eb15d78a096e7068f125b10307101fbd.ppt
- Количество слайдов: 30

MEDICARE AND CLINICAL LABS: What is new for 2010 and 2011 CCLA Annual Meeting San Diego November 3, 2010

WE WILL DISCUSS • • • National and Local Lab Policies Requesting Reconsideration Revisiting Documentation Needs Enrollment and PECOS: Needs for Referring docs to be in system Tests in Dialysis composite rates Coverage for New Lab Tests Meet with CMDs & Others Thoughts on Pricing & Coding Q&A

NATIONAL COVERAGE DECISIONS • National: NCDs come from CMS – – Based on scientific studies & data collected Presented often at MCAC-open meetings Notice and comment welcome Reconsiderations always possible – – May specify services always covered May specify services never covered Published in CMS Coverage Manual May change as science changes, new studies emerge, or as laws change. • NCDs cover entire country

LOCAL COVERAGE DECISIONS • Local: LCDs from 1 or more states/areas – Written by local CMDs about situations that are data based & need control or educational instruction – Presented at state CACs open to medical and specialty societies representatives – Presented at open meetings to all comers – Notice and comment always welcome – Reconsiderations always possible • LCDs cover a Medicare Jurisdiction (e. g. , J-1) – Discuss and describe medical necessity – Usually give codes & conditions for payment but that information in accompanying articles – May state frequency of service and diagnoses and always published locally and nationally

HOW LOCAL IS LOCAL? • Jurisdiction Size – Usually 3 or more states – CMS reducing jurisdictions from 15 to 10 in next few years • Cooperation within a Contractor • Cooperation of CMDs • CMS tends to prefer sameness of policies across jurisdictions – Recent meeting in Baltimore to make policies more uniform in construction – New jurisdiction transitions make policies similar over time
WHEN LOCAL COVERAGE BECOMES NATIONAL • When all tests done is a single laboratory within single state • Home Brew—the test performance does not cross state lines • Does not need FDA approval • All billing from single location – Past 14 day rule – CMS looking again at 14 day rule • With absence of national policy local coverage essentially becomes national coverage

LOCAL J 1 COVERAGE DECISIONS • Example of J-1 LCDs (Currently 84 “B” LCDs) – – – – Category III codes Circulating tumor cell assays Cytogenic studies Free PSA Mammaprint Oncologic in-vitro chemoresponse assays Oncotype DX Flow cytometry and immunohistochemistry • Some Part A LCDs (institutional) may also apply • Local articles may also specify lab use without a definite policy---to help with billing and coding – We suggest codes, uses and frequencies – We discuss what we look for in new tests

REQUESTING LCD RECONSIDERATIONS • Send in writing to local Contractor (Palmetto) – Specific address for reconsiderations on our web site – Specific address of CMDs • Add supporting scientific evidence – – Literature in peer reviewed journals Expert opinion from credible sources Guidelines / statements from specialty societies Results of medium or long term studies • Be specific in requests – CPT, ICD-9, organ systems or special circumstances • Be conscious of vested interests • Contractor must respond in 30 days to valid reconsideration requests

REVISITING DOCUMENTATION • Contractors are reviewing lab tests • Includes MACs, CERT, and RACs • What Contractors are finding: – Although signatures on lab slips not required, there must be evidence that tests were ordered and appropriate by treating physician – Chart notes must show intent / reason for test – Test must be reasonable and necessary for diagnosis or therapy • Some lab tests have LCD / NCD with edits – Inadequate documentation by doctor may lead to post-payment denial by CERT or RAC

ORDERING-/-REFERRING RULES FOR MEDICARE • • MD Clin. Nurse Specialist DO Clin. Psychologist Dental Surgery Nurse Midwife Dental Medicine Clin. Social Worker Podiartist Nurse Practitioner Optometrist Chiropractor Physician Assistant

ORDERING PHYSICIAN • All Claims Ordered / Referred Must Have: – NPI of ordering provider – Number in PECOS system – Specialty as listed • Grace Period – Initial: 10/5/09 to 12/31/10 warning message on remittance – ACA Reform: 6/04/10 and after: claim rejected if referring individual not in PECOS or MAC list • CMS is not enforcing as yet---will enforce sometime before 1/3/11
OTHER ENROLLMENT • Revalidation of all physicians not already in PECOS (Provider Enrollment Chain Online System) • Revalidation of some labs & IDTFs • Need to update any changes within 30 days – in PECOS or paper change – Address, phone, suite – New members in group – Other changes • If no claims to Medicare in one year— physician is automatically disenrolled

HOW TO TELL IF PROVIDER IS PROPERLY ENROLLED • If referring provider currently not enrolled, you will get a notice on your electronic remittance forms---and remind provider to re-enroll • You can find PECOS enrollees on CMS internet: – http: //www. cms. gov/Medicare. Provider. Sup. Enroll/06_Me dicare. Orderingand. Referring. asp#Top. Of. Page – Download in PDF or ZIP form and “search” • Another source from a private company internet site – – http: //www. oandp. com/pecos/ This DME company updates files when CMS updates their files

ENROLLMENT PROBLEMS PERSIST • Who is not enrolled or re-enrolled in PECOS – Older physicians who have not changed anything – Military, VA doctors, fellows in specialty training • Thousands of physicians still not enrolled • It usually takes 60 -90 days to process a new enrollment – This is due to the complexity of enrolling and the need to review all the data and make certain data is not in conflict (e. g. , 855 i— 855 r---855 c, signatures, etc. ) – Please contact your providers not currently in PECOS and encourage them to re-enroll promptly • By Jan 3, 2011 if providers are not enrolled we will reject the lab claim

DIALYSIS HAS NEW PROSPECTIVE PAYMENT • New composite rates include: – – – Additional drugs Additional lab tests Quality control items • New rates start 1/1/2011 • Labs will probably re-contract with dialysis facilities when they perform tests in composite rate and no longer separately reimbursable

• • • LABS SUBJECT TO CONSOLIDATED BILLING 82040 82108 82306 82310 82330 82374 82379 82435 82565 82570 Assay of serum albumin Assay of aluminum Vitamin d, 25 hydroxy Assay of calcium, Ionized Assay, blood carbon dioxide Assay of carnitine Assay of blood chloride Assay of creatinine Assay of urine creatinine
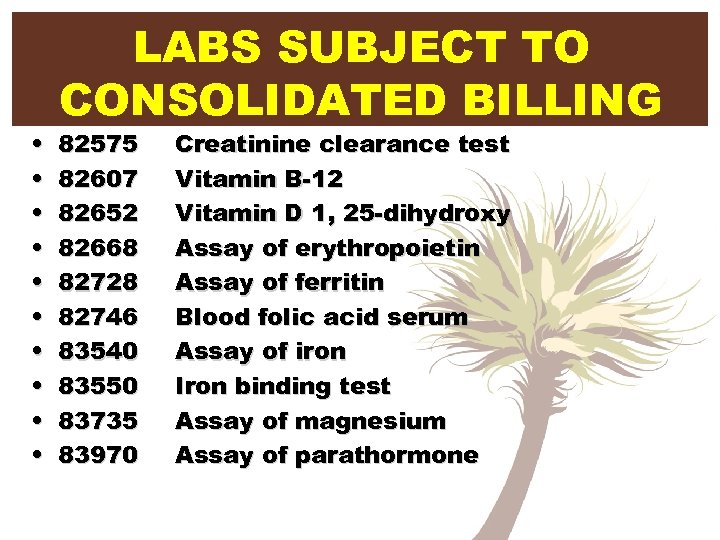
• • • LABS SUBJECT TO CONSOLIDATED BILLING 82575 82607 82652 82668 82728 82746 83540 83550 83735 83970 Creatinine clearance test Vitamin B-12 Vitamin D 1, 25 -dihydroxy Assay of erythropoietin Assay of ferritin Blood folic acid serum Assay of iron Iron binding test Assay of magnesium Assay of parathormone
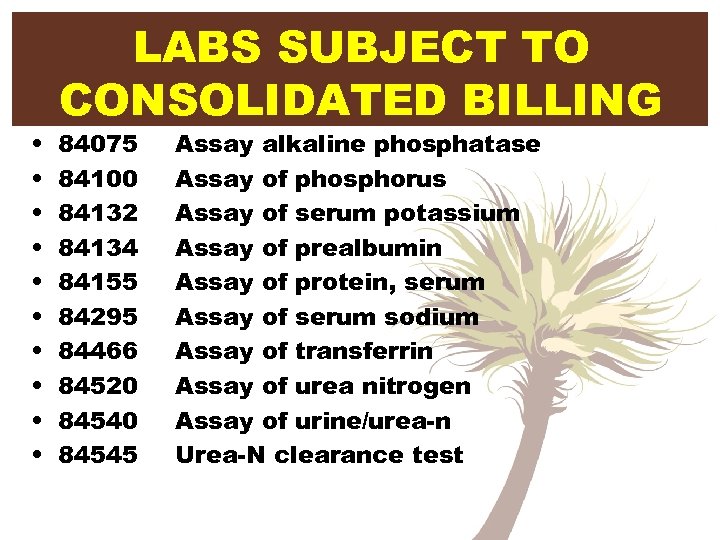
• • • LABS SUBJECT TO CONSOLIDATED BILLING 84075 84100 84132 84134 84155 84295 84466 84520 84545 Assay alkaline phosphatase Assay of phosphorus Assay of serum potassium Assay of prealbumin Assay of protein, serum Assay of serum sodium Assay of transferrin Assay of urea nitrogen Assay of urine/urea-n Urea-N clearance test
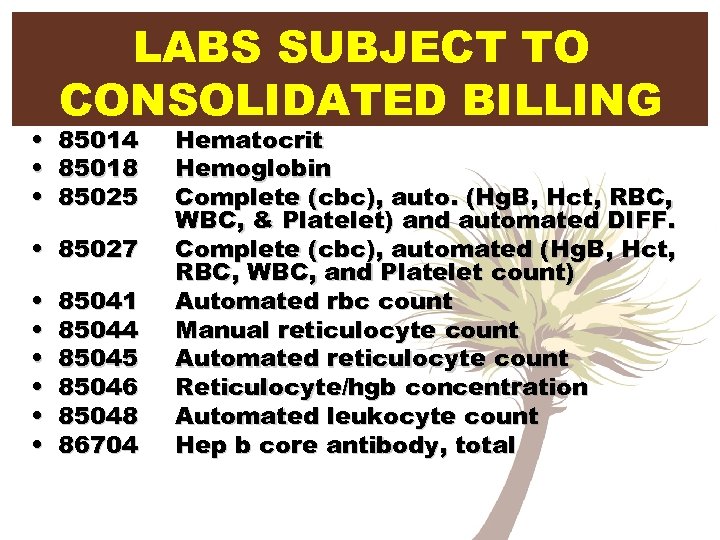
• • • LABS SUBJECT TO CONSOLIDATED BILLING 85014 85018 85025 • 85027 • • • 85041 85044 85045 85046 85048 86704 Hematocrit Hemoglobin Complete (cbc), auto. (Hg. B, Hct, RBC, WBC, & Platelet) and automated DIFF. Complete (cbc), automated (Hg. B, Hct, RBC, WBC, and Platelet count) Automated rbc count Manual reticulocyte count Automated reticulocyte count Reticulocyte/hgb concentration Automated leukocyte count Hep b core antibody, total
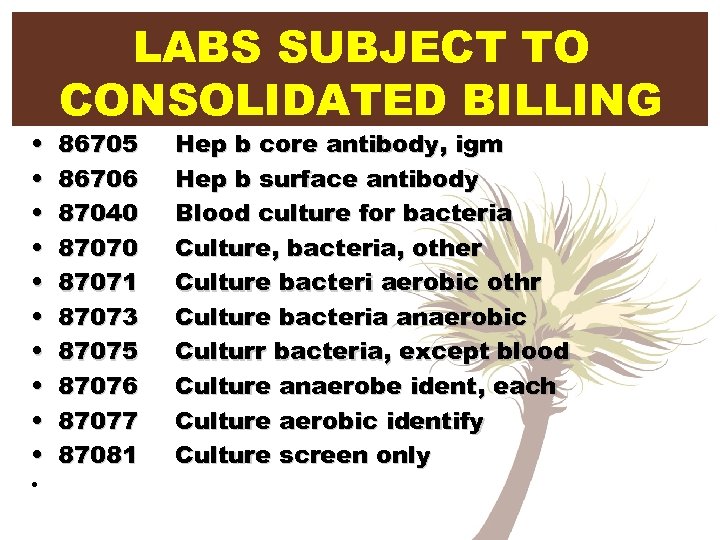
• • • LABS SUBJECT TO CONSOLIDATED BILLING 86705 86706 87040 87071 87073 87075 87076 87077 87081 Hep b core antibody, igm Hep b surface antibody Blood culture for bacteria Culture, bacteria, other Culture bacteri aerobic othr Culture bacteria anaerobic Culturr bacteria, except blood Culture anaerobe ident, each Culture aerobic identify Culture screen only
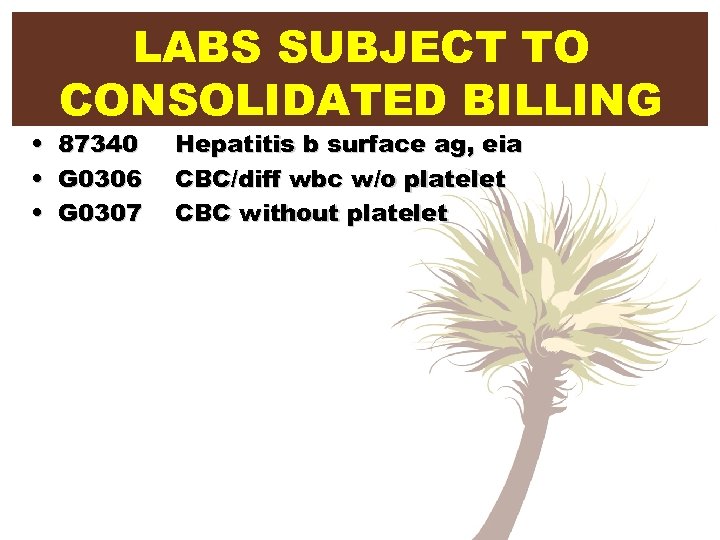
LABS SUBJECT TO CONSOLIDATED BILLING • 87340 • G 0306 • G 0307 Hepatitis b surface ag, eia CBC/diff wbc w/o platelet CBC without platelet
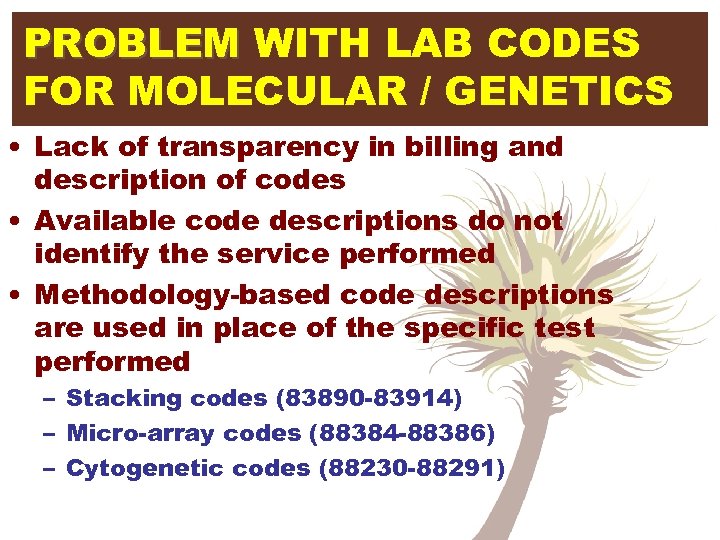
PROBLEM WITH LAB CODES FOR MOLECULAR / GENETICS • Lack of transparency in billing and description of codes • Available code descriptions do not identify the service performed • Methodology-based code descriptions are used in place of the specific test performed – Stacking codes (83890 -83914) – Micro-array codes (88384 -88386) – Cytogenetic codes (88230 -88291)
PROBLEM WITH LAB CODES • Using combinations of these codes do not allow us to track what test was actually done • Reimbursing for tests we did not know we were covering---all across the country • AMA CPT is working on at least a partial classification system as we speak, but: – Details uncertain – Two tiers of codes planned – Time frame for publication problematic…even for first tier of codes • New tests using combinations of these codes are appearing weekly and labs are asking for reimbursement

THE CONCEPT OF VALIDITY AND UTILITY • Before we cover a new test it must have validity and utility • Validity involves the quality of the test as a test and includes – Sensitivity – Specificity – Reproducibility • Utility is whether the new test gives prognostic or diagnostic information useful for the treating physician in decision making for the patient – Selecting a treatment course – Making a clinical decision (NOT JUST CONFIRMING WHAT IS ALREADY KNOWN)

CONCEPT OF SCIENCE VERSUS CREATIVE WRITING • We realize true research requires time and significant financial investment---but we need the facts…just the facts – Peer reviewed articles in responsible journals – Double blind, placebo controlled, crossover studies with statistical significant results are the best form of review when available. Not always available!! – Individual case reviews and small groups of patients studied over short periods of time are least convincing Usually these are earliest papers available!! – White papers from specialty societies, Tech assessments, advice from clinical experts NOT under financial control of company…all are favorably considered – Requests by May take time for societies to release!! individual physicians are variable

CONCEPT OF SCIENCE VERSUS CREATIVE WRITING • Sometimes the facts are harder to believe – Internal, unpublished data from inside the company developing the test – Individual case reports that sporadically appear in journals --- often “throw away” journals – Poster sessions and abstracts of a few patients with no real controls – Letters from doctors who want the test but do not have scientific reasons for wanting them • Dr. Jeter will discuss evidence based decision making that Palmetto will use in evaluating new tests

MEETING WITH LOCAL MEDICAL DIRECTOR • Palmetto GBA CMDs are very busy – – – Policies, articles, coverage Med Review and chart adjudication Education, outreach to societies / groups Contact with CMS & other organizations Transition to new Jurisdiction • Our CMDs will find time for meeting – In Person: office, hotel, other location – Telephone, Web, etc. may be more efficient • Time is always a consideration – Send info, data, literature in advance – Allows CMDs to be prepared, shortens meeting, allows quicker resolution – Must fit between CMDs travel, outreach, teleconferences with CMS and home office – PLEASE Send info, data, literature in advance !! It saves everyone time

HELP US WITH PRICING • Show us the pricing if test is contractor priced – Prefer single pricing vs code stacking – Reality versus imagination: v What is included in pricing v What should not be included • Can it be cross walked to existing CPT codes – Easier to determine prices – May use NOC codes at first to describe its use – We will compare prices to similar tests • If new test we need to perform GAP Filling – We will need steps to perform test – We will need prices of supplies and work – We will need price per test or per group of tests – We do not pay for standards, duplicates, and controls for each test

QUESTIONS THAT SOMETIMES CONFOUND ME • Is this a ME TOO TEST – Companies marketing very similar but not identical tests – Should we cover all or only the “best” ? Is it the science or the marketing of the test that determines use • Does this replace an earlier test? If so should we only cover one test or the other but not both • Does it give new information or rehash old and re -confirm what a doctor already knows • Do physician practitioners really know if one test is better than another without valid CER (Comparative Effectiveness Research) It is rare to have head to head competition between tests

BUT WE DO HAVE EXPERTS TO HELP US • Dr Jeter, out in house CMD and PATHOLOGIST • Experts from the molecular genetics field • Experts from the California Association of Pathologists • And we hope you would be our experts too! Thank you!! Do you have any questions?
eb15d78a096e7068f125b10307101fbd.ppt