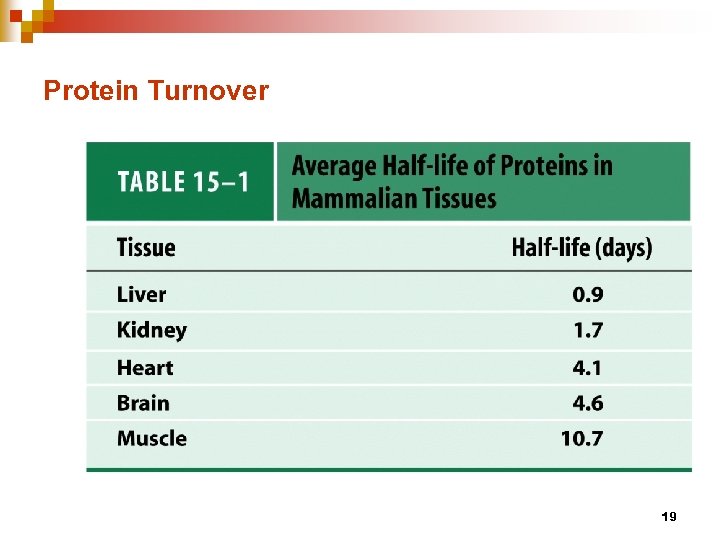
Protein Turnover 19
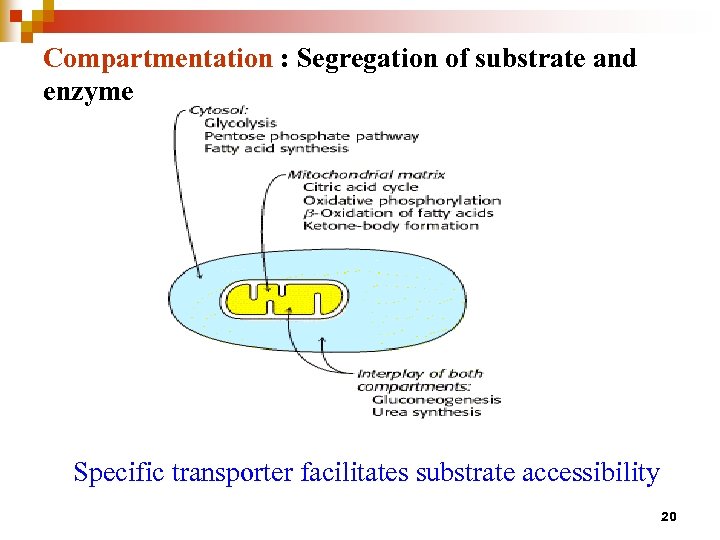
Compartmentation : Segregation of substrate and enzyme Specific transporter facilitates substrate accessibility 20
![All enzymes are sensitive to [S] 21 All enzymes are sensitive to [S] 21](https://present5.com/presentation/76aacfb021748451f3412436f14851fd/image-21.jpg)
All enzymes are sensitive to [S] 21
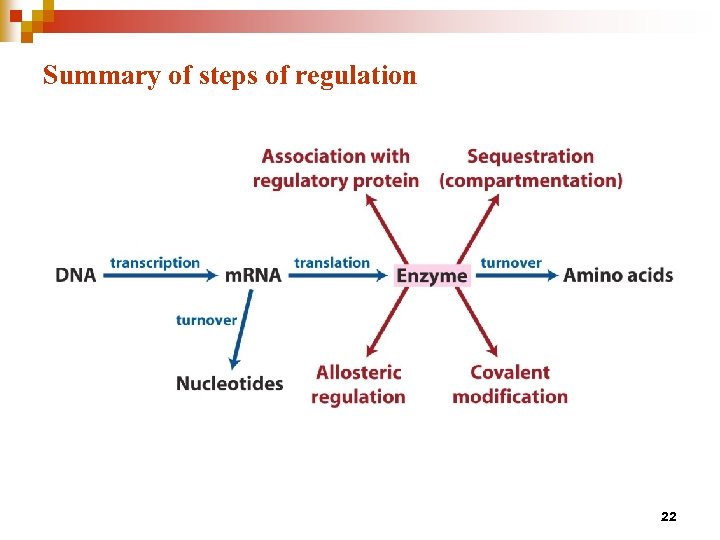
Summary of steps of regulation 22
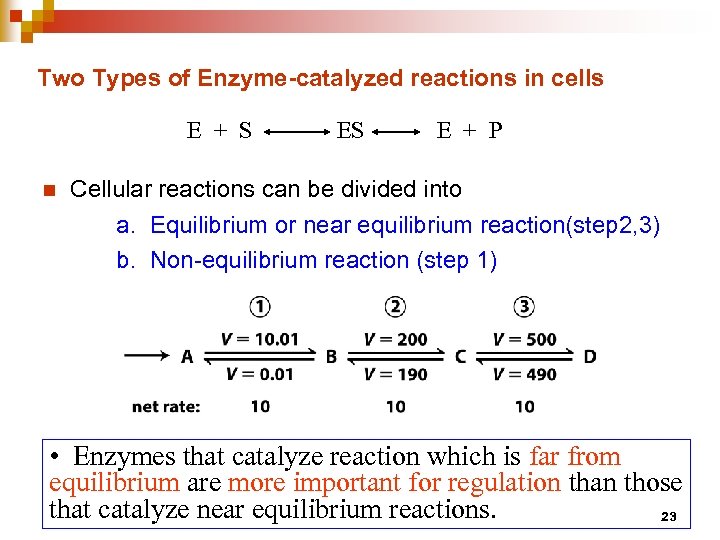
Two Types of Enzyme-catalyzed reactions in cells E + S n ES E + P Cellular reactions can be divided into a. Equilibrium or near equilibrium reaction(step 2, 3) b. Non-equilibrium reaction (step 1) • Enzymes that catalyze reaction which is far from equilibrium are more important for regulation than those that catalyze near equilibrium reactions. 23
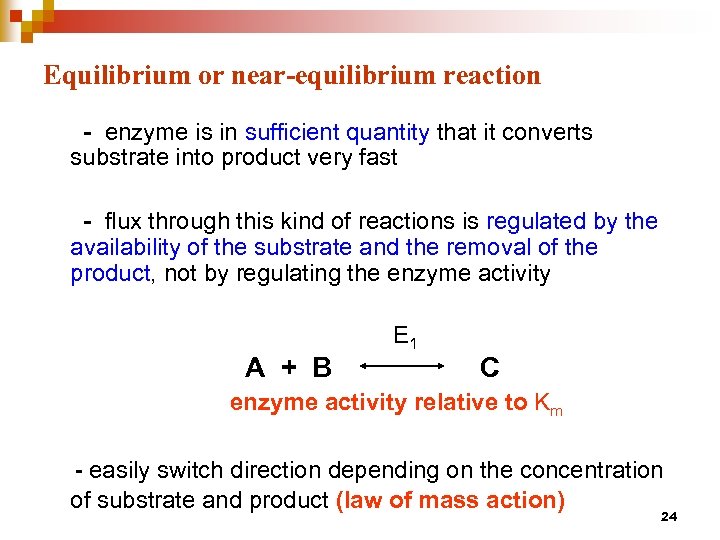
Equilibrium or near-equilibrium reaction - enzyme is in sufficient quantity that it converts substrate into product very fast - flux through this kind of reactions is regulated by the availability of the substrate and the removal of the product, not by regulating the enzyme activity A + B E 1 C enzyme activity relative to Km - easily switch direction depending on the concentration of substrate and product (law of mass action) 24
![Effect of [substrate] • rate increases as [substrate] increases • no further increase occurs Effect of [substrate] • rate increases as [substrate] increases • no further increase occurs](https://present5.com/presentation/76aacfb021748451f3412436f14851fd/image-25.jpg)
Effect of [substrate] • rate increases as [substrate] increases • no further increase occurs after all enzyme molecules are saturated with substrate 25
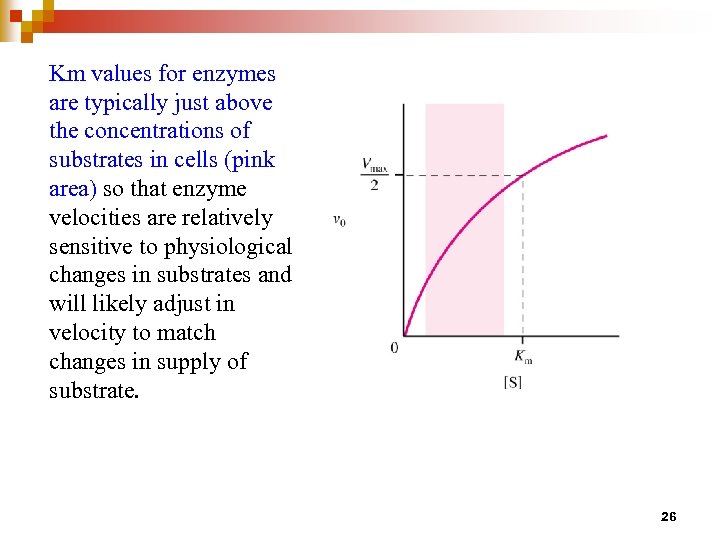
Km values for enzymes are typically just above the concentrations of substrates in cells (pink area) so that enzyme velocities are relatively sensitive to physiological changes in substrates and will likely adjust in velocity to match changes in supply of substrate. 26
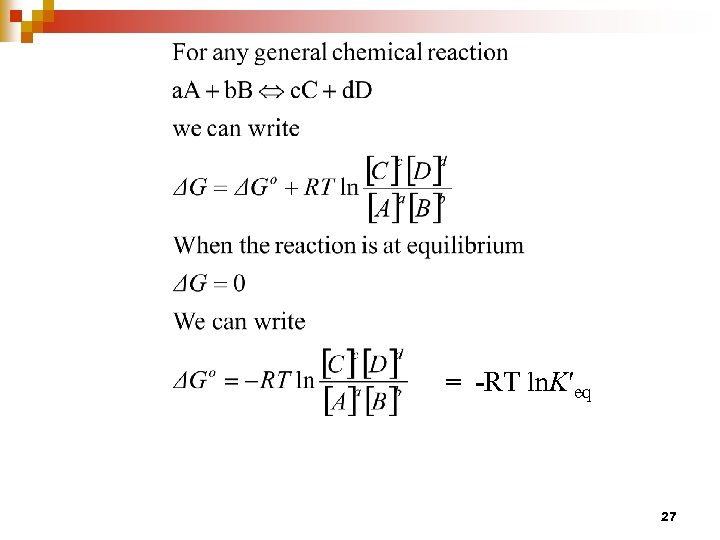
= -RT ln. K′eq 27
![To identify equilibrium or non-equilibrium reactions Mass action ratio Q = [C][D] / [A][B] To identify equilibrium or non-equilibrium reactions Mass action ratio Q = [C][D] / [A][B]](https://present5.com/presentation/76aacfb021748451f3412436f14851fd/image-28.jpg)
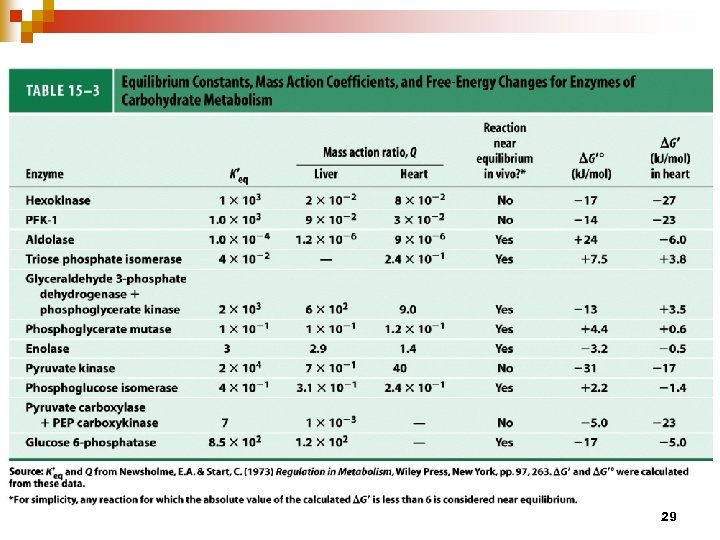
To identify equilibrium or non-equilibrium reactions Mass action ratio Q = [C][D] / [A][B] When Q and K′eq are within 1 to 2 orders of magnitude of each other, the reaction is near equilibrium e. g. 6 of the 10 steps in the glycolytic pathway (Table 15 -3) 28
29
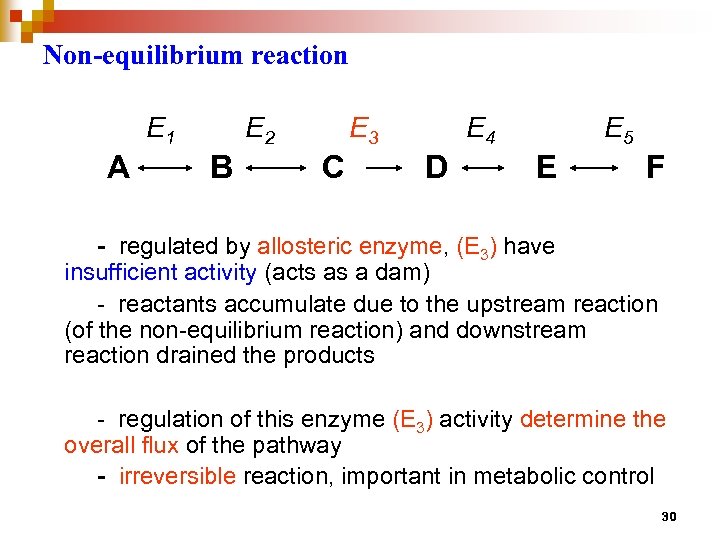
Non-equilibrium reaction E 1 A E 2 B E 3 C E 4 D E 5 E F - regulated by allosteric enzyme, (E 3) have insufficient activity (acts as a dam) - reactants accumulate due to the upstream reaction (of the non-equilibrium reaction) and downstream reaction drained the products - regulation of this enzyme (E 3) activity determine the overall flux of the pathway - irreversible reaction, important in metabolic control 30
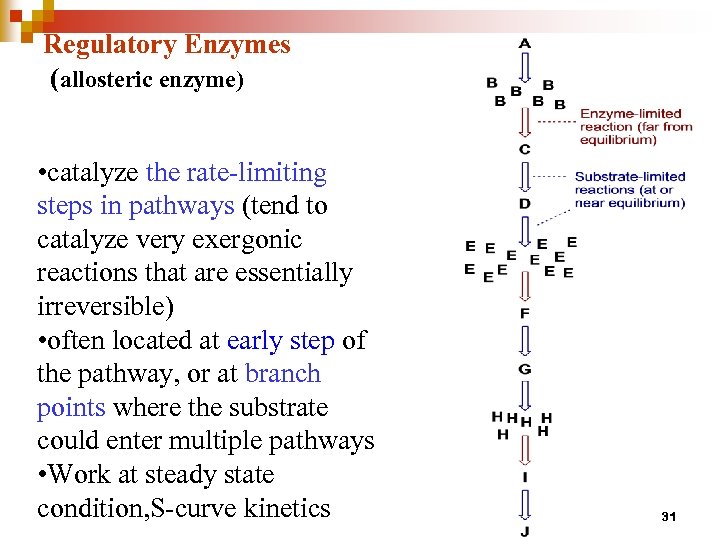
Regulatory Enzymes (allosteric enzyme) • catalyze the rate-limiting steps in pathways (tend to catalyze very exergonic reactions that are essentially irreversible) • often located at early step of the pathway, or at branch points where the substrate could enter multiple pathways • Work at steady state condition, S-curve kinetics 31

Equilibrium versus Steady State • Equilibrium no net flux in any direction A B Steady state constant flux in one direction A B C n 32
![Adenine nucleotides play special roles in metabolic regulation n Maintaining constant [ATP] is so Adenine nucleotides play special roles in metabolic regulation n Maintaining constant [ATP] is so](https://present5.com/presentation/76aacfb021748451f3412436f14851fd/image-33.jpg)
Adenine nucleotides play special roles in metabolic regulation n Maintaining constant [ATP] is so important for cells ]ATP] in a typical cell of animal tissues is 5 m. M, and many ATPusing enzymes have Km 0. 1 -1 m. M If ATP drops, these E are not saturated with ATP→ reaction rates ↓, cells cannot survive this kinetic effect on so many reactions
![Thermodynamic effect of lowered [ATP] n To get cellular work, ATP is converted to Thermodynamic effect of lowered [ATP] n To get cellular work, ATP is converted to](https://present5.com/presentation/76aacfb021748451f3412436f14851fd/image-34.jpg)
Thermodynamic effect of lowered [ATP] n To get cellular work, ATP is converted to ADP or AMP, the ATP/ADP or ATP/AMP has important effect on all reactions that use these cofactors [AMP] is a more sensitive indicator of an energetic state of cells than [ATP]. In the cell, [ATP] (5 m. M) is far higher than [AMP] ( 0. 1 m. M). When ATP is used (e. g. muscle contraction), AMP is produced by ATP→ADP, then 2 ADP Adenylate kinase AMP + ATP n If [ATP] ↓ 10%, increase in [AMP] is greater than that of ADP n
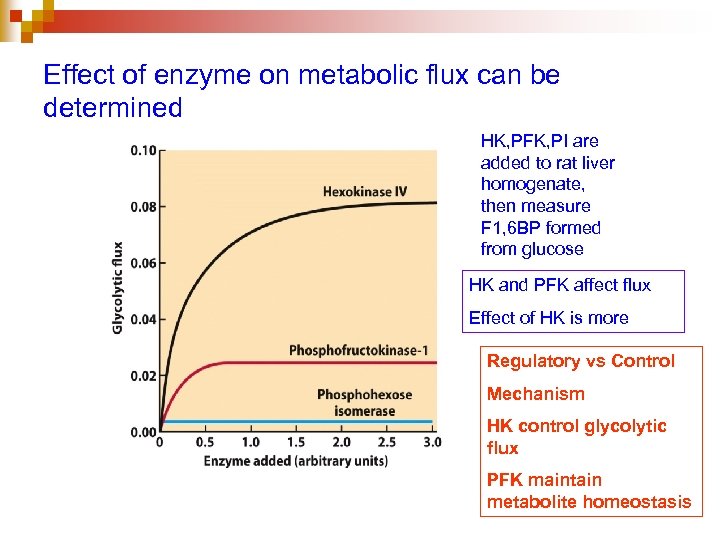
Effect of enzyme on metabolic flux can be determined HK, PFK, PI are added to rat liver homogenate, then measure F 1, 6 BP formed from glucose HK and PFK affect flux Effect of HK is more Regulatory vs Control Mechanism HK control glycolytic flux PFK maintain metabolite homeostasis
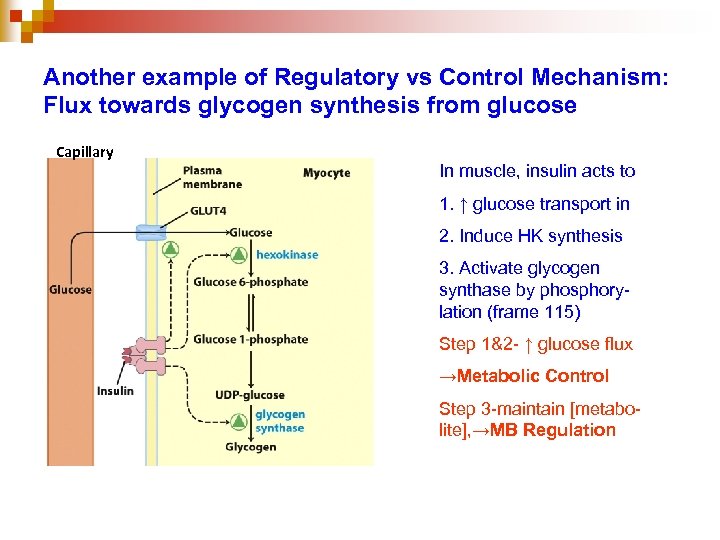
Another example of Regulatory vs Control Mechanism: Flux towards glycogen synthesis from glucose Capillary In muscle, insulin acts to 1. ↑ glucose transport in 2. Induce HK synthesis 3. Activate glycogen synthase by phosphorylation (frame 115) Step 1&2 - ↑ glucose flux →Metabolic Control Step 3 -maintain [metabolite], →MB Regulation
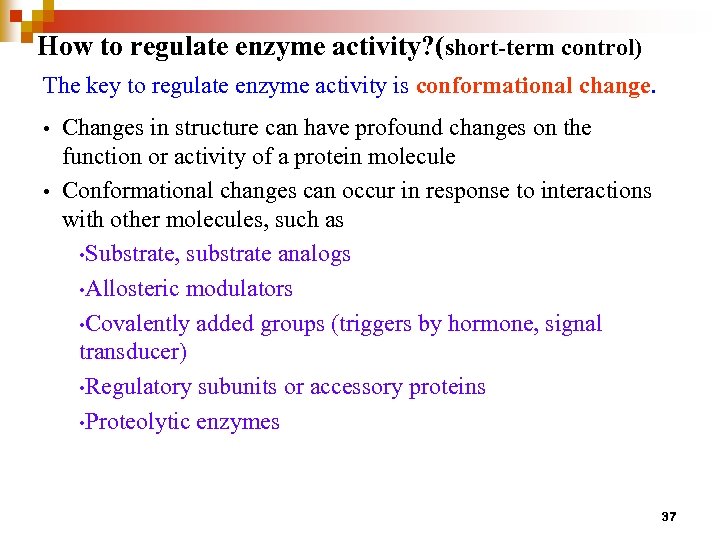
How to regulate enzyme activity? (short-term control) The key to regulate enzyme activity is conformational change. • Changes in structure can have profound changes on the function or activity of a protein molecule • Conformational changes can occur in response to interactions with other molecules, such as • Substrate, substrate analogs • Allosteric modulators • Covalently added groups (triggers by hormone, signal transducer) • Regulatory subunits or accessory proteins • Proteolytic enzymes 37
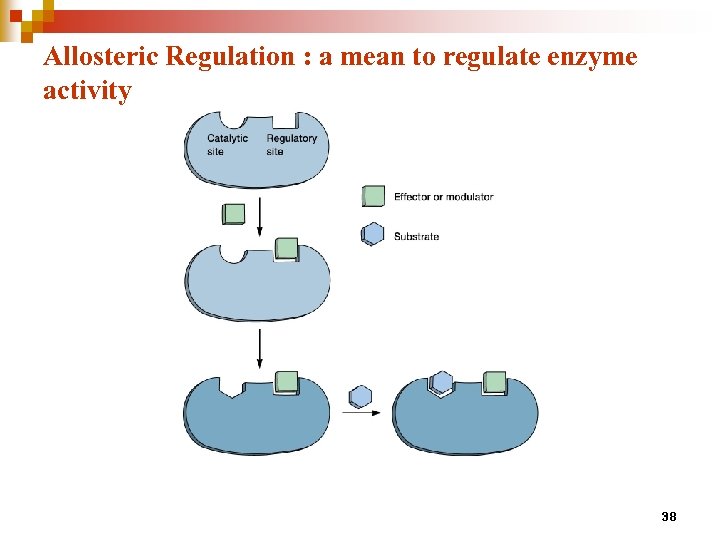
Allosteric Regulation : a mean to regulate enzyme activity 38
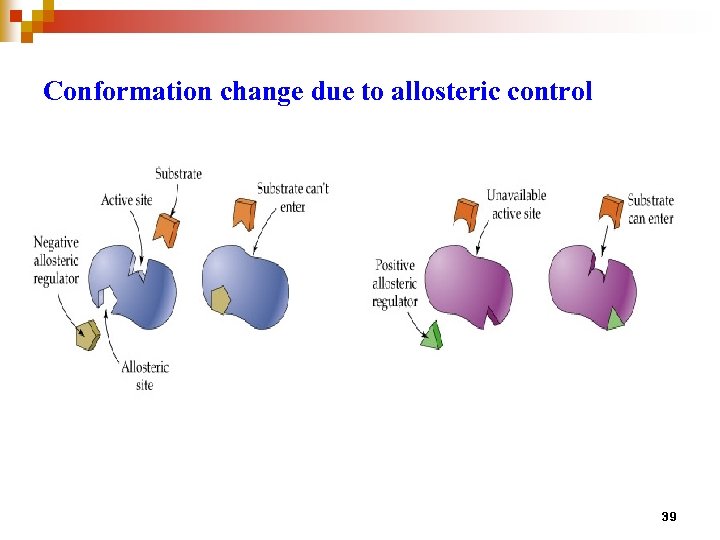
Conformation change due to allosteric control 39
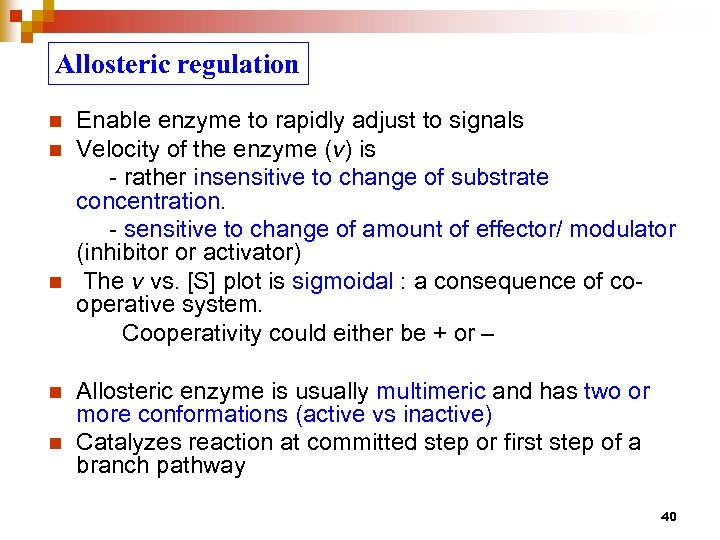
Allosteric regulation n n Enable enzyme to rapidly adjust to signals Velocity of the enzyme (v) is - rather insensitive to change of substrate concentration. - sensitive to change of amount of effector/ modulator (inhibitor or activator) The v vs. [S] plot is sigmoidal : a consequence of cooperative system. Cooperativity could either be + or – Allosteric enzyme is usually multimeric and has two or more conformations (active vs inactive) Catalyzes reaction at committed step or first step of a branch pathway 40
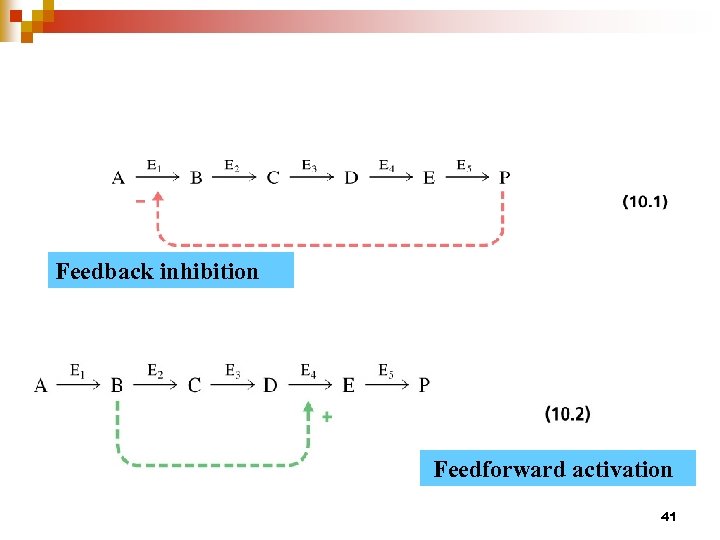
Feedback inhibition Feedforward activation 41
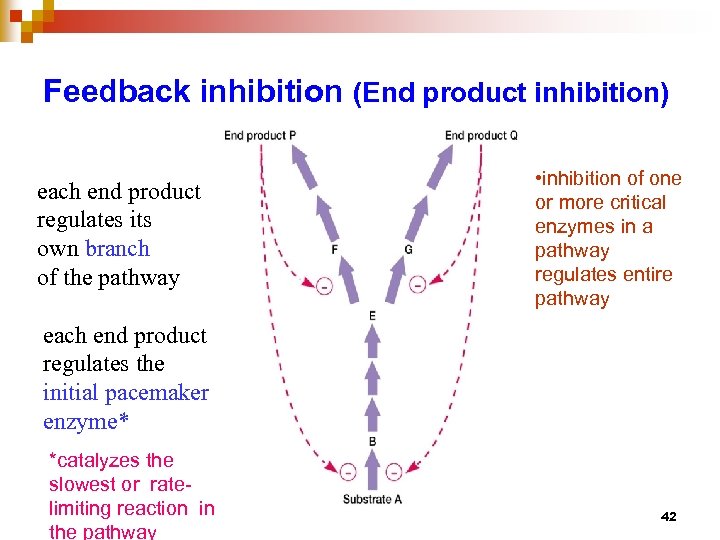
Feedback inhibition (End product inhibition) each end product regulates its own branch of the pathway • inhibition of one or more critical enzymes in a pathway regulates entire pathway each end product regulates the initial pacemaker enzyme* *catalyzes the slowest or ratelimiting reaction in the pathway 42
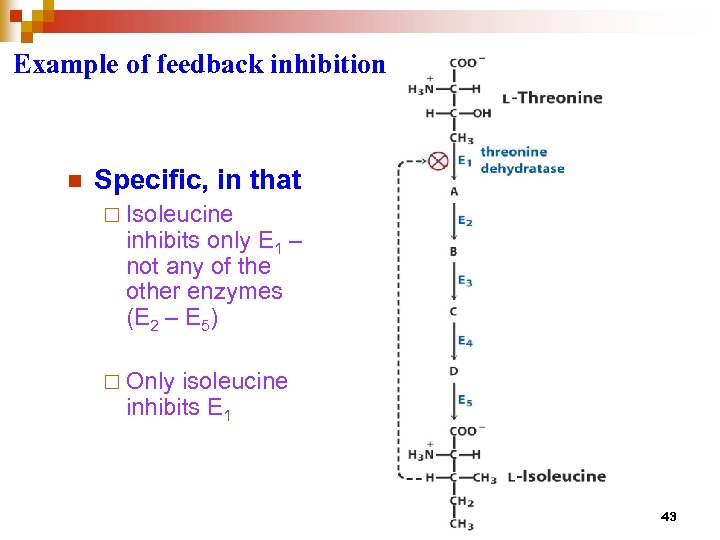
Example of feedback inhibition n Specific, in that ¨ Isoleucine inhibits only E 1 – not any of the other enzymes (E 2 – E 5) ¨ Only isoleucine inhibits E 1 43
Feed forward activation 44
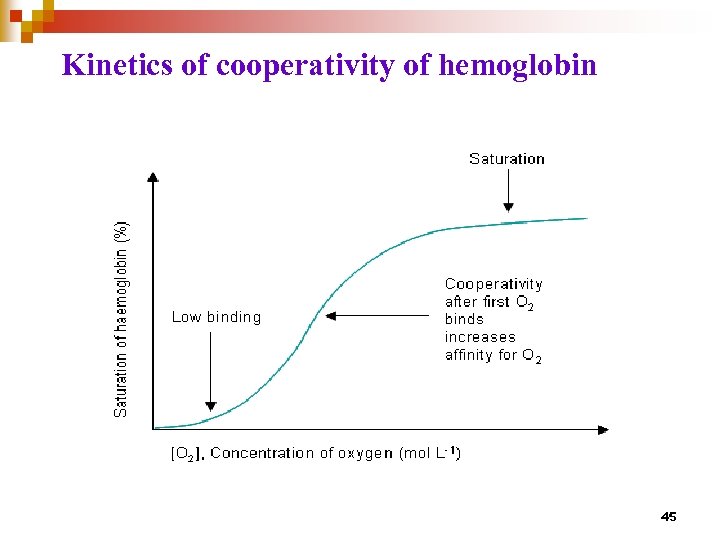
Kinetics of cooperativity of hemoglobin 45

Kinetics of allosteric enzyme • A sigmoid rather than hyperbolic curve for a plot of vo vs. [S] suggests allosteric, or cooperative interactions among subunits • Modulating activators and/or inhibitors can cause major shifts in these curves • Cooperativity can be : Homotropic : when S is also A (e. g. O 2 for Hb case) Heterotropic : when M is not S, M can be A (positive cooperativity) or I (negative cooperativity) 46
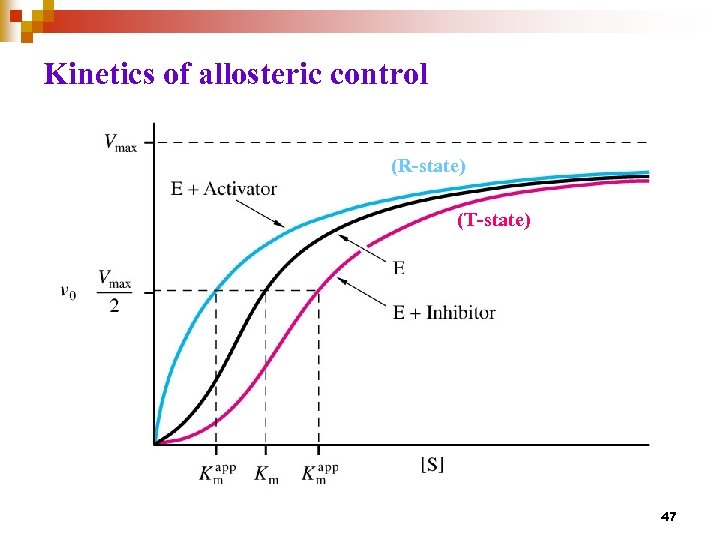
Kinetics of allosteric control (R-state) (R state) (T-state) 47
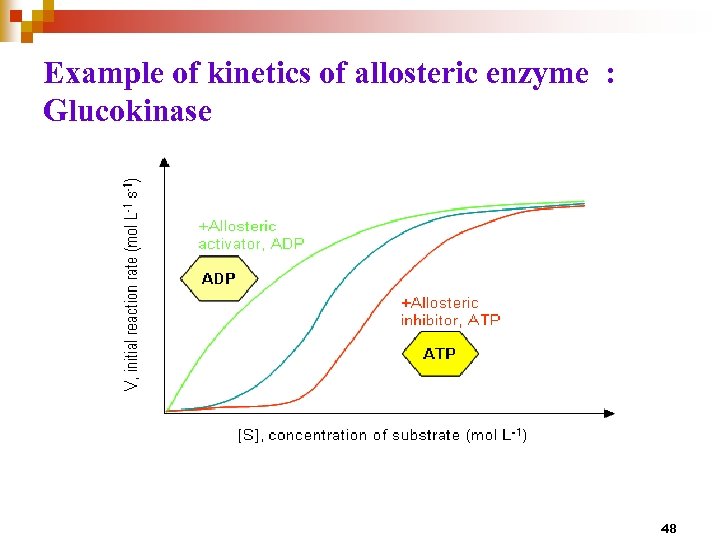
Example of kinetics of allosteric enzyme : Glucokinase 48
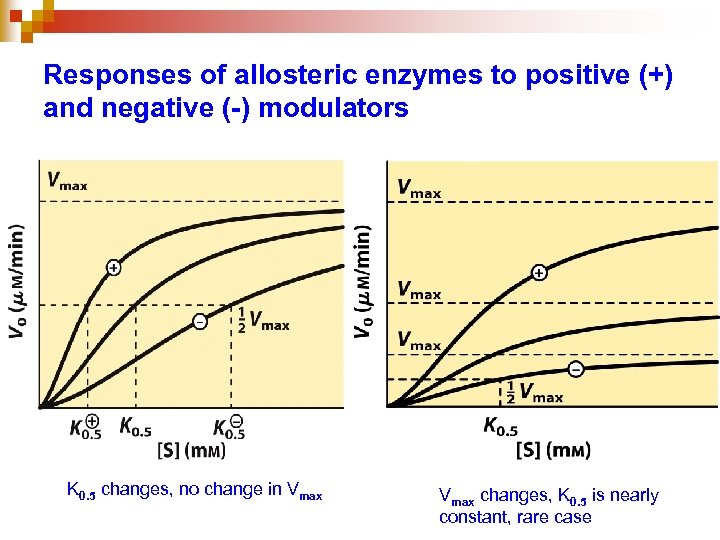
Responses of allosteric enzymes to positive (+) and negative (-) modulators K 0. 5 changes, no change in Vmax changes, K 0. 5 is nearly constant, rare case
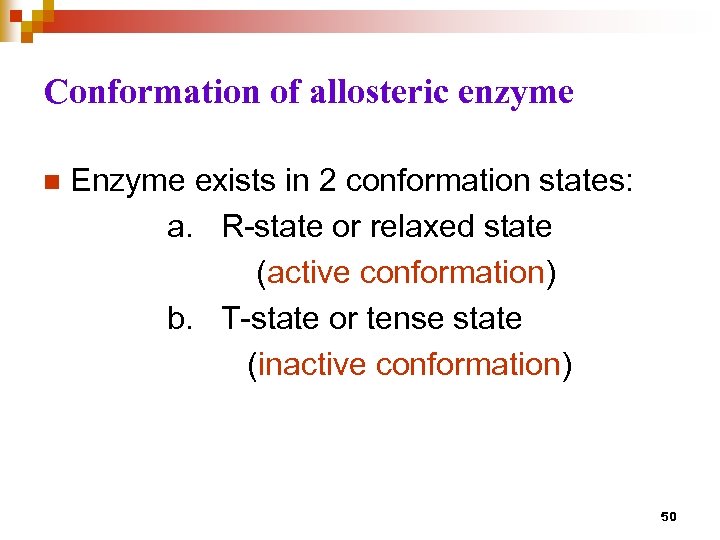
Conformation of allosteric enzyme n Enzyme exists in 2 conformation states: a. R-state or relaxed state (active conformation) b. T-state or tense state (inactive conformation) 50
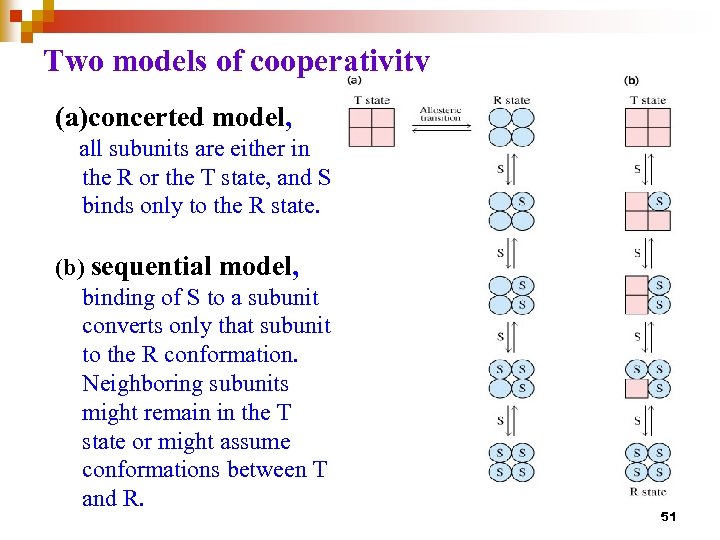
Two models of cooperativity (a)concerted model, all subunits are either in the R or the T state, and S binds only to the R state. (b) sequential model, binding of S to a subunit converts only that subunit to the R conformation. Neighboring subunits might remain in the T state or might assume conformations between T and R. 51
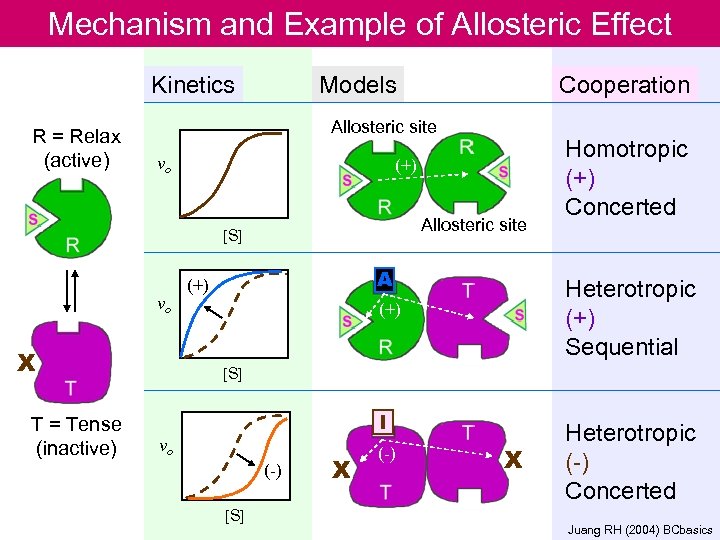
Mechanism and Example of Allosteric Effect Kinetics R = Relax (active) Models Allosteric site vo (+) Allosteric site [S] vo X T = Tense (inactive) Cooperation A (+) Homotropic (+) Concerted Heterotropic (+) Sequential (+) [S] I vo (-) [S] X (-) X Heterotropic (-) Concerted 52 Juang RH (2004) BCbasics
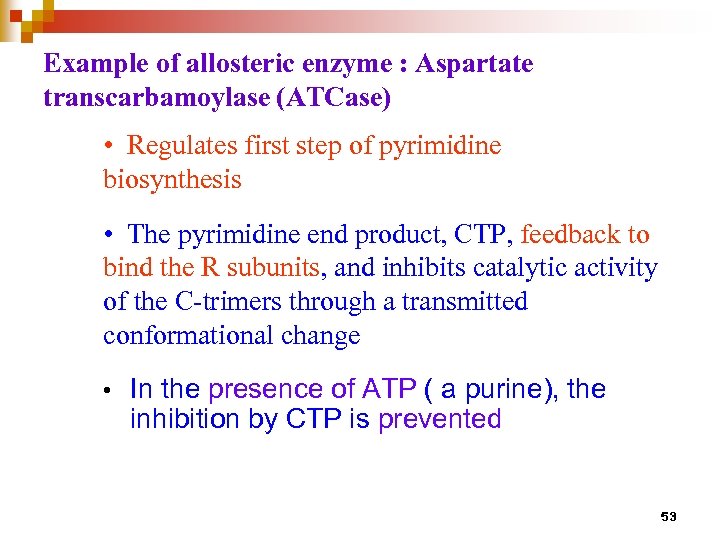
Example of allosteric enzyme : Aspartate transcarbamoylase (ATCase) • Regulates first step of pyrimidine biosynthesis • The pyrimidine end product, CTP, feedback to bind the R subunits, and inhibits catalytic activity of the C-trimers through a transmitted conformational change • In the presence of ATP ( a purine), the inhibition by CTP is prevented 53
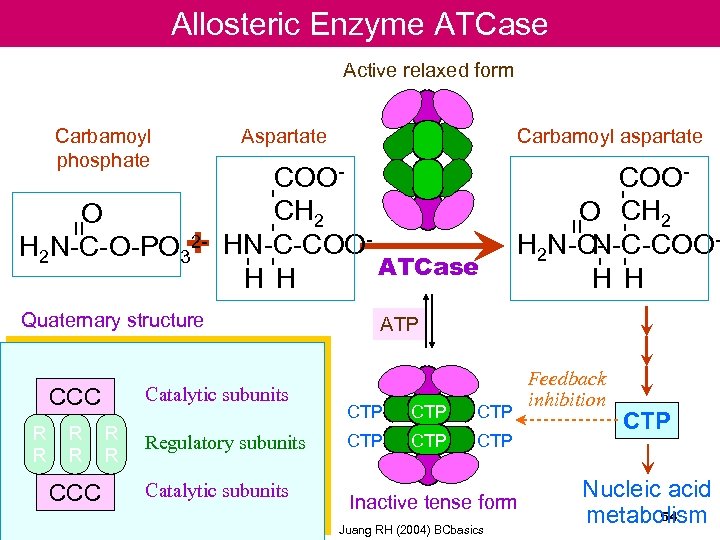
Allosteric Enzyme ATCase Active relaxed form Catalytic subunits R R CCC R R Regulatory subunits Catalytic subunits COOO CH 2 N-C-COOH 2 N-CH H - - - COOCH 2 HN-C-COOATCase H H Quaternary structure CCC Carbamoyl aspartate = = O 2 H 2 N-C-O-PO 3+ Aspartate - - - Carbamoyl phosphate ATP CTP CTP CTP Inactive tense form Juang RH (2004) BCbasics Feedback inhibition CTP Nucleic acid 54 metabolism
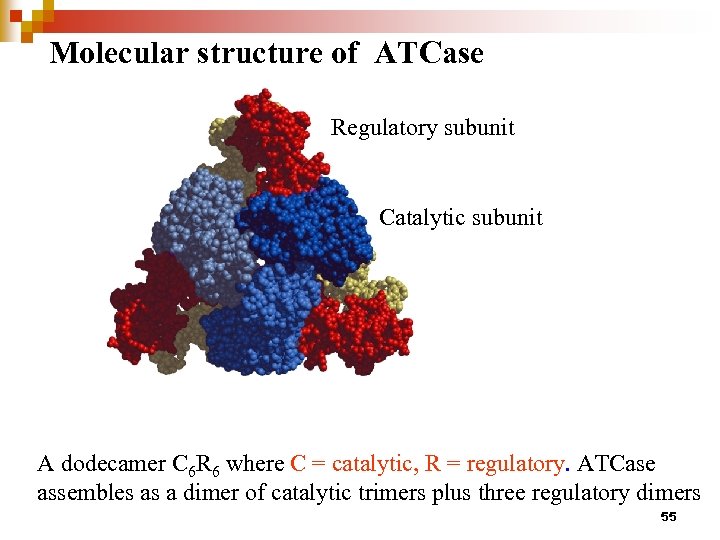
Molecular structure of ATCase Regulatory subunit Catalytic subunit A dodecamer C 6 R 6 where C = catalytic, R = regulatory. ATCase assembles as a dimer of catalytic trimers plus three regulatory dimers 55
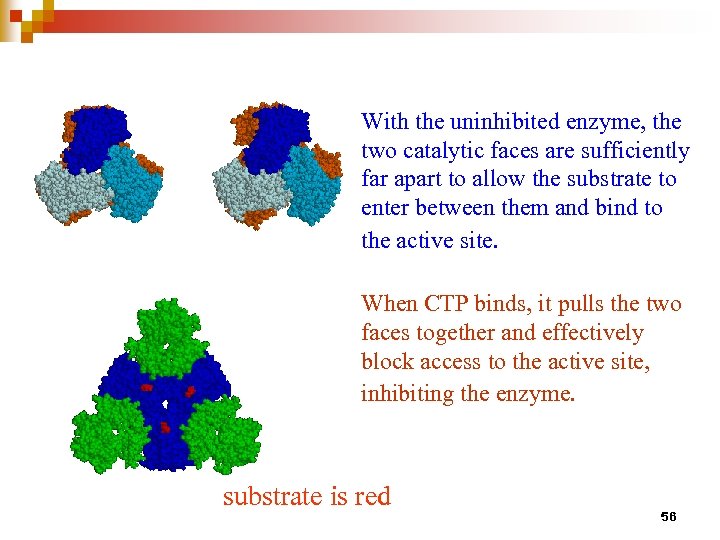
With the uninhibited enzyme, the two catalytic faces are sufficiently far apart to allow the substrate to enter between them and bind to the active site. When CTP binds, it pulls the two faces together and effectively block access to the active site, inhibiting the enzyme. substrate is red 56

Regulation of enzyme activity through covalent modification • reversible addition or removal of a chemical group which is covalently attached to protein (enzyme) • main examples are • phosphorylation – dephosphorylation • adenylation / uridylation / ADP- ribosylation • methylation 57
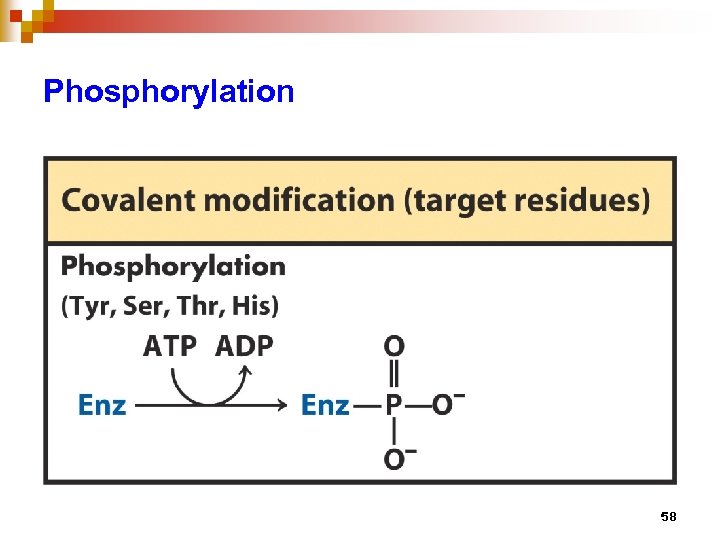
Phosphorylation 58
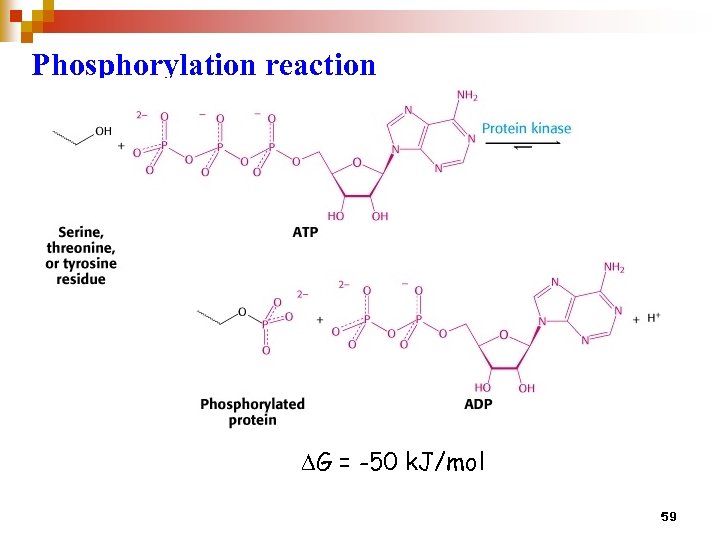
Phosphorylation reaction DG = -50 k. J/mol 59
The importance of Phosphorylation • The most common reversible covalent modification of proteins • Catalyzed by kinases • Adds two negative charges, altering electrostatic interactions • Phosphate groups also are effective at hydrogen bonding. • Phosphorylation may alter substrate binding and/or catalytic activity • Large negative ΔG for phosphorylation. About half of the -50 k. J/mol is conserved in the phosphorylated protein (the other half goes to making the reaction irreversible). 60
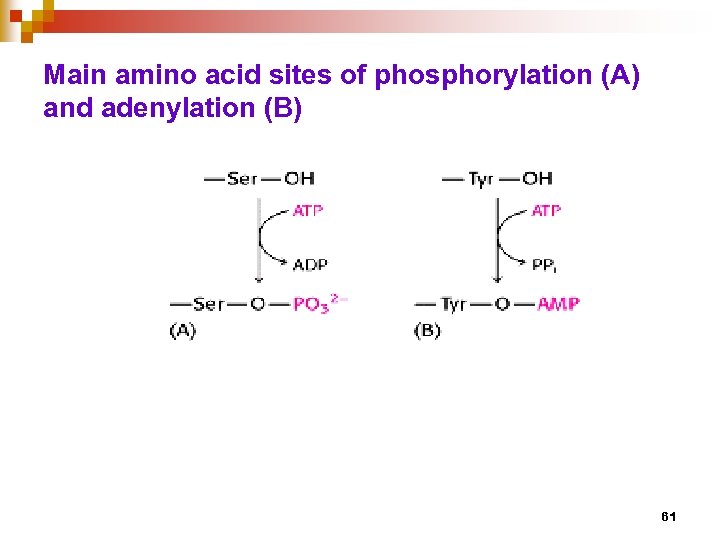
Main amino acid sites of phosphorylation (A) and adenylation (B) 61
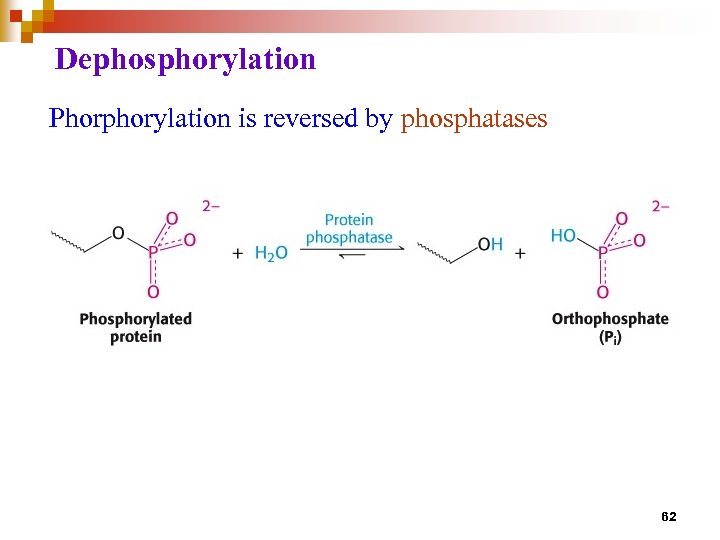
Dephosphorylation Phorphorylation is reversed by phosphatases 62
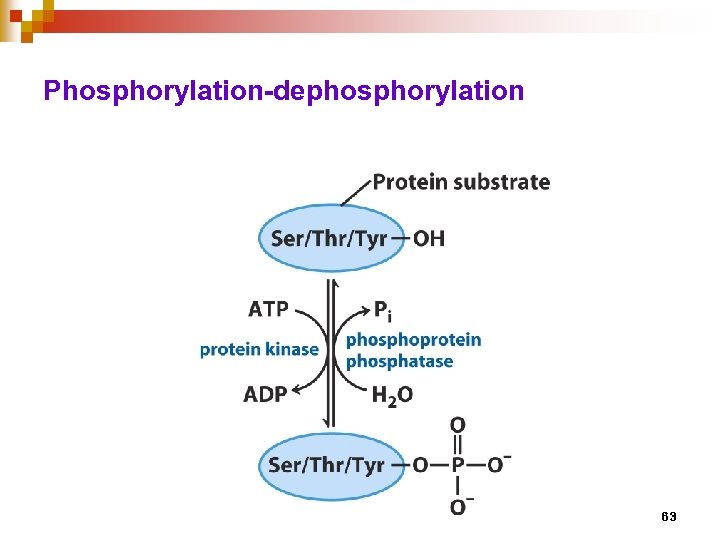
Phosphorylation-dephosphorylation 63
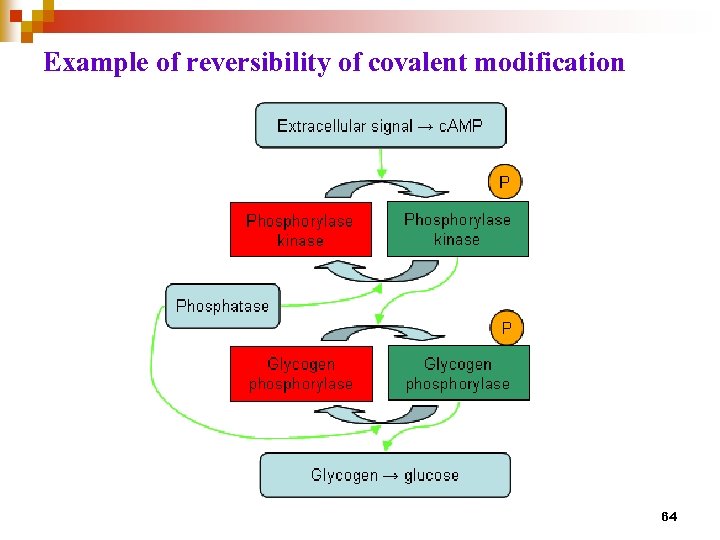
Example of reversibility of covalent modification 64
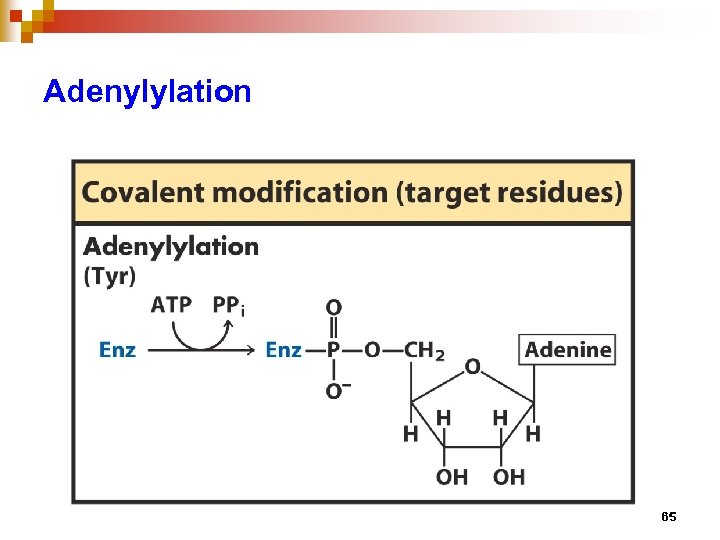
Adenylylation 65
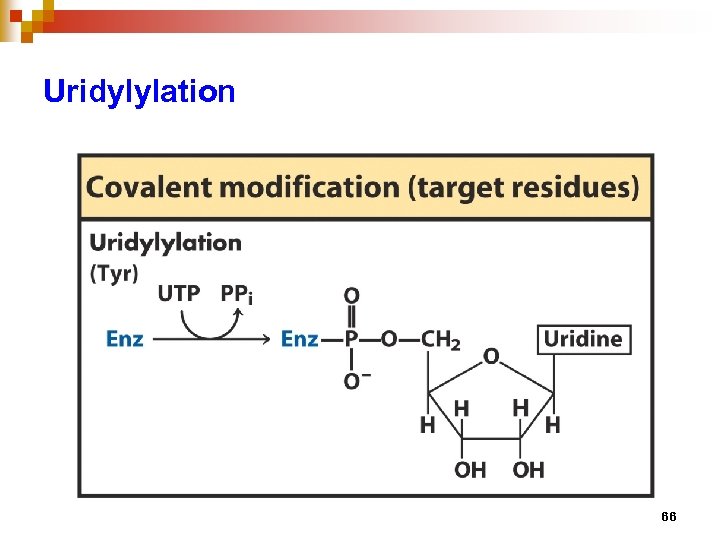
Uridylylation 66
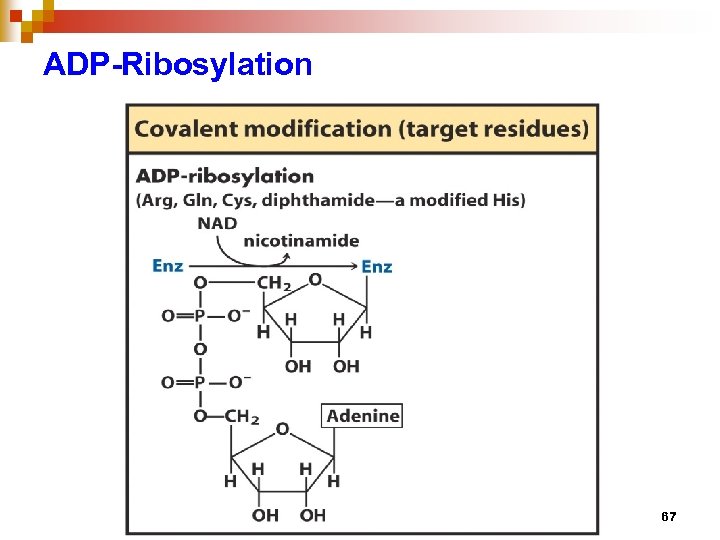
ADP-Ribosylation 67
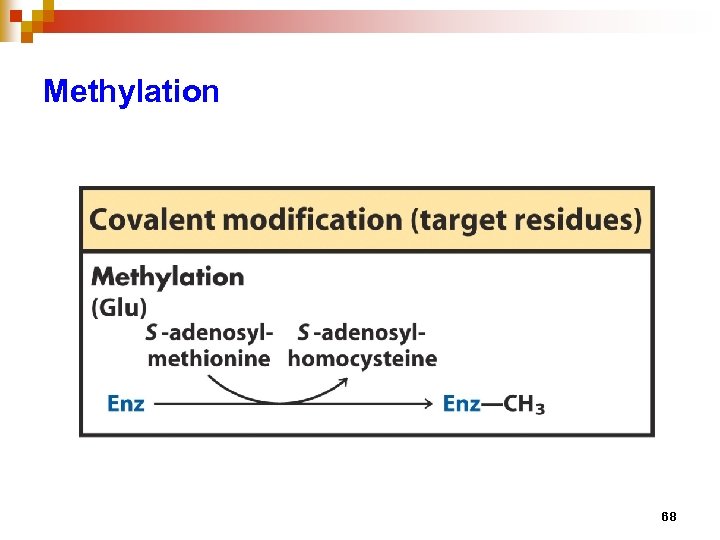
Methylation 68
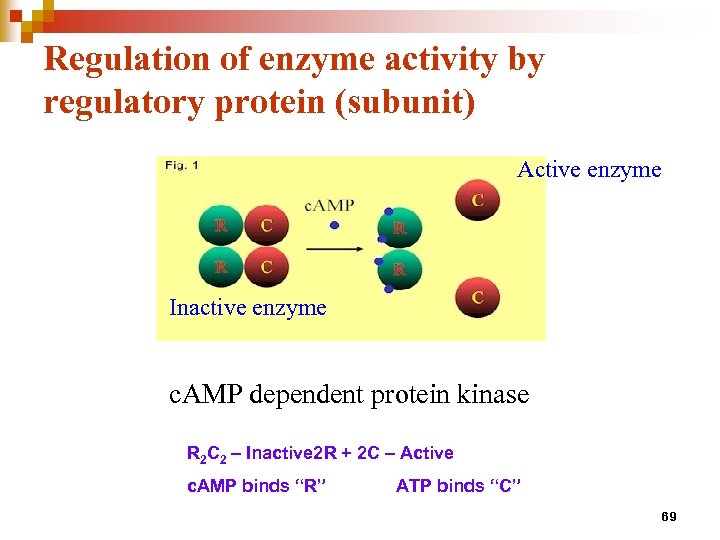
Regulation of enzyme activity by regulatory protein (subunit) Active enzyme Inactive enzyme c. AMP dependent protein kinase R 2 C 2 – Inactive 2 R + 2 C – Active c. AMP binds “R” ATP binds “C” 69
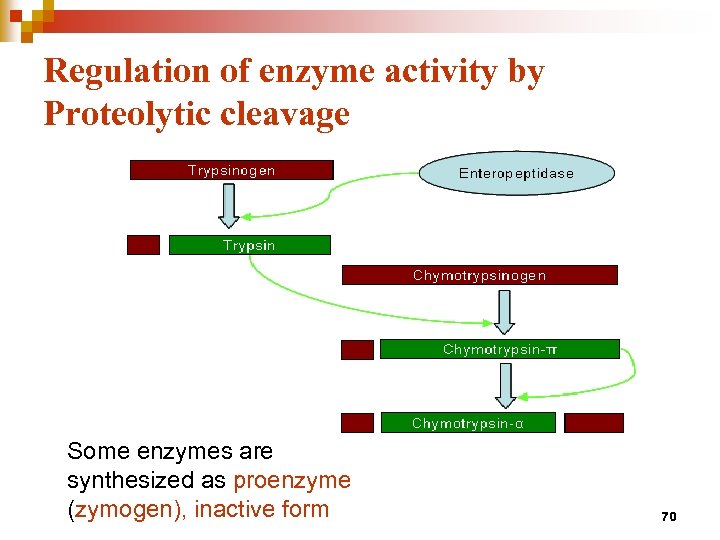
Regulation of enzyme activity by Proteolytic cleavage Some enzymes are synthesized as proenzyme (zymogen), inactive form 70
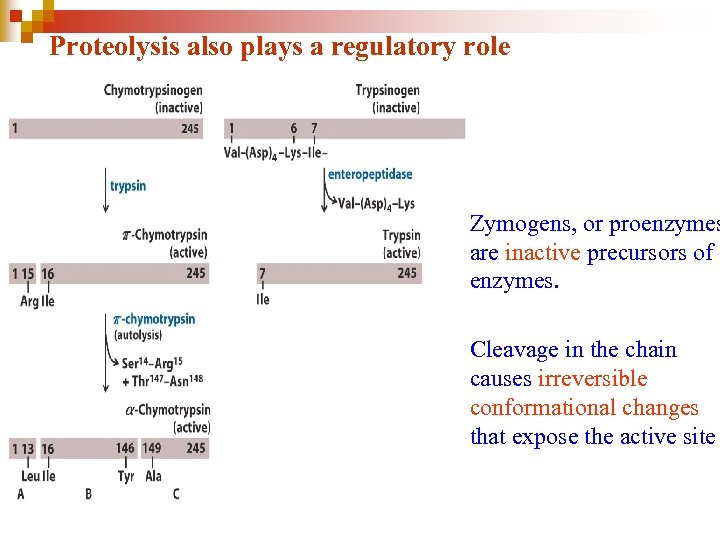
Proteolysis also plays a regulatory role • Zymogens, or proenzymes are inactive precursors of enzymes. • Cleavage in the chain causes irreversible conformational changes that expose the active site
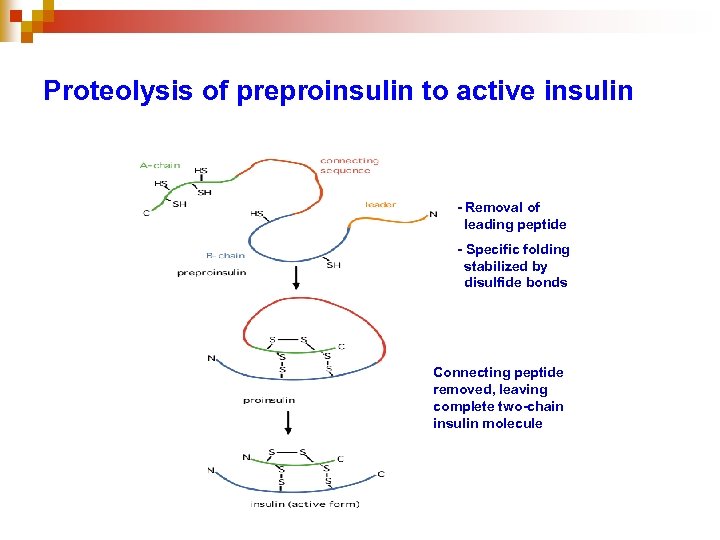
Proteolysis of preproinsulin to active insulin - Removal of leading peptide - Specific folding stabilized by disulfide bonds Connecting peptide removed, leaving complete two-chain insulin molecule

PART II Regulation of carbohydrate metabolism 73

Carbohydrate, Lipid and Protein metabolisms are tightly integrated as Energy Metabolism Main pathways are: n n n n 1. Glycolysis 2. Gluconeogenesis 3. Glycogen degradation and synthesis 4. Fatty acid degradation and synthesis 5. Citric acid cycle 6. Oxidative phosphorylation 7. Pentose phosphate pathway 8. Amino acid degradation and synthesis 74

Intrinsic vs Extrinsic Regulation n Intrinsic regulation by cell metabolites n Extrinsic regulation by external factors, i. e. hormones, stress, food, etc. 75
Intrinsic Regulation n Molecules such as NAD+, NADH, ATP, ADP, AMP are important allosteric effectors and intrinsic regulators of cellular metabolism. ●the concentrations of ATP-ADP-AMP mirror the energy charge of the cell Energy charge (EC) = [ATP] + ½ [ADP] [ATP] + [ADP] + [AMP] high EC favors biosynthesis, low EC favors catabolism ●the NAD(P)/NAD(P)H ratio also regulates metabolism low [NADH] promotes catabolism, high [NADH] = high ATP 76
![If [ATP] is low, degradative pathways are stimulated. If [ATP] is high, degradative pathways If [ATP] is low, degradative pathways are stimulated. If [ATP] is high, degradative pathways](https://present5.com/presentation/76aacfb021748451f3412436f14851fd/image-77.jpg)
If [ATP] is low, degradative pathways are stimulated. If [ATP] is high, degradative pathways are inhibited. Degradation Synthesis Regulation of Synthesis and Degradation of glucose and glycogen depend on the energy state of the cell 77
Extrinsic Regulation n Hormones are a higher order of regulation involving communication between cells, tissues, and the environment. n Hormones interact with receptors and set off a cascade of molecular events which: • stimulate or repress the activity of key enzymes. and / or • stimulate or repress the transcription of specific genes. 78
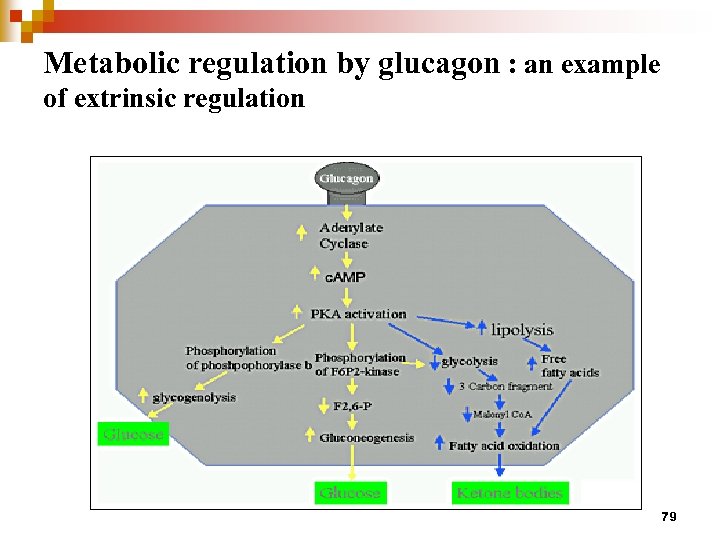
Metabolic regulation by glucagon : an example of extrinsic regulation 79
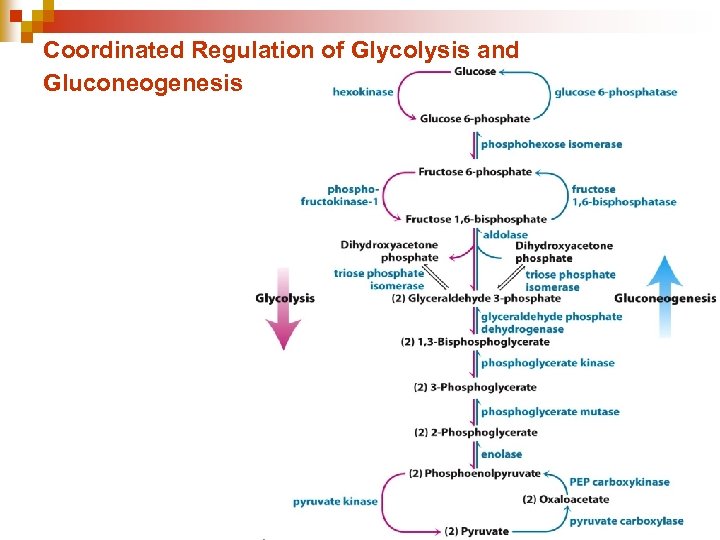
Coordinated Regulation of Glycolysis and Gluconeogenesis
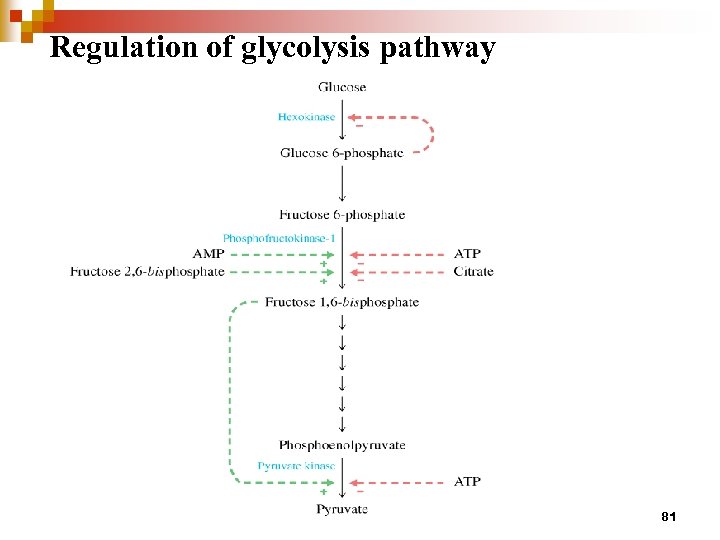
Regulation of glycolysis pathway 81
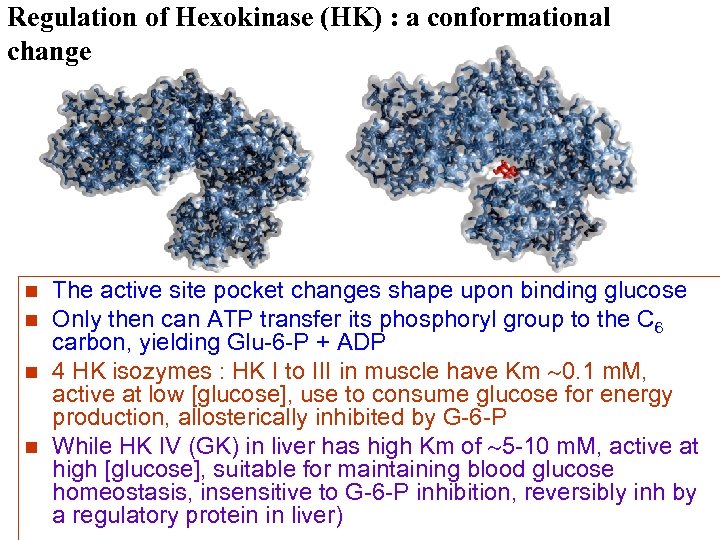
Regulation of Hexokinase (HK) : a conformational change n n The active site pocket changes shape upon binding glucose Only then can ATP transfer its phosphoryl group to the C 6 carbon, yielding Glu-6 -P + ADP 4 HK isozymes : HK I to III in muscle have Km 0. 1 m. M, active at low [glucose], use to consume glucose for energy production, allosterically inhibited by G-6 -P While HK IV (GK) in liver has high Km of 5 -10 m. M, active at high [glucose], suitable for maintaining blood glucose homeostasis, insensitive to G-6 -P inhibition, reversibly inh by a regulatory protein in liver)
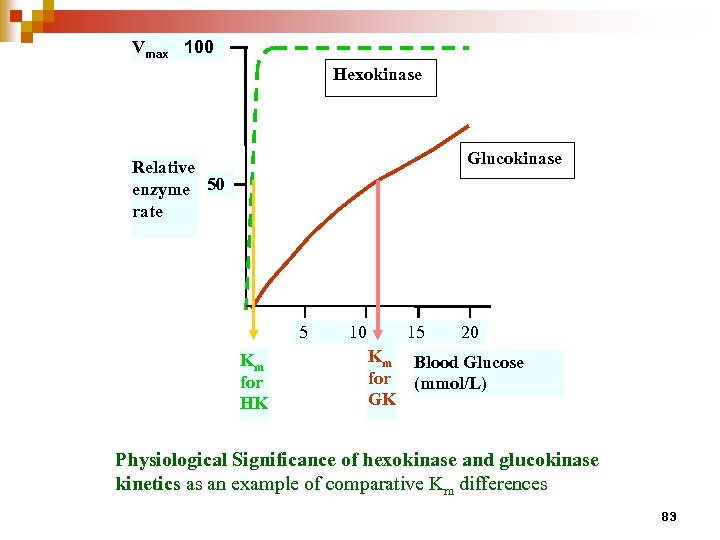
Vmax 100 Hexokinase Glucokinase Relative enzyme 50 rate 5 Km for HK 10 15 Km for GK 20 Blood Glucose (mmol/L) Physiological Significance of hexokinase and glucokinase kinetics as an example of comparative Km differences 83
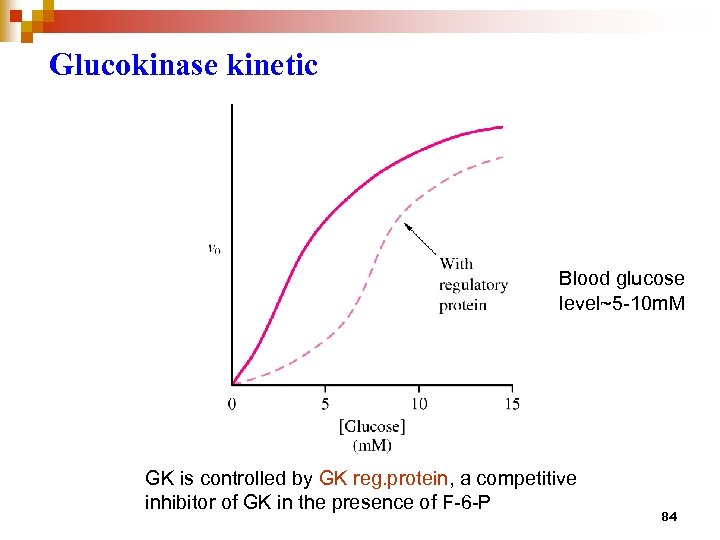
Glucokinase kinetic Blood glucose level~5 -10 m. M GK is controlled by GK reg. protein, a competitive inhibitor of GK in the presence of F-6 -P 84
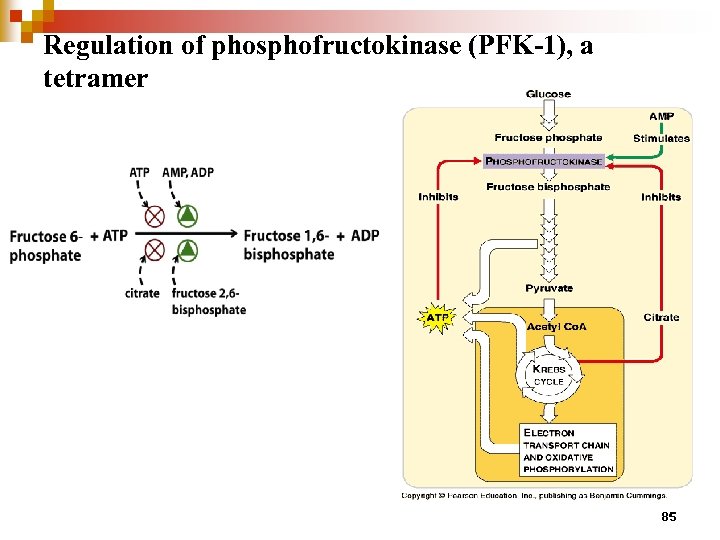
Regulation of phosphofructokinase (PFK-1), a tetramer 85
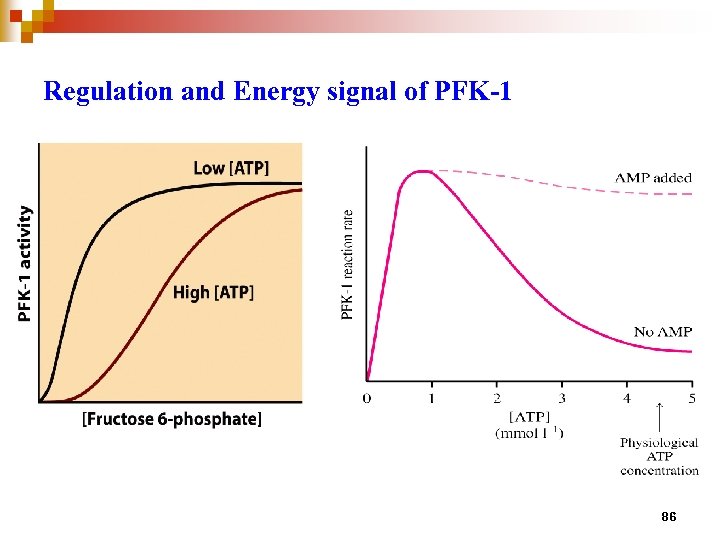
Regulation and Energy signal of PFK-1 86
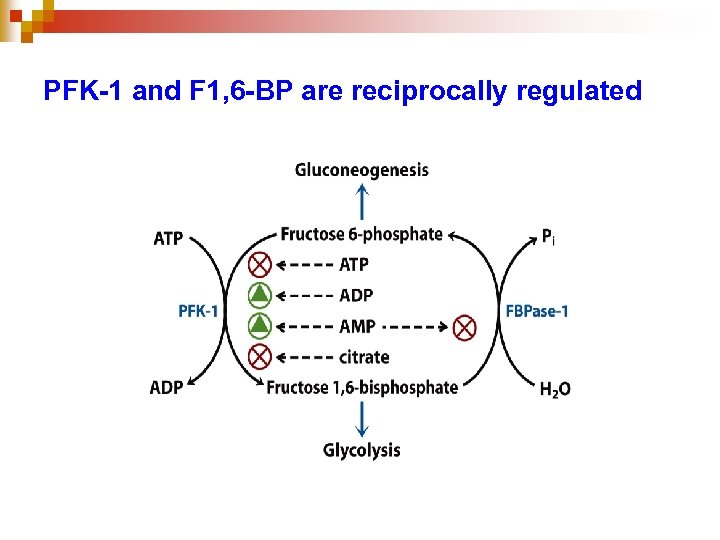
PFK-1 and F 1, 6 -BP are reciprocally regulated
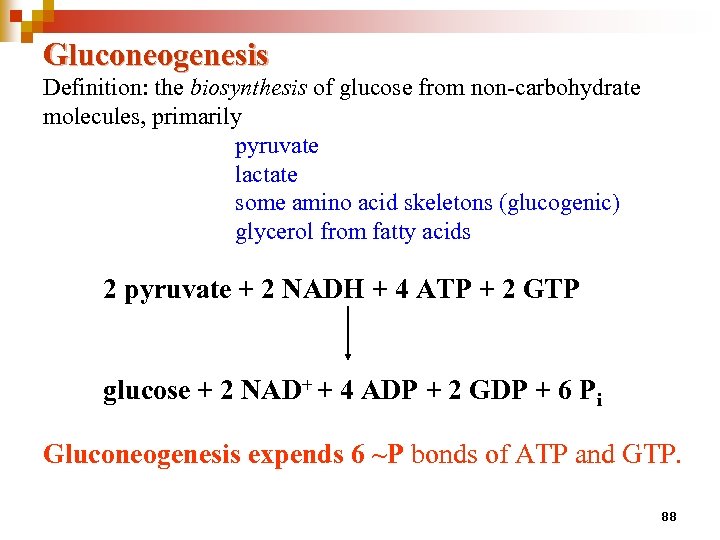
Gluconeogenesis Definition: the biosynthesis of glucose from non-carbohydrate molecules, primarily pyruvate lactate some amino acid skeletons (glucogenic) glycerol from fatty acids 2 pyruvate + 2 NADH + 4 ATP + 2 GTP glucose + 2 NAD+ + 4 ADP + 2 GDP + 6 Pi Gluconeogenesis expends 6 ~P bonds of ATP and GTP. 88

CONTROL: • gluconeogenesis serves as an alternative source of glucose when supplies are low and is largely controlled by diet. • high carbohydrate in meal reduce gluconeogenesis and starvation increases. • gluconeogenesis and glycolysis are controlled in reciprocal fashion. Two main points : 1. control PFK-1 and F 1, 6 -BPase 2. control fate of pyruvate at PDH or Pyr carboxylase 89
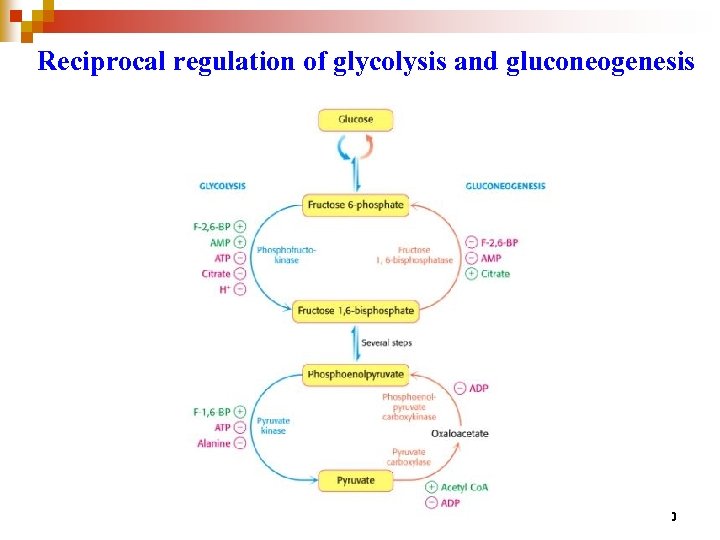
Reciprocal regulation of glycolysis and gluconeogenesis 90
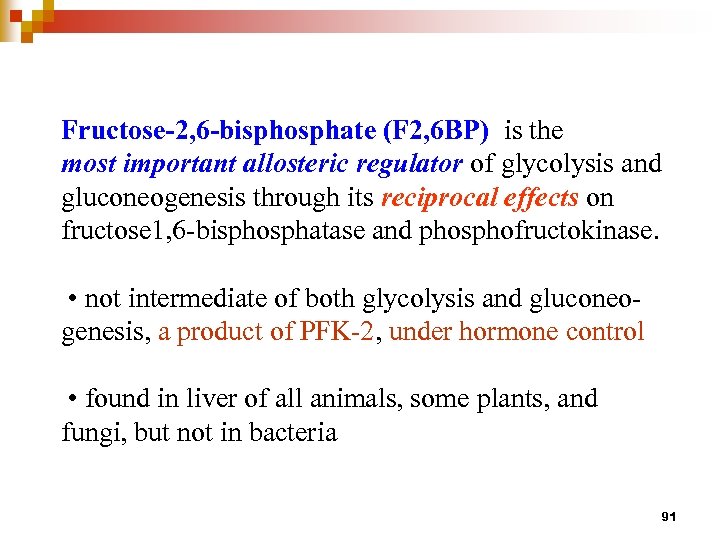
Fructose-2, 6 -bisphosphate (F 2, 6 BP) is the most important allosteric regulator of glycolysis and gluconeogenesis through its reciprocal effects on fructose 1, 6 -bisphosphatase and phosphofructokinase. • not intermediate of both glycolysis and gluconeogenesis, a product of PFK-2, under hormone control • found in liver of all animals, some plants, and fungi, but not in bacteria 91
92
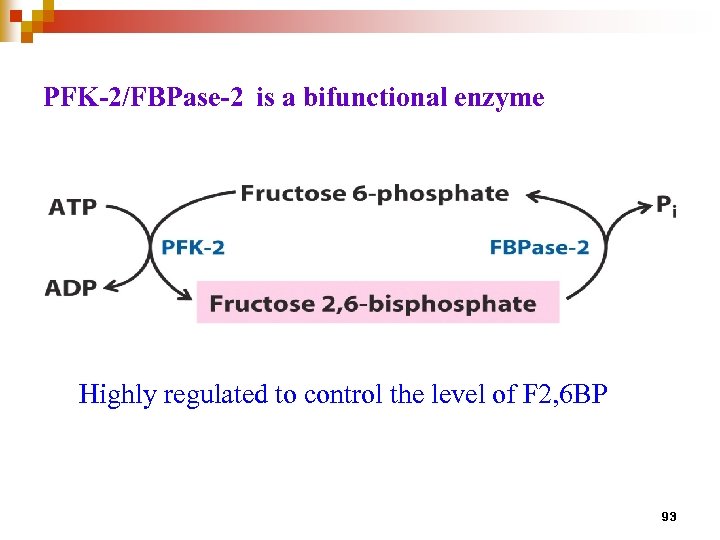
PFK-2/FBPase-2 is a bifunctional enzyme Highly regulated to control the level of F 2, 6 BP 93
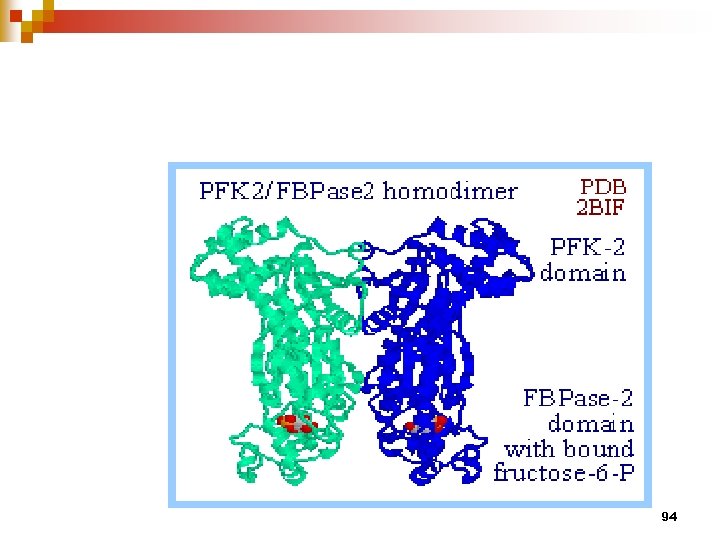
94
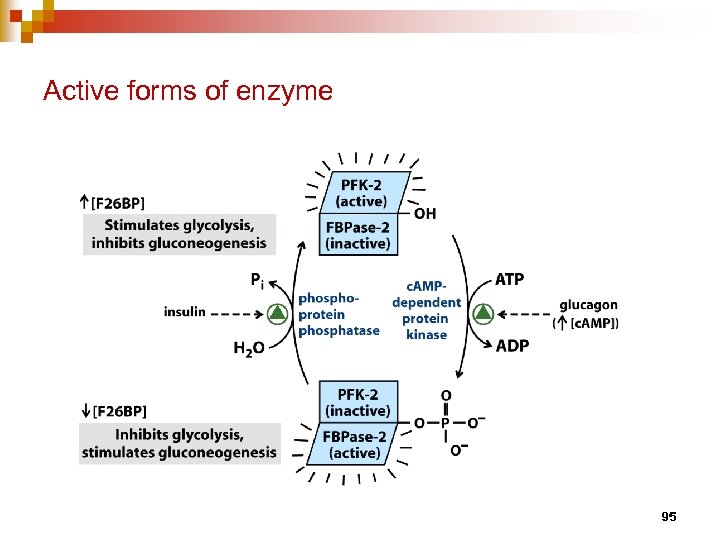
Active forms of enzyme 95
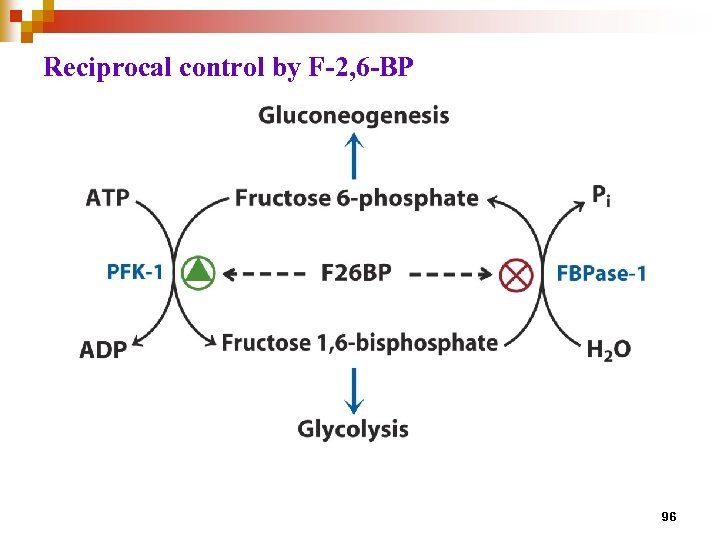
Reciprocal control by F-2, 6 -BP 96
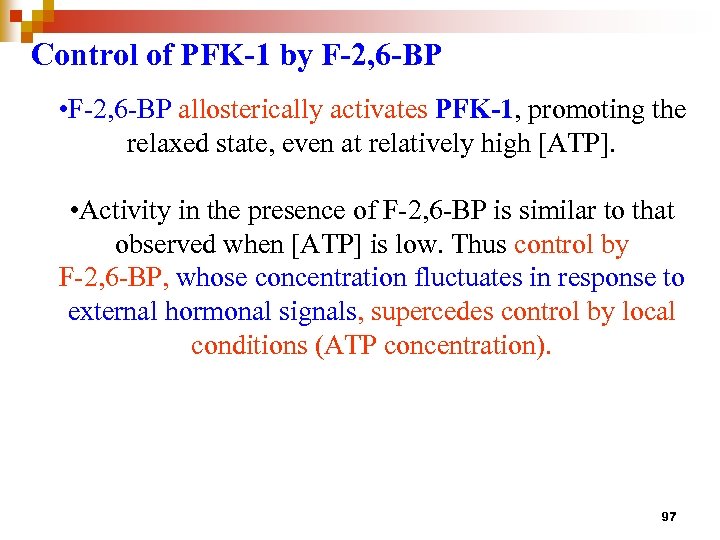
Control of PFK-1 by F-2, 6 -BP • F-2, 6 -BP allosterically activates PFK-1, promoting the relaxed state, even at relatively high [ATP]. • Activity in the presence of F-2, 6 -BP is similar to that observed when [ATP] is low. Thus control by F-2, 6 -BP, whose concentration fluctuates in response to external hormonal signals, supercedes control by local conditions (ATP concentration). 97
Pyruvate kinase (PK) n n n catalyzes substrate level phosphorylation, PEP Pyr 3 isozymes in vertebrates, allosterically inh by ATP, Ac. Co. A and long chain fatty acids isoenzyme L (liver) – additionally regulated by glucagon via phosphorylation isoenzyme M (muscle) feedforward activated by F 1, 6 BP, feedback inhibition by ATP 98
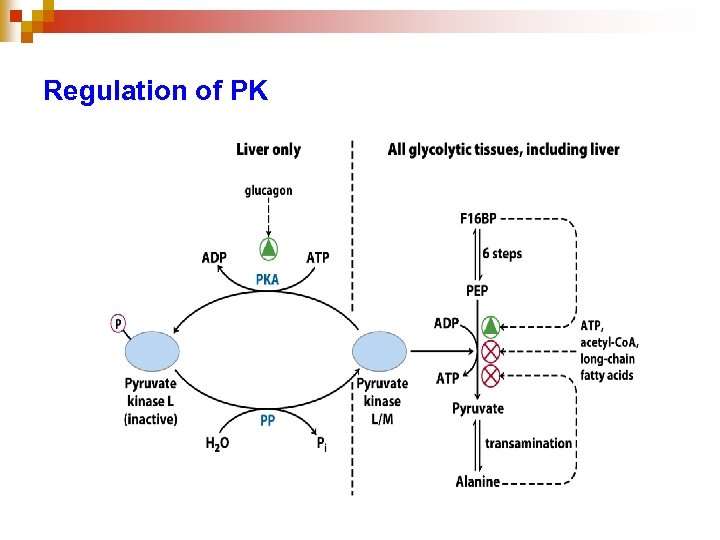
Regulation of PK
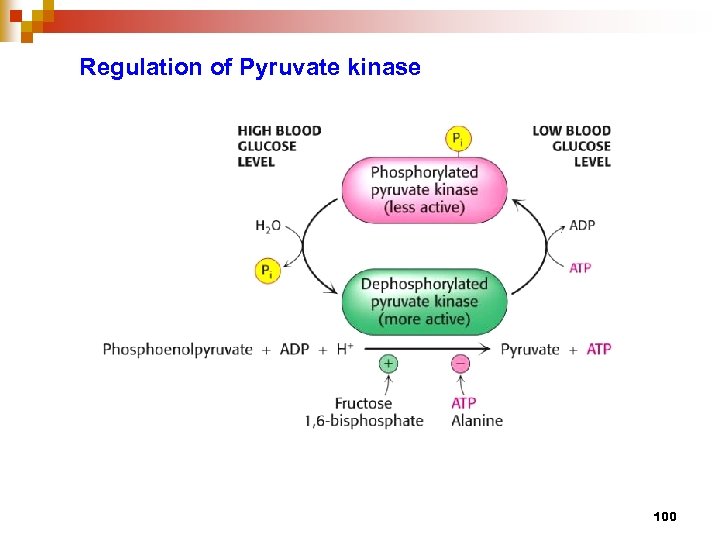
Regulation of Pyruvate kinase 100
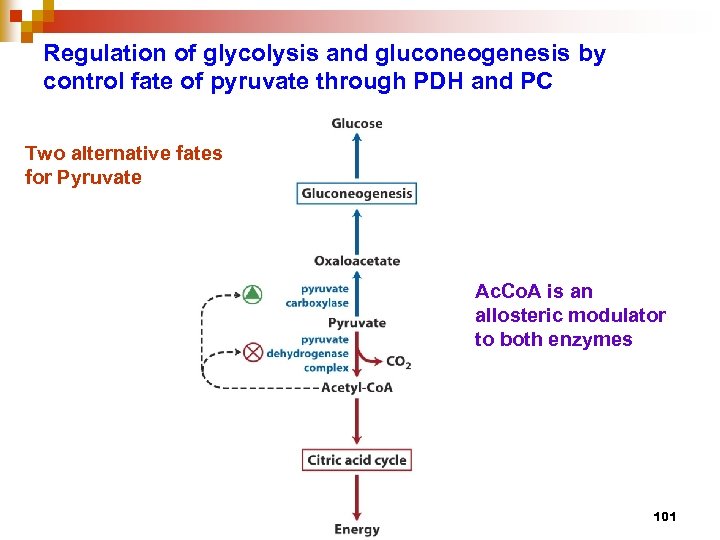
Regulation of glycolysis and gluconeogenesis by control fate of pyruvate through PDH and PC Two alternative fates for Pyruvate Ac. Co. A is an allosteric modulator to both enzymes 101
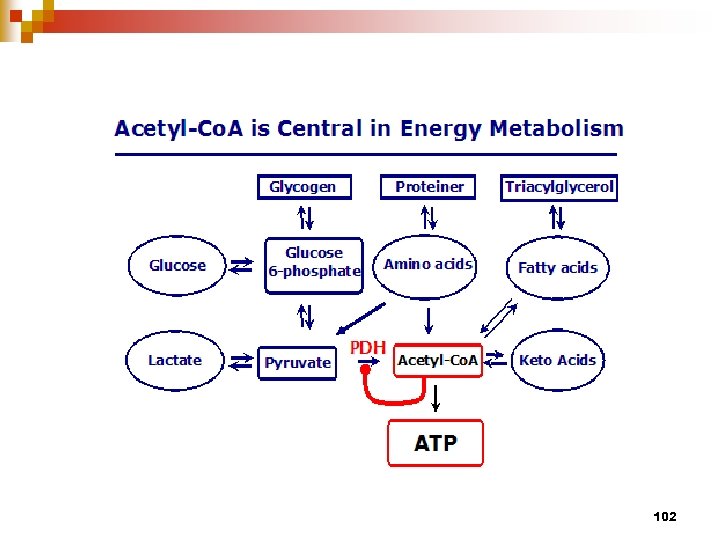
102
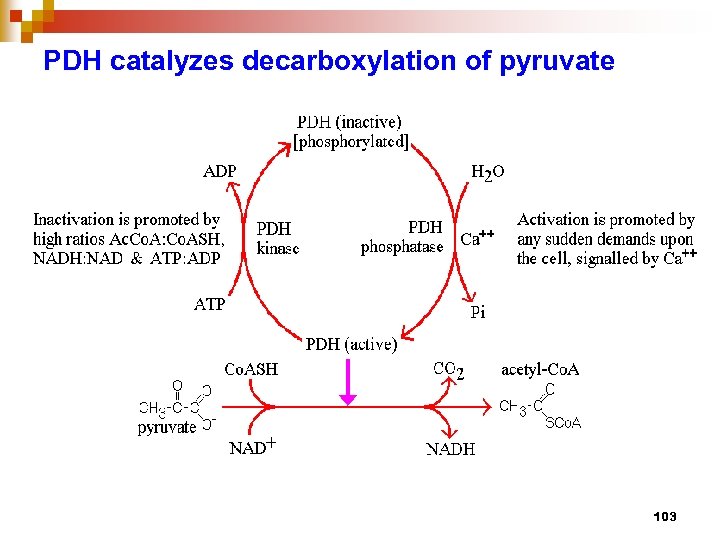
PDH catalyzes decarboxylation of pyruvate 103
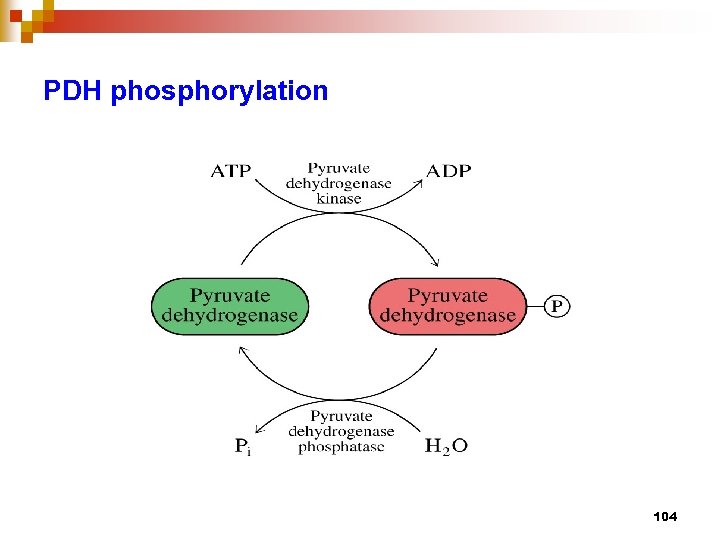
PDH phosphorylation 104

PDH multienzyme complex n n consists of 3 enzymes, E 1 – Pyr DH tetramer, E 2 – dihydrolipoamide (DHLA) acetyl transferase, E 3 – DHLA DH involves 5 coenzymes / prosthetic group : Co. ASH, NAD+, TPP, Lipoate, FAD bind E 1, E 2, and E 3, respectively, then pyruvate comes in, transfer acetyl group to E 2 -lipoyl, then Co. ASH comes to receive Ac. And released as Ac. Co. A , finally NAD comes in to receive H+ from FADH 2 bound at E 3 all 3 enzymes are controlled by covelent modi. and allosteric reg. through 2 regulatory enzymes : PDH kinase and PDH phosphatase 105
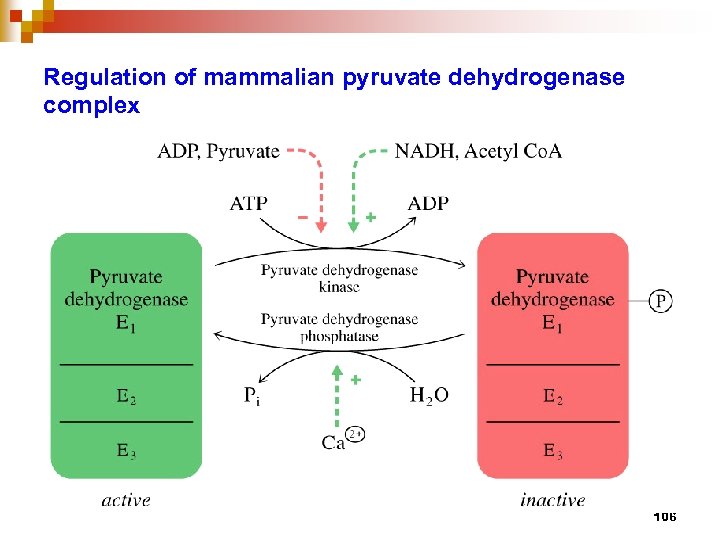
Regulation of mammalian pyruvate dehydrogenase complex 106
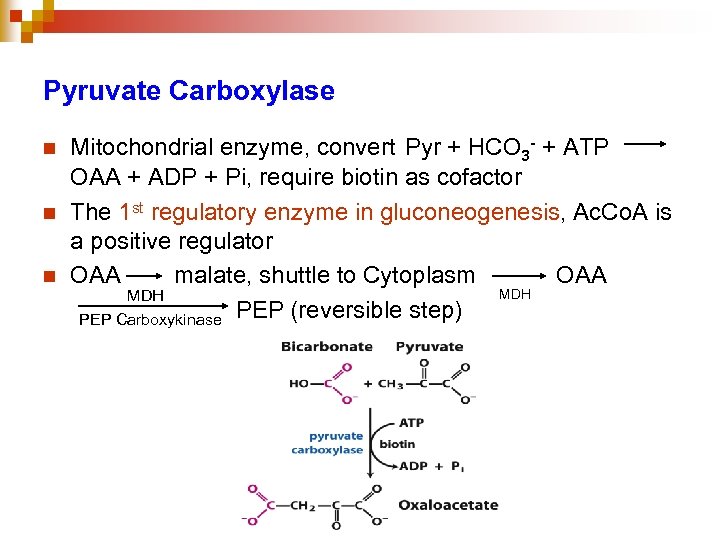
Pyruvate Carboxylase n n n Mitochondrial enzyme, convert Pyr + HCO 3 - + ATP OAA + ADP + Pi, require biotin as cofactor The 1 st regulatory enzyme in gluconeogenesis, Ac. Co. A is a positive regulator OAA malate, shuttle to Cytoplasm OAA MDH PEP Carboxykinase PEP (reversible step)
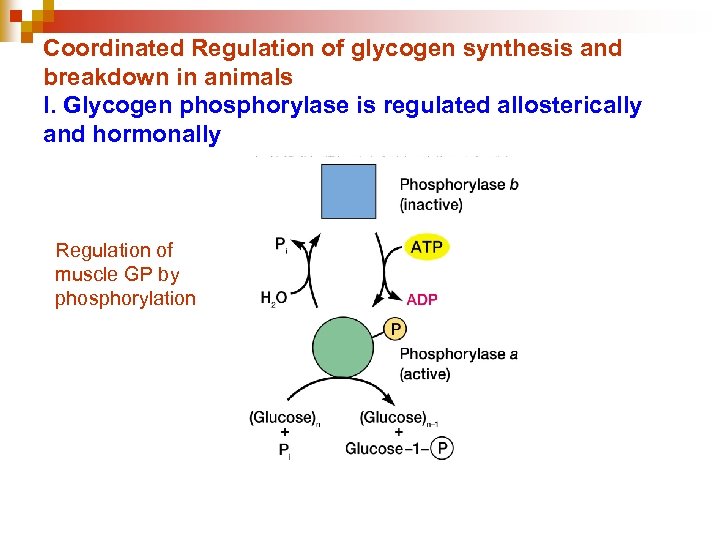
Coordinated Regulation of glycogen synthesis and breakdown in animals I. Glycogen phosphorylase is regulated allosterically and hormonally Regulation of muscle GP by phosphorylation
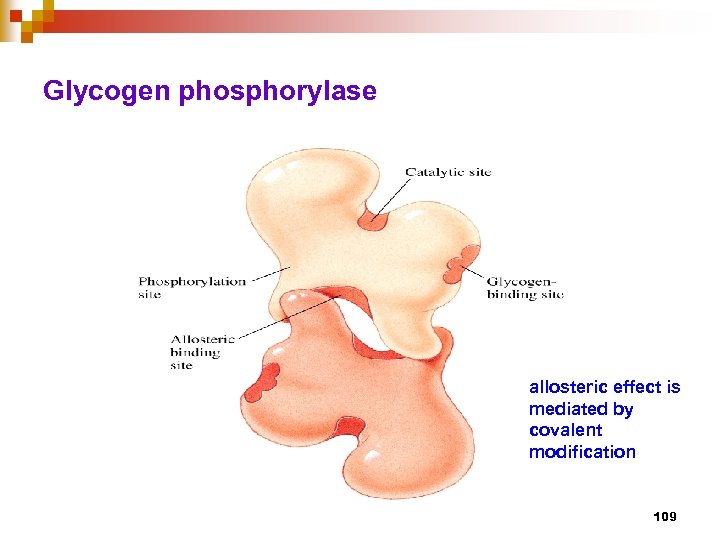
Glycogen phosphorylase allosteric effect is mediated by covalent modification 109
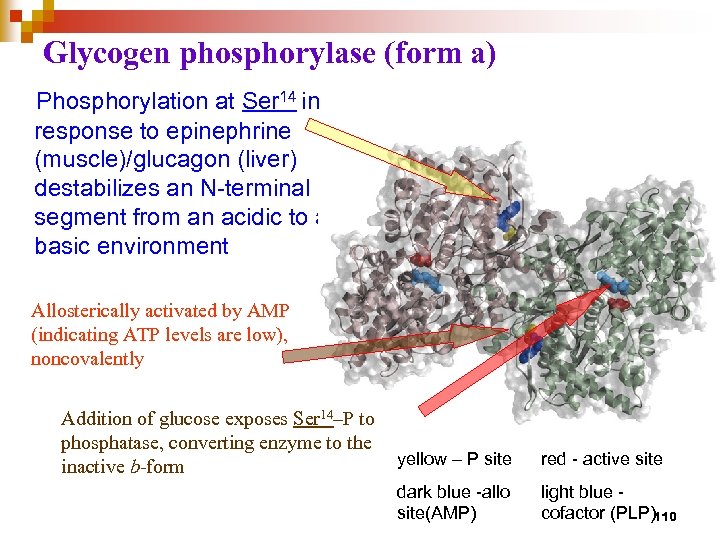
Glycogen phosphorylase (form a) Phosphorylation at Ser 14 in response to epinephrine (muscle)/glucagon (liver) destabilizes an N-terminal segment from an acidic to a basic environment Allosterically activated by AMP (indicating ATP levels are low), noncovalently Addition of glucose exposes Ser 14–P to phosphatase, converting enzyme to the inactive b-form yellow – P site red - active site dark blue -allo site(AMP) light blue cofactor (PLP)110
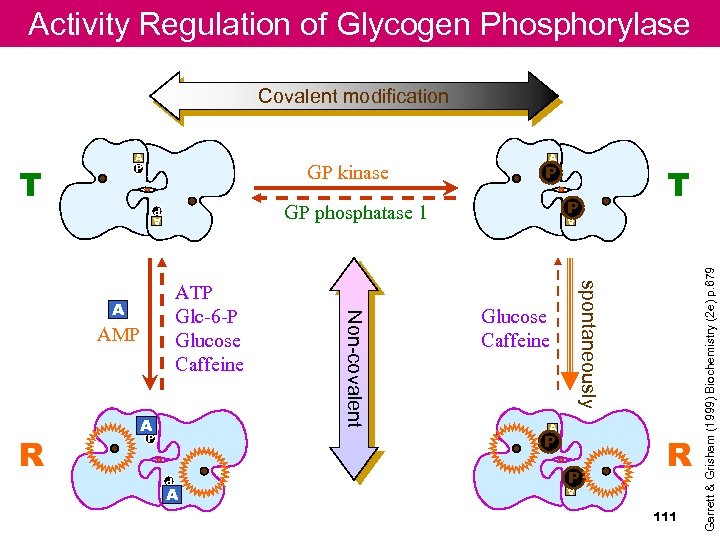
Activity Regulation of Glycogen Phosphorylase Covalent modification P GP phosphatase 1 A P A A Glucose Caffeine A P P A P A P P T spontaneously AMP Non-covalent ATP Glc-6 -P Glucose Caffeine A R P A P T GP kinase A P R 111 Garrett & Grisham (1999) Biochemistry (2 e) p. 679 A P
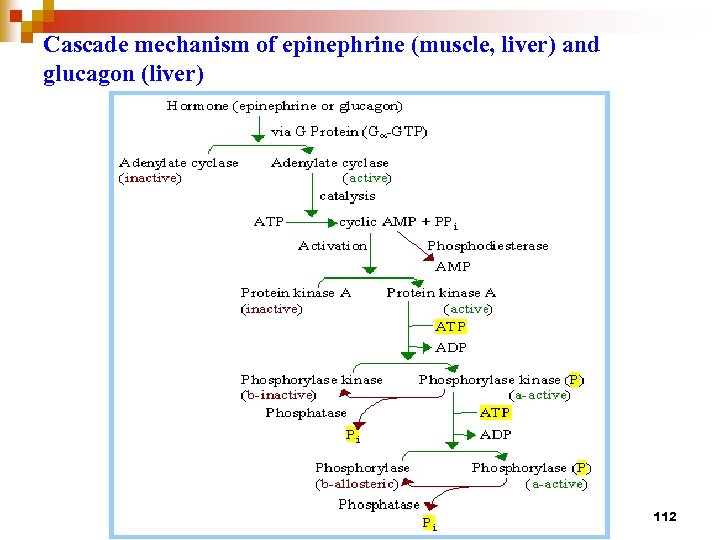
Cascade mechanism of epinephrine (muscle, liver) and glucagon (liver) 112
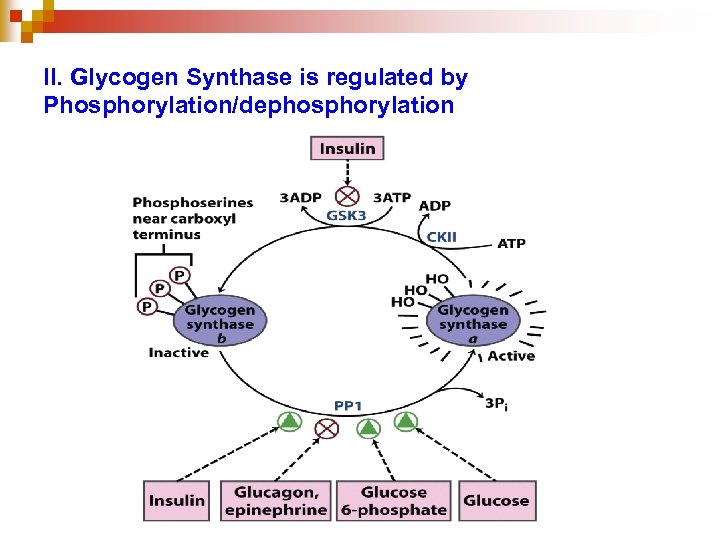
II. Glycogen Synthase is regulated by Phosphorylation/dephosphorylation
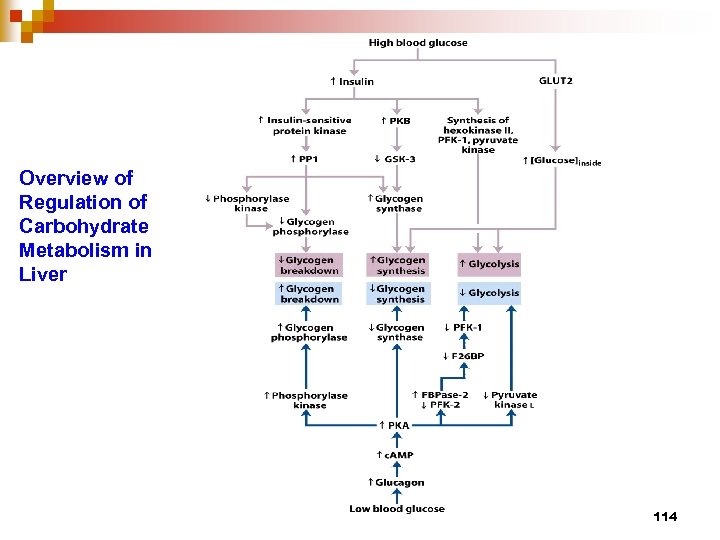
Overview of Regulation of Carbohydrate Metabolism in Liver 114
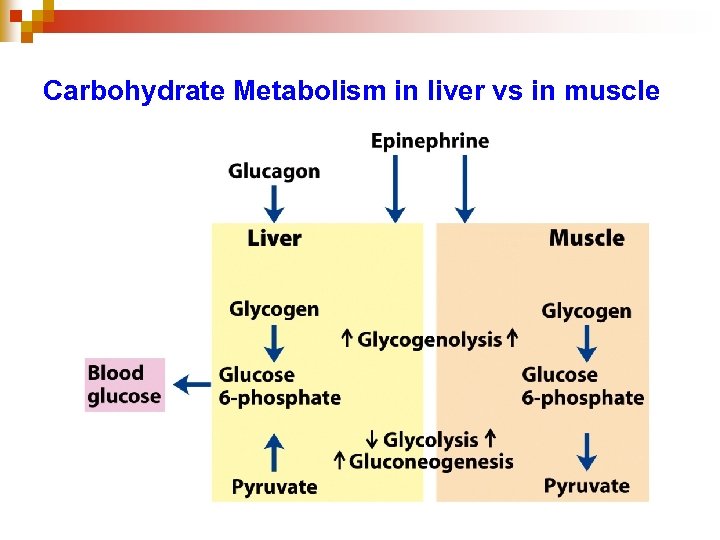
Carbohydrate Metabolism in liver vs in muscle

References n n 1. Lehninger Principles of Biochemistry, 5 th Edition, 2008, D. L. Nelson and M. M. Cox, W. H. Freeman & Co. , NY, Chapter 15, 6 2. Biochemistry, 3 rd ed. , 2004, D. Voet and J. G. Voet, Biochemistry John Wiley & Sons, Inc. , USA. , Chapter 10, 16 -18 116
76aacfb021748451f3412436f14851fd.ppt