c867327faecb6351d069d24f1ae332e0.ppt
- Количество слайдов: 145

Meeting of the Advisory Committee on Heritable Disorders in Newborns and Children Bethesda Marriott Pooks Hill September 24, 2009

Agenda - Price 1. The Nationwide Health Information Network – 2. 3. 4. 5. 6. 7. 8. 9. Ginger Price, Office of the National Coordinator for Health Information Technology Status of the Implementation of the Newborn Screening Use Case Newborn Screening Codes & Terminology and an Approach to a Standard Report Payload Newborn Screening Web Portal Concept Break Measures for Quality Internal Review Workgroup: Nomination of Alpha Thalassemia: Hemoglobin H Disease to the ACHDNC’s Recommended Uniform Screening Panel Committee Discussion Adjourn

Nationwide Health Information Network Overview Ginger Price Program Director, Nationwide Health Information Network Office of the National Coordinator for Health IT Presentation to the Secretary’s Advisory Committee on Heritable Disorders September 24, 2009

Agenda • ONC ARRA Activities – Meaningful Use – State Grant Program • Nationwide Health Information Program – Overview • Questions

ONC ARRA Activities Meaningful Use State Health Information Exchange Cooperative Agreement Program

Meaningful use • The challenge for health IT is one of changing sociology changing the fundamental way we collect, organize and use health information. • Achieving the realization that the value of health IT is in the way it is used – using it in a meaningful way on a day to day basis. • Over time, the definition of meaningful use will become more demanding; requirements increase between 2011 and 2013 and again between 2013 and 2015. • In the summer of this year, the HIT Policy Committee provided its final recommendations regarding the definition of meaningful use. CMS is drafting the Meaningful Use Notice of Proposed Rule Making. • The meaningful use definitions should be finalized in 2010.
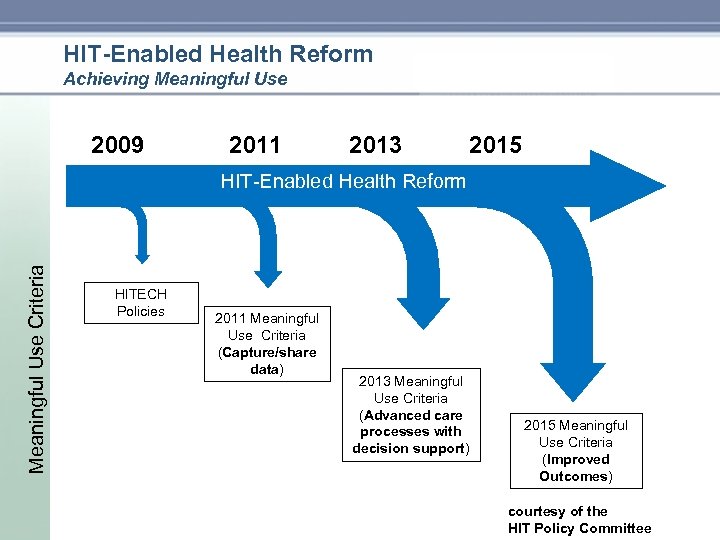
HIT-Enabled Health Reform Achieving Meaningful Use 2009 2011 2013 2015 Meaningful Use Criteria HIT Enabled Health Reform HITECH Policies 2011 Meaningful Use Criteria (Capture/share data) 2013 Meaningful Use Criteria (Advanced care processes with decision support) 2015 Meaningful Use Criteria (Improved Outcomes) courtesy of the HIT Policy Committee
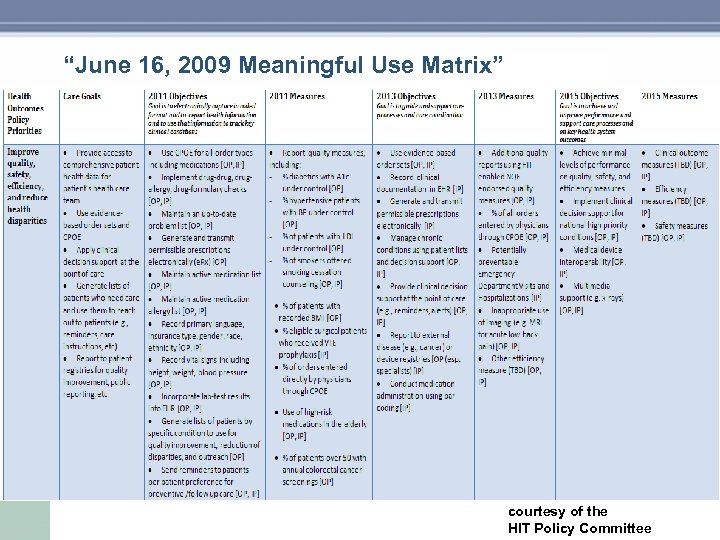
“June 16, 2009 Meaningful Use Matrix” courtesy of the HIT Policy Committee

Timeline for Next 12 Months • 3 Q 09: Develop process for updating meaningful use objectives and measures – Tag 2011 measures relevant to specialties • 4 Q 09: Conduct informational hearings to inform 2013 and 2015 criteria development • 1 Q 10: Update 2013 and 2015 criteria • 2 Q 10: Work with HIT Standards committee to ascertain availability of relevant standards • 3 Q 10: Refine 2013 meaningful use criteria • 4 Q 10: Assess industry preparedness for meeting 2011 and initial 2013 meaningful use criteria courtesy of the HIT Policy Committee
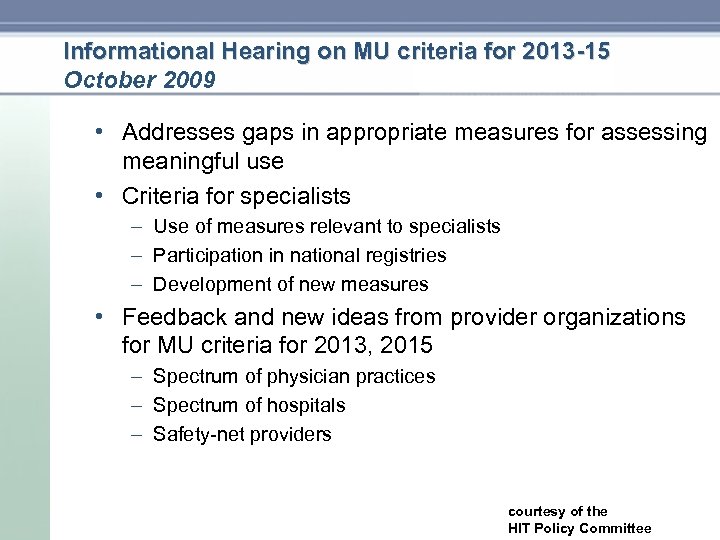
Informational Hearing on MU criteria for 2013 -15 October 2009 • Addresses gaps in appropriate measures for assessing meaningful use • Criteria for specialists – Use of measures relevant to specialists – Participation in national registries – Development of new measures • Feedback and new ideas from provider organizations for MU criteria for 2013, 2015 – Spectrum of physician practices – Spectrum of hospitals – Safety net providers courtesy of the HIT Policy Committee
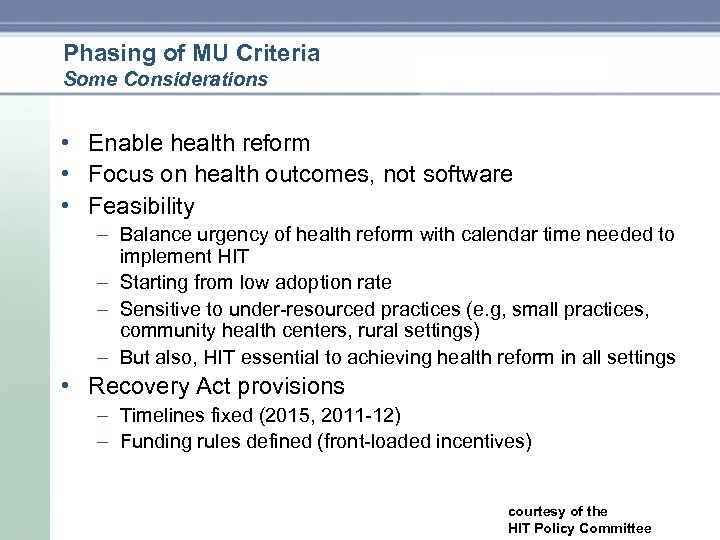
Phasing of MU Criteria Some Considerations • Enable health reform • Focus on health outcomes, not software • Feasibility – Balance urgency of health reform with calendar time needed to implement HIT – Starting from low adoption rate – Sensitive to under resourced practices (e. g, small practices, community health centers, rural settings) – But also, HIT essential to achieving health reform in all settings • Recovery Act provisions – Timelines fixed (2015, 2011 12) – Funding rules defined (front loaded incentives) courtesy of the HIT Policy Committee

Supporting Meaningful use • Experience supports the finding that meaningful use of Health IT isn’t easy and requires ongoing help to implement and maximize use. HITECH recognized this need as well. • There are two important grant programs, totally approximately $1. 2 B of ONC’s $2 B in discretionary funds, to assist and support ongoing implementation of health IT supporting meaningful use. • State Health Information Exchange Cooperative Agreement Program • Health Information Technology Extension Program • Keep up to date on state Health Information Exchange Cooperative Agreement, the Health Information Technology Extension Program and Meaningful Use, visit: • http: //healthit. hhs. gov select “Health. IT/Recovery”
State Health Information Exchange Cooperative Agreement Program • The HITECH Act amends Title XXX of the Public Health Service Act by adding Section 3013, State Grants to Promote Health Information Technology. Section 3013 provides for state grants to promote health information technology. • Over the next several months, cooperative agreements will be awarded through the State Health Information Exchange Cooperative Agreement Program to states and qualified State Designated Entities (SDEs) to develop and advance mechanisms for information sharing across the health care system. • Under these State cooperative agreements $564 million will be awarded to support efforts to achieve widespread and sustainable health information exchange (HIE) within and among states through the meaningful use of certified Electronic Health Records (EHRs). • The Centers for Medicare & Medicaid Services will issue proposed criteria for meaningful use by the end of 2009.

State Health Information Exchange Cooperative Agreement Program • To help potential applicants and other interested parties better understand the federal grants process, The Office of the National Coordinator for Health Information Technology (ONC) has prepared a Getting Started Grants Primer. This document highlights the key steps needed to find apply for grants. • The following links lead to government wide web sites related to federal grants and other federal funding opportunities. – Grants. gov – Fed. Biz. Opps. gov • ONC is also initiating a series of Section 3013 State Cooperative Agreements Program Technical Assistance Calls to provide resources and answer questions for those interested in responding to this fundig opportunity.
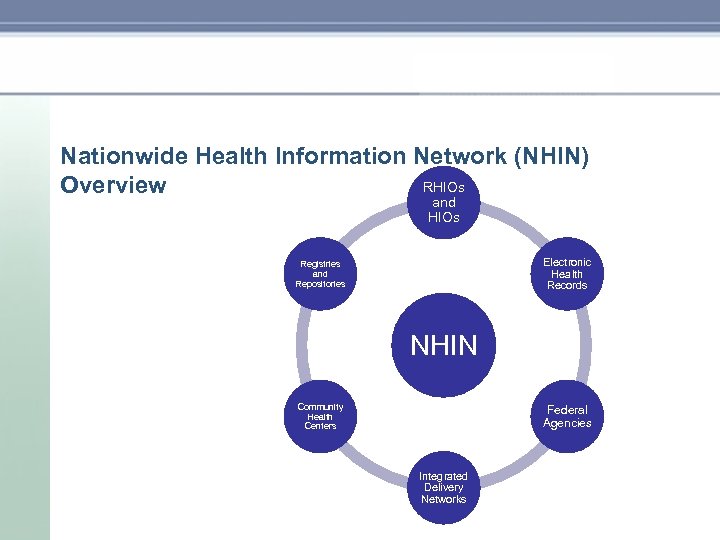
Nationwide Health Information Network (NHIN) RHIOs Overview and HIOs Electronic Health Records Registries and Repositories NHIN Community Health Centers Federal Agencies Integrated Delivery Networks

Nationwide Health Information Network (NHIN) The widespread availability and low cost of the Internet make it an attractive option for the secure exchange of health information. However, internet-based exchanges present two critical challenges: • Patient privacy, security and trust must be maintained, and • Information exchange should be “interoperable” between systems, so that information generated in one system can be used and understood by another. The NHIN was designed to address these challenges: • Privacy, Security and Trust: the NHIN creates a “trusted” network where there is: o o o • Assurance that parties can be trusted (Governance, Directory, Certificates) Assurance that patient preferences are being adhered to Assurance that the transmission across the internet is secure. Interoperability: the NHIN includes a set of technical protocols, industry standards and very specific implementation guides that enable NHIN participants to read and understand the health information that is exchanged with minimal or no “point to point” coordination
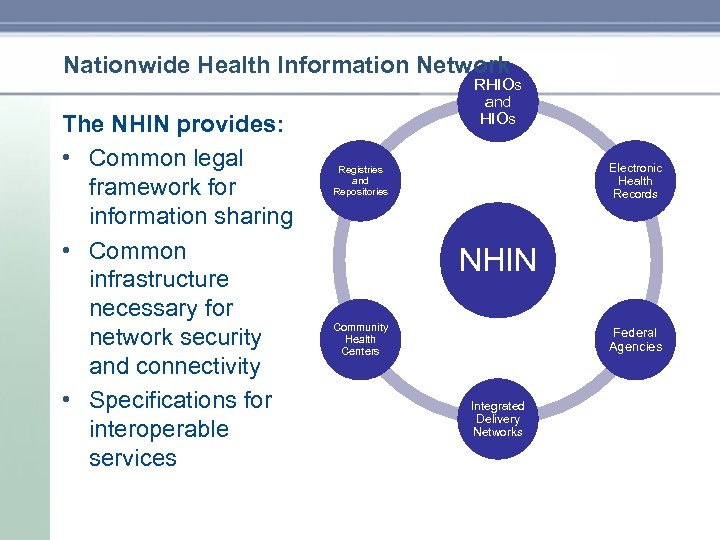
Nationwide Health Information Network The NHIN provides: • Common legal framework for information sharing • Common infrastructure necessary for network security and connectivity • Specifications for interoperable services RHIOs and HIOs Electronic Health Records Registries and Repositories NHIN Community Health Centers Federal Agencies Integrated Delivery Networks
![NHIN Architecture N • H • I • N [en eych ahy en] noun. NHIN Architecture N • H • I • N [en eych ahy en] noun.](https://present5.com/presentation/c867327faecb6351d069d24f1ae332e0/image-18.jpg)
NHIN Architecture N • H • I • N [en eych ahy en] noun. A self governed cooperative The NHIN is the network that ties other health networks together in a common, interoperable infrastructure.

NHIN Architectural Principles Highly distributed: Patient health information is retained at the local health information exchange level Local autonomy: Each HIO must make their own determinations with respect to the release of patient information Focus only on inter-organizational health exchange: The NHIN does not attempt to standardize implementations of the NHIN services and interfaces, only the communications between HIOs Use public internet: The NHIN is not a separate physical network, but a set of protocols and standards that run on the existing internet infrastructure Platform neutral: The NHIN has adopted a stack (web services) that can be implemented using many operating systems and programming languages
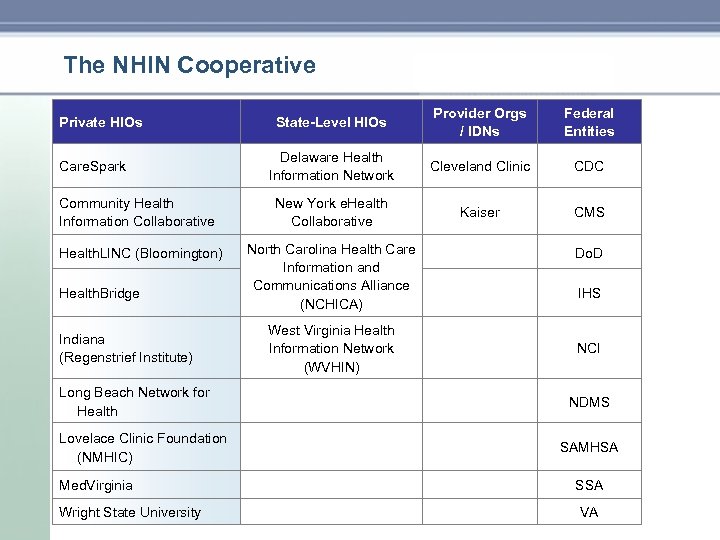
The NHIN Cooperative Private HIOs Care. Spark State-Level HIOs Delaware Health Information Network Community Health Information Collaborative New York e. Health Collaborative Health. LINC (Bloomington) North Carolina Health Care Information and Communications Alliance (NCHICA) Provider Orgs / IDNs Federal Entities Cleveland Clinic CDC Kaiser CMS Do. D IHS NCI Long Beach Network for Health NDMS Lovelace Clinic Foundation (NMHIC) SAMHSA Med. Virginia SSA VA Health. Bridge Indiana (Regenstrief Institute) Wright State University West Virginia Health Information Network (WVHIN)

Demonstration Projects • NHIN limited production pilots are critical to the success of demonstrating how standards and specifications are implemented as working operational solutions for health information exchange. • Med. Virginia and SSA entered into the first limited production pilot in February, 2009. • Other organizations planning to demonstrate health information exchange via the NHIN in the coming months include: • • • Kaiser Permanente Department of Veterans Affairs Department of Defense Centers for Disease Control and Prevention SSA Contract Awardees • The next NHIN pilot project demonstrations will include onboarding pilot partners into the NHIN trusted community, performing conformance and interoperability testing, issuing a digital certificate, and adding them into the NHIN service registry.

Supporting New Features • The NHIN is implementing processes to elicit and prioritize new information exchange features from the Health IT community. • Beyond the NHIN Core services, new features have recently been submitted for consideration: 1. 2. 3. 4. 5. CDC Population Health Data Submission – CDC requested a new profile to gather population health data from Information Exchanges. CMS Transfer of Care – CMS request a new profile to enable HIOs to transmit transfer of care reports to CMS via the NHIN, FDA Analytic Query Service – FDA request for new service for analytic purposes. CMS Quality Reporting – Request for NHIN capability to support CMS’ Physician Quality Reporting Initiative CDC Public Health Alerting – Request for NHIN capability to provide alerts to providers on public health alerts and interventions • NHIN is responding to requests to allow input and review of technical artifacts.

Going Forward • The NHIN will showcase demonstrations and network operational capabilities in early 2010. For more information about the NHIN: • Go to http: //healthit. hhs. gov and click on “Nationwide Health Information Network • For regular updates, join the Health IT Listserv at https: //list. nih. gov/archives/health it. html. Click on "Join or Leave the List, or Update Options. " • Questions? Contact us at nhin@hhs. gov
Agenda 1. - Zuckerman The Nationwide Health Information Network 2. Status of the Implementation of the Newborn Screening Use Case – 3. 4. 5. 6. 7. 8. 9. Alan Zuckerman, M. D. , Georgetown University Newborn Screening Codes & Terminology and an Approach to a Standard Report Payload Newborn Screening Web Portal Concept Break Measures for Quality Internal Review Workgroup: Nomination of Alpha Thalassemia: Hemoglobin H Disease to the ACHDNC’s Recommended Uniform Screening Panel Committee Discussion Adjourn

Title Newborn Screening Use Case Update: Moving Newborn Screening Into the Electronic Age Alan E Zuckerman MD FAAP Georgetown University Medical Center Contractor HHS/OS/ONC aez@georgetown. edu September 25, 2009 25
Overview • Updates from HITSP, PHII, IHE – The HITSP Newborn Screening Interoperability Specification will be completed in January 2010 – The APHL has approved the Newborn Screening HL 7 Implementation Guide prepared by PHII – IHE has finalized their Newborn Screening White Paper • HITSP Requirements Design Standards Selection (RDSS) • Inspection Testing of the Draft Interoperability Specification – Re use of constructs from other Use Cases • Anticipated Use of SNOMED and LOINC • Filter Paper Data Capture of lab test ordering information • Considerations for ACHDNC
Requirements Design Standards Selection • The Requirements Design Standards Selection (RDSS) Document is the first milestone in the HITSP development process and provides specific solutions for each aspect of the original use case that remains fixed • The RDSS will be open for public comment Sept 18 – Oct 16 • A key feature of the RDSS is the listing of the Data Requirements for each Information Exchange. The community will: – need to review the data captured on the dried blood spot filter paper as part of the test ordering data requirements – need to review the separate data requirements for newborn hearing screening – need to review the data requirements for newborn screening lab reports
Interoperability Specification Inspection Testing • Draft Interoperability Specification (IS) based on RDSS document completed by Oct 30, 2009 • Inspection testing and public comments due Dec 4, 2009 • Final Interoperability Specification expected completion Jan 2010 • Key components of IS will make extensive re use of material from other use cases (e. g. EHR Lab).
Anticipated Use of SNOMED and LOINC • Migration from ICD 9 CM to SNOMED coded problem lists and required use of LOINC coded lab reports is anticipated to be part of certification criteria for EHR under ARRA and implementation for NBS is part of the use case. • SNOMED coded problem lists will be a requirement by 2015 and will begin use soon • Use of LOINC codes to report both analytes (measures) and genetic test interpretation by condition using the methods developed for the Personalized Healthcare Use Case and electronic reporting of genetic testing • Newborn Screening Codes project at NLM will maintain the SNOMED and LOINC codes required for newborn screening test ordering, result reporting, and some follow up activities
NBS Laboratory Test Order Data Fields • • • Rational for Data Collection and Analysis – The data will help identify an inclusive set of requirements for lab test ordering that will work for all states – The goal is not to produce one standard national form or terminology but to assure that all state requirements are included Methods – Filter paper forms from 50 states and DC were scanned and all fields tabulated with identification of synonyms and value sets – Cluster analysis created three groups of fields that were most prevalent, frequently occurring, and used in only a few states Next Steps – Data Verification
Newborn Screening (NBS) Data Field Analysis - Explanation Data Elements were plotted by two sets of values: NBF: Newborn Factors (y-axis) -Indicates potential volume of data for each data element -Calculated by summing the number of live births in the states where the data element is found, based on the National Newborn Screening and Genetics Resource Center (NNSGRC) 2006 data set. Sample calculation: if the baby’s middle name is found in NY (10 births) MD (5 births) and CA (15 births) the NBF factor would be 30. Frequency (x-axis) -Indicates degree of usage as a key data element among states -Measures of the occurrence of the data element among the states, Elements appearing in the top right quadrant are the most prevalent, with a common occurrence among states and a potentially very large volume of data nationally. Conversely elements occurring in the lower left are relatively uncommon and have a lower potential for data volume. Elements centered on a blue circle are representations of several data elements that are part of the same category.
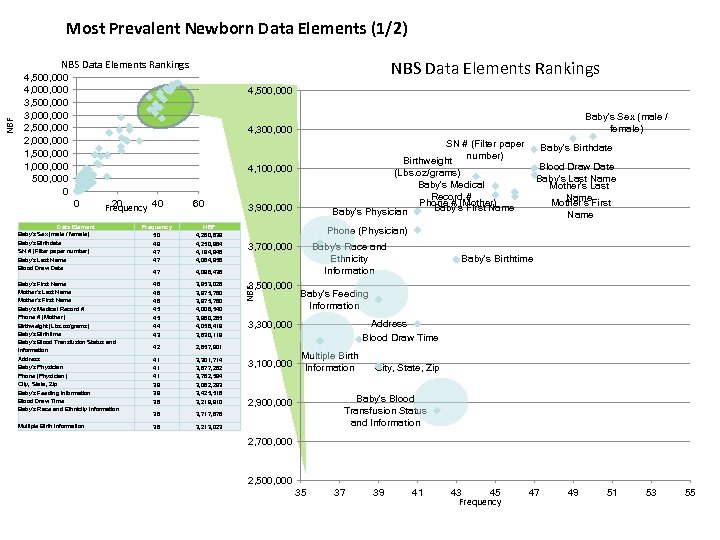
Most Prevalent Newborn Data Elements (1/2) NBS Data Elements Rankings 4, 500, 000 4, 000 3, 500, 000 3, 000 2, 500, 000 2, 000 1, 500, 000 1, 000 500, 000 0 4, 500, 000 Baby's Sex (male / female) 4, 300, 000 SN # (Filter paper number) Birthweight (Lbs. oz/grams) Baby's Medical Record # Phone # (Mother) Baby's First Name Baby's Physician 4, 100, 000 0 20 Frequency 40 Data Element Baby's Sex (male / female) Baby's Birthdate SN # (Filter paper number) Baby's Last Name Blood Draw Date 60 Frequency 50 49 47 47 NBF 4, 260, 839 4, 250, 964 4, 194, 948 4, 064, 958 47 4, 098, 438 Baby's First Name Mother's Last Name Mother's First Name Baby's Medical Record # Phone # (Mother) Birthweight (Lbs. oz/grams) Baby's Birthtime Baby's Blood Transfusion Status and Information 46 46 46 45 45 44 43 3, 953, 028 3, 975, 780 4, 006, 540 3, 960, 265 4, 056, 419 3, 630, 119 42 2, 857, 901 Address Baby's Physician Phone (Physician) City, State, Zip Baby's Feeding Information Blood Draw Time Baby's Race and Ethnicity Information 41 41 41 39 39 38 3, 301, 714 3, 877, 282 3, 782, 594 3, 082, 293 3, 425, 516 3, 219, 910 36 3, 717, 878 Multiple Birth Information 36 3, 900, 000 3, 213, 023 Baby's Birthdate Blood Draw Date Baby's Last Name Mother's First Name Phone (Physician) 3, 700, 000 3, 500, 000 NBF NBS Data Elements Rankings Baby's Race and Ethnicity Information Baby's Feeding Information Address Blood Draw Time 3, 300, 000 3, 100, 000 Baby's Birthtime Multiple Birth Information City, State, Zip Baby's Blood Transfusion Status and Information 2, 900, 000 2, 700, 000 2, 500, 000 35 37 39 41 43 45 Frequency 47 49 51 53 55
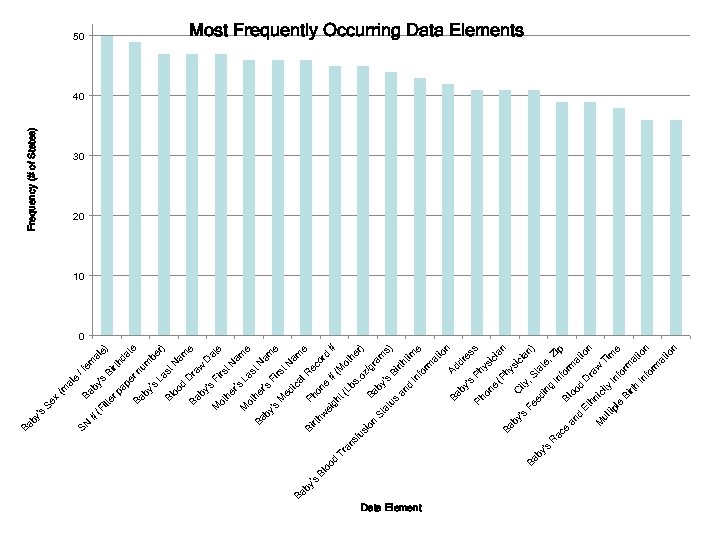
# ilt er (F e) al da rth Bi /f em rn pe pa 's by e (m al Ba x Se Ba te um by be 's La r) st Bl N oo am d D e Ba ra w by D 's at Fi M e rs ot t. N he r's am M La e ot st he Ba N r's am by Fi 's e rs M t. N ed am ic Ba al e by R Bi Ph ec 's rth on or Bl w e d oo ei # # gh d (M Tr t( ot an Lb he sf s. r) us oz io /g Ba n ra by St m at 's s) us Bi rth an tim d In e fo rm at io n Ad Ba dr by es 's s Ph Ph ys on ic e ia (P n Ba hy si by C ci ity 's an Fe , S Ba ) ed ta by te in 's , Z g R In ip ac fo e Bl rm an oo at d d io Et D n ra hn w M ic Ti ity ul m tip In e le fo Bi rm rth at io In n fo rm at io n SN by 's Ba Frequency (# of States) 50 Most Frequently Occurring Data Elements 40 30 20 10 0 Data Element
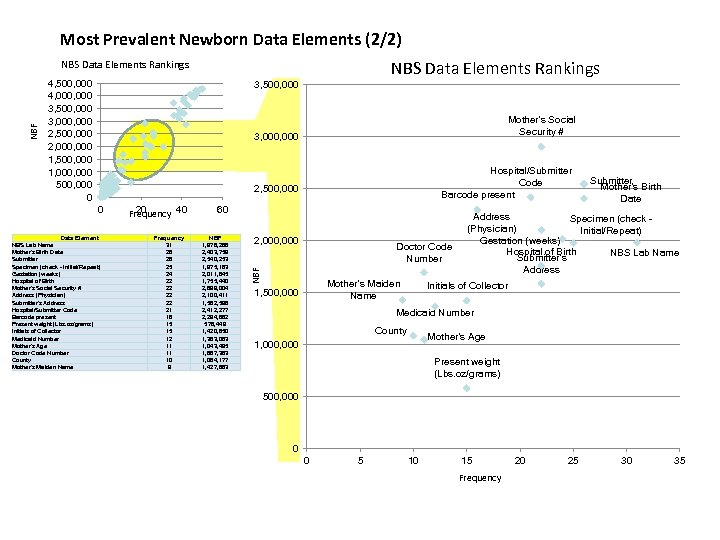
Most Prevalent Newborn Data Elements (2/2) NBS Data Elements Rankings 4, 500, 000 4, 000 3, 500, 000 3, 000 2, 500, 000 2, 000 1, 500, 000 1, 000 500, 000 0 3, 500, 000 Mother's Social Security # 3, 000 Hospital/Submitter Code Barcode present 2, 500, 000 0 Data Element NBS Lab Name Mother's Birth Date Submitter Specimen (check Initial/Repeat) Gestation (weeks) Hospital of Birth Mother's Social Security # Address (Physician) Submitter's Address Hospital/Submitter Code Barcode present Present weight (Lbs. oz/grams) Initials of Collector Medicaid Number Mother's Age Doctor Code Number County Mother's Maiden Name 20 Frequency 40 Frequency 31 26 26 25 24 22 22 21 16 15 15 12 11 11 10 9 60 NBF 1, 978, 266 2, 403, 759 2, 540, 253 1, 975, 183 2, 011, 845 1, 755, 440 2, 899, 004 2, 100, 411 1, 582, 596 2, 412, 277 2, 294, 882 578, 449 1, 420, 650 1, 363, 063 1, 043, 495 1, 687, 363 1, 084, 177 1, 427, 863 2, 000 Doctor Code Number NBF NBS Data Elements Rankings Mother's Maiden Name 1, 500, 000 Submitter Mother's Birth Date Address Specimen (check (Physician) Initial/Repeat) Gestation (weeks) Hospital of Birth NBS Lab Name Submitter's Address Initials of Collector Medicaid Number County Mother's Age 1, 000 Present weight (Lbs. oz/grams) 500, 000 0 0 5 10 15 Frequency 20 25 30 35
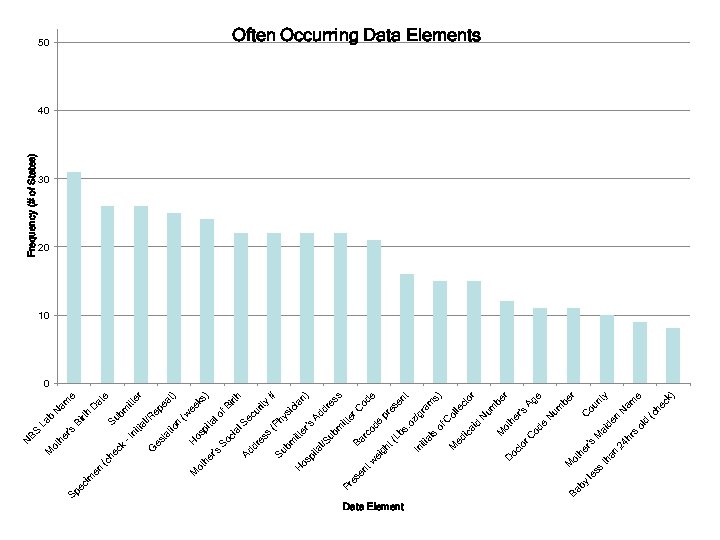
Data Element by Ba ss le th an s d ol ck he (c ) am e N r y ou nt C be Ag e N um en ai d M e 's r or um be N he r ot od C hr 24 's to r er ot h D oc M id le ct ol t en am s) gr z/ of C ic a ed M al s iti In . o es pr s es od e er C # n) ia Ad dr od e bs (L rc Ba gh t ei w M en t 's ic hy s y h irt rit ec u m itt ub l/S (P er bm itt os pi ta H Su s es al S ci So ) ee ks (w t) er ea lo f. B ta pi os H n at io l/R ep bm itt Su at e D e am N rth Bi b La tia ni -I es t dr Ad 's er G ck he (c ot h M en im es Pr Sp ec r's he M ot N BS Frequency (# of States) 50 Often Occurring Data Elements 40 30 20 10 0

Considerations for ACHDNC • Participate in public comments on the Requirements Design Standards Selection Document RDSS – Due Oct 16, 2009 • Participate in the inspection testing (public comment) on the Interoperability Specification – Due Dec 4, 2009 • Propose a mechanism to assign identifier numbers to all newborn screening laboratories (not all have CLIA numbers) • Endorse implementation of the Interoperability Specification at the next ACHDNC Meeting Jan 21 22, 2010 • Review NLM Newborn Screening Codes using SNOMED and LOINC – On going

Agenda 1. 2. - Fung The Nationwide Health Information Network Status of the Implementation of the Newborn Screening Use Case 3. Newborn Screening Codes & Terminology and an Approach to a Standard Report Payload – 4. 5. 6. 7. 8. 9. Kin Wah Fung, M. D. , National Library of Medicine Newborn Screening Web Portal Concept Break Measures for Quality Internal Review Workgroup: Nomination of Alpha Thalassemia: Hemoglobin H Disease to the ACHDNC’s Recommended Uniform Screening Panel Committee Discussion Adjourn

Newborn Screening Codes & Terminology and an Approach to a Standard Report Payload Kin Wah Fung, MD Clement Mc. Donald, MD Lister Hill National Center for Biomedical Communications National Library of Medicine ACHDNC Meeting Sept 2009

Outline of presentation Goals Ø Work done so far Ø • • • Ø Standardization of content Standardization of messaging format Demonstration of new website Work ahead
Goals To promote and facilitate the use of electronic health data standards in recording and transmitting newborn screening test results Ø Benefits: Ø • • speed the delivery of newborn screening reports facilitate the care and follow-up of infants with positive test results enable the use (and comparison) of data from different laboratories support the development of strategies for improving the newborn screening process

Prerequisites for standardized electronic reporting Ø Content • Ø Standard codes for test names, analytes, conditions screened and other categorical answers Messaging format • Standard messaging format to convey the content electronically

Use of national and international code standards A coding and terminology framework is essential to standardizing laboratory reporting and enabling interoperability of information exchange across Electronic Health Record (EHR) platforms Ø Coding standards used: Ø • • • LOINC SNOMED CT ICD-9 -CM, ICD-10 -CM Enzyme codes OMIM codes

LOINC Logical Observation Identifiers Name and Codes Ø Supported by NLM and Regenstrief Foundation ( Indianapolis) Ø Universal code for identifying measurement (e. g. laboratory tests) and results in HL 7 messages Ø Used widely in U. S. and internationally Ø No cost license in perpetuity Ø
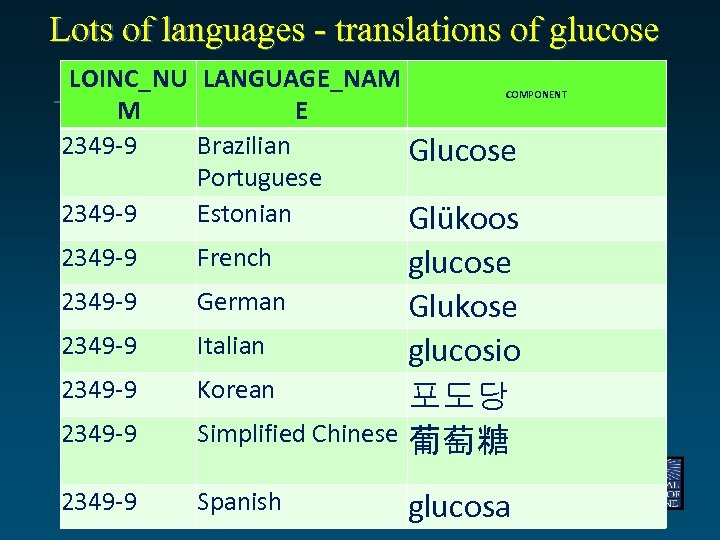
Lots of languages - translations of glucose LOINC_NU LANGUAGE_NAM COMPONENT M E 2349 -9 Brazilian Glucose Portuguese 2349 -9 Estonian Glükoos 2349 -9 glucose German Glukose Italian glucosio Korean 포도당 Simplified Chinese 葡萄糖 2349 -9 Spanish 2349 -9 French glucosa
LOINC Web site –download everything 20009 06 03 Clem Mc. Donald - Lister Hill Center

SNOMED CT Systematized Nomenclature of Medicine – Clinical Terms Ø Originally developed by the College of American Pathologists Ø • • Ø ownership transferred to the International Health Terminology Standards Development Organisation (IHTSDO) 12 member countries including US, Canada, UK, Australia, Netherlands, Sweden and Spain. Emergent international clinical terminology standard

SNOMED CT Very comprehensive – over 300, 000 concepts Ø multilingual clinical health care terminology designed for use in Electronic Health Record systems and in health data exchange Ø available free of charge in IHTSDO member countries, including the U. S. , in low-income countries as defined by the World Bank, and for qualified research projects in any country. Ø

ICD-9 -CM International Classification of Diseases, Ninth Revision, Clinical Modification Ø the official system of assigning codes to diagnoses associated with hospital utilization and public health reporting in the U. S. Ø One of the Health Insurance Portability and Accountability Act (HIPAA) code sets Ø Planned transition to ICD-10 -CM by 2013 Ø
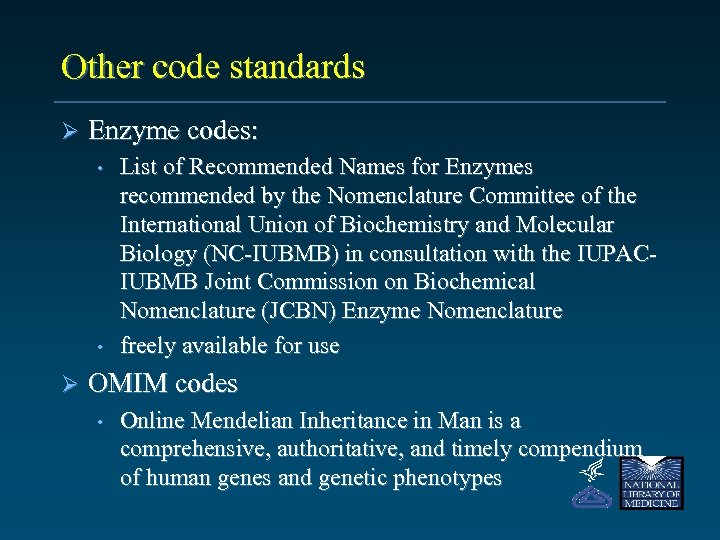
Other code standards Ø Enzyme codes: • • Ø List of Recommended Names for Enzymes recommended by the Nomenclature Committee of the International Union of Biochemistry and Molecular Biology (NC-IUBMB) in consultation with the IUPACIUBMB Joint Commission on Biochemical Nomenclature (JCBN) Enzyme Nomenclature freely available for use OMIM codes • Online Mendelian Inheritance in Man is a comprehensive, authoritative, and timely compendium of human genes and genetic phenotypes
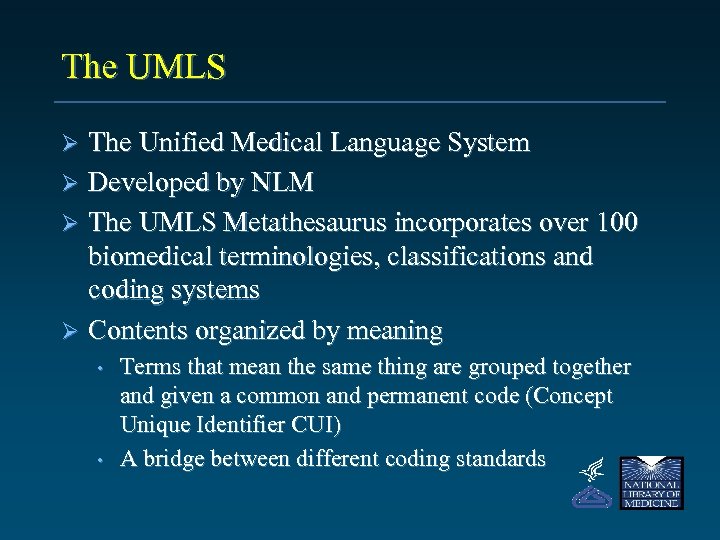
The UMLS The Unified Medical Language System Ø Developed by NLM Ø The UMLS Metathesaurus incorporates over 100 biomedical terminologies, classifications and coding systems Ø Contents organized by meaning Ø • • Terms that mean the same thing are grouped together and given a common and permanent code (Concept Unique Identifier CUI) A bridge between different coding standards

What NLM has done Collect the lists of tests, analytes, conditions and categorical answers, some of them are already mapped to standard coding systems Ø Fill in gaps where standard codes exist, add UMLS CUIs Ø Make these lists available on an NLM website (http: //newbornscreeningcodes. nlm. nih. gov), together with guidance and rationale for their use produced by the AHIC Committee on Newborn Screening, and other useful links Ø Maintain these lists and guidance as they are revised over time Ø

Messaging standard Encourage the use of HL 7 as the standard for reporting of Newborn Screening results Ø Facilitate the development of a standard specification for the payload part of the message that uses the codes and approaches proposed by the AHIC committee Ø

HL 7 Health Level Seven is an international messaging standard for the healthcare domain Ø HL 7 version 2 - almost universally available in large practices, laboratories, hospitals Ø Federal government requires HL 7 vs. 2. 5 for laboratory reporting Ø Used widely internationally as well - Germany, Netherlands, France, Japan, etc Ø
HL 7 basics Ø An HL 7 message is composed of segments, each segment conveys a specific type of information e. g. • • MSH – message header segment PID – patient identification and demographic information OBR – information about observation requests (e. g. laboratory and radiology orders) OBX – information about observations (e. g. laboratory results)

HL 7 basics Each type of message has specified syntax so that information can be sent and received unambiguously Ø HL 7 also has pre-defined data types e. g. Ø • • • DT – Date e. g. CCYYMMDD PN - Person name e. g. Last name ^ first name ^ middle ^ suffix CE – Coded e. g. Code 1 ^ print text ^ Code system
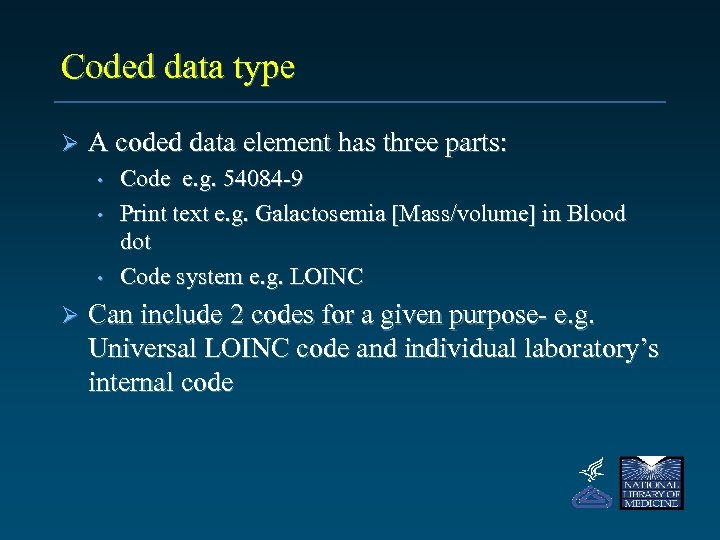
Coded data type Ø A coded data element has three parts: • • • Ø Code e. g. 54084 -9 Print text e. g. Galactosemia [Mass/volume] in Blood dot Code system e. g. LOINC Can include 2 codes for a given purpose- e. g. Universal LOINC code and individual laboratory’s internal code
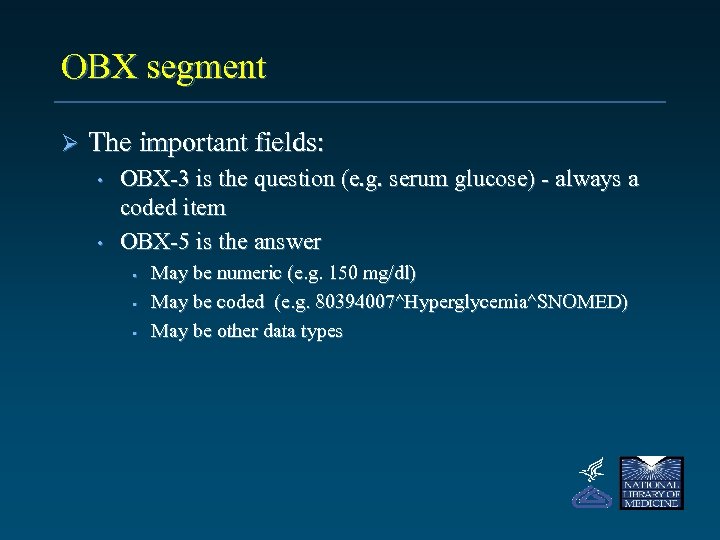
OBX segment Ø The important fields: • • OBX-3 is the question (e. g. serum glucose) - always a coded item OBX-5 is the answer • • • May be numeric (e. g. 150 mg/dl) May be coded (e. g. 80394007^Hyperglycemia^SNOMED) May be other data types

Put it all together for hemogram report – Red > measurement, Orange > value Patient level PID|||0999999^6^M 10||TEST^PATIENT^||1992022 5|F||B|4050 SW WAYWARD BLVD | Order/report level O BR|||H 9759 -0^REG_LAB|20725^Hemogram^LOINC Discrete Results OBX|2|NM||789 - 8^RBC^LN||4. 9|M/mm 3| 4. 0 -5. 4 OBX|3|NM|718 -7^HGB^LN||12. 4|g/d. L|12. 0 5. 0||||F| OBX|4|NM||20570 -8^HCT^LN||50|%|35 -49|H|||F| OBX|5|NM||30428 -7^MCV^LN||81|f. L|80 -94||||F|b
Proposed “Rules of engagement” NBS Labs would report quantitative and categorical results labeled with the appropriate LOINC code from the NBS LOINC catalogue. Ø They would report quantitative measures numbers with the agreed upon units specified in the NBS LOINC catalogue Ø The categorical results (e. g. S-Beta thalassemia from hemoglobin electrophoresis studies) would be reported as SNOMED CT codes Ø
A mock-up HL 7 message for NBS It is based on real but completely de-identified message data from Georgia Ø Message structure Ø • • The wrapper segments (MSH, PID etc. ) The payload • • • The OBX segments are grouped and indented below the OBRs e. g. Acylcarnitine tests Includes both interpretations and quantitative measures Each discrete interpretation/measurement is reported in a separate OBX and identified by a LOINC code

Galactosemia panel Ø OBR|1|2001178109|10 A 1912102|540799^Galactosemia Newborn Screening Panel^LOINC|… y • • OBX|1|ST|46737 -3^ Galactosemia interp^ LOINC|1|Test for enzyme defect: INCONCLUSIVE|| |*|||F|||20090714143552 OBX|2|ST|54084 -9^Galactose [Mass/volume] in Blood dot (filter paper)^LOINC|1| 1. 6|mg/d. L|<11||||F|||20090714143552

Customizing test names Ø Regarding names in reports • • • The test names are LOINC long common names These could be revised per a consensus of NBS labs HL 7 has option for including laboratory’s code and names in addition to the universal LOINC codes and names
NLM’s NBS web site – Launched 9/16

Web site functions Ø Down load the AHIC tables Ø Customized reports of table contents Ø Links to related documents and resources

Download tables
Customized views of tables Ø Four different views: • • Ø Conditions only Analytes (and measurements) only Conditions linked to analytes Analytes linked to conditions Additional filters: • Conditions • • • Condition categories e. g. hearing loss, amino acid disorders ACHDNC core or secondary conditions Analytes • • Analyte categories e. g. amino acid, organic acid Derived measures

Four different views
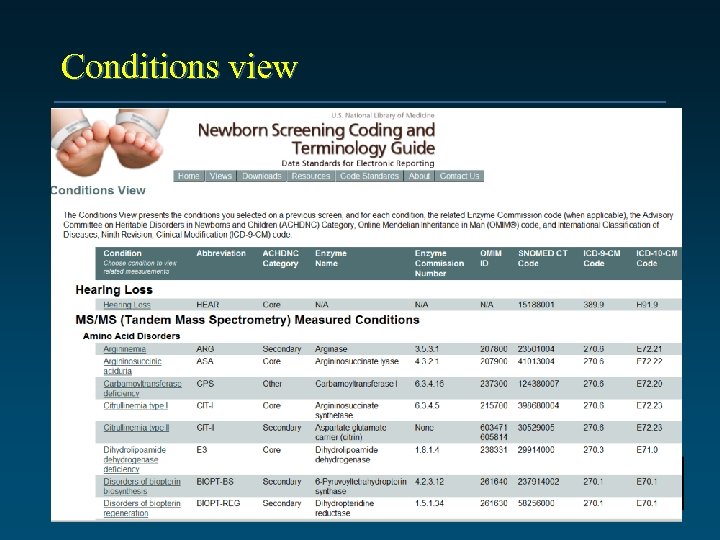
Conditions view

Analytes/measurements view
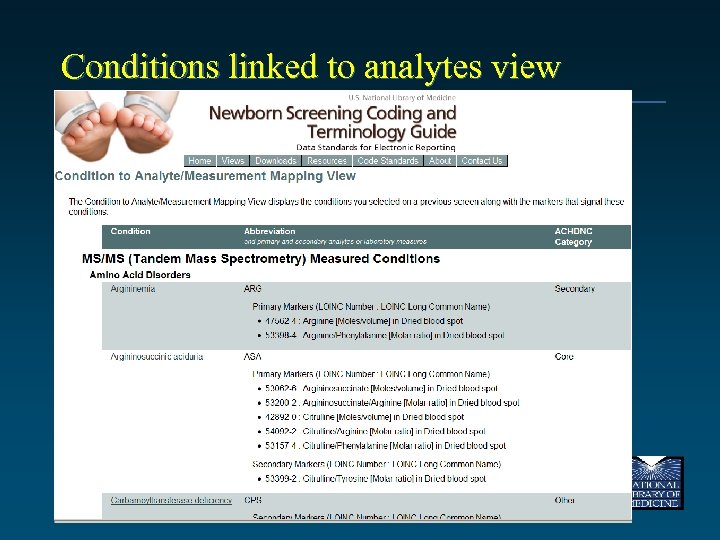
Conditions linked to analytes view
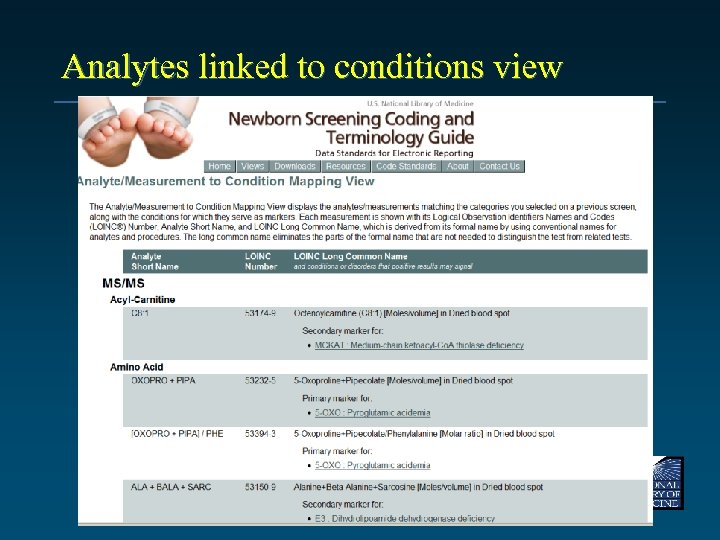
Analytes linked to conditions view

Filters for conditions

Filters for analytes/measurements

Link to other resources

Information on code standards

Work in progress…. .
Card variables Additional information collected about the baby e. g. birth weight, transfusion history Ø Some may be covered in other HL 7 segments e. g. PID segment Ø Others will be sent in OBX segments Ø Will need additional LOINC panel and observation codes – but first have to agree upon a core set of data elements Ø

Use of special HL 7 functionalities Ø Hide function of OBX-13 • • Ø Can flag some results to be hidden from routine clinical care displays Results will still be available for management and research purposes Delivery of a printed image of the report • • HL 7 can deliver a full formatted report within an OBX segment Specific LOINC codes will be assigned to each kind of report

Other work Build a more complete example and guide of HL 7 messaging Ø Get agreement on requirement of additional interpretation variables Ø

Thank you! 80

Agenda 1. 2. 3. - Downing The Nationwide Health Information Network Status of the Implementation of the Newborn Screening Use Case Newborn Screening Codes & Terminology and an Approach to a Standard Report Payload 4. Newborn Screening Web Portal Concept – 5. 6. 7. 8. 9. Gregory Downing D. O. , Ph. D. and Constanze Coon, Ph. D. , Personalized Health Care Initiative, OS/HHS Break Measures for Quality Internal Review Workgroup: Nomination of Alpha Thalassemia: Hemoglobin H Disease to the ACHDNC’s Recommended Uniform Screening Panel Committee Discussion Adjourn

Title Newborn Screening Web Portal Concept – Service-oriented Architecture Constanze Coon, Ph. D Gregory Downing, DO, Ph. D OS, Dept. Health and Human Services September 25, 2009

Agenda • Overview of current state of newborn screening electronic information exchange • Value proposition of newborn screening electronic information exchange • Proposal of approach to promote state information exchange adoption: Newborn Screening Web Portal

Newborn Screening Health IT Overview Purpose • To improve quality of care for newborns by enabling early detection of and intervention for heritable disorders. Special considerations and challenges • Public health screening in conjunction with primary care delivery • Continuity of care from birth center to primary care and follow up care. • Pediatric and maternal health – integration of pre natal, post natal and infant healthcare Resources available to initiate electronic information exchange of NBS data • Use Case • Coding and Terminology Guide • Information Package • Privacy and Security Policy Guidance
Current Limitations for Electronic Information Exchange in Newborn Screening • Public health information exchange systems are nascent (NHIN etc. ). • Overall limited capability to exchange lab orders and results – progress in some states (i. e. Iowa, Texas, Delaware and New York) However: – Provides opportunity for connecting Newborn Screening with (i. e. immunization to build comprehensive EHR). – Presents a case of transfer of care (birth center to primary care provider) and could serve as a template for other scenarios. – Supports population health activities including research and program evaluation. – Supported by massive federal investment (HITECH) in infrastructure and adoption.

Rationale and Proposal for Newborn Screening Web Portal Web-portal based information exchange addresses both the importance of Newborn Screening as well as electronic information exchange opportunities. • an area of public health importance. • mandated by all states. • a ‘leading edge’ area for clinical application of genetic knowledge. An effective electronic communication strategy would both improve NBS-based care and potentially serve as a model for: • Use of health information storage and exchange to support pediatric / lifelong care • Communication among various elements of the health care system • Integration of practice and public health information Electronic storage and distribution of NBS data would also: • Provide new resources for research • Lay a foundation for use of genetic information in clinical care • Expand consumer access to information and medical decision making
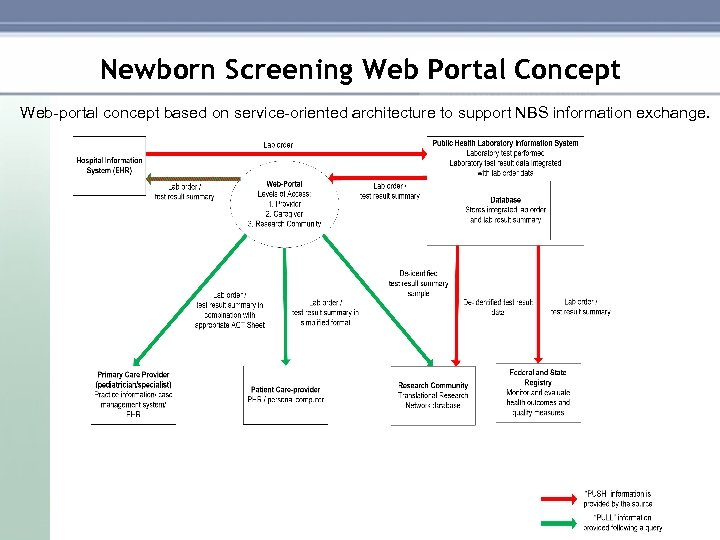
Newborn Screening Web Portal Concept Web portal concept based on service oriented architecture to support NBS information exchange.

Expected Outcomes and Benefits to Patients and Parents: • Availability of complete newborn screening information • Portability of patient record • Improved coordination of care • Improved child safety (adverse event avoidance) • Reduced loss to follow up Benefits to Primary Care Providers: • Actionable information for primary care providers, public health and specialists • Reduced time and effort entering data manually • Improved timely and appropriate data sharing to support follow up care and treatment. • Non –duplication of services Benefits to State Health Department / Public Health Laboratory: • Preserve the systems that healthcare professionals already use • Reduced cost of specimen processing due to more effective recording and reporting. • Connect local systems into regional network • Provide a centralized data exchange • Deliver benefits to users early in the deployment process • Reduced cost and deployment time compared to fully integrated HIE

Next Steps • Identification of community based coalitions including state(s) or regional collaboratives to lead demonstration project • Funding resources available for web portal development Ø ARRA/HITECH Section 3013 Ø ARRA/HITECH Section 3011 Ø CMS Medicaid Incentive Payments

BREAK

Agenda 1. 2. 3. 4. 5. - Scholle The Nationwide Health Information Network Status of the Implementation of the Newborn Screening Use Case Newborn Screening Codes & Terminology and an Approach to a Standard Report Payload Newborn Screening Web Portal Concept Break 6. Measures for Quality – 7. 8. 9. Sarah Scholle, Dr. PH, National Center for Quality Assurance Internal Review Workgroup: Nomination of Alpha Thalassemia: Hemoglobin H Disease to the ACHDNC’s Recommended Uniform Screening Panel Committee Discussion Adjourn

NCQA’s Efforts to Improve Quality Measurement in Child Health Care Title Sarah Hudson Scholle Assistant Vice President, Research Advisory Committee on Heritable Disorders In Newborns and Children September 24, 2009

Agenda • NCQA • Strategy for Child Health Quality • Measures for newborn screening and follow-up • Efforts to expand measurement in care coordination and women’s health 93

NCQA: A Brief Introduction • Private, independent non-profit health care quality oversight organization founded in 1990 • Committed to measurement, transparency and accountability • Unites diverse groups around common goal: improving health care quality 94

NCQA: Committed to measurement, transparency, accountability Quality measurement means: • Use of objective measures based on evidence • Results that are comparable across organizations • Impartial third-party evaluation and audit • Public Reporting NCQA’s quality programs include: • Accreditation of health plans using performance data • HEDIS clinical measures • CAHPS consumer survey • Measurement of quality in provider groups • Physician Recognition 95

What is NCQA’s HEDIS? The Healthcare Effectiveness Data and Information Set: • Process and outcomes measures • Standardized member experience surveys • Used by commercial, Medicare, and Medicaid plans alike • Allows plan-to-plan comparisons by quality, not just by price 96
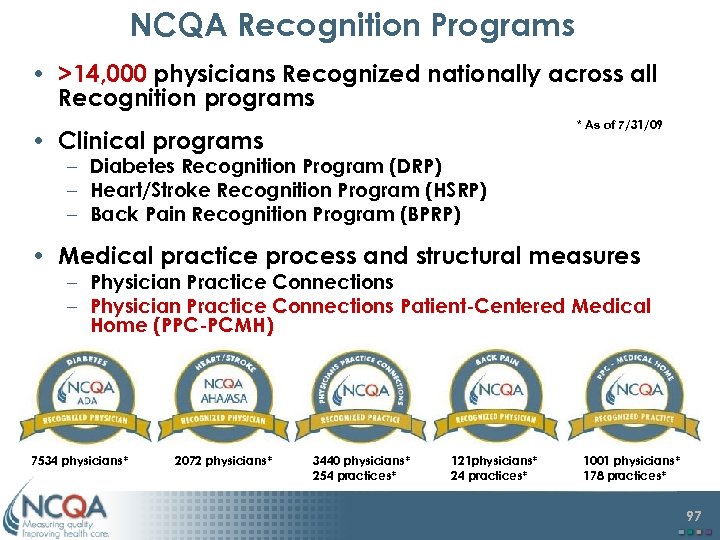
NCQA Recognition Programs • >14, 000 physicians Recognized nationally across all Recognition programs * As of 7/31/09 • Clinical programs – Diabetes Recognition Program (DRP) – Heart/Stroke Recognition Program (HSRP) – Back Pain Recognition Program (BPRP) • Medical practice process and structural measures – Physician Practice Connections Patient-Centered Medical Home (PPC-PCMH) 7534 physicians* 2072 physicians* 3440 physicians* 254 practices* 121 physicians* 24 practices* 1001 physicians* 178 practices* 97

Long-Term Vision for Improving Child Health Quality Measurement • Develop measurement strategy to increase attention to child health outcomes – School readiness, workforce readiness, family productivity • Explore opportunities for assessing return on investment and for communicating with stakeholders • Identify opportunities to use new and emerging technologies to build a new infrastructure for monitoring child health • Build strategic partnerships to achieve vision and complement other efforts 98
Comprehensive Well Care Measures • Specify measures for age-appropriate strategy for comprehensive well child care • Conduct a field test at the health plan and physician levels • Examine the impact of alternative eligibility criteria and methods for calculating performance rates • Replace current, nonspecific measures for well-child visits Supported by The Commonwealth Fund 99
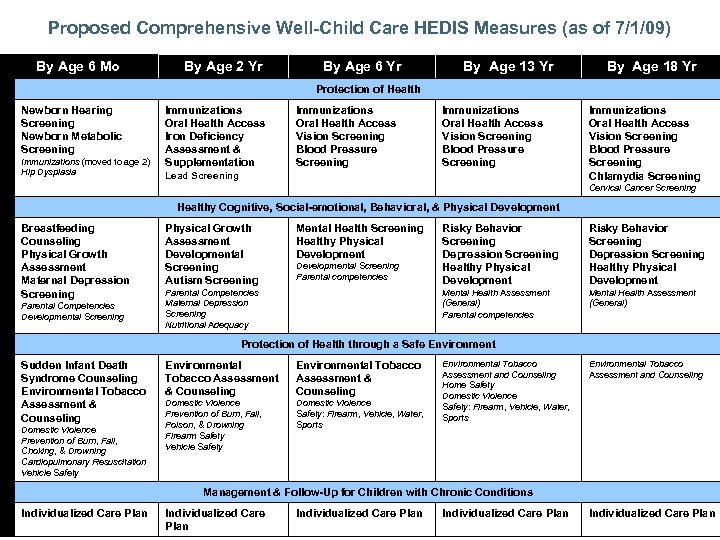
Proposed Comprehensive Well-Child Care HEDIS Measures (as of 7/1/09) HEDIS Measures By Age 6 Mo By Age 2 Yr By Age 6 Yr By Age 13 Yr By Age 18 Yr Protection of Health Newborn Hearing Screening Newborn Metabolic Screening Immunizations (moved to age 2) Hip Dysplasia Immunizations Oral Health Access Iron Deficiency Assessment & Supplementation Immunizations Oral Health Access Vision Screening Blood Pressure Screening Lead Screening Immunizations Oral Health Access Vision Screening Blood Pressure Screening Chlamydia Screening Cervical Cancer Screening Healthy Cognitive, Social-emotional, Behavioral, & Physical Development Breastfeeding Counseling Physical Growth Assessment Maternal Depression Screening Parental Competencies Developmental Screening Physical Growth Assessment Developmental Screening Autism Screening Mental Health Screening Healthy Physical Development Parental Competencies Maternal Depression Screening Nutritional Adequacy Risky Behavior Screening Depression Screening Healthy Physical Development Mental Health Assessment (General) Parental competencies Developmental Screening Parental competencies Risky Behavior Screening Depression Screening Healthy Physical Development Mental Health Assessment (General) Protection of Health through a Safe Environment Sudden Infant Death Syndrome Counseling Environmental Tobacco Assessment & Counseling Domestic Violence Prevention of Burn, Fall, Choking, & Drowning Cardiopulmonary Resuscitation Vehicle Safety Environmental Tobacco Assessment & Counseling Domestic Violence Prevention of Burn, Fall, Poison, & Drowning Firearm Safety Vehicle Safety Domestic Violence Safety: Firearm, Vehicle, Water, Sports Environmental Tobacco Assessment and Counseling Home Safety Domestic Violence Safety: Firearm, Vehicle, Water, Sports Environmental Tobacco Assessment and Counseling Management & Follow-Up for Children with Chronic Conditions Individualized Care Plan Individualized Care Plan
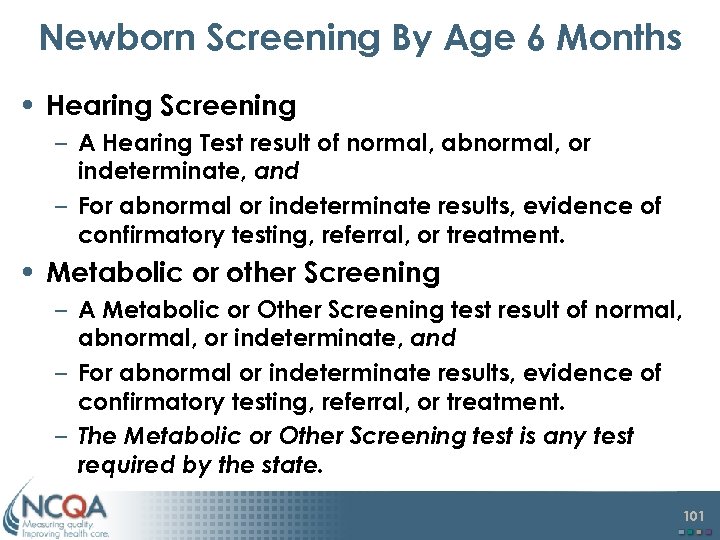
Newborn Screening By Age 6 Months • Hearing Screening – A Hearing Test result of normal, abnormal, or indeterminate, and – For abnormal or indeterminate results, evidence of confirmatory testing, referral, or treatment. • Metabolic or other Screening – A Metabolic or Other Screening test result of normal, abnormal, or indeterminate, and – For abnormal or indeterminate results, evidence of confirmatory testing, referral, or treatment. – The Metabolic or Other Screening test is any test required by the state. 101

Individualized Care Plan • Separate document outlining important health information for children with chronic conditions – – – – Current list of allergies, diagnoses, and medications Treatment plan Goals for self-management Other clinicians/agencies involved in the child’s health care Instructions for when to seek urgent care Information on the next scheduled appointment Evidence that the plan was discussed with the family or caregivers – Evidence that the plan was given to the family or caregivers 102

Timeline for 2009 • Complete specifications and field test plan • Conduct field test in up to 20 physician practices and 6 programs including Medicaid and CHIP plans and state PCCM program • Prepare report and recommendations for revised measures specification by end of 2009 • Present to NCQA’s Committee on Performance Measurement in January 2010 103

Care Coordination for Vulnerable Children • To identify an approach for measurement and feasible implementation strategies for monitoring and improving care coordination for children with or at risk of developmental delay Supported by The Commonwealth Fund 104
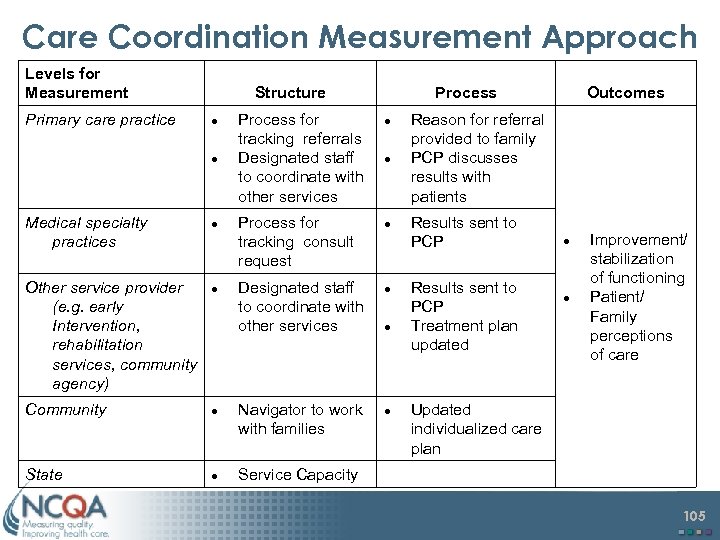
Care Coordination Measurement Approach Levels for Measurement Primary care practice Structure Medical specialty practices Process for tracking referrals Designated staff to coordinate with other services Reason for referral provided to family PCP discusses results with patients Process for tracking consult request Results sent to PCP Outcomes Other service provider (e. g. early Intervention, rehabilitation services, community agency) Designated staff to coordinate with other services Results sent to PCP Treatment plan updated Community Navigator to work with families Improvement/ stabilization of functioning Patient/ Family perceptions of care Updated individualized care plan State Service Capacity 105

Project Implementation • Interviews with key informants to identify measurement approach • Convene a multi-stakeholder advisory panel • Conduct site visits to states to gain feedback and suggestions on the care coordination measurement approach • Prepare and disseminate recommendations for future measure testing and implementation 106

Extend Population Health Measurement to Women’s Health Care • Phase 1: Convene a small working meeting to prioritize prioritizing measurement opportunities – Jointly sponsored by AMA PCPI Multi-stakeholder: consumers, physicians, nursemidwives, health plans, employers, state and federal Medicaid officials, and researchers. Supported by CDC and HRSA – – Evidence Review Specifications Field testing Public comment – – • Phase 2: Develop and test measures 107

End For more information: Sarah Hudson Scholle, MPH, Dr. PH scholle@ncqa. org 202 955 1726 www. ncqa. org 108

Agenda 1. 2. 3. 4. 5. 6. - Frempong The Nationwide Health Information Network Status of the Implementation of the Newborn Screening Use Case Newborn Screening Codes & Terminology and an Approach to a Standard Report Payload Newborn Screening Web Portal Concept Break Measures for Quality 7. Internal Review Workgroup: Nomination of Alpha Thalassemia: Hemoglobin H Disease to the ACHDNC’s Recommended Uniform Screening Panel – 8. 9. Kwaku Ohene-Frempong, M. D. , Committee Member Committee Discussion Adjourn

Nomination of Alpha Thalassemia: Hemoglobin H Disease Kwaku Ohene Frempong, MD University of Pennsylvania The Children’s Hospital of Philadelphia

Nomination of Alpha Thalassemia: Hemoglobin H Disease Name of Proponent: Elliott Vichinsky, MD Organization: Children’s Hospital Oakland Date: 4/28/09 Condition: Alpha thalassemia / Hb H Type of Disorder: Hemoglobinopathy Screening Method: Newborn, Dried Blood Spot Treatment strategy: Early referral for comprehensive care before onset of illness
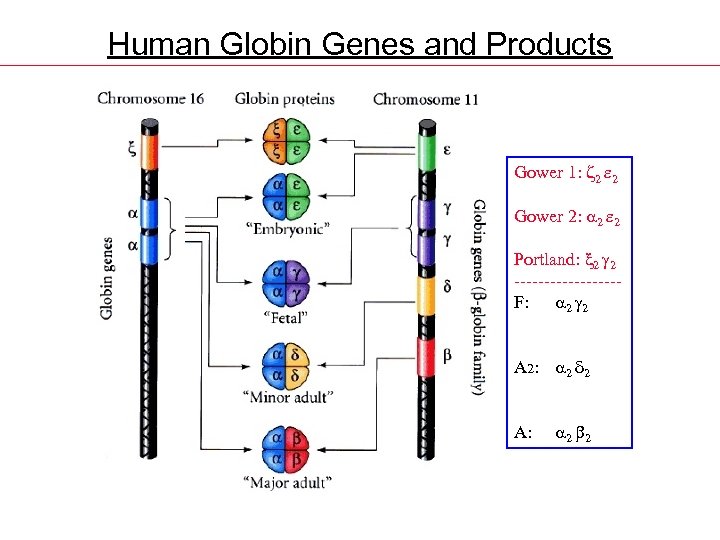
Human Globin Genes and Products Gower 1: z 2 e 2 Gower 2: 2 e 2 Portland: x 2 2 ---------F: 2 2 A 2: 2 d 2 A: 2 2
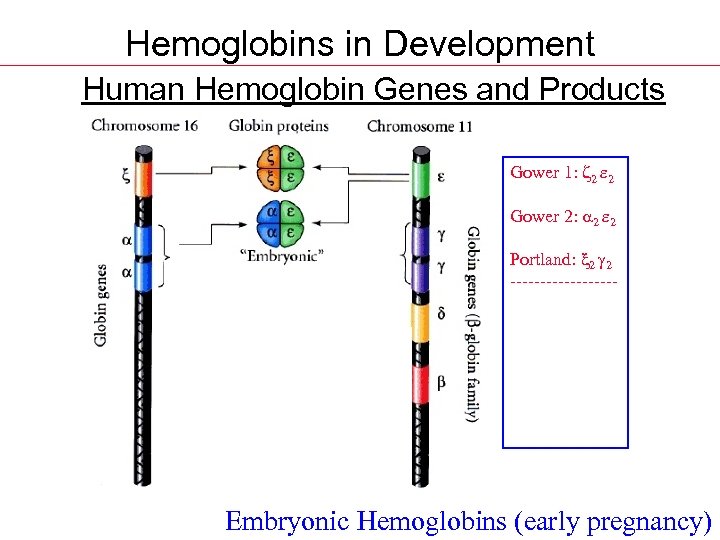
Hemoglobins in Development Human Hemoglobin Genes and Products Gower 1: z 2 e 2 Gower 2: 2 e 2 Portland: x 2 2 ---------F: 2 2 A 2: 2 d 2 A: 2 2 Embryonic Hemoglobins (early pregnancy)
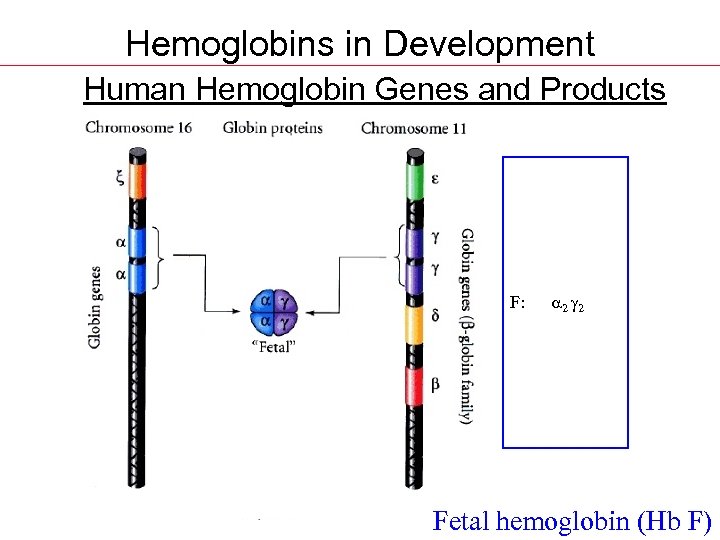
Hemoglobins in Development Human Hemoglobin Genes and Products Gower 1: z 2 e 2 Gower 2: 2 e 2 Portland: x 2 2 ---------F: 2 2 A 2: 2 d 2 A: 2 2 Fetal hemoglobin (Hb F)
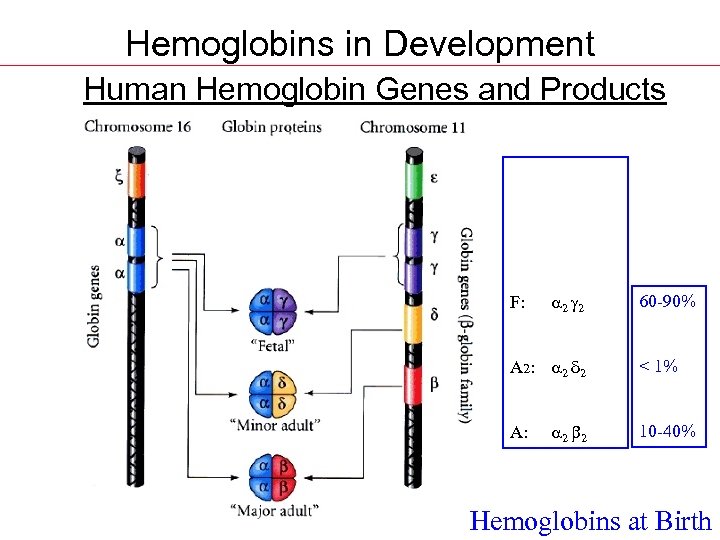
Hemoglobins in Development Human Hemoglobin Genes and Products Gower 1: z 2 e 2 Gower 2: 2 e 2 Portland: x 2 2 ---------F: 2 2 60 -90% A 2: 2 d 2 < 1% A: 2 2 10 -40% Hemoglobins at Birth
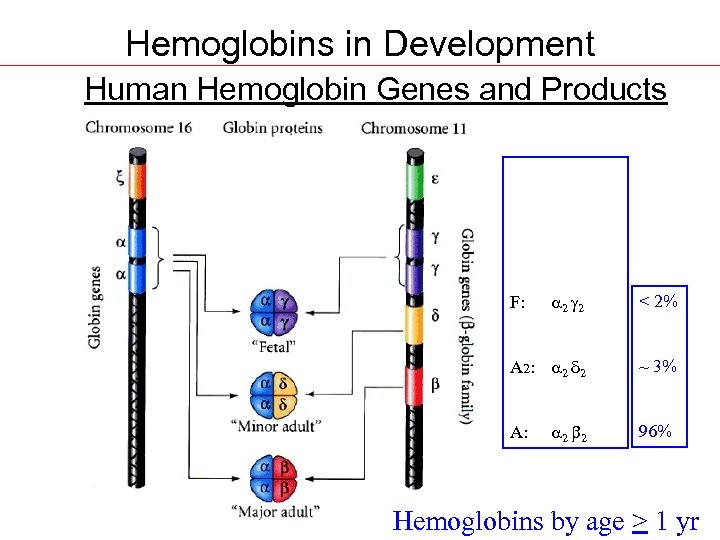
Hemoglobins in Development Human Hemoglobin Genes and Products Gower 1: z 2 e 2 Gower 2: 2 e 2 Portland: x 2 2 ---------F: 2 2 < 2% A 2: 2 d 2 ~ 3% 2 2 96% A: Hemoglobins by age > 1 yr

Alpha thalassemia results from a deficiency in globin production Globin Imbalance Normal Hb Globin composition F A Thalassemia Abnormal Hb Excess Bart's H
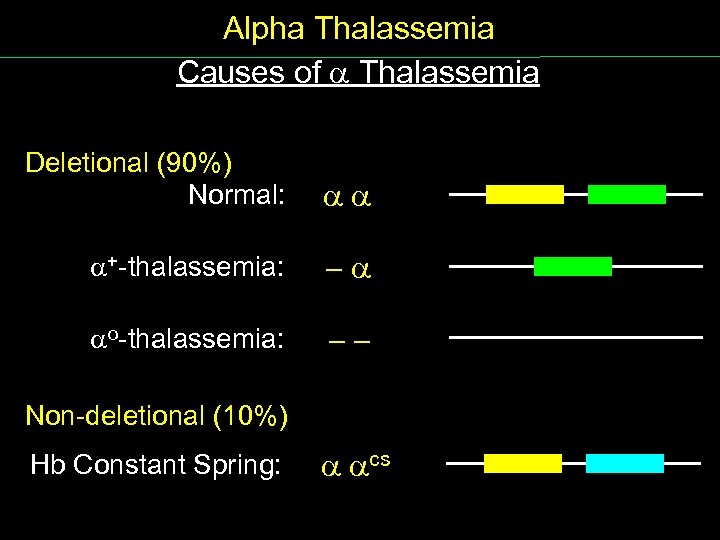
Alpha Thalassemia Causes of Thalassemia Deletional (90%) Normal: + thalassemia: - o thalassemia: - - Non deletional (10%) Hb Constant Spring: cs
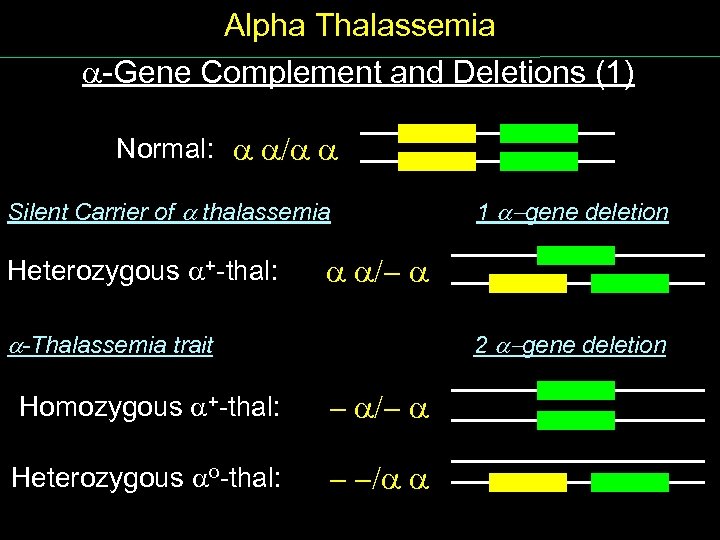
Alpha Thalassemia Gene Complement and Deletions (1) Normal: / Silent Carrier of thalassemia Heterozygous + thal: 1 -gene deletion /- -Thalassemia trait 2 -gene deletion Homozygous + thal: - /- Heterozygous o thal: - -/
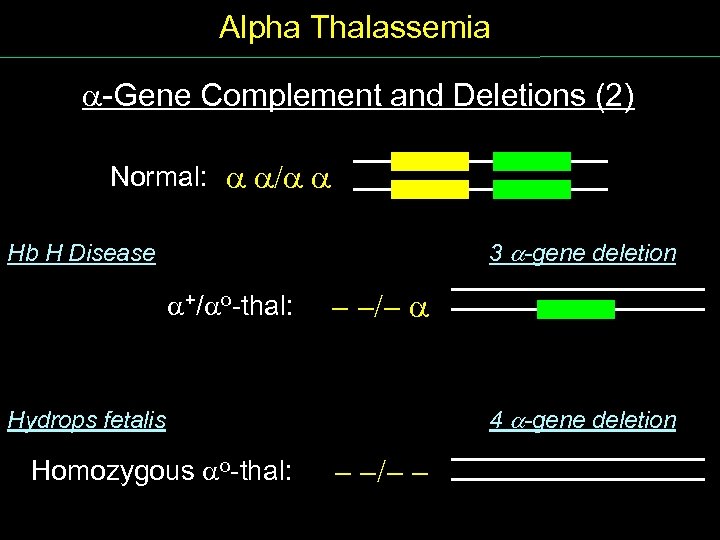
Alpha Thalassemia Gene Complement and Deletions (2) Normal: / 3 -gene deletion Hb H Disease +/ o thal: - -/- 4 -gene deletion Hydrops fetalis Homozygous o thal: - -/- -
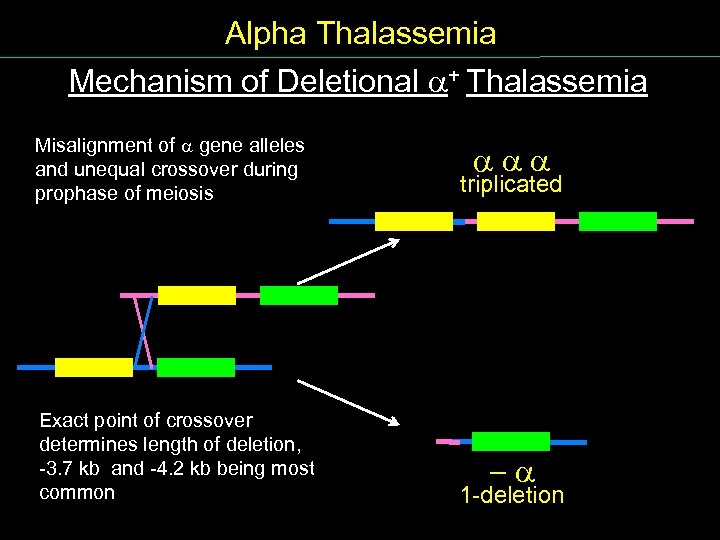
Alpha Thalassemia Mechanism of Deletional + Thalassemia Misalignment of gene alleles and unequal crossover during prophase of meiosis Exact point of crossover determines length of deletion, 3. 7 kb and 4. 2 kb being most common triplicated - 1 deletion
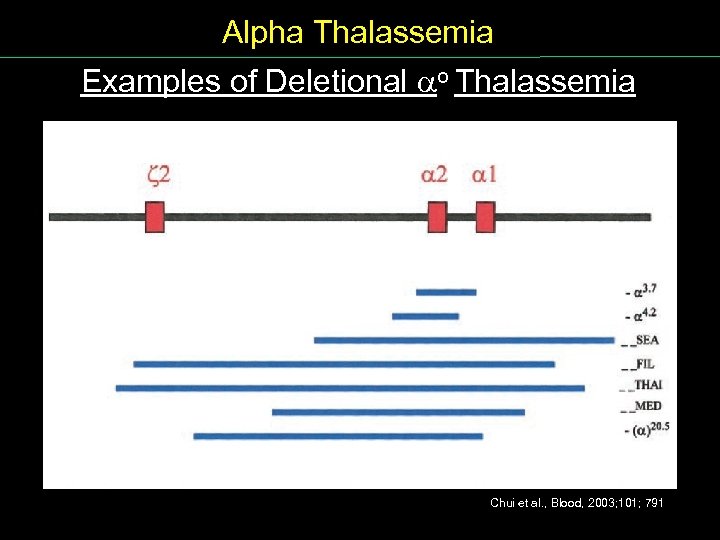
Alpha Thalassemia Examples of Deletional o Thalassemia Chui et al. , Blood, 2003; 101; 791
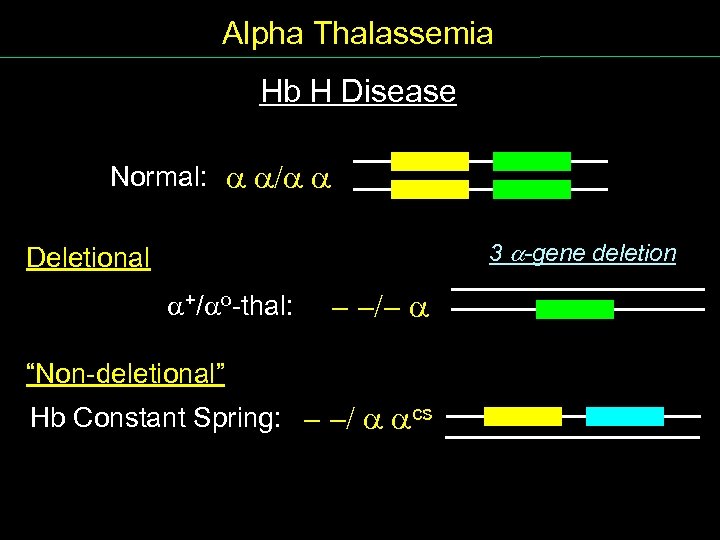
Alpha Thalassemia Hb H Disease Normal: / 3 -gene deletion Deletional +/ o thal: - -/- “Non deletional” Hb Constant Spring: - -/ cs
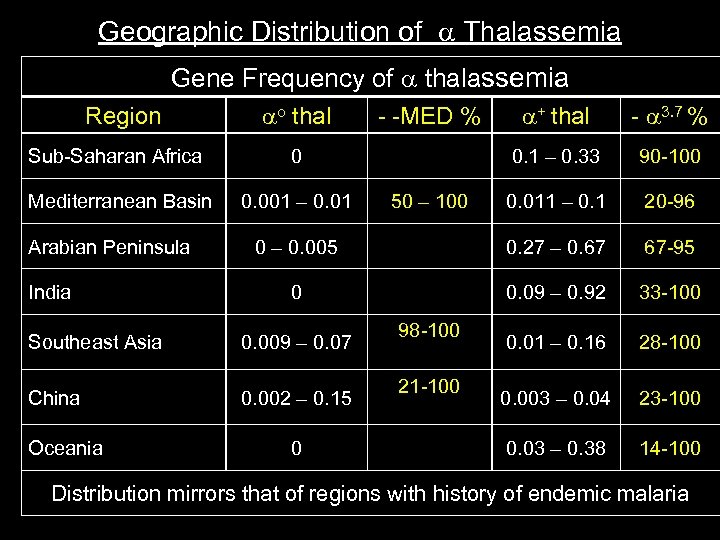
Geographic Distribution of Thalassemia Gene Frequency of thalassemia Region o thal + thal 3. 7 % 0. 1 – 0. 33 90 100 0. 011 – 0. 1 20 96 0 – 0. 005 0. 27 – 0. 67 67 95 0 0. 09 – 0. 92 33 100 0. 01 – 0. 16 28 100 0. 003 – 0. 04 23 100 0. 03 – 0. 38 14 100 Sub Saharan Africa 0 Mediterranean Basin 0. 001 – 0. 01 MED % Arabian Peninsula India Southeast Asia 0. 009 – 0. 07 China 0. 002 – 0. 15 Oceania 0 50 – 100 98 100 21 100 Distribution mirrors that of regions with history of endemic malaria
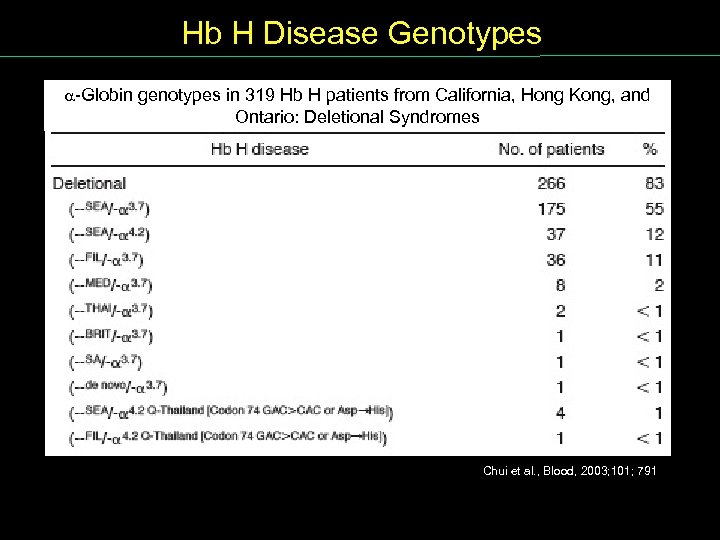
Hb H Disease Genotypes Globin genotypes in 319 Hb H patients from California, Hong Kong, and Ontario: Deletional Syndromes Chui et al. , Blood, 2003; 101; 791
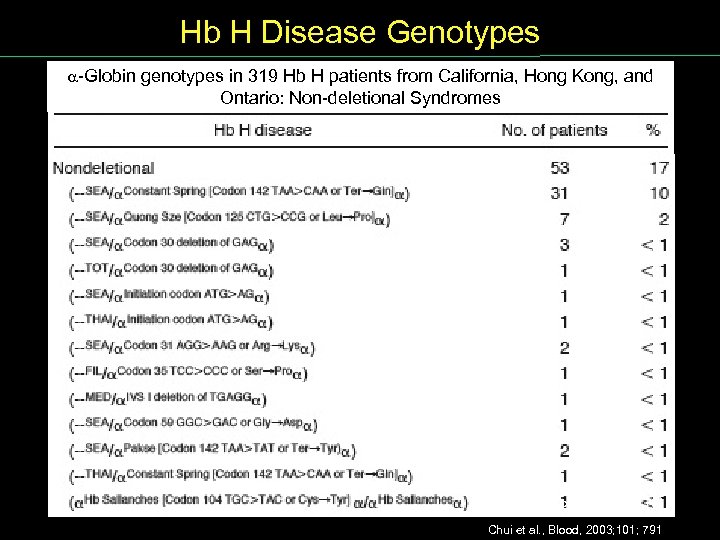
Hb H Disease Genotypes Globin genotypes in 319 Hb H patients from California, Hong Kong, and Ontario: Non deletional Syndromes Chui et al. , Blood, 2003; 101; 791

Hemoglobin H Disease Pathophysiology 1. Deficiency of globin m. RNA and globin chains • / m. RNA ratio < 0. 5 • / globin chain ratio, 0. 2 to 0. 7 2. Fetal development: Excess globin chains for 4 tetramers (Hb Bart’s) 3. Switch from Hb F to Hb A predominance: Excess globin chains for 4 tetramers (Hb H) 4. Hb H • High O 2 affinity, insignificant tissue O 2 delivery • Unstable, oxidized to form precipitates (Hb H inclusion bodies) • Hb H precipitates cause • early erythroid cell death • ineffective erythropoiesis • membrane damage leading to hemolysis
Hemoglobin H Disease Clinical Presentation Wasi, 1974: Thailand: 1, 002 patients (200 adults, 502 children) • Age at presentation: Birth to 74 years • Infants: Near normal Hb at birth, no hepatosplenomegaly Chen, 2000: • Hong Kong: 114 patients • Only 24% presented with symptoms; 76% discovered incidentally • 13% growth failure in 13%, < 3 rd % Most patients discovered through routine hematologic tests or with symptoms of acute or chronic hemolytic anemia: pallor, fatigue, jaundice
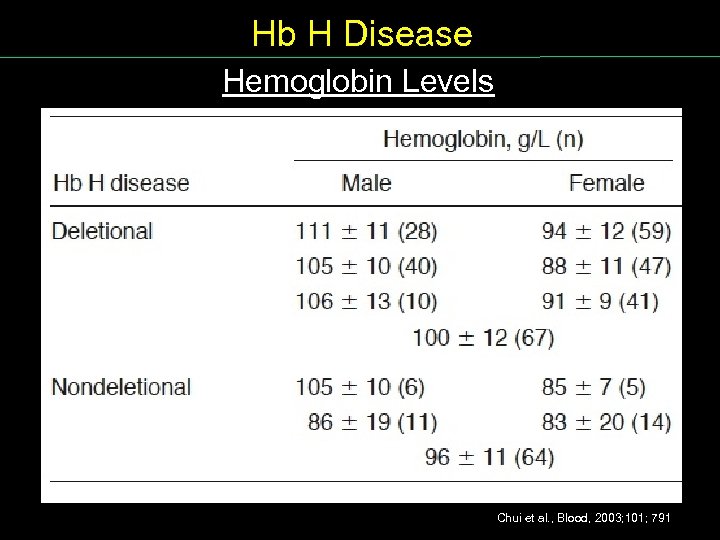
Hb H Disease Hemoglobin Levels Chui et al. , Blood, 2003; 101; 791
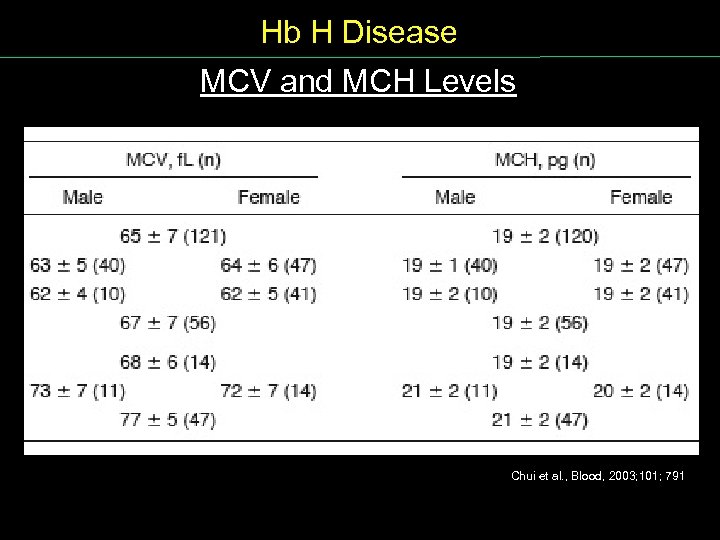
Hb H Disease MCV and MCH Levels Chui et al. , Blood, 2003; 101; 791
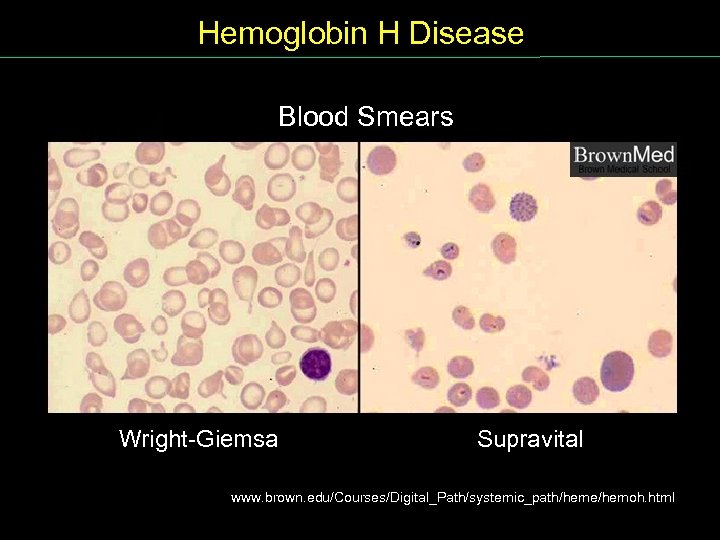
Hemoglobin H Disease Blood Smears Wright Giemsa Supravital www. brown. edu/Courses/Digital_Path/systemic_path/heme/hemoh. html

Hemoglobin H Disease Clinical Course Wide clinical spectrum • Acute anemia with febrile illness, oxidising agents usually hemolytic, but also erythroid aplasia • Splenomegaly common, hepatomegaly uncommon • Iron overload, common in SEA uncommon in Mediterranean genotypes, not related to transfusion but increased absorption • Cholelithiasis • Anemia may worsen in pregnancy; pre eclampsia, and CHF reported

Hemoglobin H Disease Treatment Primarily preventive and supportive • Folic acid supplementation • Education about signs of acute anemia, palpation of spleen • Avoidance of oxidative medications • No iron therapy, unless iron deficiency is documented • Monitoring for iron overload; iron chelation therapy if needed • Episodic red cell transfusion as needed for acute illness • Chronic transfusion therapy • Close monitoring of pregnant women • Splenectomy for those with hypersplenism
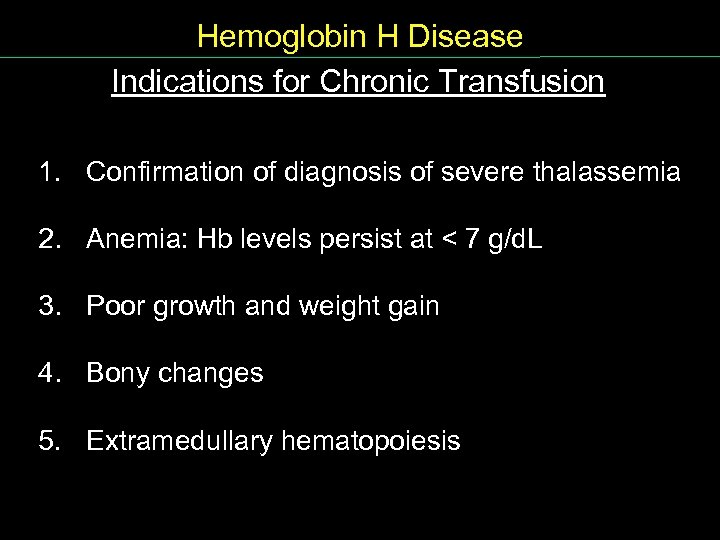
Hemoglobin H Disease Indications for Chronic Transfusion 1. Confirmation of diagnosis of severe thalassemia 2. Anemia: Hb levels persist at < 7 g/d. L 3. Poor growth and weight gain 4. Bony changes 5. Extramedullary hematopoiesis
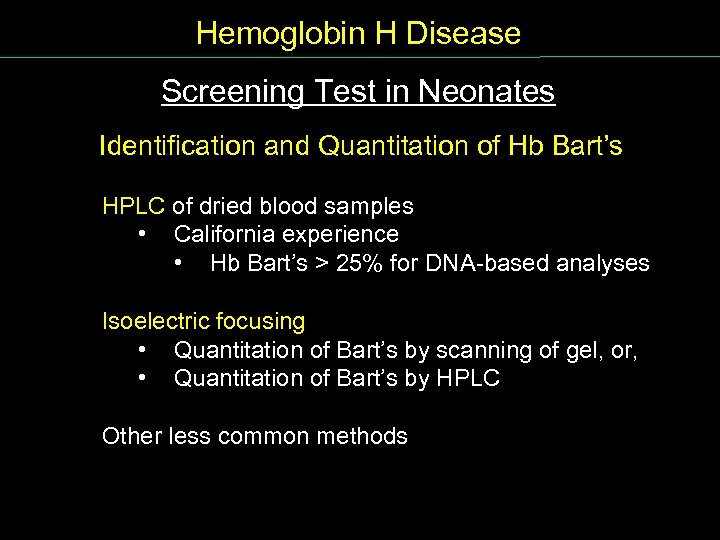
Hemoglobin H Disease Screening Test in Neonates Identification and Quantitation of Hb Bart’s HPLC of dried blood samples • California experience • Hb Bart’s > 25% for DNA based analyses Isoelectric focusing • Quantitation of Bart’s by scanning of gel, or, • Quantitation of Bart’s by HPLC Other less common methods
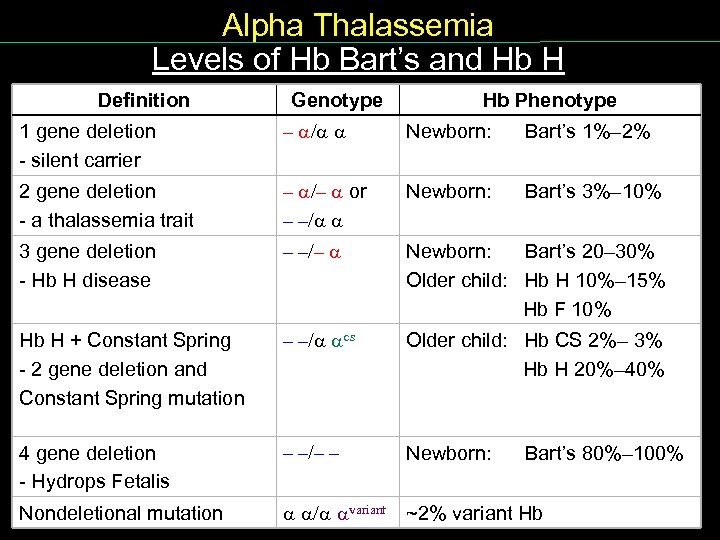
Alpha Thalassemia Levels of Hb Bart’s and Hb H Definition Levels Hb. Bart’s Genotype Hb Phenotype 1 gene deletion silent carrier – / Newborn: Bart’s 1%– 2% 2 gene deletion a thalassemia trait – /– or – –/ Newborn: Bart’s 3%– 10% 3 gene deletion Hb H disease – –/– Newborn: Bart’s 20– 30% Older child: Hb H 10%– 15% Hb F 10% Hb H + Constant Spring 2 gene deletion and Constant Spring mutation – –/ cs Older child: Hb CS 2%– 3% Hb H 20%– 40% 4 gene deletion Hydrops Fetalis – –/– – Newborn: Bart’s 80%– 100% Nondeletional mutation / variant ~2% variant Hb
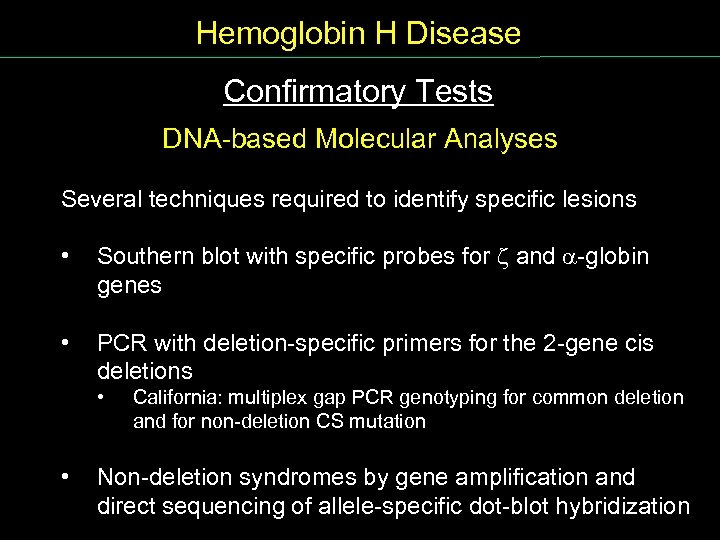
Hemoglobin H Disease Confirmatory Tests Conf Tests DNA based Molecular Analyses Several techniques required to identify specific lesions • Southern blot with specific probes for z and globin genes • PCR with deletion specific primers for the 2 gene cis deletions • • California: multiplex gap PCR genotyping for common deletion and for non deletion CS mutation Non deletion syndromes by gene amplification and direct sequencing of allele specific dot blot hybridization

Screenshot
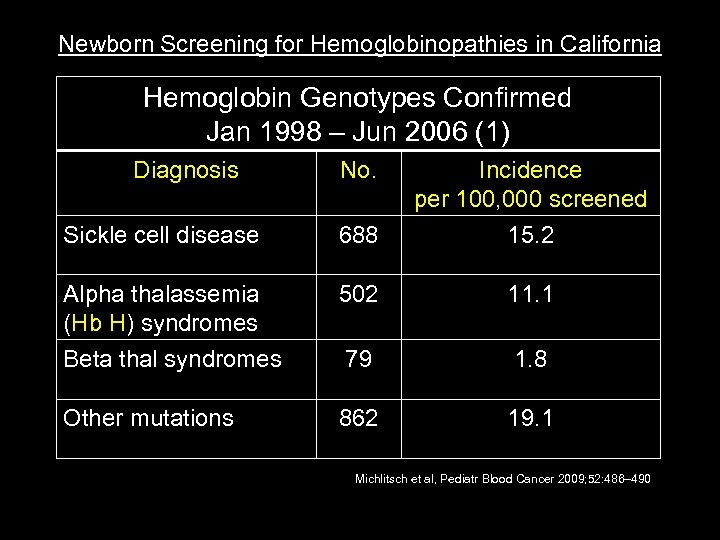
Newborn Screening for Hemoglobinopathies in California NB Screen CA Hemoglobin Genotypes Confirmed Jan 1998 – Jun 2006 (1) Diagnosis No. Incidence per 100, 000 screened Sickle cell disease 688 15. 2 Alpha thalassemia (Hb H) syndromes 502 11. 1 Beta thal syndromes 79 1. 8 Other mutations 862 19. 1 Michlitsch et al, Pediatr Blood Cancer 2009; 52: 486– 490
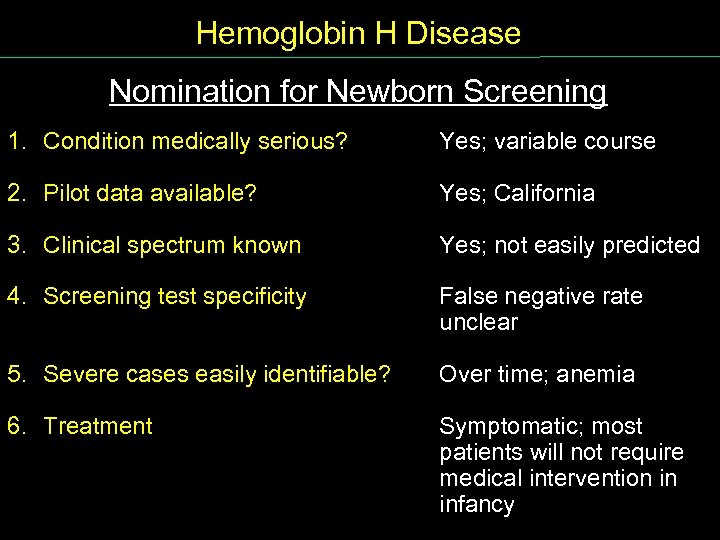
Hemoglobin H Disease Nomination for Newborn Screening Nomination NB Screen 1. Condition medically serious? Yes; variable course 2. Pilot data available? Yes; California 3. Clinical spectrum known Yes; not easily predicted 4. Screening test specificity False negative rate unclear 5. Severe cases easily identifiable? Over time; anemia 6. Treatment Symptomatic; most patients will not require medical intervention in infancy
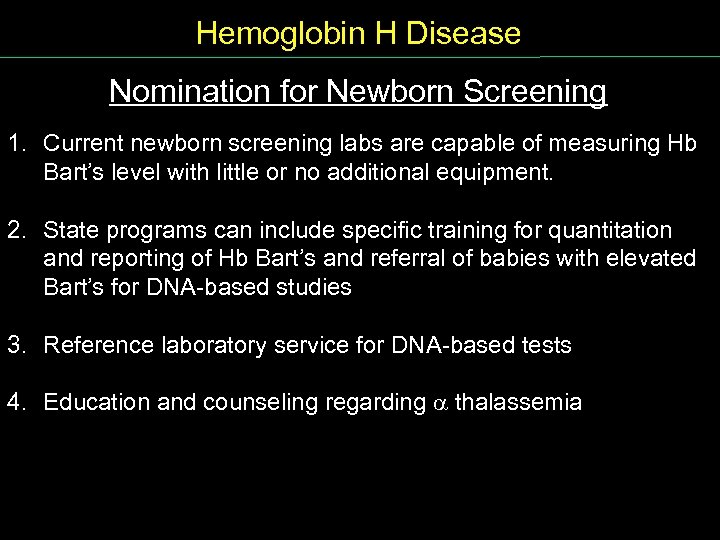
Hemoglobin H Disease Nomination for Newborn Screening 1. Current newborn screening labs are capable of measuring Hb Bart’s level with little or no additional equipment. 2. State programs can include specific training for quantitation and reporting of Hb Bart’s and referral of babies with elevated Bart’s for DNA based studies 3. Reference laboratory service for DNA based tests 4. Education and counseling regarding thalassemia Nomination NB Screen

Agenda - Discussion 1. The Nationwide Health Information Network 2. Status of the Implementation of the Newborn Screening Use Case 3. Newborn Screening Codes & Terminology and an Approach to a Standard Report Payload 4. Newborn Screening Web Portal Concept 5. Break 6. Measures for Quality 7. Internal Review Workgroup: Nomination of Alpha Thalassemia: Hemoglobin H Disease to the ACHDNC’s Recommended Uniform Screening Panel 8. Committee Discussion 9. Adjourn

Meeting Adjourned

The meeting will start momentarily.

The meeting will resume momentarily.
c867327faecb6351d069d24f1ae332e0.ppt