64029fc1e215ff7bb4d517c2c1f80102.ppt
- Количество слайдов: 16
 Medicines Transparency Alliance: Under New Management Dr Tim Reed Director, Health Action International (Global) International Me. TA Secretariat Dr Gilles Forte Coordinator, Programmes Coordination, Policy and Information, WHO International Me. TA Secretariat Me. TA 25 05 09 1
Medicines Transparency Alliance: Under New Management Dr Tim Reed Director, Health Action International (Global) International Me. TA Secretariat Dr Gilles Forte Coordinator, Programmes Coordination, Policy and Information, WHO International Me. TA Secretariat Me. TA 25 05 09 1
 Me. TA Hypotheses H 1: Transparency of the pharmaceutical sector will bring about efficiencies in medicines supply chain and increase access to medicines H 2: All stakeholders with an interest in the outcome of the medicines market must be brought together and engage in a policy dialogue that should foster transparency and accountability
Me. TA Hypotheses H 1: Transparency of the pharmaceutical sector will bring about efficiencies in medicines supply chain and increase access to medicines H 2: All stakeholders with an interest in the outcome of the medicines market must be brought together and engage in a policy dialogue that should foster transparency and accountability
 Me. TA Core Principles l l l Governments are ‘responsible’ for providing access to health care, including access to essential medicines Stronger and more transparent systems and improved supply chain management will increase access Increasing equitable access to medicines improves health and enables other human development objectives to be achieved Improved information about medicines can inform public debate, and provide a basis for better policy A multi-stakeholder approach that involves all sectors – private, public and civil society - will lead to greater accountability
Me. TA Core Principles l l l Governments are ‘responsible’ for providing access to health care, including access to essential medicines Stronger and more transparent systems and improved supply chain management will increase access Increasing equitable access to medicines improves health and enables other human development objectives to be achieved Improved information about medicines can inform public debate, and provide a basis for better policy A multi-stakeholder approach that involves all sectors – private, public and civil society - will lead to greater accountability
 Me. TA Scope l Me. TA aims at improving access to quality medicines by increasing transparency of the pharmaceutical sector through collection of reliable data, valid analysis, and then disclosure for advocacy and policy dialogue among stakeholders e. g. private sector/public sector/civil society. l l 7 pilot countries: Ghana, Jordan, Kyrgyzstan, Peru, Philippines, Uganda and Zambia. Pilot Phase 2008 -2010. Me. TA Councils established in every country and work plans were developed, implemented and monitored.
Me. TA Scope l Me. TA aims at improving access to quality medicines by increasing transparency of the pharmaceutical sector through collection of reliable data, valid analysis, and then disclosure for advocacy and policy dialogue among stakeholders e. g. private sector/public sector/civil society. l l 7 pilot countries: Ghana, Jordan, Kyrgyzstan, Peru, Philippines, Uganda and Zambia. Pilot Phase 2008 -2010. Me. TA Councils established in every country and work plans were developed, implemented and monitored.
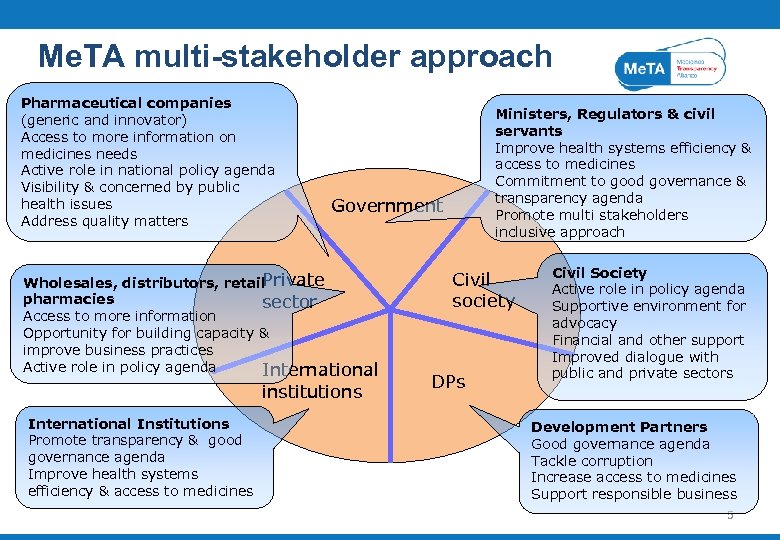 Me. TA multi-stakeholder approach Pharmaceutical companies (generic and innovator) Access to more information on medicines needs Active role in national policy agenda Visibility & concerned by public health issues Address quality matters Government Private Wholesales, distributors, retail pharmacies sector Access to more information Opportunity for building capacity & improve business practices Active role in policy agenda International institutions International Institutions Promote transparency & good governance agenda Improve health systems efficiency & access to medicines Ministers, Regulators & civil servants Improve health systems efficiency & access to medicines Commitment to good governance & transparency agenda Promote multi stakeholders inclusive approach Civil society DPs Civil Society Active role in policy agenda Supportive environment for advocacy Financial and other support Improved dialogue with public and private sectors Development Partners Good governance agenda Tackle corruption Increase access to medicines Support responsible business 5
Me. TA multi-stakeholder approach Pharmaceutical companies (generic and innovator) Access to more information on medicines needs Active role in national policy agenda Visibility & concerned by public health issues Address quality matters Government Private Wholesales, distributors, retail pharmacies sector Access to more information Opportunity for building capacity & improve business practices Active role in policy agenda International institutions International Institutions Promote transparency & good governance agenda Improve health systems efficiency & access to medicines Ministers, Regulators & civil servants Improve health systems efficiency & access to medicines Commitment to good governance & transparency agenda Promote multi stakeholders inclusive approach Civil society DPs Civil Society Active role in policy agenda Supportive environment for advocacy Financial and other support Improved dialogue with public and private sectors Development Partners Good governance agenda Tackle corruption Increase access to medicines Support responsible business 5
 Government at country level l l l Ministries of Health Medicines Regulators Government Insurance Funds Government Procurement Chief Pharmacists/Medical Officers et al …
Government at country level l l l Ministries of Health Medicines Regulators Government Insurance Funds Government Procurement Chief Pharmacists/Medical Officers et al …
 Private sector at country level l l Multinationals and Innovators Generic importers Local Manufacturers Wholesalers, distributors Retail pharmacies Drug shops Private health care providers Mission Sector Insurance companies et al …
Private sector at country level l l Multinationals and Innovators Generic importers Local Manufacturers Wholesalers, distributors Retail pharmacies Drug shops Private health care providers Mission Sector Insurance companies et al …
 Civil Society at country level l l l Health NGOs/CSOs (domestic & international) Medicines NGOs/CSOs Transparency NGOs Patient Groups Consumer Groups et al …
Civil Society at country level l l l Health NGOs/CSOs (domestic & international) Medicines NGOs/CSOs Transparency NGOs Patient Groups Consumer Groups et al …
 What could be disclosed? Medicines Registration and Quality Assurance Data l Market registration procedures l Registration status of all medicines l Good Manufacturing Practice (GMP) outcomes for domestic and foreign manufacturers l Quality assurance processes in public and non-profit tenders l Quality assurance data during registration or procurement l Routine quality testing and adverse event monitoring
What could be disclosed? Medicines Registration and Quality Assurance Data l Market registration procedures l Registration status of all medicines l Good Manufacturing Practice (GMP) outcomes for domestic and foreign manufacturers l Quality assurance processes in public and non-profit tenders l Quality assurance data during registration or procurement l Routine quality testing and adverse event monitoring
 What could be disclosed? Medicines Availability l Volume and value of medicines procured in the public and non-profit sectors l Volume and value of medicines supplied in the private sector l Availability of medicines to consumers l Routine audits for public, private, and non-profit medicines outlets
What could be disclosed? Medicines Availability l Volume and value of medicines procured in the public and non-profit sectors l Volume and value of medicines supplied in the private sector l Availability of medicines to consumers l Routine audits for public, private, and non-profit medicines outlets
 What could be disclosed? Medicines Prices l Consumer and ex-manufacture prices of medicines in the public, private, and non-profit sectors l Public sector medicines procurement prices l Medicines price components in the public, non-profit, and private sectors l Pharmaceutical patents held in-country Medicines use and Promotion l Standard treatment guidelines l Essential medicines list l Medicines promotion regulations, policies, and industry practices
What could be disclosed? Medicines Prices l Consumer and ex-manufacture prices of medicines in the public, private, and non-profit sectors l Public sector medicines procurement prices l Medicines price components in the public, non-profit, and private sectors l Pharmaceutical patents held in-country Medicines use and Promotion l Standard treatment guidelines l Essential medicines list l Medicines promotion regulations, policies, and industry practices
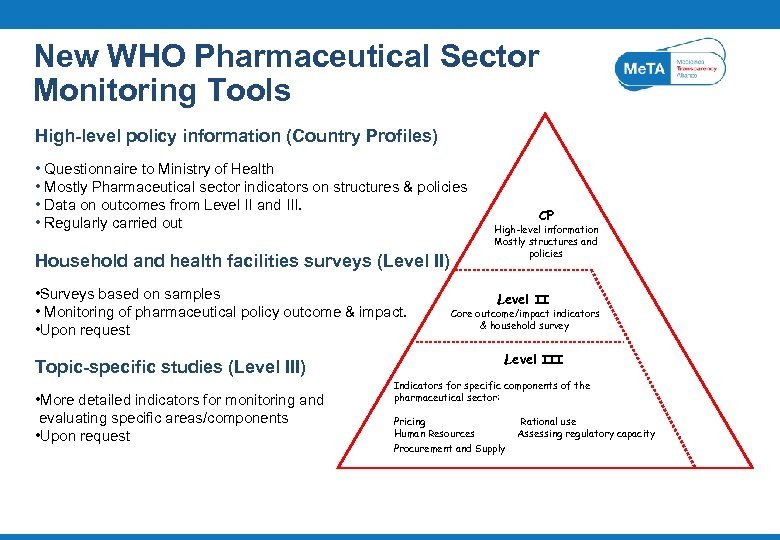 New WHO Pharmaceutical Sector Monitoring Tools High-level policy information (Country Profiles) • Questionnaire to Ministry of Health • Mostly Pharmaceutical sector indicators on structures & policies • Data on outcomes from Level II and III. • Regularly carried out Household and health facilities surveys (Level II) • Surveys based on samples • Monitoring of pharmaceutical policy outcome & impact. • Upon request Topic-specific studies (Level III) • More detailed indicators for monitoring and evaluating specific areas/components • Upon request CP High-level information Mostly structures and policies Level II Core outcome/impact indicators & household survey Level III Indicators for specific components of the pharmaceutical sector: Pricing Human Resources Procurement and Supply Rational use Assessing regulatory capacity
New WHO Pharmaceutical Sector Monitoring Tools High-level policy information (Country Profiles) • Questionnaire to Ministry of Health • Mostly Pharmaceutical sector indicators on structures & policies • Data on outcomes from Level II and III. • Regularly carried out Household and health facilities surveys (Level II) • Surveys based on samples • Monitoring of pharmaceutical policy outcome & impact. • Upon request Topic-specific studies (Level III) • More detailed indicators for monitoring and evaluating specific areas/components • Upon request CP High-level information Mostly structures and policies Level II Core outcome/impact indicators & household survey Level III Indicators for specific components of the pharmaceutical sector: Pricing Human Resources Procurement and Supply Rational use Assessing regulatory capacity
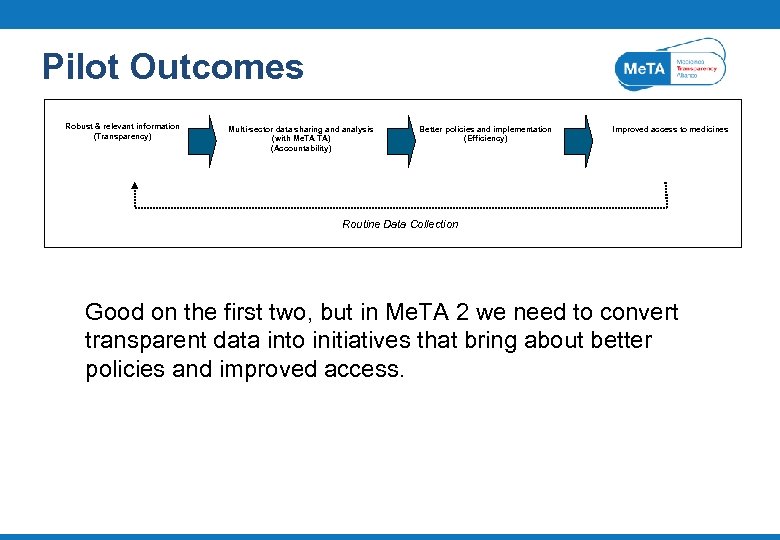 Pilot Outcomes Robust & relevant information (Transparency) Multi-sector data sharing and analysis (with Me. TA TA) (Accountability) Better policies and implementation (Efficiency) Improved access to medicines Routine Data Collection Good on the first two, but in Me. TA 2 we need to convert transparent data into initiatives that bring about better policies and improved access.
Pilot Outcomes Robust & relevant information (Transparency) Multi-sector data sharing and analysis (with Me. TA TA) (Accountability) Better policies and implementation (Efficiency) Improved access to medicines Routine Data Collection Good on the first two, but in Me. TA 2 we need to convert transparent data into initiatives that bring about better policies and improved access.
 Pilot Successes l l The National Medicines Regulatory Authorities of Kyrgyzstan, Uganda and Zambia have created web-sites on which they make available key information: registered medicines list, list of authorized wholesalers, etc. Peru developed a database of medicines prices in public and private pharmacies. The system allows consumers to compare the prices and choose where to buy. This increased competition is meant to reduce prices of medicines. Jordan conducted studies on access to medicines in health facilities and households. The studies indicated issues with rational use of medicines and therefore Me. TA has supported the country to develop Standard Treatment Guidelines for key diseases. In the Philippines Me. TA contributed to the enactment of the "Cheaper Medicines Act" 2008 and to the establishment of an e-procurement system for medicines.
Pilot Successes l l The National Medicines Regulatory Authorities of Kyrgyzstan, Uganda and Zambia have created web-sites on which they make available key information: registered medicines list, list of authorized wholesalers, etc. Peru developed a database of medicines prices in public and private pharmacies. The system allows consumers to compare the prices and choose where to buy. This increased competition is meant to reduce prices of medicines. Jordan conducted studies on access to medicines in health facilities and households. The studies indicated issues with rational use of medicines and therefore Me. TA has supported the country to develop Standard Treatment Guidelines for key diseases. In the Philippines Me. TA contributed to the enactment of the "Cheaper Medicines Act" 2008 and to the establishment of an e-procurement system for medicines.
 Pilot Lessons • Multi-stakeholder working is a new concept – not easy – it takes patience, understanding, diplomacy and tact • Identifying champions in each sector can greatly expedite the process of multi-stakeholder engagement and transparency • Each sector needs to “give & take” to build consensus • Conflict of Interest identification - transparency • The Me. TA process needs to be country-led but with guidance • Gaining consensus and understanding requires a constant and frank exchange of views • Tools for gathering baseline data on access do already exist; a new tool to measure multi-stakeholder collaboration has been developed; new innovative ‘useable’ tools required
Pilot Lessons • Multi-stakeholder working is a new concept – not easy – it takes patience, understanding, diplomacy and tact • Identifying champions in each sector can greatly expedite the process of multi-stakeholder engagement and transparency • Each sector needs to “give & take” to build consensus • Conflict of Interest identification - transparency • The Me. TA process needs to be country-led but with guidance • Gaining consensus and understanding requires a constant and frank exchange of views • Tools for gathering baseline data on access do already exist; a new tool to measure multi-stakeholder collaboration has been developed; new innovative ‘useable’ tools required
 Me. TA 2: What’s changed Pilot Model: l Non-technical International Me. TA Secretariat (IMS) based in UK, external consultant driven technical support l International Consultants appointed in UK to facilitate in-country work l Short timeline – Two years l Financing and Sustainability Me. TA 2: l IMS joint ownership WHO and HAI l In-country technical support by WHO l In-country Civil Society support by HAI l Longer timeline - Four years l Sustainability model l Expansion into new countries? Year three ….
Me. TA 2: What’s changed Pilot Model: l Non-technical International Me. TA Secretariat (IMS) based in UK, external consultant driven technical support l International Consultants appointed in UK to facilitate in-country work l Short timeline – Two years l Financing and Sustainability Me. TA 2: l IMS joint ownership WHO and HAI l In-country technical support by WHO l In-country Civil Society support by HAI l Longer timeline - Four years l Sustainability model l Expansion into new countries? Year three ….


