 Medical Devices Quality Management System in Agnitio CHEN, Chichen Auditing Office
Medical Devices Quality Management System in Agnitio CHEN, Chichen Auditing Office
 Medical Devices QMS • Taiwan (ISO 13485: 2003) – GMP, Pharmaceutical Good Manufacturing Practice Regulations (藥事法、藥物製造 廠 設廠標準、藥物優良製造準則) • Medical Device Product Approval • International Standard – ISO 13485: 2003 • CE Marking for European Union
Medical Devices QMS • Taiwan (ISO 13485: 2003) – GMP, Pharmaceutical Good Manufacturing Practice Regulations (藥事法、藥物製造 廠 設廠標準、藥物優良製造準則) • Medical Device Product Approval • International Standard – ISO 13485: 2003 • CE Marking for European Union
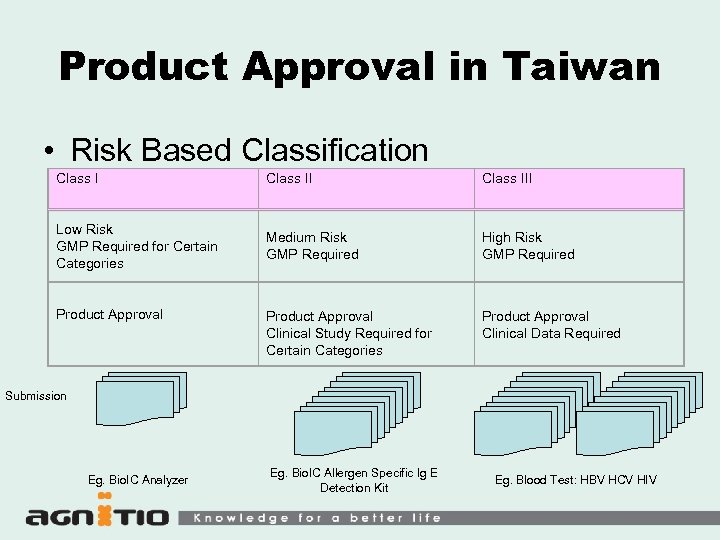 Product Approval in Taiwan • Risk Based Classification Class III Low Risk GMP Required for Certain Categories Medium Risk GMP Required High Risk GMP Required Product Approval Clinical Study Required for Certain Categories Product Approval Clinical Data Required Product Approval Submission Eg. Bio. IC Analyzer Eg. Bio. IC Allergen Specific Ig E Detection Kit Eg. Blood Test: HBV HCV HIV
Product Approval in Taiwan • Risk Based Classification Class III Low Risk GMP Required for Certain Categories Medium Risk GMP Required High Risk GMP Required Product Approval Clinical Study Required for Certain Categories Product Approval Clinical Data Required Product Approval Submission Eg. Bio. IC Analyzer Eg. Bio. IC Allergen Specific Ig E Detection Kit Eg. Blood Test: HBV HCV HIV
 Implementation in Agnitio • TW GMP – 2006: 1 st Registration – 2009: Reassessment – 2012: Reassessment (every 3 years) Registration No. 1015060972
Implementation in Agnitio • TW GMP – 2006: 1 st Registration – 2009: Reassessment – 2012: Reassessment (every 3 years) Registration No. 1015060972
 Implementation in Agnitio • ISO 13485 By BSI British Standards Institution – 2008: 1 st Registration – Annual Reassessment Registration No. MD 525664
Implementation in Agnitio • ISO 13485 By BSI British Standards Institution – 2008: 1 st Registration – Annual Reassessment Registration No. MD 525664
 Implementation in Agnitio • TW Product Approval, 8 certificates – 1 Class II: Bio. IC Allergen Specific Ig E Detection Kit – 7 Class I: Bio. IC Analyzer / DNA Extraction Kit / Automatic Platform for Magnetic System • 1 CE Marking: Bio. IC Allergen Specific Ig E Detection Kit • China : in process
Implementation in Agnitio • TW Product Approval, 8 certificates – 1 Class II: Bio. IC Allergen Specific Ig E Detection Kit – 7 Class I: Bio. IC Analyzer / DNA Extraction Kit / Automatic Platform for Magnetic System • 1 CE Marking: Bio. IC Allergen Specific Ig E Detection Kit • China : in process
 Implementation in Agnitio • TW Product Approval – Class I: Bio. IC Analyzer Registration No. DOH-MD-(I)-No. 001997
Implementation in Agnitio • TW Product Approval – Class I: Bio. IC Analyzer Registration No. DOH-MD-(I)-No. 001997
 Implementation in Agnitio • TW Product Approval – Class II: Bio. IC Allergen Specific Ig E Detection Kit Registration No. DOH-MD-No. 002717
Implementation in Agnitio • TW Product Approval – Class II: Bio. IC Allergen Specific Ig E Detection Kit Registration No. DOH-MD-No. 002717
 Implementation in Agnitio • CE Marking: Bio. IC Allergen Specific Ig E Detection Kit
Implementation in Agnitio • CE Marking: Bio. IC Allergen Specific Ig E Detection Kit
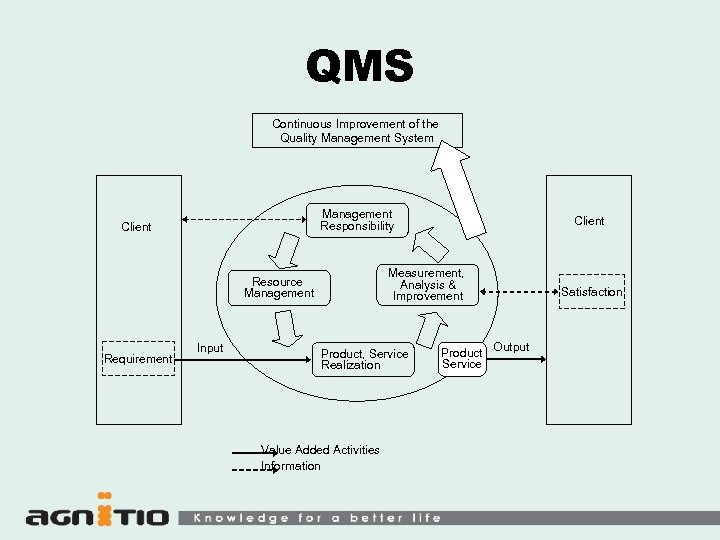 QMS Continuous Improvement of the Quality Management System Management Responsibility Client Measurement, Analysis & Improvement Resource Management Requirement Input Client Product, Service Realization Value Added Activities Information Product Service Satisfaction Output
QMS Continuous Improvement of the Quality Management System Management Responsibility Client Measurement, Analysis & Improvement Resource Management Requirement Input Client Product, Service Realization Value Added Activities Information Product Service Satisfaction Output
 Infrastructures • Document Control Center • Management Representative: Dr. CHANG, Ping • Pluridisciplinaire Team (EE, ME, Bio, BME…) • Clean Room Facilities, Class 100 K • In-House Setup for Manufacturing & QC
Infrastructures • Document Control Center • Management Representative: Dr. CHANG, Ping • Pluridisciplinaire Team (EE, ME, Bio, BME…) • Clean Room Facilities, Class 100 K • In-House Setup for Manufacturing & QC
 Continuous Improvement • Design Control / Risk Management ISO 14971: 2009 • Monitoring & Measuring • Feedback from Customers • Analysis of Data • Corrective & Preventive Actions Plan - Do - Check - Act
Continuous Improvement • Design Control / Risk Management ISO 14971: 2009 • Monitoring & Measuring • Feedback from Customers • Analysis of Data • Corrective & Preventive Actions Plan - Do - Check - Act
 Thank you !!!
Thank you !!!