861edf073fcb495c8e9889a5616fea99.ppt
- Количество слайдов: 52
Medical biotechnology - an overview Maria Judit. Molnar Institute of Medical Genomics and Rare Diseases Semmelweis University Budapest, Hungary
TOPICS Biotechnology in medicine Milestones in medical biotechnology Achievements, paradigm shifts Current trends and research Conclusions 2

Biotechnology in medicine Biologic medicinal product Biologic al product = Biotech drug Biological drug Biologic ech t Bio uct rod p 3
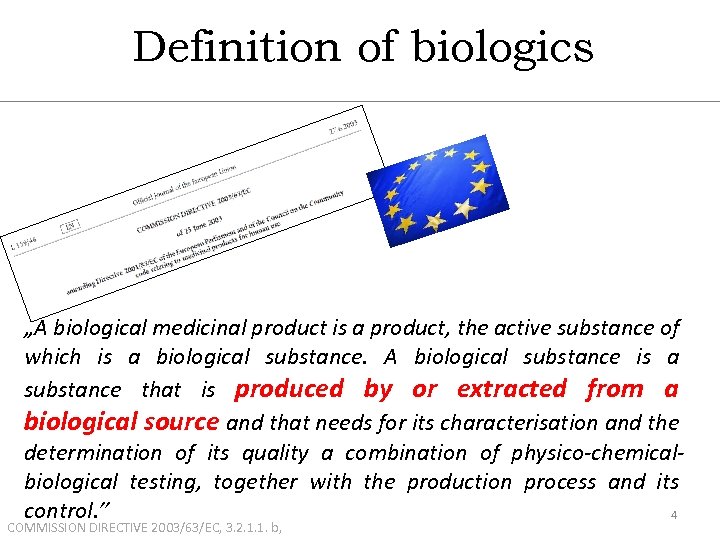
Definition of biologics „A biological medicinal product is a product, the active substance of which is a biological substance. A biological substance is a substance that is produced by or extracted from a biological source and that needs for its characterisation and the determination of its quality a combination of physico-chemicalbiological testing, together with the production process and its control. ” 4 COMMISSION DIRECTIVE 2003/63/EC, 3. 2. 1. 1. b,
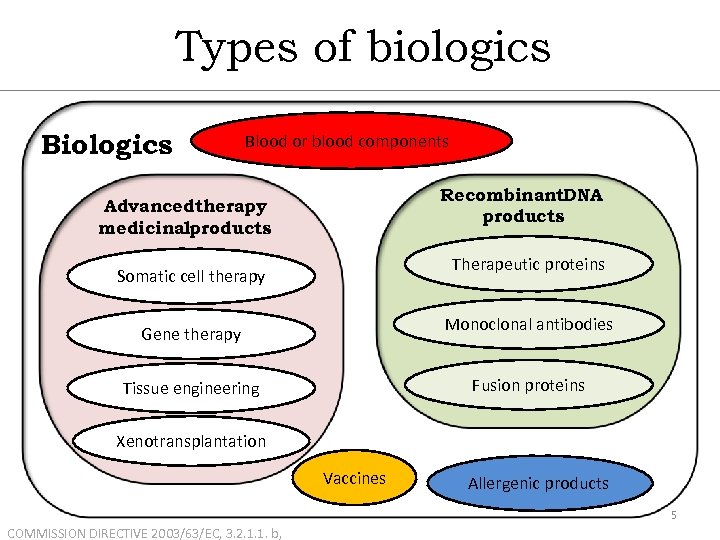
Types of biologics Blood or blood components Recombinant. DNA products Advancedtherapy medicinalproducts Therapeutic proteins Somatic cell therapy Monoclonal antibodies Gene therapy Fusion proteins Tissue engineering Xenotransplantation Vaccines Allergenic products 5 COMMISSION DIRECTIVE 2003/63/EC, 3. 2. 1. 1. b,
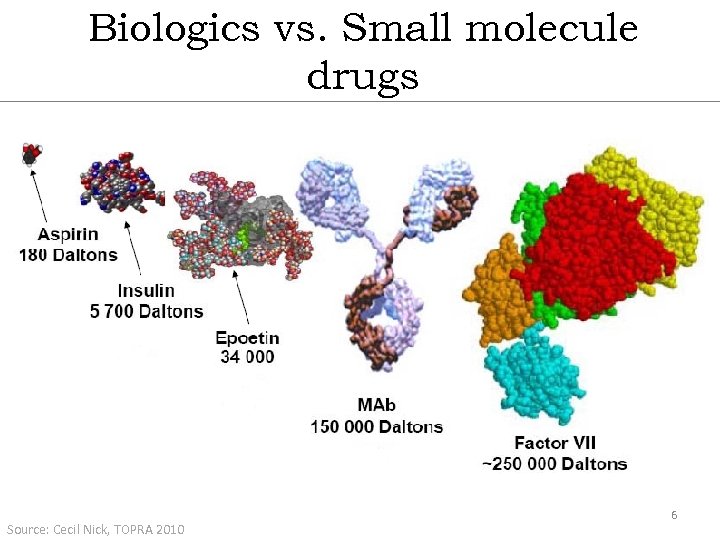
Biologics vs. Small molecule drugs Source: Cecil Nick, TOPRA 2010 6
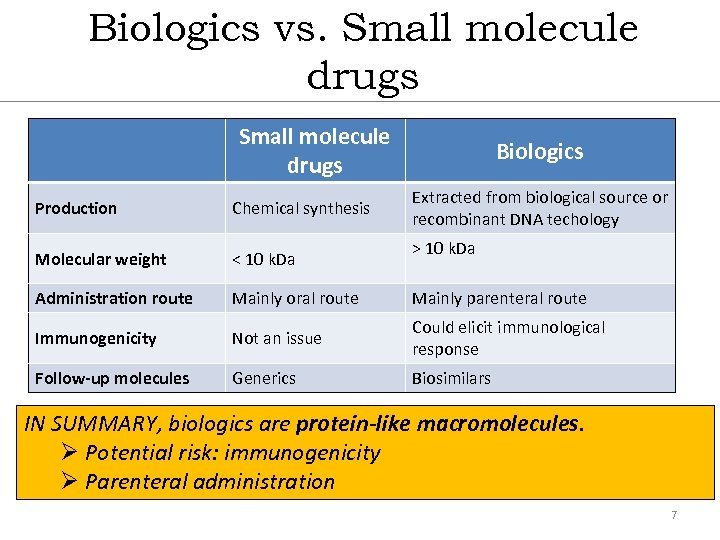
Biologics vs. Small molecule drugs Biologics Extracted from biological source or recombinant DNA techology Production Chemical synthesis Molecular weight < 10 k. Da Administration route Mainly oral route Mainly parenteral route Immunogenicity Not an issue Could elicit immunological response Follow-up molecules Generics Biosimilars > 10 k. Da IN SUMMARY, biologics are protein-like macromolecules. Ø Potential risk: immunogenicity Ø Parenteral administration 7

Biotechnology in medicine Milestones in medical biotechnology Achievements, paradigm shifts Current trends and research Conclusions 8
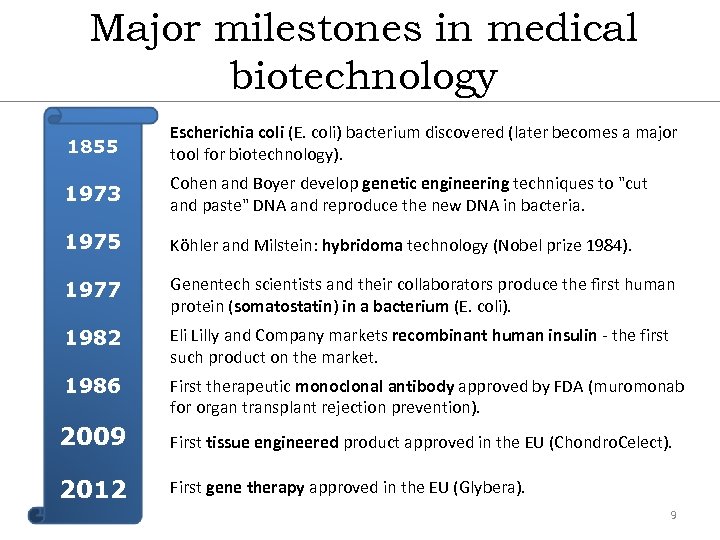
Major milestones in medical biotechnology 1855 Escherichia coli (E. coli) bacterium discovered (later becomes a major tool for biotechnology). 1973 Cohen and Boyer develop genetic engineering techniques to "cut and paste" DNA and reproduce the new DNA in bacteria. 1975 Köhler and Milstein: hybridoma technology (Nobel prize 1984). 1977 Genentech scientists and their collaborators produce the first human protein (somatostatin) in a bacterium (E. coli). 1982 Eli Lilly and Company markets recombinant human insulin - the first such product on the market. 1986 First therapeutic monoclonal antibody approved by FDA (muromonab for organ transplant rejection prevention). 2009 First tissue engineered product approved in the EU (Chondro. Celect). 2012 First gene therapy approved in the EU (Glybera). 9
Recombinant DNA products Y, R THEIR TO ES AC ID A F BES IKE IN K L TE OR PRO. E. coli S W ED EINS ELL SIR OT D C DE E PR ON THE WN CL NG O CI DU O PR 10
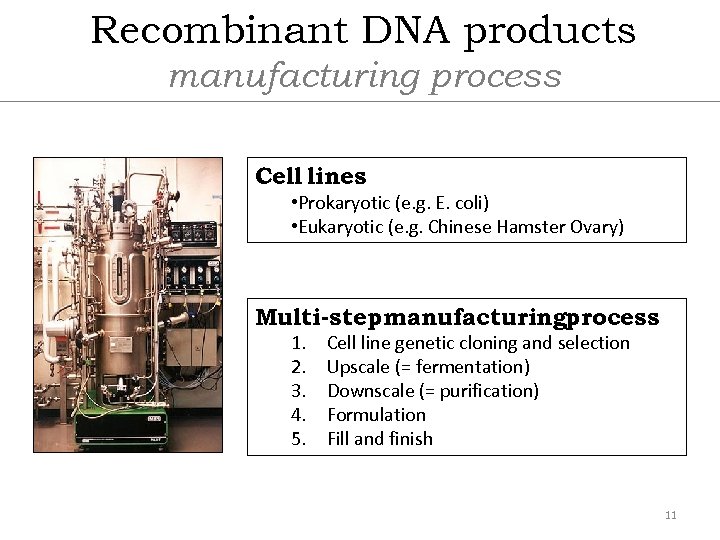
Recombinant DNA products manufacturing process Cell lines • Prokaryotic (e. g. E. coli) • Eukaryotic (e. g. Chinese Hamster Ovary) Multi-stepmanufacturingprocess 1. 2. 3. 4. 5. Cell line genetic cloning and selection Upscale (= fermentation) Downscale (= purification) Formulation Fill and finish 11
Biotechnology in medicine Milestones in medical biotechnology Achievements, paradigm shifts Current trends and research Conclusions 12
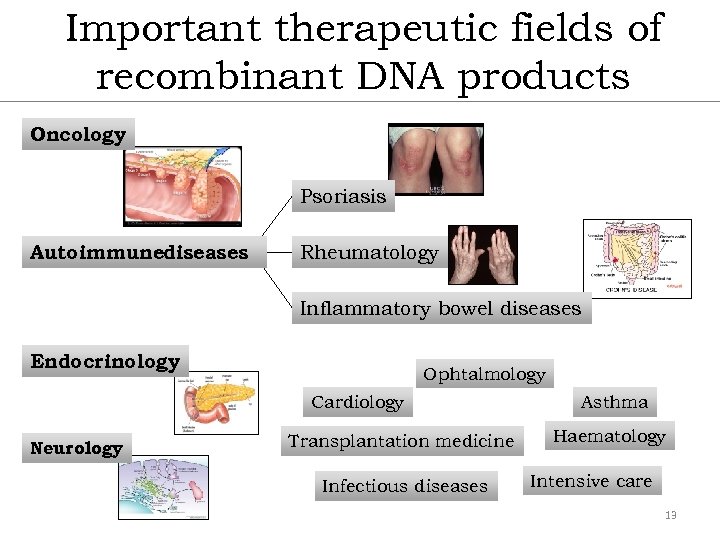
Important therapeutic fields of recombinant DNA products Oncology Psoriasis Autoimmunediseases Rheumatology Inflammatory bowel diseases Endocrinology Ophtalmology Cardiology Neurology Transplantation medicine Infectious diseases Asthma Haematology Intensive care 13
ENDOCRINOLOGY Insulin From the 1920’s insulin was derived from porcine / cattle pancreas for therapeutic use (organotherapy) 1982 first recombinant insulin on the European market 2014 multiple recombinant insulin products on the market ØRapid-acting ØShort-acting ØIntermediate-acting ØLong-acting ØPre-mixed 14

ENZYMES Gaucher’s disease and imiglucerase Gaucher’s disease • lysosomal disease • deficit of the enzyme glucocerebrosidase • accumulation of glucocerebrosides • autosomal recessive trait Therapy of Gaucher’s disease • enzyme substitution therapy • e. g. imiglucerase 15
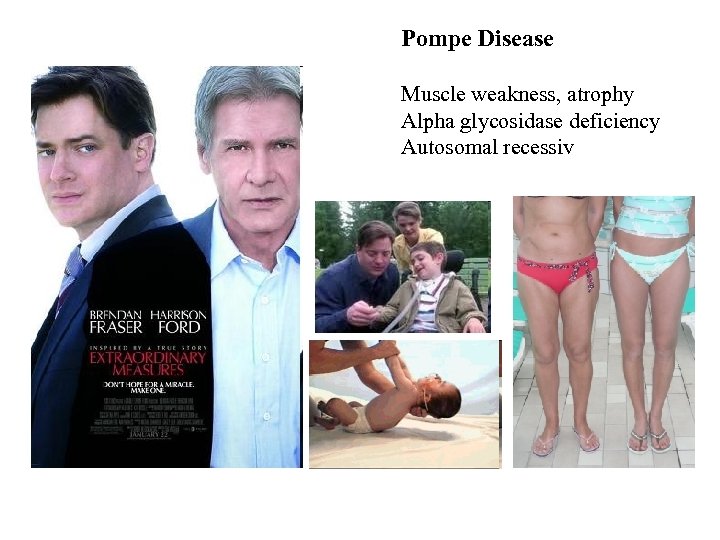
Pompe Disease Muscle weakness, atrophy Alpha glycosidase deficiency Autosomal recessiv
Source: International Society for Mannosidosis & Related Diseases
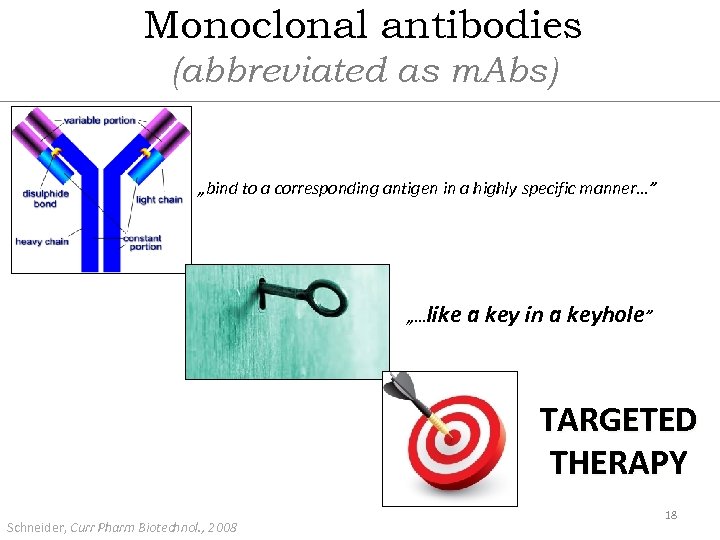
Monoclonal antibodies (abbreviated as m. Abs) „bind to a corresponding antigen in a highly specific manner…” „…like a key in a keyhole” TARGETED THERAPY Schneider, Curr Pharm Biotechnol. , 2008 18
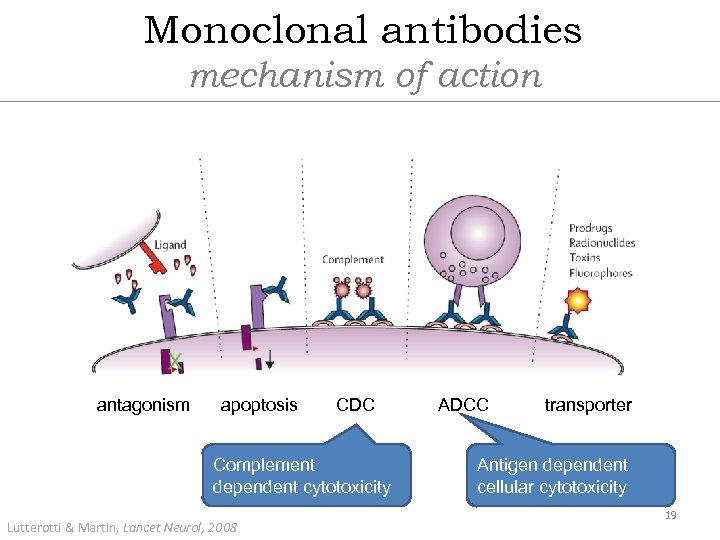
Monoclonal antibodies mechanism of action antagonism apoptosis CDC Complement dependent cytotoxicity Lutterotti & Martin, Lancet Neurol, 2008 ADCC transporter Antigen dependent cellular cytotoxicity 19
ONCOLOGY Trastuzumab Conventionalchemotherapy • like „carpet bombing” –> frequent adverse events Monoclonalantibodies(m. Abs) • targeted therapy Ø better outcomes Ø less adverse events An example from oncology Trastuzumab : • indication: HER 2 positive metastatic breast cancer • HER 2 is a type of growth factor receptor • HER 2 positive histology: in approx. ¼ of the cases • trastuzumab targets HER 2 and kills cancer cells Source: Herceptin®, European Public Assessment Report 20
ONCOLOGY + AUTOIMMUNE DISEASE CD 20 • antigen present on mature B cells • not present on pre-B cells and plasma cells • B cells are key players in immune responses more specifically in humoral immunity Rituximab • monoclonal antibody (m. Ab) • targeting CD 20 with high specificity, and depletes B cells Indicationsof rituximab • Oncological diseases • non-Hodgkin’s lymphoma • chronic lymphocytic leukemia • Autoimmune diseases • rheumatoid arthritis • specific types of vasculitis Source: Mab. Thera®, European Public Assessment Report 21
AUTOIMMUNE DISEASE TNF-alfa • cytokine • plays important role in inflammatory processes Etanercept • fusion protein • Fc part of a m. Ab + 2 pieces of TNF-receptors • targeting TNF-alfa Indicationsof etanercept • rheumatoid arthritis • juvenile idiopathic arthritis • ankylosing spondylitis • psoriatic arthritis • plaque psoriasis • paediatric plaque psoriasis Source: Enbrel®, European Public Assessment Report 22
Vaccines Production Ø derived from natural source Ø recombinant DNA technology Prophylactic vaccines Ø against infectious diseases Ø e. g. Hepatitis B vaccination Therapeuticvaccines Ø e. g. cancer vaccines Ø intensive research is ongoing 23

Blood and blood components Whole blood Blood components Ø red blood cells Ø white blood cells Ø plasma Ø clotting factors Ø platelets For the treatmentof Ø anaemia Ø thrombocytopenia Ø clotting deficiencies 24
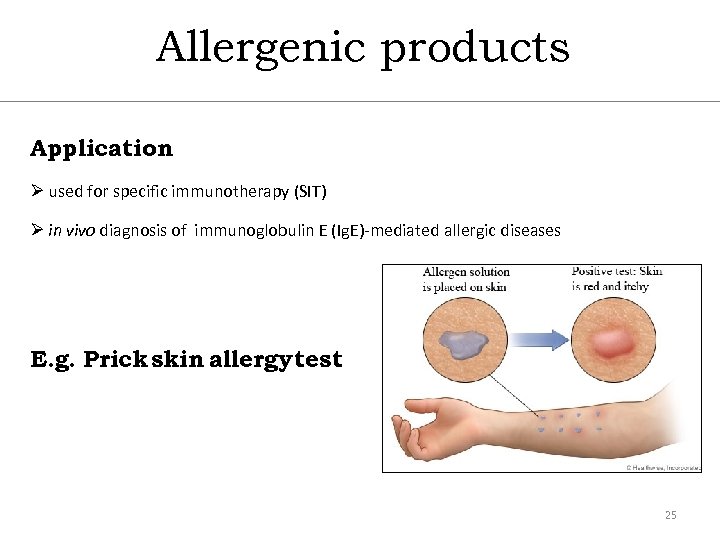
Allergenic products Application Ø used for specific immunotherapy (SIT) Ø in vivo diagnosis of immunoglobulin E (Ig. E)-mediated allergic diseases E. g. Prick skin allergy test 25

Gene therapy „contain genes that lead to a therapeutic effect. They work by 'recombinant' genes into cells, inserting usually to treat a variety of diseases, including genetic disorders, cancer or long-term diseases. A recombinant gene is a stretch of DNA that is created in the laboratory, bringing together DNA from different sources” • First gene therapy approved in EU • Recurrent pancreatitis (lipoprotein lipase deficiency) • Vector: adeno-associated virus Source: European Medicines Agency homepage 26

The history of the gene therapy 1977 - A gene was successfully delivered into mammalian cells 1990 - First human gene therapy was approved: SCID 1999 - J. Gelsinger with OTC deficiency died from organ failure after gene therapy 2000 - A. Fischer cured children with SCID using retroviral vector, 2 of the children developed leukemia. FDA halted the use of retroviruses in the US 2006 - Patients was successfully treated with metastatic melanoma using killer T cells genetically retargeted to attack the cancer cells 2006 - Succesfull gene-based th. for the treatment of HIV: lentiviral vector for delivery of an antisense gene againts HIV envelope 2009 - Researchers succeeded at halting adrenoleukodystrophy, using a vector derived from HIV to deliver the gene for the missing enzyme
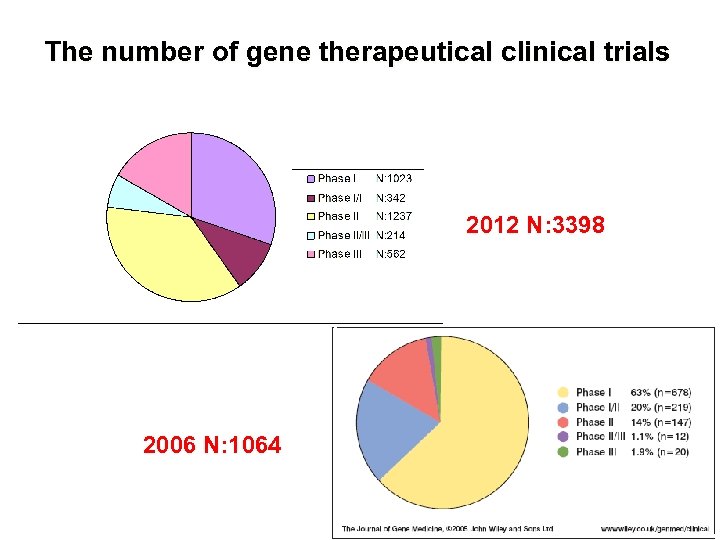
The number of gene therapeutical clinical trials 2012 N: 3398 2006 N: 1064
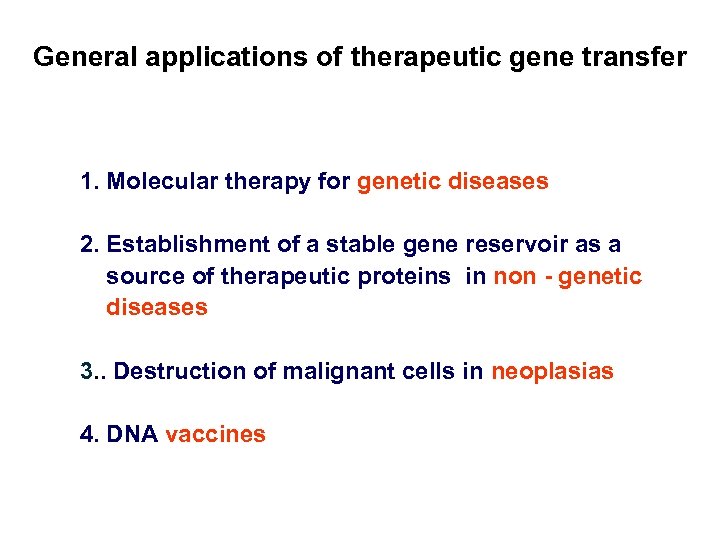
General applications of therapeutic gene transfer 1. Molecular therapy for genetic diseases 2. Establishment of a stable gene reservoir as a source of therapeutic proteins in non - genetic diseases 3. . Destruction of malignant cells in neoplasias 4. DNA vaccines
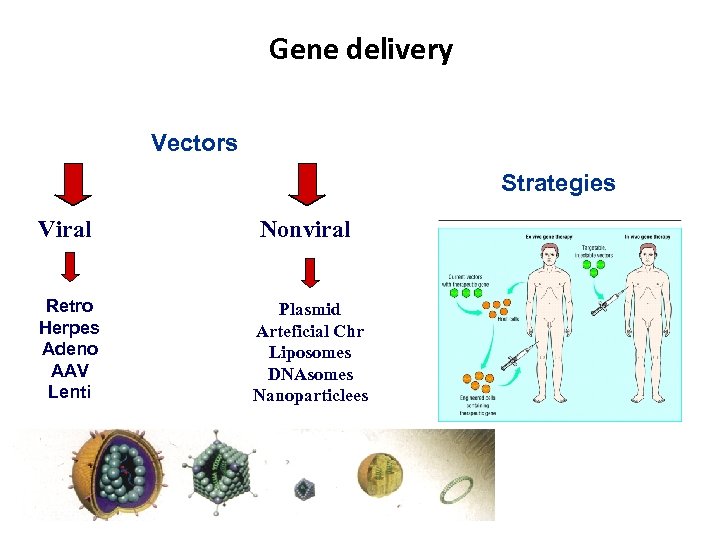
Gene delivery Vectors Strategies Viral Nonviral Retro Herpes Adeno AAV Lenti Plasmid Arteficial Chr Liposomes DNAsomes Nanoparticlees
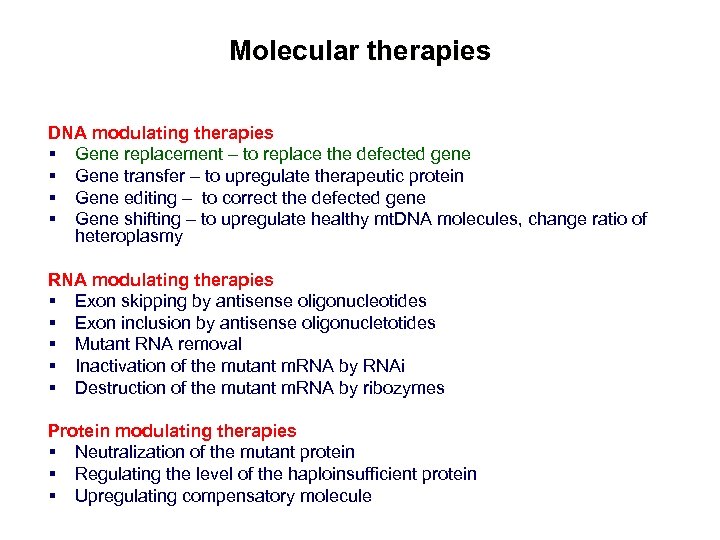
Molecular therapies DNA modulating therapies § Gene replacement – to replace the defected gene § Gene transfer – to upregulate therapeutic protein § Gene editing – to correct the defected gene § Gene shifting – to upregulate healthy mt. DNA molecules, change ratio of heteroplasmy RNA modulating therapies § Exon skipping by antisense oligonucleotides § Exon inclusion by antisense oligonucletotides § Mutant RNA removal § Inactivation of the mutant m. RNA by RNAi § Destruction of the mutant m. RNA by ribozymes Protein modulating therapies § Neutralization of the mutant protein § Regulating the level of the haploinsufficient protein § Upregulating compensatory molecule
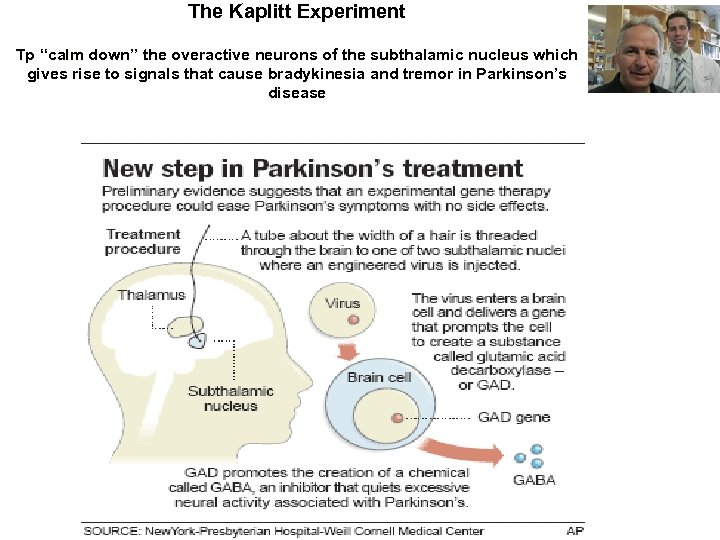
The Kaplitt Experiment Tp “calm down” the overactive neurons of the subthalamic nucleus which gives rise to signals that cause bradykinesia and tremor in Parkinson’s disease
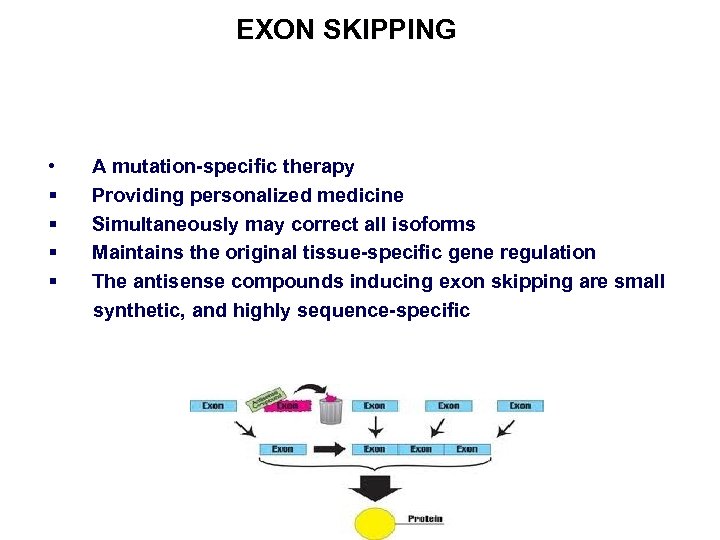
EXON SKIPPING • § § A mutation-specific therapy Providing personalized medicine Simultaneously may correct all isoforms Maintains the original tissue-specific gene regulation The antisense compounds inducing exon skipping are small synthetic, and highly sequence-specific
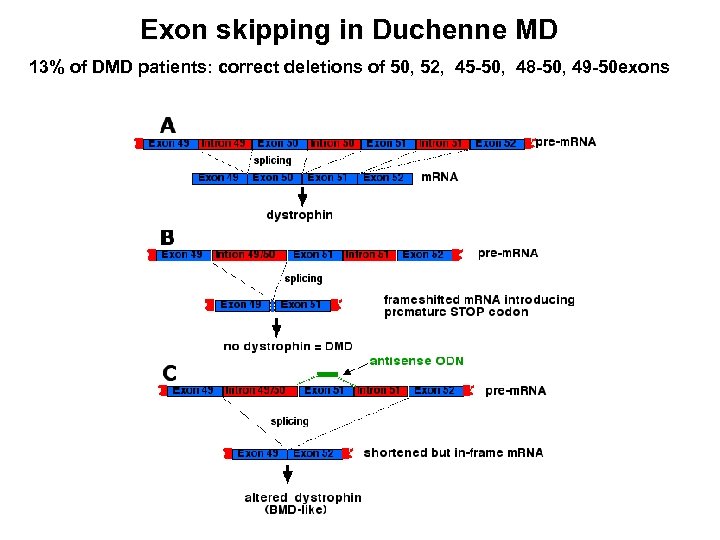
Exon skipping in Duchenne MD 13% of DMD patients: correct deletions of 50, 52, 45 -50, 48 -50, 49 -50 exons

Progress in AON exon skipping therapy in DMD Timelines PRO 051 Prosensa/GSK 2 OME AON 2007 2008 2009 2010 2011 2012 2013 Ph I Im. Ph I/II Systemic adm, iv, weekly Ph I/II Study extension. Ph I Non ambulant Ph II Dosing Ph III Efficacy Eteplirsen AVI PMO AON Ph I Im. Ph I/II Syst. admin. sc, 3 w Ph I/II Dosing
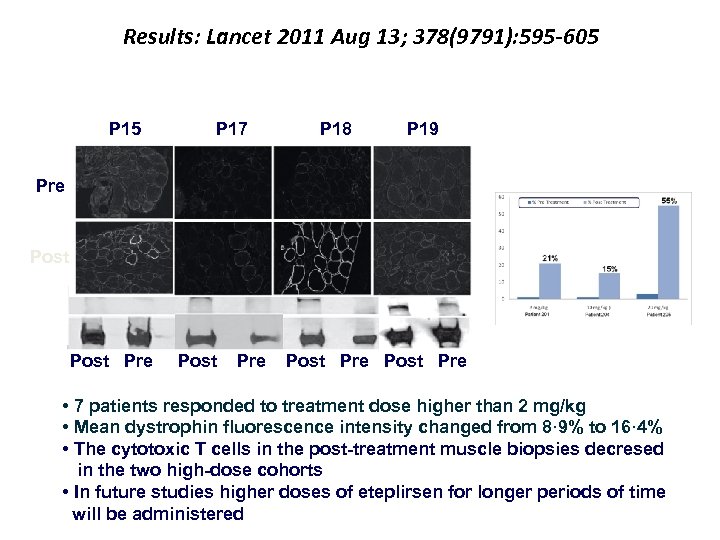
Results: Lancet 2011 Aug 13; 378(9791): 595 -605 P 15 Pre P 15 P 17 P 18 P 19 P 18 Post Pre Post Pre • 7 patients responded to treatment dose higher than 2 mg/kg • Mean dystrophin fluorescence intensity changed from 8· 9% to 16· 4% • The cytotoxic T cells in the post-treatment muscle biopsies decresed in the two high-dose cohorts • In future studies higher doses of eteplirsen for longer periods of time will be administered
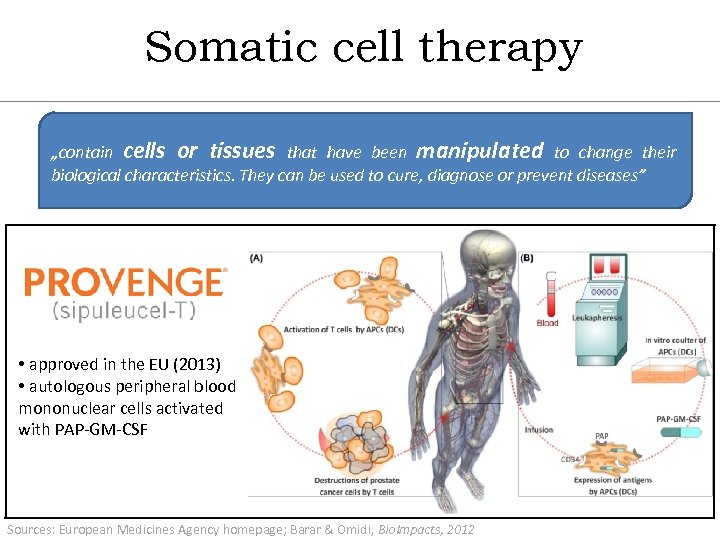
Somatic cell therapy „contain cells or tissues that have been manipulated to change their biological characteristics. They can be used to cure, diagnose or prevent diseases” • approved in the EU (2013) • autologous peripheral blood mononuclear cells activated with PAP-GM-CSF Sources: European Medicines Agency homepage; Barar & Omidi, Bio. Impacts, 2012 37
Stem cells „cells with self-renewing capacity i. e. the capability to generate daughter cells and multi-lineage differentiation capacity. Stem cells are capable of proliferation as stem cells in an undifferentiated form” Embryonicstem cells Adult or somatic stem cells • Bone marrow (hematopoietic) stem cells • Mesenchymal stromal / stem cells • Tissue-specific progenitor cells with a more restricted differentiation capacity responsible for normal tissue renewal and turnover (neurons, intestine, skin, lung and muscle) Induced pluripotentstem cells Geneticallymodifiedstem cells Source: EMA/CAT/571134/2009 38
Stem cells Potentialapplicationsof stem cells Ø metabolic, degenerative and inflammatory diseases Ø repair and regeneration of damaged or lost tissues Ø treatment of cancer Umbilical cord stem cell preservation e. g. Re. Neuron’s Re. N 001 ØPhase 1 trial is ongoing, phase 2 trial application submitted ØFor the treatment of ischemic stroke Source: EMA/CAT/571134/2009; Re. Neuron homepage 39
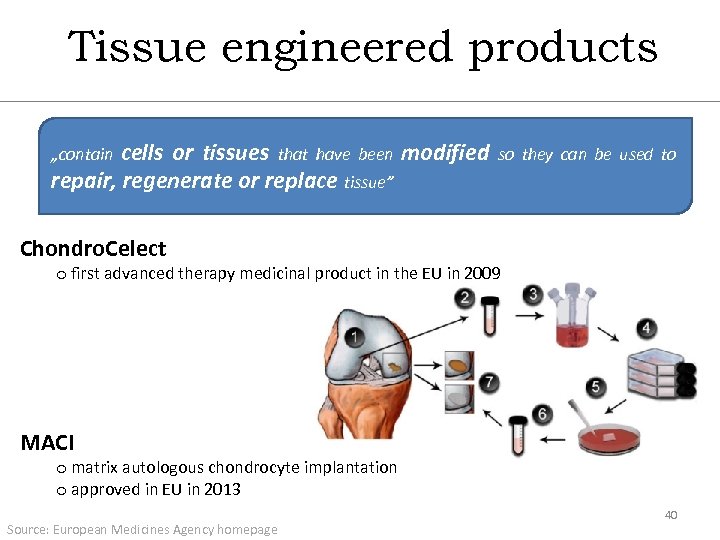
Tissue engineered products cells or tissues that have been modified repair, regenerate or replace tissue” „contain so they can be used to Chondro. Celect o first advanced therapy medicinal product in the EU in 2009 MACI o matrix autologous chondrocyte implantation o approved in EU in 2013 Source: European Medicines Agency homepage 40
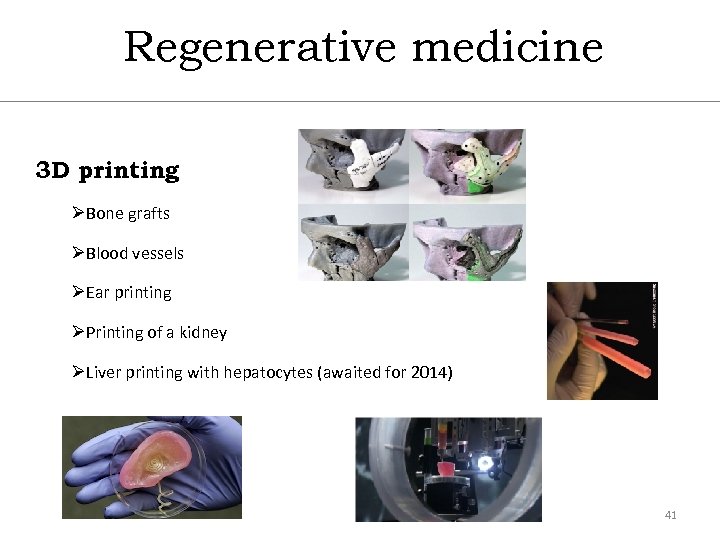
Regenerative medicine 3 D printing ØBone grafts ØBlood vessels ØEar printing ØPrinting of a kidney ØLiver printing with hepatocytes (awaited for 2014) 41

Biotechnology in medicine Milestones in medical biotechnology Achievements, paradigm shifts Current trends and research Conclusions 42

Personalized medicine "the right patient with the right drug at the right dose at the right time. " To date more than 100 drugs approved by FDA have information on pharmacogenetic biomarkers in the labelling. Examples of individualized tailoredtherapies / Ø Metastatic breast cancer • Drug: trastuzumab (Herceptin®) • Biomarker: HER 2 positivity of tumor Ø Duchenne muscular dystrophy • exon skipping Source: FDA homepage 43
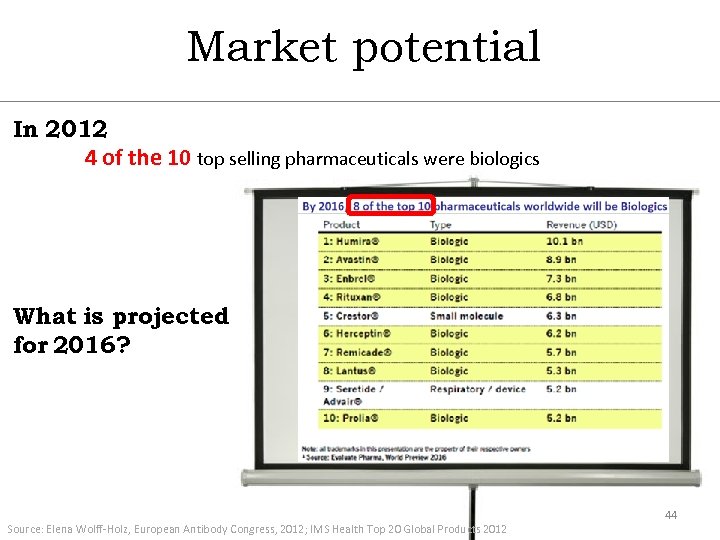
Market potential In 2012 4 of the 10 top selling pharmaceuticals were biologics What is projected for 2016? Source: Elena Wolff-Holz, European Antibody Congress, 2012; IMS Health Top 20 Global Products 2012 44
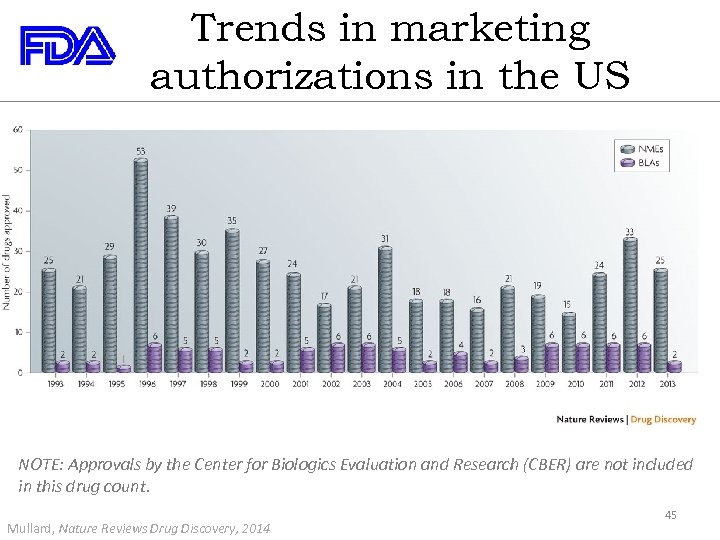
Trends in marketing authorizations in the US NOTE: Approvals by the Center for Biologics Evaluation and Research (CBER) are not included in this drug count. Mullard, Nature Reviews Drug Discovery, 2014 45
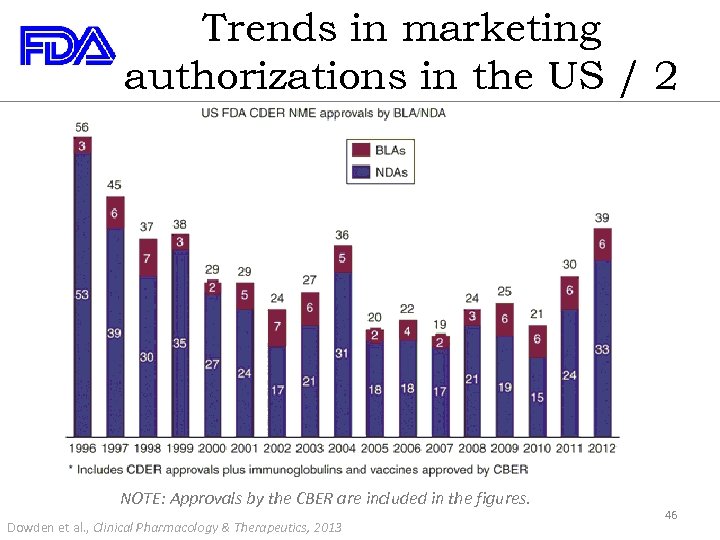
Trends in marketing authorizations in the US / 2 NOTE: Approvals by the CBER are included in the figures. Dowden et al. , Clinical Pharmacology & Therapeutics, 2013 46
Costs and risks associated to biotech drug development Ø Approx. $ 1 billion out-of-pocket money for 1 new drug. ≈ Ø Biotech product development is somewhat more expensive than small molecule development ($1241 vs $899 million). Golden Gate bridge, San Francisco Ø Average research success rate for clinical development: 1: 6. Ø Biotech products are somewhat better in terms of pre-market success rate compared to small molecules. Ø Approx. 8 -12 years of research for 1 new drug. Di. Masi et al. , Manage. Decis. Econ. 2007; Di. Masi et al. , Nature, 2010; goldengatebridge. org (USD currency in 2003) 47
Recent metrics on biotech Research & Development From 2001 to 2012 (11 years)… Ø the number of biotech products in clinical development grew 155%, from 355 to 907. Ø financing of biotech research increased 10 -fold, from $10. 5 billion to $103 billion. Ø worldwide growth in biotechnology product sales grew 353%, from $36 billion to $163 billion. In 2012, the 21 largest pharmaceutical companies had 429 biotech products in clinical development, of which 58% were monoclonal antibody products. CSDD, Impact Report, November/December 2013 48

Biotechnology in medicine Milestones in medical biotechnology Achievements, paradigm shifts Current trends and research Conclusions 49

Conclusions • Some diseases can now be cured / controlled effectively with biologics, and become part of the standard of care. • Biologics are important tools of targeted therapy and help to fulfil the principles of personalized medicine. • Intensive research is ongoing for new biotech therapies. • Hungary is strong in the research and production of biotechnological treatments. • Further innovative products are expected in the future especially in the field of the regenerative medicine. 50

Thank you for your attention! 51

Questions & Answers 52
861edf073fcb495c8e9889a5616fea99.ppt