16c3294d1cbd33e17e61df9a54ffd125.ppt
- Количество слайдов: 24
 Medidata Rave Start-Up Information
Medidata Rave Start-Up Information
 Medidata Rave® Medidata Rave is a registered trademark of Medidata Solutions Worldwide • A web based electronic data collection system (EDC) being implemented across NCI CTEP • Only requires a web browser and internet connection • Data will be entered at the site by a Rave Clinical Research Associate (CRA role) • No paper case report forms submitted to RTOG • Sites will have access to multiple clinical trial group protocols through a single point of entry 2
Medidata Rave® Medidata Rave is a registered trademark of Medidata Solutions Worldwide • A web based electronic data collection system (EDC) being implemented across NCI CTEP • Only requires a web browser and internet connection • Data will be entered at the site by a Rave Clinical Research Associate (CRA role) • No paper case report forms submitted to RTOG • Sites will have access to multiple clinical trial group protocols through a single point of entry 2
 Rave Account Activation DETAILS ON CTSU WEB SITE UNDER RAVE TAB • Once your site has RSS authorization and users have been registered with a CTEP-IAM account, users will receive an email invitation from: i. Medidata-Notification@mdsol. com to set up their Rave account. Make sure this email is not in a Junk or Spam mailbox. • Click on the link inside the email (this link does expire in 30 days) and follow the instructions provided. Remember your login name and password for future access to Rave. • If you have already participated in another Rave study with another group, then you only need to obtain your Rave account once. 3
Rave Account Activation DETAILS ON CTSU WEB SITE UNDER RAVE TAB • Once your site has RSS authorization and users have been registered with a CTEP-IAM account, users will receive an email invitation from: i. Medidata-Notification@mdsol. com to set up their Rave account. Make sure this email is not in a Junk or Spam mailbox. • Click on the link inside the email (this link does expire in 30 days) and follow the instructions provided. Remember your login name and password for future access to Rave. • If you have already participated in another Rave study with another group, then you only need to obtain your Rave account once. 3
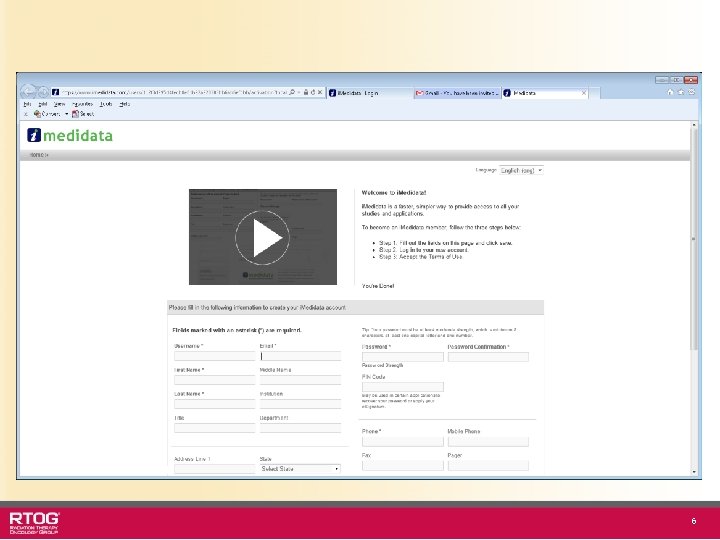
 Steps for Registering for the RTOG-1115 Study www. ctsu. org – Rave tab • • • Obtain authorization for your site through the Regulatory Support System (RSS). This web-enabled system is managed by the CTSU Obtain CTEP-IAM accounts for site coordinators and investigators. Patients are registered for this study using the Oncology Patient Enrollment Network (OPEN) after sites have been authorized in RSS (submission of regulatory documents). The enrollment information entered in OPEN will automatically be posted to the RTOG-1115 Rave database. Access to i. Medidata and Rave is controlled through the CTEP-IAM system and through role assignments made in the RSS system. The first time your site receives approval for a Rave study, you will receive an i. Medidata Invitation email that will enable you to login to that Rave database as either a CRA or an Investigator, depending on the role that was assigned in RSS. Review the www. ctsu. org website for detailed information on RSS, CTEPIAM, Rave, and OPEN. 4
Steps for Registering for the RTOG-1115 Study www. ctsu. org – Rave tab • • • Obtain authorization for your site through the Regulatory Support System (RSS). This web-enabled system is managed by the CTSU Obtain CTEP-IAM accounts for site coordinators and investigators. Patients are registered for this study using the Oncology Patient Enrollment Network (OPEN) after sites have been authorized in RSS (submission of regulatory documents). The enrollment information entered in OPEN will automatically be posted to the RTOG-1115 Rave database. Access to i. Medidata and Rave is controlled through the CTEP-IAM system and through role assignments made in the RSS system. The first time your site receives approval for a Rave study, you will receive an i. Medidata Invitation email that will enable you to login to that Rave database as either a CRA or an Investigator, depending on the role that was assigned in RSS. Review the www. ctsu. org website for detailed information on RSS, CTEPIAM, Rave, and OPEN. 4
 Rave Account Activation Invitation Email (EXAMPLE) Dear User, Welcome to i. Medidata. com -- a faster, simpler way to access all your Medidata Rave® studies, Medidata applications and online discussions in one place. You've been invited to activate your new account. Please click on the following link: http: //www. imedidata. com/users/. . . . /activation? locale=eng If clicking the link above does not work, copy and paste the URL in a new browser window instead. If you are a current user of Rave and would like a brief tutorial on connecting your Rave account to i. Medidata, please click on the following video: http: //www. imedidata. com/Web. Help. Videos_ENG/init. htm Thank you for using i. Medidata. For more information please visit http: //www. imedidata. com. For support with your new account, please email helpdesk@mdsol. com. This is a post-only mailing. Replies to this message are not monitored or answered. Medidata Solutions Worldwide 5
Rave Account Activation Invitation Email (EXAMPLE) Dear User, Welcome to i. Medidata. com -- a faster, simpler way to access all your Medidata Rave® studies, Medidata applications and online discussions in one place. You've been invited to activate your new account. Please click on the following link: http: //www. imedidata. com/users/. . . . /activation? locale=eng If clicking the link above does not work, copy and paste the URL in a new browser window instead. If you are a current user of Rave and would like a brief tutorial on connecting your Rave account to i. Medidata, please click on the following video: http: //www. imedidata. com/Web. Help. Videos_ENG/init. htm Thank you for using i. Medidata. For more information please visit http: //www. imedidata. com. For support with your new account, please email helpdesk@mdsol. com. This is a post-only mailing. Replies to this message are not monitored or answered. Medidata Solutions Worldwide 5
 6
6
 7
7
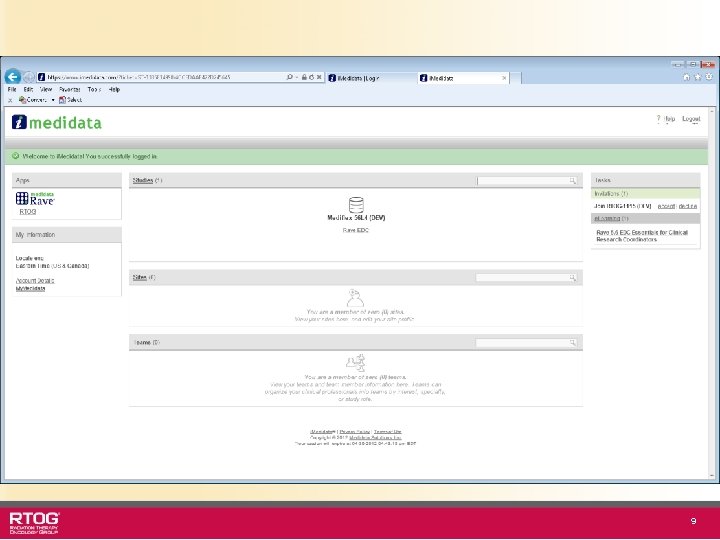
 Accept Study Invitation and Perform e. Learnings • After login, there will be an invitation to join the RTOG 1115 study (in the TASKS section). Accept this invitation. • A list of e. Learning modules will also be on your welcome screen (if applicable) underneath TASKS. • Click each module to run the associated course. After completion of required course(s), you will have access to your assigned study. • Credit for taking the e. Learning modules will apply to all Cooperative Groups’ Rave studies. • Users have required e. Learning modules and optional e. Learning modules 8
Accept Study Invitation and Perform e. Learnings • After login, there will be an invitation to join the RTOG 1115 study (in the TASKS section). Accept this invitation. • A list of e. Learning modules will also be on your welcome screen (if applicable) underneath TASKS. • Click each module to run the associated course. After completion of required course(s), you will have access to your assigned study. • Credit for taking the e. Learning modules will apply to all Cooperative Groups’ Rave studies. • Users have required e. Learning modules and optional e. Learning modules 8
 9
9
 e. Learning Modules • Modules for CRAs – EDC Essentials for Clinical Research Coordinators (required) – 45 min – EDC Inspection Readiness for Clinical Sites (optional) – 30 min – Data Privacy Considerations for Clinical Sites (optional) – 50 min • Modules for Investigators – EDC Essentials for Investigators with Data Entry (required) – 50 min – EDC Inspection Readiness for Clinical Sites (optional) – 30 min – Data Privacy Considerations for Clinical Sites (optional) – 50 min 10
e. Learning Modules • Modules for CRAs – EDC Essentials for Clinical Research Coordinators (required) – 45 min – EDC Inspection Readiness for Clinical Sites (optional) – 30 min – Data Privacy Considerations for Clinical Sites (optional) – 50 min • Modules for Investigators – EDC Essentials for Investigators with Data Entry (required) – 50 min – EDC Inspection Readiness for Clinical Sites (optional) – 30 min – Data Privacy Considerations for Clinical Sites (optional) – 50 min 10
 SUBJECT ENROLLMENT Like magic once you have enrolled your patient through the OPEN system all of this information will then appear in the Rave system. In our current EDC system, the enrollment information is represented by the A 0 form. In Rave the enrollment information will appear as (5) forms: 1. Subject Enrollment 2. Step Information 3. Treatment Assignment 4. Demography 5. Eligibility Checklist 11
SUBJECT ENROLLMENT Like magic once you have enrolled your patient through the OPEN system all of this information will then appear in the Rave system. In our current EDC system, the enrollment information is represented by the A 0 form. In Rave the enrollment information will appear as (5) forms: 1. Subject Enrollment 2. Step Information 3. Treatment Assignment 4. Demography 5. Eligibility Checklist 11
 Access Study RTOG-1115 Select the EDC Link to Perform EDC Functions 12
Access Study RTOG-1115 Select the EDC Link to Perform EDC Functions 12
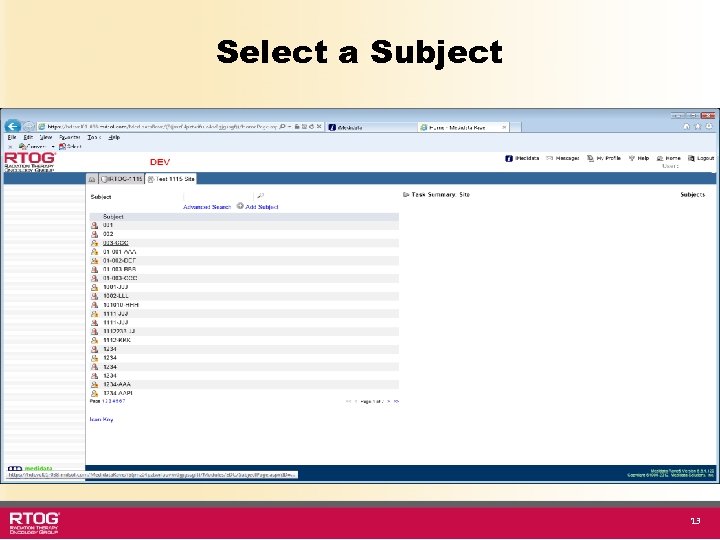 Select a Subject 13
Select a Subject 13
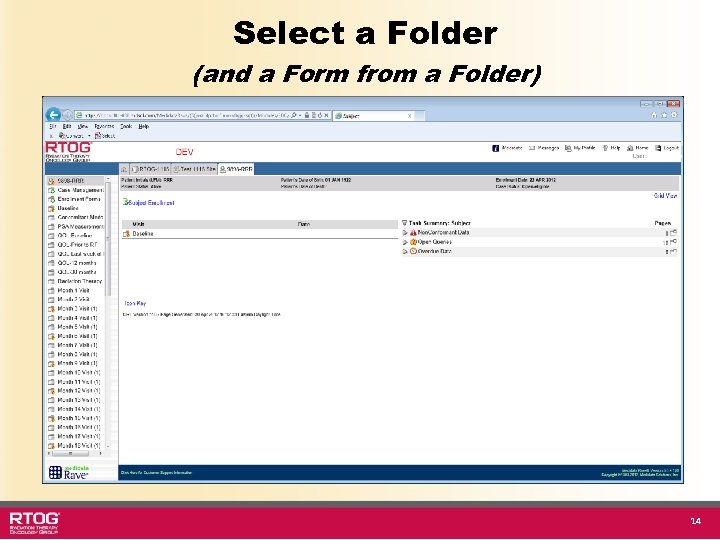 Select a Folder (and a Form from a Folder) 14
Select a Folder (and a Form from a Folder) 14
 New Folders and Forms will Appear Automatically • Refer to Section 12 of the RTOG-1115 protocol for detailed information regarding what folders and forms are used for this study. • As information is added to forms, additional forms and folders may appear for the subject (patient). • For example, when the Work Up form is completed in the Baseline folder (with a valid response to the Performance Status field) and the “Save” button is pressed, then the next series of folders and forms will be populated for that subject. • Another example, if in the Month 3 Visit folder, the user indicates on the Follow Up form that adverse events have occurred or continued, then an Adverse Events form will be added to the Month 3 Visit folder and a message will appear reminding you to complete the adverse event form after you complete and save the current form. 15
New Folders and Forms will Appear Automatically • Refer to Section 12 of the RTOG-1115 protocol for detailed information regarding what folders and forms are used for this study. • As information is added to forms, additional forms and folders may appear for the subject (patient). • For example, when the Work Up form is completed in the Baseline folder (with a valid response to the Performance Status field) and the “Save” button is pressed, then the next series of folders and forms will be populated for that subject. • Another example, if in the Month 3 Visit folder, the user indicates on the Follow Up form that adverse events have occurred or continued, then an Adverse Events form will be added to the Month 3 Visit folder and a message will appear reminding you to complete the adverse event form after you complete and save the current form. 15
 OLD vs NEW (RAVE) EDC • In our current EDC system if a ‘filter question’ is answered yes—additional information would then appear once you clicked on ‘next’ and have to be answered • In RAVE if a ‘filter question’ is answered as yes, you will not immediately see the additional info to be answered—once the save button is clicked then another form will be available in the ‘forms list’ to report the information 16
OLD vs NEW (RAVE) EDC • In our current EDC system if a ‘filter question’ is answered yes—additional information would then appear once you clicked on ‘next’ and have to be answered • In RAVE if a ‘filter question’ is answered as yes, you will not immediately see the additional info to be answered—once the save button is clicked then another form will be available in the ‘forms list’ to report the information 16
 FILTER QUESTIONS IN RAVE AN EXAMPLE The Follow Up form asks if the patient had ‘a new primary cancer or MDS diagnosed’ and the answer is ‘yes’—the diagnosed form will continue to ask questions until you ‘save’ the form. A message will appear above the question that states: If patient is diagnosed with a new primary, then complete the New Primary Cancer form (after completion of this form). Once this is done you will then notice that there is a New Primary Cancer form available for data entry. On this ‘new form’ you will complete the ‘site’ and the ‘date of diagnosis’ 17
FILTER QUESTIONS IN RAVE AN EXAMPLE The Follow Up form asks if the patient had ‘a new primary cancer or MDS diagnosed’ and the answer is ‘yes’—the diagnosed form will continue to ask questions until you ‘save’ the form. A message will appear above the question that states: If patient is diagnosed with a new primary, then complete the New Primary Cancer form (after completion of this form). Once this is done you will then notice that there is a New Primary Cancer form available for data entry. On this ‘new form’ you will complete the ‘site’ and the ‘date of diagnosis’ 17
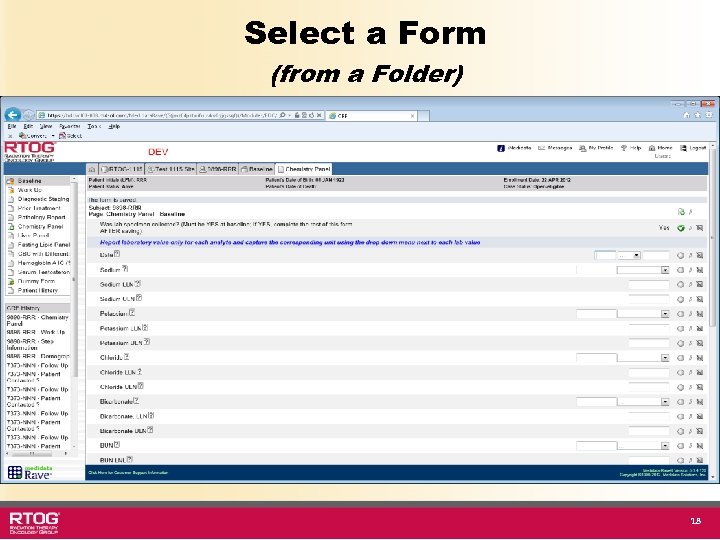 Select a Form (from a Folder) 18
Select a Form (from a Folder) 18
 REPORTING DATES • In our current EDC system we ask that all dates be reported as MM/DD/YYYY—for example: 06/15/2012 • In Rave all dates are reported as DD/MMM/YYYY—for example: 15/Jun/2012 19
REPORTING DATES • In our current EDC system we ask that all dates be reported as MM/DD/YYYY—for example: 06/15/2012 • In Rave all dates are reported as DD/MMM/YYYY—for example: 15/Jun/2012 19
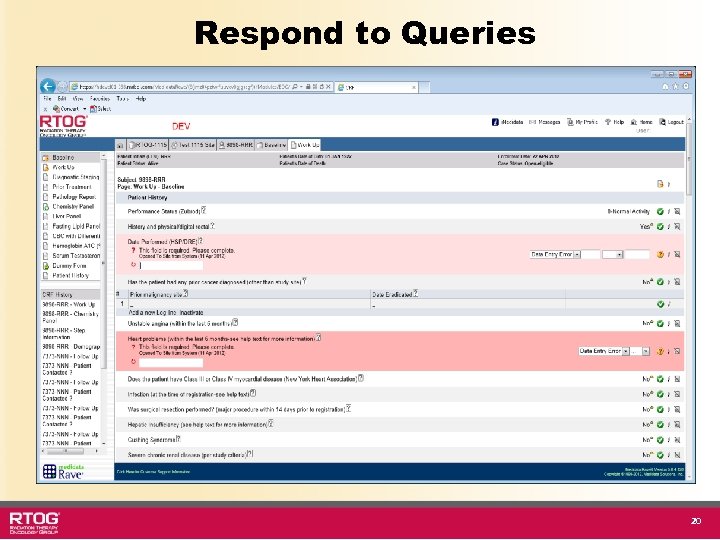 Respond to Queries 20
Respond to Queries 20
 REVISION REASONS PLEASE NOTE: The ‘default’ reason for making a revision is data entry error (this reason will always appear). There is a drop down box that also allows you to select other reasons for making a revision such as: • Per query • New information If you do not utilize the drop down box then all your revision reasons will be the same. 21
REVISION REASONS PLEASE NOTE: The ‘default’ reason for making a revision is data entry error (this reason will always appear). There is a drop down box that also allows you to select other reasons for making a revision such as: • Per query • New information If you do not utilize the drop down box then all your revision reasons will be the same. 21
 QUERIES • Currently queries are mailed to you after data are entered into our EDC system and pass through a validation program (runs overnight). • In Rave once you click the SAVE button, you will be notified immediately if you have data that did not pass system edit checks. 22
QUERIES • Currently queries are mailed to you after data are entered into our EDC system and pass through a validation program (runs overnight). • In Rave once you click the SAVE button, you will be notified immediately if you have data that did not pass system edit checks. 22
 A 5 DEMOGRAPY FORM VS PATIENT HISTORY FORM • In our current RTOG studies we collect the A 5 form, which is a voluntary form completed by the patient or his designee. • In Rave, this same form will be collected; however, the name of the form has been changed to the Patient History Form 23
A 5 DEMOGRAPY FORM VS PATIENT HISTORY FORM • In our current RTOG studies we collect the A 5 form, which is a voluntary form completed by the patient or his designee. • In Rave, this same form will be collected; however, the name of the form has been changed to the Patient History Form 23
 Questions ? • CTSU Help Desk – 9: 00 AM-5: 30 PM EST - (1 -888 -823 -5923) • Support for Rave Navigation, Rave Functionality, i. Medidata Navigation, e. Learning Assistance, Account Access • https: //www. ctsu. org/RAVE/ – Extended hours 5: 30 -8: 30 PM • General Rave Navigation and General Account Support • https: //www. ctsu. org/public/CTSUContact. aspx • RTOG – Protocol Specific Questions go to the Data Managers 24
Questions ? • CTSU Help Desk – 9: 00 AM-5: 30 PM EST - (1 -888 -823 -5923) • Support for Rave Navigation, Rave Functionality, i. Medidata Navigation, e. Learning Assistance, Account Access • https: //www. ctsu. org/RAVE/ – Extended hours 5: 30 -8: 30 PM • General Rave Navigation and General Account Support • https: //www. ctsu. org/public/CTSUContact. aspx • RTOG – Protocol Specific Questions go to the Data Managers 24


