4d48186c5e5c71497689461a26f91b8d.ppt
- Количество слайдов: 18
 Medical Device User Fee and Modernization Act of 2002 (MDUFMA) Blood Products Advisory Committee December 12, 2002 Mary Elizabeth Jacobs, Ph. D. Associate Director for Regulatory Affairs OBRR, CBER
Medical Device User Fee and Modernization Act of 2002 (MDUFMA) Blood Products Advisory Committee December 12, 2002 Mary Elizabeth Jacobs, Ph. D. Associate Director for Regulatory Affairs OBRR, CBER
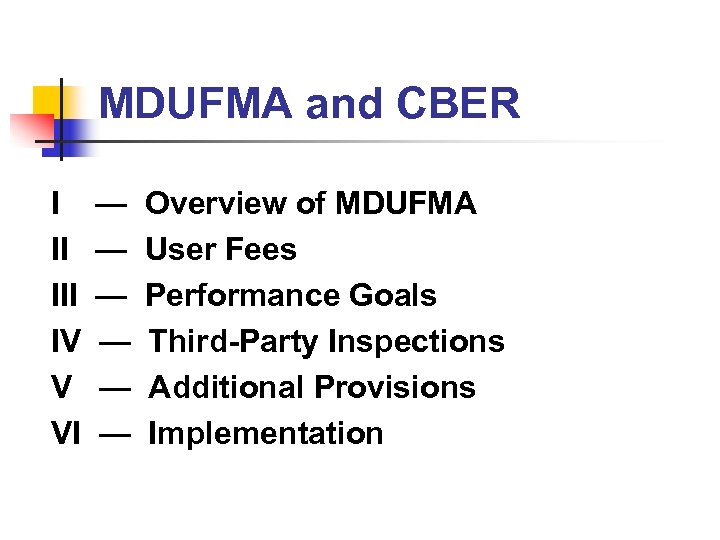 MDUFMA and CBER I II IV V VI — — — Overview of MDUFMA User Fees Performance Goals Third-Party Inspections Additional Provisions Implementation
MDUFMA and CBER I II IV V VI — — — Overview of MDUFMA User Fees Performance Goals Third-Party Inspections Additional Provisions Implementation
 How to Obtain Additional Information - General n First and foremost, periodically visit the MDUFMA website for general guidance, reference materials, and new information: www. fda. gov/cdrh/mdufma n Send an e-mail to: MDUFMA@cdrh. fda. gov
How to Obtain Additional Information - General n First and foremost, periodically visit the MDUFMA website for general guidance, reference materials, and new information: www. fda. gov/cdrh/mdufma n Send an e-mail to: MDUFMA@cdrh. fda. gov
 Additional information – CBER specific n Check CBER Website: n n www. fda. gov/cber/devices/mdufma Email CBER: n n Manufacturers: matt@cber. fda. gov Consumers, health care professionals: octma@cber. fda. gov
Additional information – CBER specific n Check CBER Website: n n www. fda. gov/cber/devices/mdufma Email CBER: n n Manufacturers: matt@cber. fda. gov Consumers, health care professionals: octma@cber. fda. gov
 Background n n Developed in consultation with industry Bipartisan House and Senate support Explicitly recognizes need for additional medical device resources Signed into law October 26, 2002 — implementation clock is ticking
Background n n Developed in consultation with industry Bipartisan House and Senate support Explicitly recognizes need for additional medical device resources Signed into law October 26, 2002 — implementation clock is ticking
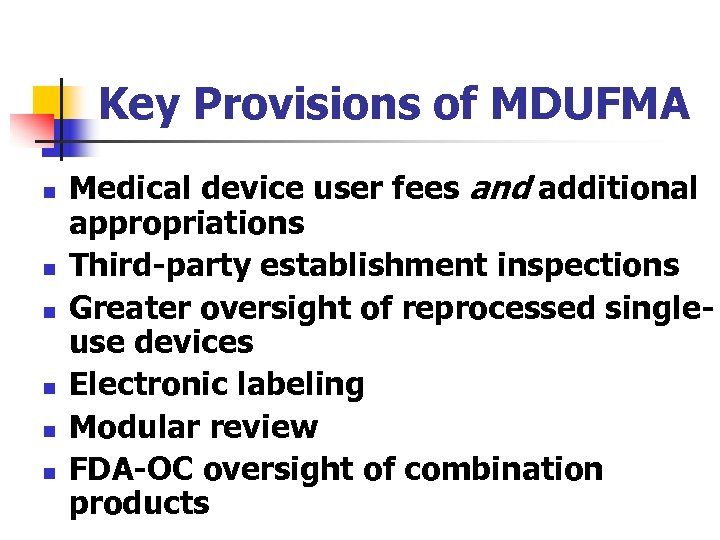 Key Provisions of MDUFMA n n n Medical device user fees and additional appropriations Third-party establishment inspections Greater oversight of reprocessed singleuse devices Electronic labeling Modular review FDA-OC oversight of combination products
Key Provisions of MDUFMA n n n Medical device user fees and additional appropriations Third-party establishment inspections Greater oversight of reprocessed singleuse devices Electronic labeling Modular review FDA-OC oversight of combination products
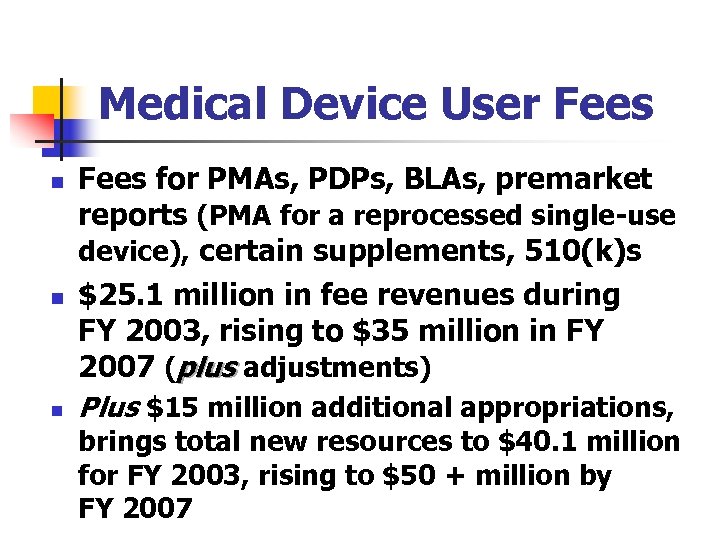 Medical Device User Fees n n n Fees for PMAs, PDPs, BLAs, premarket reports (PMA for a reprocessed single-use device), certain supplements, 510(k)s $25. 1 million in fee revenues during FY 2003, rising to $35 million in FY 2007 (plus adjustments) Plus $15 million additional appropriations, brings total new resources to $40. 1 million for FY 2003, rising to $50 + million by FY 2007
Medical Device User Fees n n n Fees for PMAs, PDPs, BLAs, premarket reports (PMA for a reprocessed single-use device), certain supplements, 510(k)s $25. 1 million in fee revenues during FY 2003, rising to $35 million in FY 2007 (plus adjustments) Plus $15 million additional appropriations, brings total new resources to $40. 1 million for FY 2003, rising to $50 + million by FY 2007
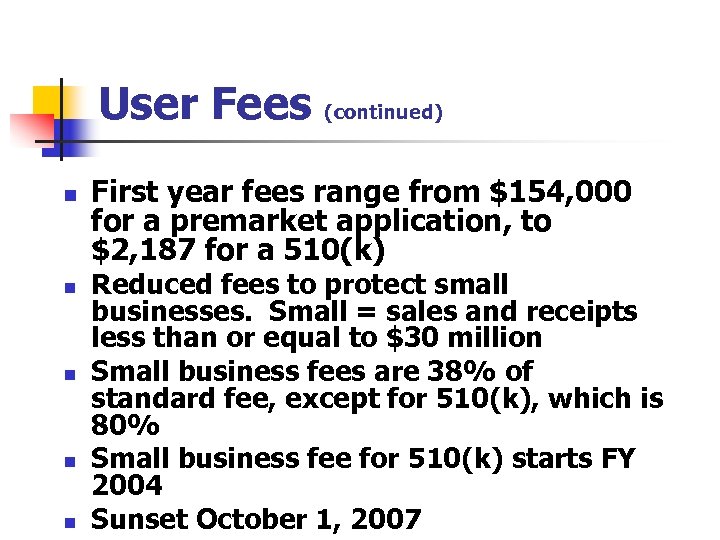 User Fees (continued) n n n First year fees range from $154, 000 for a premarket application, to $2, 187 for a 510(k) Reduced fees to protect small businesses. Small = sales and receipts less than or equal to $30 million Small business fees are 38% of standard fee, except for 510(k), which is 80% Small business fee for 510(k) starts FY 2004 Sunset October 1, 2007
User Fees (continued) n n n First year fees range from $154, 000 for a premarket application, to $2, 187 for a 510(k) Reduced fees to protect small businesses. Small = sales and receipts less than or equal to $30 million Small business fees are 38% of standard fee, except for 510(k), which is 80% Small business fee for 510(k) starts FY 2004 Sunset October 1, 2007
 Fee Exemptions, Waivers n n n No fee if applicant is Federal or State government, unless device is to be marketed First premarket application by a small business is free First premarket report by submitter of PMA for reprocessed device is free No fee for third-party 510(k) No fee if solely for pediatric use
Fee Exemptions, Waivers n n n No fee if applicant is Federal or State government, unless device is to be marketed First premarket application by a small business is free First premarket report by submitter of PMA for reprocessed device is free No fee for third-party 510(k) No fee if solely for pediatric use
 Performance Goals n n Overall, aiming to improve FDA performance by 25% Goals are defined in letter from DHHS Secretary Thompson to Congress Combination of cycle goals (all) and decision goals (PMA, 510(k)) Measured in FDA days
Performance Goals n n Overall, aiming to improve FDA performance by 25% Goals are defined in letter from DHHS Secretary Thompson to Congress Combination of cycle goals (all) and decision goals (PMA, 510(k)) Measured in FDA days
 Performance Goals n (continued) For BLAs, by FY 2005 -7, review and act on: n n 75 -90% of submissions in 10 months 75 -90% of Class II resubmissions in six months 75 -90% of Class I resubmissions in two months 75 -90% of manufacturing supplements in four months
Performance Goals n (continued) For BLAs, by FY 2005 -7, review and act on: n n 75 -90% of submissions in 10 months 75 -90% of Class II resubmissions in six months 75 -90% of Class I resubmissions in two months 75 -90% of manufacturing supplements in four months
 Performance Goals n n (continued) For PMAs, by FY 2005 -7, cycle goals, e. g. , first action “major deficiency” letter in 150 days (from 180 days) in 7090% For PMAs, decision goal, FY 2007 50% to have FDA decision within 180 days; will be re-evaluated during FY 2006 n n Public meeting Notify Congress by August 1, 2006
Performance Goals n n (continued) For PMAs, by FY 2005 -7, cycle goals, e. g. , first action “major deficiency” letter in 150 days (from 180 days) in 7090% For PMAs, decision goal, FY 2007 50% to have FDA decision within 180 days; will be re-evaluated during FY 2006 n n Public meeting Notify Congress by August 1, 2006
 Performance Goals n n (continued) For 510(k)s, by FY 2005 -7, cycle goals, e. g. , first action “additional information” letter in 75 days (from 90 days) in 7090% For 510(k)s, by FY 2005 -6 75% to have FDA decision within 90 days; FY 2007 goal of 80% will be re-evaluated n n public meeting Notify Congress
Performance Goals n n (continued) For 510(k)s, by FY 2005 -7, cycle goals, e. g. , first action “additional information” letter in 75 days (from 90 days) in 7090% For 510(k)s, by FY 2005 -6 75% to have FDA decision within 90 days; FY 2007 goal of 80% will be re-evaluated n n public meeting Notify Congress
 Third-Party Inspections n n n Most complex provisions of the new law FDA-accredited third-party may inspect a manufacturer of class II and class III devices if strict eligibility requirements are met by the establishment and the selected third-party Inspections permitted are QS/GMP only; pre-approval, Bi. Mo, and “for cause” inspections remain exclusive FDA purview
Third-Party Inspections n n n Most complex provisions of the new law FDA-accredited third-party may inspect a manufacturer of class II and class III devices if strict eligibility requirements are met by the establishment and the selected third-party Inspections permitted are QS/GMP only; pre-approval, Bi. Mo, and “for cause” inspections remain exclusive FDA purview
 Third-Party Inspections (continued) n n FDA must publish accreditation criteria by April 24, 2003 Establishment markets in U. S. and abroad Most recent FDA inspection must have been classified as NAI or VAI FDA must periodically inspect (normally one out of three)
Third-Party Inspections (continued) n n FDA must publish accreditation criteria by April 24, 2003 Establishment markets in U. S. and abroad Most recent FDA inspection must have been classified as NAI or VAI FDA must periodically inspect (normally one out of three)
 Additional Provisions n n n Combination products — reviews coordinated by new office in the Office of Commissioner Electronic labeling — in some circumstances Sect. 205 one year report to Congress on the “timeliness and effectiveness” of premarket reviews by centers other than the Center for Devices and Radiological Health
Additional Provisions n n n Combination products — reviews coordinated by new office in the Office of Commissioner Electronic labeling — in some circumstances Sect. 205 one year report to Congress on the “timeliness and effectiveness” of premarket reviews by centers other than the Center for Devices and Radiological Health
 Implementation n n Developing basic reference materials Implementation teams n n n For all major provisions Stakeholder team Training
Implementation n n Developing basic reference materials Implementation teams n n n For all major provisions Stakeholder team Training
 Stakeholder Involvement n n Annual public meeting beginning FY 2004 to review progress in implementing MDUFMA Consultation on specific policies, e. g. , bundling, performance goals for modular PMAs
Stakeholder Involvement n n Annual public meeting beginning FY 2004 to review progress in implementing MDUFMA Consultation on specific policies, e. g. , bundling, performance goals for modular PMAs


