c2782607d1b0b6e94bf798a2d3d391ad.ppt
- Количество слайдов: 27

Medical Device Integration Steve Merritt Infrastructure Engineer Baystate Health

Why Are We Here? • • • Background Barriers to adoption Momentum moving us forward How IHE is breaking down these barriers Lets get to work! Panel discussion

Key Benefits of Point of Care Medical Device Interoperability • More accurate data (10 to 20% errors introduced with data transcription) – Improved patient safety and care outcomes – Improved discharge decisions – Improved Case Management, Infection Prevention and QA • More “real time” data available to MD, clinicians and care managers – – – More clinically sound diagnosis and orders Earlier initiative of appropriate interventions and therapies Prevention of undetected patient deterioration (“failure to rescue”) More “proactive” patient management ( LOS, reimbursement) Better outcomes • Increased MD productivity and satisfaction • Increased Nursing productivity and satisfaction – 1 to 1. 5 hrs day savings per RN or CAN • Outcomes data warehousing

Interoperability: A Brief History of Time • Pre 1990’s – analog outputs (e. g. 0 -3 V) • 1990’s – DICOM: Imaging devices – HL 7: Healthcare informatics, ADT, orders, results – ISO/IEEE 11073: Medical devices • 2000’s – – IHE HITSP Continua ASTM F 29

Standards Smorgasbord

Barriers: Market Forces • Healthcare organization financial priorities – Where is the ROI for medical device interoperability? – Each solution must be justified financially – Reimbursement drivers – Are you willing to pay more for standards? • Would anyone buy an ultrasound without “DICOM” • Vendors marketing one-size-fits-all – Do they really make financial sense? – Don’t listen to these marketing or sales guys • Talk to people who have actually implemented • “Sure, we can interface these widgets to your EMR”

Safely, Effectively • Rigorous validation, verification, and testing of medical devices is required • This slows development to market timelines • We’re creating complex systems of systems requiring analysis

Complex Problems • Most healthcare organizations do not have the staff to understand requirements of medical device interoperability – Sure it “interfaces” does to your EMR but what does that mean? • We need to simplify the integration requirements – Vendor salespeople wouldn’t be able to blow as much smoke • Imaging devices as an example

Cultural • Clinical Engineering and Information Systems have traditionally worked in silo’s • Clinical Systems Engineer – A Hybrid employee • Trend is partnering CE with IT – Neither one can do this alone • AAMI-ACCE-HIMSS CE-IT Community

What Is Driving Us Today? • Trend to organizations and government initiatives to move this forward. • These are not Standards Delivery Organizations (SDO’s) – HITSP (Healthcare Information Technology Standards Panel) • Wide focus on harmonizing and integrating standards across healthcare – Continua • Focus on Personal Health Devices – IHE • Patient Care Devices Domain • Where at least one actor is a regulated point-of-care medical device

IHE PCD: Simplify Specs!
PCD Overview • Sponsored by HIMSS and ACCE • The IHE Patient Care Device Domain, working with regional and national deployment committees, will apply the proven, Use Case driven IHE processes to: – Deliver the technical framework for the IHE-PCD; – Test conformance with published IHE-PCD profiles using test plans, tools and scripts at Connectathons; and – Demonstrate marketable solutions at public trade shows. • IHE-PCD profiles: – Improve patient safety and clinical efficacy, – Reduce healthcare delivery cost by improving efficiency, reliability, and operational flexibility for healthcare providers, – Enable innovative patient care capabilities, and – Expand the international marketplace for patient care device vendors.
![PCD Status • [ACM] Alarm Communication Management enables the remote communication of point-of-care medical PCD Status • [ACM] Alarm Communication Management enables the remote communication of point-of-care medical](https://present5.com/presentation/c2782607d1b0b6e94bf798a2d3d391ad/image-13.jpg)
PCD Status • [ACM] Alarm Communication Management enables the remote communication of point-of-care medical device alarm conditions ensuring the right alarm with the right priority to the right individuals with the right content (e. g. , evidentiary data). • [DEC] Device Enterprise Communication supports publication of information acquired from point-of-care medical devices to applications such as clinical information systems and electronic health record systems, using a consistent messaging format and device semantic content. • [PIV] Point-of-care Infusion Verification supports communication of a 5 -Rights validated medication delivery / infusion order from a BCMA system to an infusion pump or pump management system, thus "closing the loop. “ • DPI, MEM, WCM, IDCO
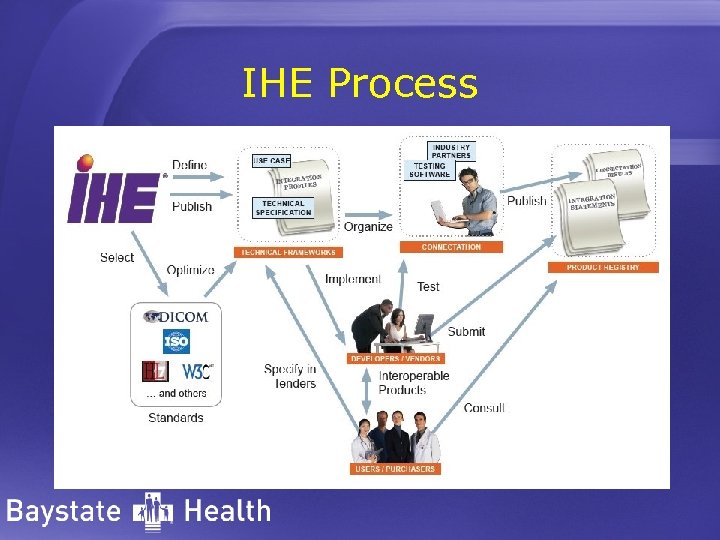
IHE Process
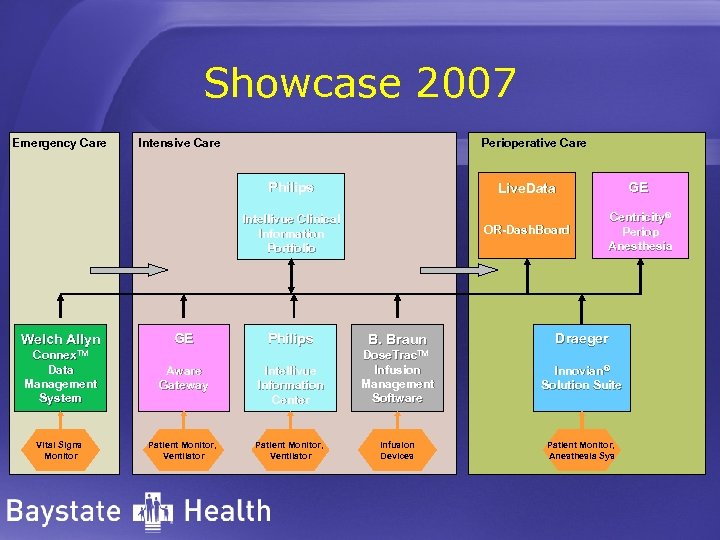
Showcase 2007 Emergency Care Intensive Care Perioperative Care Philips Live. Data OR-Dash. Board Intellivue Clinical Information Portfolio Welch Allyn GE Philips Connex. TM B. Braun GE Centricity® Periop Anesthesia Draeger Dose. Trac. TM Data Management System Aware Gateway Intellivue Information Center Infusion Management Software Innovian® Solution Suite Vital Signs Monitor Patient Monitor, Ventilator Infusion Devices Patient Monitor, Anesthesia Sys
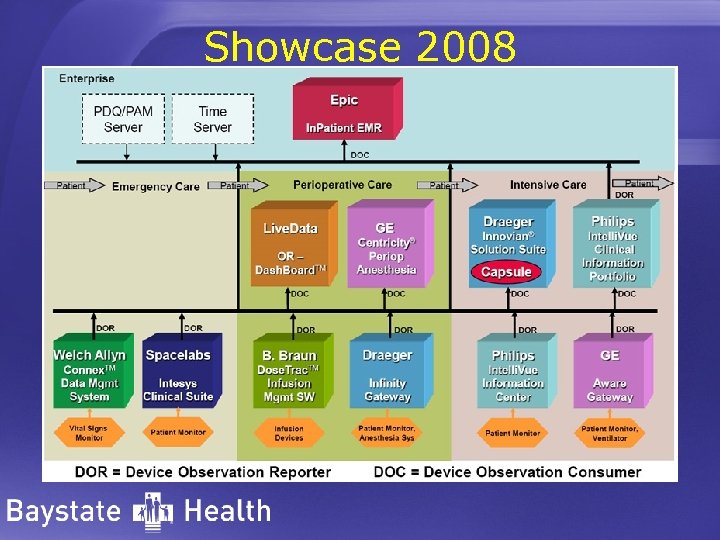
Showcase 2008
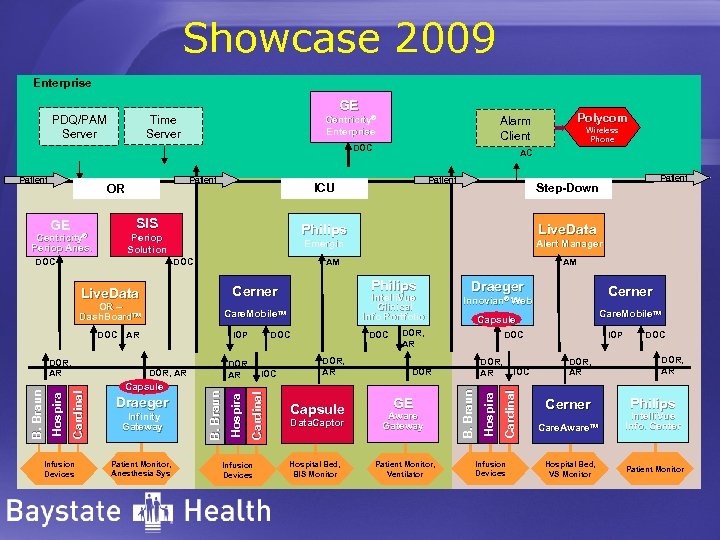
Showcase 2009 Enterprise GE Time Server PDQ/PAM Server Centricity® Enterprise DOC Patient OR Patient Monitor, Anesthesia Sys Infusion Devices DOC DOR, AR Capsule Data. Captor Hospital Bed, BIS Monitor DOC DOR, AR DOR GE Patient Monitor, Ventilator Care. Mobile. TM Capsule DOR, AR Aware Gateway Cerner Innovian® Web IOC Cardinal Infinity Gateway IOC Cardinal Draeger Hospira DOR, AR Capsule DOC Draeger Hospira IOP B. Braun Cardinal Hospira B. Braun Intelli. Vue Clinical Info Portfolio Care. Mobile. TM AR AM Philips Cerner OR – Dash. Board. TM Infusion Devices Alert Manager AM Live. Data DOR, AR Live. Data Emergin DOC Patient Step-Down Philips Periop Solution DOC Patient ICU SIS GE Centricity® Periop Anes. Wireless Phone AC B. Braun Patient Polycom Alarm Client Infusion Devices IOP DOR, AR Cerner Care. Aware. TM Hospital Bed, VS Monitor DOC DOR, AR Philips Intelli. Vue Info. Center Patient Monitor

IHE PCD Collaboration • Helping to harmonize standards • IHE PCD and Continua team up – HITSP IS 77 Remote Monitoring – Content - HL 7 v 2. 6 messages using IHE PCD-01 Vocabulary - Constrained to IEEE/ISO 11073 -20601/11073 -104 xx (PHD Device specialization) nomenclature • ICE-PAC: Collaboration with CIMIT • NIST: Test tooling

Leveraging IHE for purchasing • How do you get IHE Integration Profiles? – Specify IHE capabilities as requirements – State in the RFP which IHE Actors and Integration Profiles you want. • What do IHE Integration Profiles cost? – Nothing in most cases – Any cost should be a fraction of the overall
The business case for implementing IHE Profiles • Enables you to efficiently manage the array of integrated information systems necessary to support effective healthcare • The alternative – Building site-specific interfaces • More expensive • Requires maintaining these custom interfaces for the life of the system involved. • Integration via IHE is less costly at the start and makes future acquisitions easier to plan and execute • IHE Profiles give clear definitions of how the pieces fit together • IHE Profiles come with initial unit testing done

What Can You Do? • Plan, Evaluate, Purchase IHE Conforming Devices • In continuing discussions with vendors – at all levels – Push IHE Interoperability • Refer to lower deployment, maintenance costs – Encourage vendors’ active IHE participation • Lower development, installation, support costs – Refer to profiles • Leverage public and objective commitments • In RFPs – Refer to profiles, Conformance Statements – Use Conformance Statements to “nail down” vendor’s representations – Adopt very specific language

Sample language • “The device shall support the IHE Device Enterprise Communication (DEC) Integration Profile as the Device Observation Reporter (DOR) Actor. ” • “The pump shall support the IHE Point-of-Care Infusion Verification (PIV) Integration Profile as the Infusion Order Consumer (IOC) Actor. ” • “The device shall support the IHE Alarm Communication Management (ACM) Integration Profile as the Alarm Reporter (AR) Actor. ”

Help Break Down Barriers • IHE PCD Call for Work Item Proposals – Due 9/25 • User handbook • Join the technical workgroups • Join us at the Face to Face meetings – Oct 5 -9

All Aboard!

PANEL DISCUSSION: INDUSTRY STANDARDS WHICH STANDARDS WILL BE ADOPTED AND WHY? • 5 minutes each followed by audience questions • Julian M. Goldman, MD, Medical Director of Biomedical Engineering, Partners Health. Care System, Director, CIMIT Program on Interoperability and Medical Device Plug-and-Play Interoperability Program, Massachusetts General Hospital • John Harrington, Vice President Research and Development, Hill-Rom IT Solutions • Sudheer Matta, Product Manager, Wireless Networking Business Unit, Cisco • Dick Moberg, President, Moberg Research, Inc. • Bridget Moorman, CCE, President, BMoorman Consulting, LLC • Robert Rinck, Manager, Clinical Engineering, Spectrum Health • Vaughan Zakian, Founder & CTO, Nuvon, Inc.

My Background • Past Position: Clinical Engineering, IT Specialist • M. S. Biomedical Engineering, University of Connecticut – Focus on Clinical Engineering • Co-chair IHE Patient Care Devices Planning Committee • 2 years in Desktop and Server Support • 5 years in Clinical Engineering • 4 years as Hybrid CE-IT role • I’m not a standards guy, I would just love to see more of them

c2782607d1b0b6e94bf798a2d3d391ad.ppt