eccb14712336df827cb2cc7900159263.ppt
- Количество слайдов: 33
 Medical Device Alert & Recall Management Leo de Kryger Senior Technologist Biomedical Engineering Department The Ottawa Hospital Ottawa - Canada
Medical Device Alert & Recall Management Leo de Kryger Senior Technologist Biomedical Engineering Department The Ottawa Hospital Ottawa - Canada
 AGENDA n Definitions. n Review of “the old system”. n Why change was necessary. n How the current system was developed. n Roadway to success. n Sources of Medical Device Alerts & Recalls. n Review of commercial tracking systems.
AGENDA n Definitions. n Review of “the old system”. n Why change was necessary. n How the current system was developed. n Roadway to success. n Sources of Medical Device Alerts & Recalls. n Review of commercial tracking systems.
 Alerts & Recalls ? ? ?
Alerts & Recalls ? ? ?
 What is an Alert? n A medical device Alert describes a situation where patients, their families, visitors or staff might be at risk. n This risk might be present at all times, only under certain circumstances, or after a chain of events.
What is an Alert? n A medical device Alert describes a situation where patients, their families, visitors or staff might be at risk. n This risk might be present at all times, only under certain circumstances, or after a chain of events.
 What is a Recall? n A published notification originating from a regulatory agency or a medical device manufacturer or distributor, stating a medical device condition related to design, use or operation that may cause harm to patient or staff, or that prevents the device from providing the intended use or output.
What is a Recall? n A published notification originating from a regulatory agency or a medical device manufacturer or distributor, stating a medical device condition related to design, use or operation that may cause harm to patient or staff, or that prevents the device from providing the intended use or output.
 The “old” system n Alerts and Recalls were received by a variety of Departments and/or individuals within the institution. n Distribution and follow-up was haphazard, there was a risk that required action was either not taken, or was not taken in a timely fashion.
The “old” system n Alerts and Recalls were received by a variety of Departments and/or individuals within the institution. n Distribution and follow-up was haphazard, there was a risk that required action was either not taken, or was not taken in a timely fashion.
 Why change was necessary n Early in 2000 a patient alleged that ongoing medical problems with a suture line were due to the hospital’s use of a recalled suture during a surgical procedure. n Attempts to refute this statement were complicated due to a lack of record keeping of Alerts & Recalls. n We were fortunate that it only took us a full day to determine that the product identified by the patient was never purchased by the hospital, and that the recalled product was shipped to only one hospital in Canada 3000 Km away. n Most, if not all, of this confusion could have been avoided if we had a centralized tracking system.
Why change was necessary n Early in 2000 a patient alleged that ongoing medical problems with a suture line were due to the hospital’s use of a recalled suture during a surgical procedure. n Attempts to refute this statement were complicated due to a lack of record keeping of Alerts & Recalls. n We were fortunate that it only took us a full day to determine that the product identified by the patient was never purchased by the hospital, and that the recalled product was shipped to only one hospital in Canada 3000 Km away. n Most, if not all, of this confusion could have been avoided if we had a centralized tracking system.
 How we centralized Alerts & Recalls n Flowcharting the existing system was impossible since it was too fragmented. n We flowcharted the “Ideal Situation” as follows:
How we centralized Alerts & Recalls n Flowcharting the existing system was impossible since it was too fragmented. n We flowcharted the “Ideal Situation” as follows:
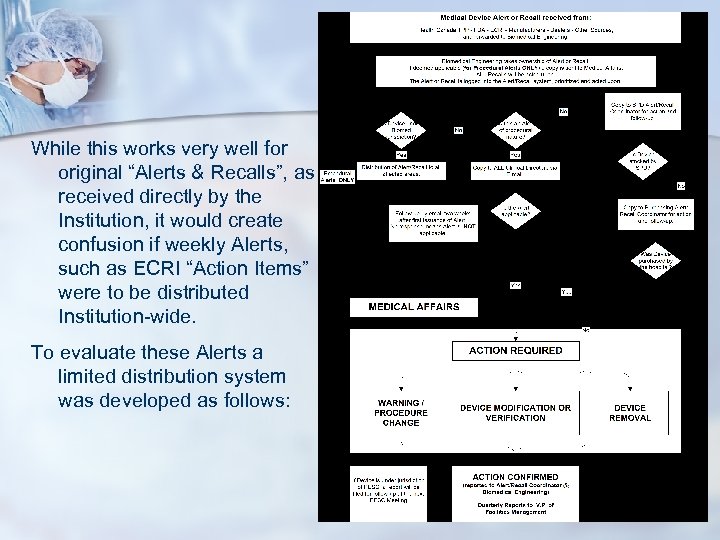 While this works very well for original “Alerts & Recalls”, as received directly by the Institution, it would create confusion if weekly Alerts, such as ECRI “Action Items” were to be distributed Institution-wide. To evaluate these Alerts a limited distribution system was developed as follows:
While this works very well for original “Alerts & Recalls”, as received directly by the Institution, it would create confusion if weekly Alerts, such as ECRI “Action Items” were to be distributed Institution-wide. To evaluate these Alerts a limited distribution system was developed as follows:
 • Since 2000 when this was charted all communication has been by means of email.
• Since 2000 when this was charted all communication has been by means of email.
 Classification of incoming Alerts & Recalls n Prefixes: n. A for Alerts n R for Recalls n FYI for “For Your Information” n Suffixes: n 1 High Risk n 2 Moderate Risk n 3 Low Risk n FYI items do not carry a suffix
Classification of incoming Alerts & Recalls n Prefixes: n. A for Alerts n R for Recalls n FYI for “For Your Information” n Suffixes: n 1 High Risk n 2 Moderate Risk n 3 Low Risk n FYI items do not carry a suffix
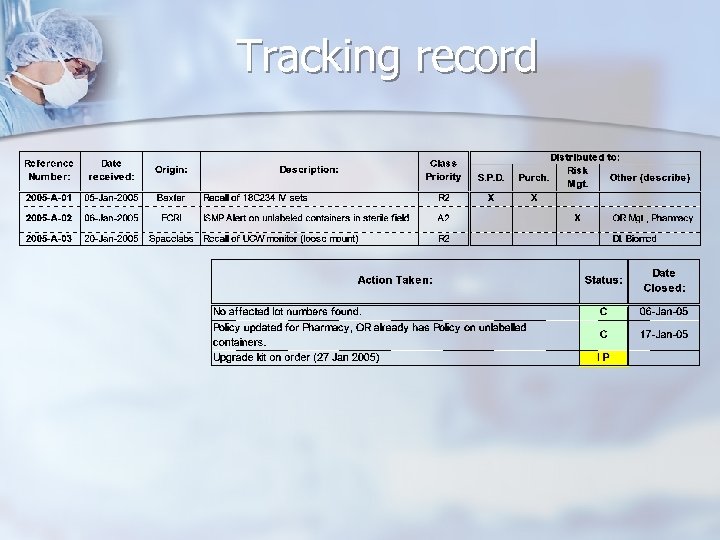 Tracking record
Tracking record
 TOH Policy
TOH Policy
 Implementation § It became evident early on that unless the program was promoted by the initial “stakeholders”, Risk Management, Purchasing, Logistics/SPD, Engineering Services (Physical Plant) and Biomedical Engineering, success could not be guaranteed. § With the Policy written, and approved by Senior Management, we embarked on a publication blitz. § Articles were written and published in “The Journal”, the internal bi-weekly newsletter for all employees. Letters were sent to all our suppliers, emails were sent to all management staff.
Implementation § It became evident early on that unless the program was promoted by the initial “stakeholders”, Risk Management, Purchasing, Logistics/SPD, Engineering Services (Physical Plant) and Biomedical Engineering, success could not be guaranteed. § With the Policy written, and approved by Senior Management, we embarked on a publication blitz. § Articles were written and published in “The Journal”, the internal bi-weekly newsletter for all employees. Letters were sent to all our suppliers, emails were sent to all management staff.



 § A dedicated email address was established: recalls-alerts@ottawahospital. on. ca § All of our suppliers were advised of this.
§ A dedicated email address was established: recalls-alerts@ottawahospital. on. ca § All of our suppliers were advised of this.
 Our experience following implementation n The internal forwarding of notifications is working as foreseen. In fact, it has improved with the event of email. Our vendors/suppliers have largely ignored the letter sent jointly by the Directors of Purchasing and Biomedical Engineering. The email address established to receive notifications in electronic format has not seen any action….
Our experience following implementation n The internal forwarding of notifications is working as foreseen. In fact, it has improved with the event of email. Our vendors/suppliers have largely ignored the letter sent jointly by the Directors of Purchasing and Biomedical Engineering. The email address established to receive notifications in electronic format has not seen any action….
 Roadway to success n Determine which Department will act as the central coordination point. n Flowchart the proposed system, based on your Institutions’ protocols and procedures. (You may have to develop an associated Policy) n Meet with all the stakeholders and review your proposals. Make sure you get “Buy-in” of all parties concerned.
Roadway to success n Determine which Department will act as the central coordination point. n Flowchart the proposed system, based on your Institutions’ protocols and procedures. (You may have to develop an associated Policy) n Meet with all the stakeholders and review your proposals. Make sure you get “Buy-in” of all parties concerned.
 n After you have developed your Policy & Procedure you will have to advise all parties concerned, within your Institution, and all your suppliers. n You will have to name a Central Coordinator, and provide a back-up in case he/she is unavailable.
n After you have developed your Policy & Procedure you will have to advise all parties concerned, within your Institution, and all your suppliers. n You will have to name a Central Coordinator, and provide a back-up in case he/she is unavailable.
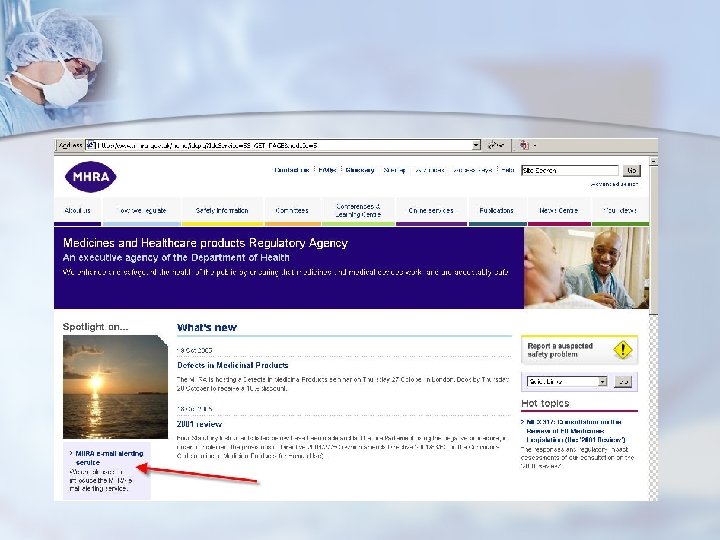
 Resources Original Notifications n ECRI weekly “Action Items” n http: //www. ecri. org/ (available for “members” only) FDA Medwatch list-server n Health Canada Medeffect list-server n http: //www. fda. gov/medwatch/ http: //www. hc-sc. gc. ca/dhp-mps/medeff/advisories-avis/index_e. html n UK MHRA list-server http: //www. mhra. gov. uk/home/idcplg? Idc. Service=SS_GET_PAGE&node. Id=217 n French AFSSAPS http: //agmed. sante. gouv. fr/htm/alertes/al 000. htm
Resources Original Notifications n ECRI weekly “Action Items” n http: //www. ecri. org/ (available for “members” only) FDA Medwatch list-server n Health Canada Medeffect list-server n http: //www. fda. gov/medwatch/ http: //www. hc-sc. gc. ca/dhp-mps/medeff/advisories-avis/index_e. html n UK MHRA list-server http: //www. mhra. gov. uk/home/idcplg? Idc. Service=SS_GET_PAGE&node. Id=217 n French AFSSAPS http: //agmed. sante. gouv. fr/htm/alertes/al 000. htm
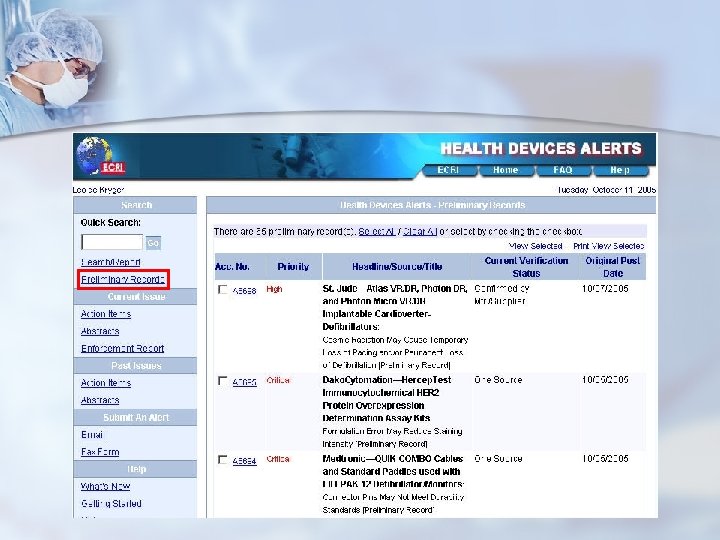
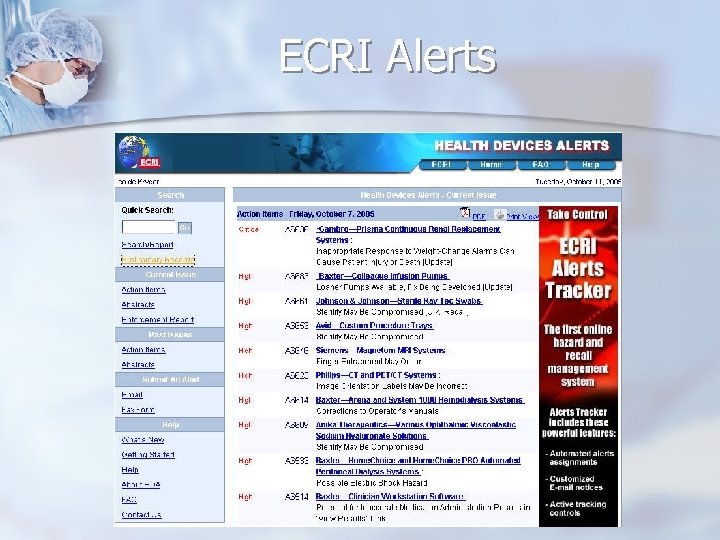 ECRI Alerts
ECRI Alerts
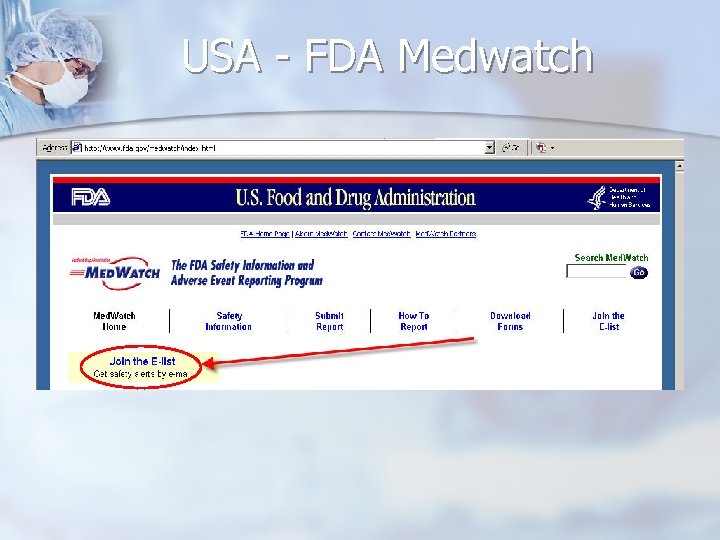 USA - FDA Medwatch
USA - FDA Medwatch
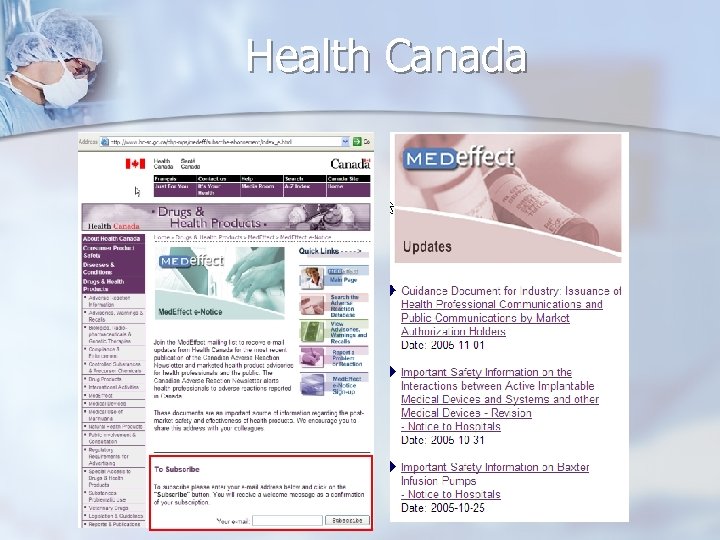 Health Canada
Health Canada
 UK - MHRA
UK - MHRA
 France - AFSSAPS
France - AFSSAPS
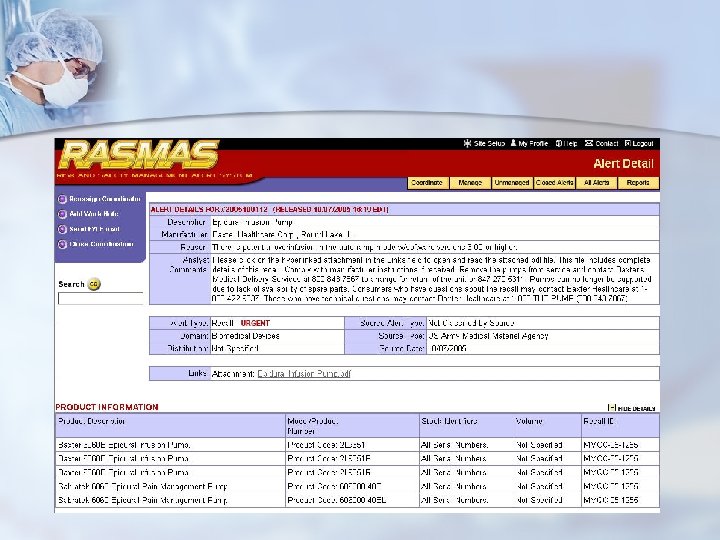
 Commercial Tracking Systems n ECRI “Alerts Tracker” n Mitretek RASMAS (Risk And Safety Management Alert System)
Commercial Tracking Systems n ECRI “Alerts Tracker” n Mitretek RASMAS (Risk And Safety Management Alert System)
 ECRI – Alerts Tracker
ECRI – Alerts Tracker
 Mitretek - RASMAS
Mitretek - RASMAS