d334457ada8773cce3793f842696dedf.ppt
- Количество слайдов: 112
 Med. DRA® Overview and Update Eisai Inc. Judy Harrison, M. D. Med. DRA® is a registered trademark of the International Federation of Pharmaceutical Manufacturers and Associations (IFPMA)
Med. DRA® Overview and Update Eisai Inc. Judy Harrison, M. D. Med. DRA® is a registered trademark of the International Federation of Pharmaceutical Manufacturers and Associations (IFPMA)
 Course Objectives/Overview To provide an introduction to: • Med. DRA’s structure, scope, and characteristics • Coding and the “Med. DRA Term Selection: Points to Consider” document • Data quality issues • Med. DRA’s application in data retrieval and analysis: the “Med. DRA Data Retrieval and Presentation: Points to Consider” document • Standardised Med. DRA Queries (SMQs) • Med. DRA maintenance and versioning MSSO-DI-6277 -11. 0. 0 © 2008 Northrop Grumman Corporation. All Rights Reserved. 2
Course Objectives/Overview To provide an introduction to: • Med. DRA’s structure, scope, and characteristics • Coding and the “Med. DRA Term Selection: Points to Consider” document • Data quality issues • Med. DRA’s application in data retrieval and analysis: the “Med. DRA Data Retrieval and Presentation: Points to Consider” document • Standardised Med. DRA Queries (SMQs) • Med. DRA maintenance and versioning MSSO-DI-6277 -11. 0. 0 © 2008 Northrop Grumman Corporation. All Rights Reserved. 2
 Med. DRA Background
Med. DRA Background
 What is Med. DRA? Med = Medical D = Dictionary for R = Regulatory A = Activities MSSO-DI-6277 -11. 0. 0 © 2008 Northrop Grumman Corporation. All Rights Reserved. 4
What is Med. DRA? Med = Medical D = Dictionary for R = Regulatory A = Activities MSSO-DI-6277 -11. 0. 0 © 2008 Northrop Grumman Corporation. All Rights Reserved. 4
 Conditions Before Med. DRA • Use of different types of terminologies • Use of terminologies for different phases of regulatory cycle • Lack of specificity of terms • Limited data retrieval options • Resulting use of non-standard terminologies developed “in-house” • Resulting high cost and time investment MSSO-DI-6277 -11. 0. 0 © 2008 Northrop Grumman Corporation. All Rights Reserved. 5
Conditions Before Med. DRA • Use of different types of terminologies • Use of terminologies for different phases of regulatory cycle • Lack of specificity of terms • Limited data retrieval options • Resulting use of non-standard terminologies developed “in-house” • Resulting high cost and time investment MSSO-DI-6277 -11. 0. 0 © 2008 Northrop Grumman Corporation. All Rights Reserved. 5
 Objectives for Med. DRA Development To provide: • An international multi-lingual terminology • Standardized communication between industry and regulators • Support of electronic submissions • Application through all phases of the development cycle MSSO-DI-6277 -11. 0. 0 © 2008 Northrop Grumman Corporation. All Rights Reserved. 6
Objectives for Med. DRA Development To provide: • An international multi-lingual terminology • Standardized communication between industry and regulators • Support of electronic submissions • Application through all phases of the development cycle MSSO-DI-6277 -11. 0. 0 © 2008 Northrop Grumman Corporation. All Rights Reserved. 6
 Objectives for Med. DRA Development (cont) To provide (cont): • Classification for a wide range of clinical information • Support for multiple medical product areas • A terminology that saves time, resources, and money MSSO-DI-6277 -11. 0. 0 © 2008 Northrop Grumman Corporation. All Rights Reserved. 7
Objectives for Med. DRA Development (cont) To provide (cont): • Classification for a wide range of clinical information • Support for multiple medical product areas • A terminology that saves time, resources, and money MSSO-DI-6277 -11. 0. 0 © 2008 Northrop Grumman Corporation. All Rights Reserved. 7
 Med. DRA Definition Med. DRA is a clinically-validated international medical terminology used by regulatory authorities and the regulated biopharmaceutical industry. The terminology is used through the entire regulatory process, from pre-marketing to post-marketing, and for data entry, retrieval, evaluation, and presentation. MSSO-DI-6277 -11. 0. 0 © 2008 Northrop Grumman Corporation. All Rights Reserved. 8
Med. DRA Definition Med. DRA is a clinically-validated international medical terminology used by regulatory authorities and the regulated biopharmaceutical industry. The terminology is used through the entire regulatory process, from pre-marketing to post-marketing, and for data entry, retrieval, evaluation, and presentation. MSSO-DI-6277 -11. 0. 0 © 2008 Northrop Grumman Corporation. All Rights Reserved. 8
 Med. DRA’s Structure and Scope
Med. DRA’s Structure and Scope
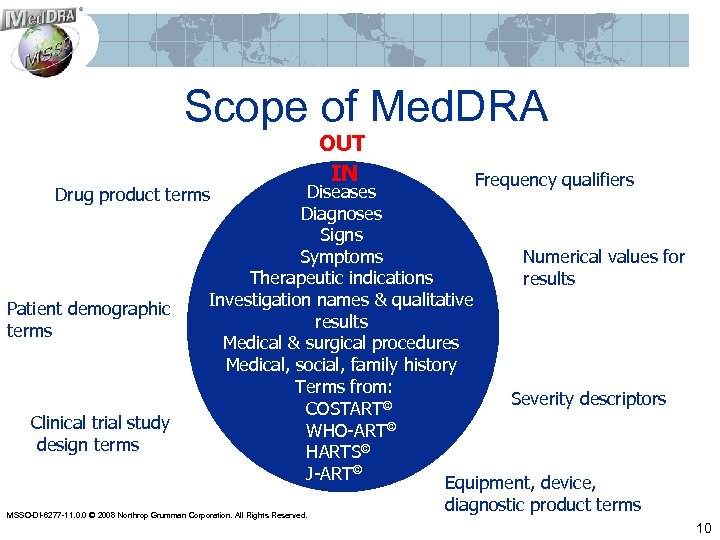 Scope of Med. DRA OUT IN Frequency qualifiers Diseases Drug product terms Diagnoses Signs Symptoms Numerical values for Therapeutic indications results Investigation names & qualitative Patient demographic results terms Medical & surgical procedures Medical, social, family history Terms from: Severity descriptors COSTART© Clinical trial study WHO-ART© design terms HARTS© J-ART© Equipment, device, diagnostic product terms MSSO-DI-6277 -11. 0. 0 © 2008 Northrop Grumman Corporation. All Rights Reserved. 10
Scope of Med. DRA OUT IN Frequency qualifiers Diseases Drug product terms Diagnoses Signs Symptoms Numerical values for Therapeutic indications results Investigation names & qualitative Patient demographic results terms Medical & surgical procedures Medical, social, family history Terms from: Severity descriptors COSTART© Clinical trial study WHO-ART© design terms HARTS© J-ART© Equipment, device, diagnostic product terms MSSO-DI-6277 -11. 0. 0 © 2008 Northrop Grumman Corporation. All Rights Reserved. 10
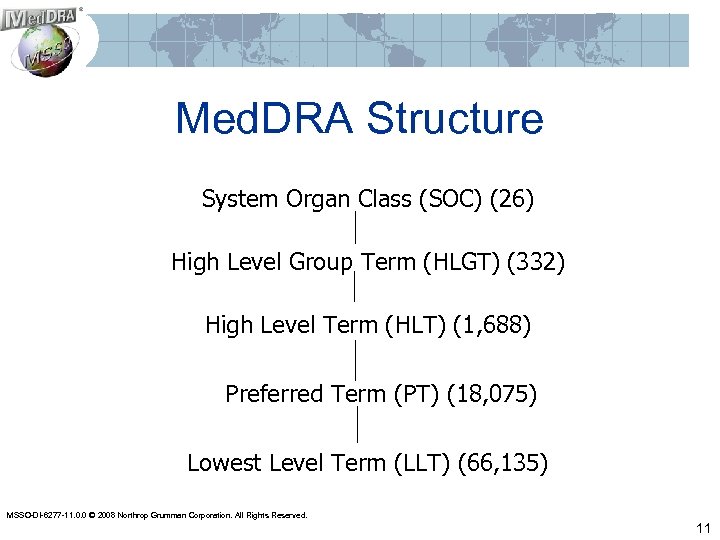 Med. DRA Structure System Organ Class (SOC) (26) High Level Group Term (HLGT) (332) High Level Term (HLT) (1, 688) Preferred Term (PT) (18, 075) Lowest Level Term (LLT) (66, 135) MSSO-DI-6277 -11. 0. 0 © 2008 Northrop Grumman Corporation. All Rights Reserved. 11
Med. DRA Structure System Organ Class (SOC) (26) High Level Group Term (HLGT) (332) High Level Term (HLT) (1, 688) Preferred Term (PT) (18, 075) Lowest Level Term (LLT) (66, 135) MSSO-DI-6277 -11. 0. 0 © 2008 Northrop Grumman Corporation. All Rights Reserved. 11
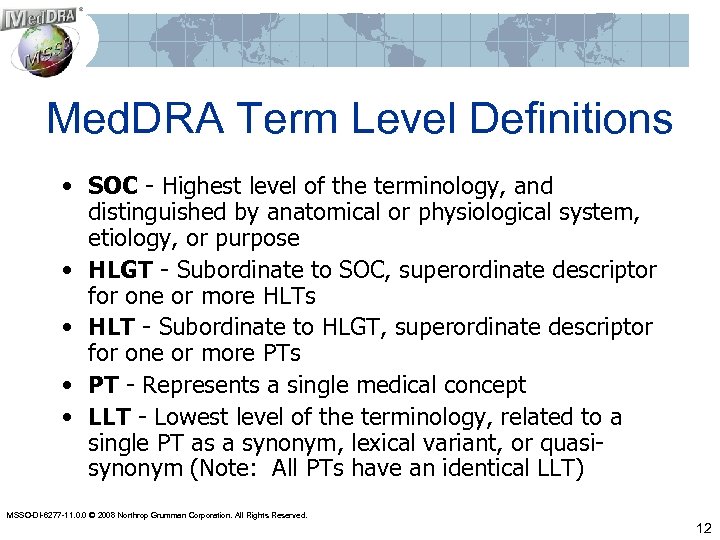 Med. DRA Term Level Definitions • SOC - Highest level of the terminology, and distinguished by anatomical or physiological system, etiology, or purpose • HLGT - Subordinate to SOC, superordinate descriptor for one or more HLTs • HLT - Subordinate to HLGT, superordinate descriptor for one or more PTs • PT - Represents a single medical concept • LLT - Lowest level of the terminology, related to a single PT as a synonym, lexical variant, or quasisynonym (Note: All PTs have an identical LLT) MSSO-DI-6277 -11. 0. 0 © 2008 Northrop Grumman Corporation. All Rights Reserved. 12
Med. DRA Term Level Definitions • SOC - Highest level of the terminology, and distinguished by anatomical or physiological system, etiology, or purpose • HLGT - Subordinate to SOC, superordinate descriptor for one or more HLTs • HLT - Subordinate to HLGT, superordinate descriptor for one or more PTs • PT - Represents a single medical concept • LLT - Lowest level of the terminology, related to a single PT as a synonym, lexical variant, or quasisynonym (Note: All PTs have an identical LLT) MSSO-DI-6277 -11. 0. 0 © 2008 Northrop Grumman Corporation. All Rights Reserved. 12
 System Organ Classes • • • • Blood and lymphatic system disorders Cardiac disorders Congenital, familial and genetic disorders Ear and labyrinth disorders Endocrine disorders Eye disorders Gastrointestinal disorders General disorders and administration site conditions Hepatobiliary disorders Immune system disorders Infections and infestations Injury, poisoning and procedural complications Investigations Metabolism and nutrition disorders • • • Musculoskeletal and connective tissue disorders Neoplasms benign, malignant and unspecified (incl cysts and polyps) Nervous system disorders Pregnancy, puerperium and perinatal conditions Psychiatric disorders Renal and urinary disorders Reproductive system and breast disorders Respiratory, thoracic and mediastinal disorders Skin and subcutaneous tissue disorders Social circumstances Surgical and medical procedures Vascular disorders MSSO-DI-6277 -11. 0. 0 © 2008 Northrop Grumman Corporation. All Rights Reserved. 13
System Organ Classes • • • • Blood and lymphatic system disorders Cardiac disorders Congenital, familial and genetic disorders Ear and labyrinth disorders Endocrine disorders Eye disorders Gastrointestinal disorders General disorders and administration site conditions Hepatobiliary disorders Immune system disorders Infections and infestations Injury, poisoning and procedural complications Investigations Metabolism and nutrition disorders • • • Musculoskeletal and connective tissue disorders Neoplasms benign, malignant and unspecified (incl cysts and polyps) Nervous system disorders Pregnancy, puerperium and perinatal conditions Psychiatric disorders Renal and urinary disorders Reproductive system and breast disorders Respiratory, thoracic and mediastinal disorders Skin and subcutaneous tissue disorders Social circumstances Surgical and medical procedures Vascular disorders MSSO-DI-6277 -11. 0. 0 © 2008 Northrop Grumman Corporation. All Rights Reserved. 13
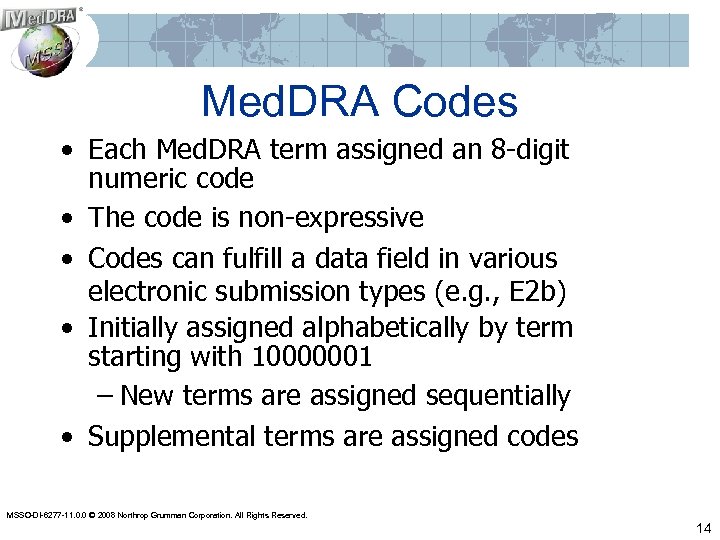 Med. DRA Codes • Each Med. DRA term assigned an 8 -digit numeric code • The code is non-expressive • Codes can fulfill a data field in various electronic submission types (e. g. , E 2 b) • Initially assigned alphabetically by term starting with 10000001 – New terms are assigned sequentially • Supplemental terms are assigned codes MSSO-DI-6277 -11. 0. 0 © 2008 Northrop Grumman Corporation. All Rights Reserved. 14
Med. DRA Codes • Each Med. DRA term assigned an 8 -digit numeric code • The code is non-expressive • Codes can fulfill a data field in various electronic submission types (e. g. , E 2 b) • Initially assigned alphabetically by term starting with 10000001 – New terms are assigned sequentially • Supplemental terms are assigned codes MSSO-DI-6277 -11. 0. 0 © 2008 Northrop Grumman Corporation. All Rights Reserved. 14
 Non-Current Terms • Non-current terms are flagged at the LLT level within Med. DRA • Not recommended for continued use • Retained within the terminology to preserve historical data for retrieval and analysis • Terms very vague, ambiguous, outdated, truncated, or misspelled • Terms derived from other terminologies that do not fit Med. DRA rules MSSO-DI-6277 -11. 0. 0 © 2008 Northrop Grumman Corporation. All Rights Reserved. 15
Non-Current Terms • Non-current terms are flagged at the LLT level within Med. DRA • Not recommended for continued use • Retained within the terminology to preserve historical data for retrieval and analysis • Terms very vague, ambiguous, outdated, truncated, or misspelled • Terms derived from other terminologies that do not fit Med. DRA rules MSSO-DI-6277 -11. 0. 0 © 2008 Northrop Grumman Corporation. All Rights Reserved. 15
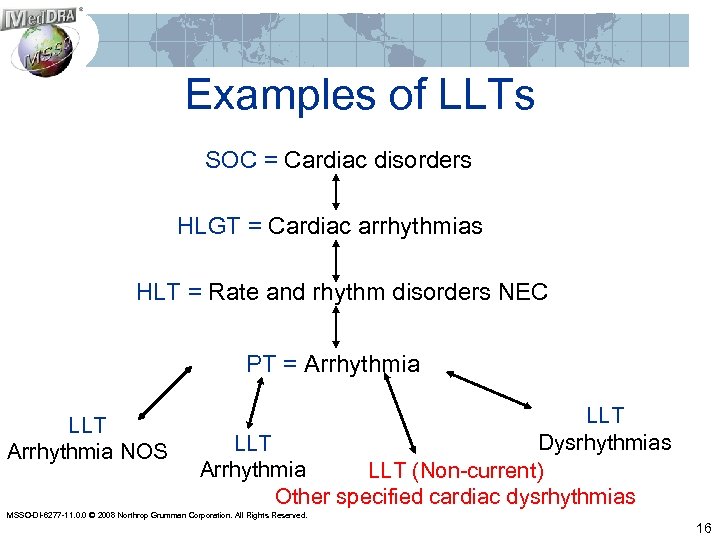 Examples of LLTs SOC = Cardiac disorders HLGT = Cardiac arrhythmias HLT = Rate and rhythm disorders NEC PT = Arrhythmia LLT Arrhythmia NOS LLT Dysrhythmias LLT Arrhythmia LLT (Non-current) Other specified cardiac dysrhythmias MSSO-DI-6277 -11. 0. 0 © 2008 Northrop Grumman Corporation. All Rights Reserved. 16
Examples of LLTs SOC = Cardiac disorders HLGT = Cardiac arrhythmias HLT = Rate and rhythm disorders NEC PT = Arrhythmia LLT Arrhythmia NOS LLT Dysrhythmias LLT Arrhythmia LLT (Non-current) Other specified cardiac dysrhythmias MSSO-DI-6277 -11. 0. 0 © 2008 Northrop Grumman Corporation. All Rights Reserved. 16
 A Multi-Axial Terminology • Multi-axial = the representation of a medical concept in multiple SOCs – Allows grouping by different classifications – Allows retrieval and presentation via different data sets • Purpose of Primary SOC – Determines which SOC will represent a PT during cumulative data outputs – Is used to support consistent data presentation for reporting to regulators MSSO-DI-6277 -11. 0. 0 © 2008 Northrop Grumman Corporation. All Rights Reserved. 17
A Multi-Axial Terminology • Multi-axial = the representation of a medical concept in multiple SOCs – Allows grouping by different classifications – Allows retrieval and presentation via different data sets • Purpose of Primary SOC – Determines which SOC will represent a PT during cumulative data outputs – Is used to support consistent data presentation for reporting to regulators MSSO-DI-6277 -11. 0. 0 © 2008 Northrop Grumman Corporation. All Rights Reserved. 17
 A Multi-Axial Terminology (cont) • A PT can be associated with one or more SOCs • One of the associations is primary; all others are secondary • A PT will have one and only one path to any particular SOC • Med. DRA can express multi-axiality at the PT, HLT, or HLGT level MSSO-DI-6277 -11. 0. 0 © 2008 Northrop Grumman Corporation. All Rights Reserved. 18
A Multi-Axial Terminology (cont) • A PT can be associated with one or more SOCs • One of the associations is primary; all others are secondary • A PT will have one and only one path to any particular SOC • Med. DRA can express multi-axiality at the PT, HLT, or HLGT level MSSO-DI-6277 -11. 0. 0 © 2008 Northrop Grumman Corporation. All Rights Reserved. 18
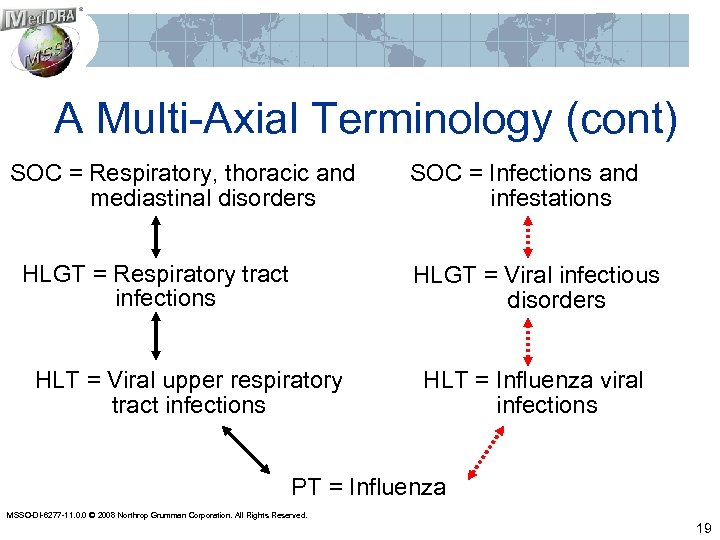 A Multi-Axial Terminology (cont) SOC = Respiratory, thoracic and mediastinal disorders HLGT = Respiratory tract infections SOC = Infections and infestations HLGT = Viral infectious disorders HLT = Viral upper respiratory tract infections HLT = Influenza viral infections PT = Influenza MSSO-DI-6277 -11. 0. 0 © 2008 Northrop Grumman Corporation. All Rights Reserved. 19
A Multi-Axial Terminology (cont) SOC = Respiratory, thoracic and mediastinal disorders HLGT = Respiratory tract infections SOC = Infections and infestations HLGT = Viral infectious disorders HLT = Viral upper respiratory tract infections HLT = Influenza viral infections PT = Influenza MSSO-DI-6277 -11. 0. 0 © 2008 Northrop Grumman Corporation. All Rights Reserved. 19
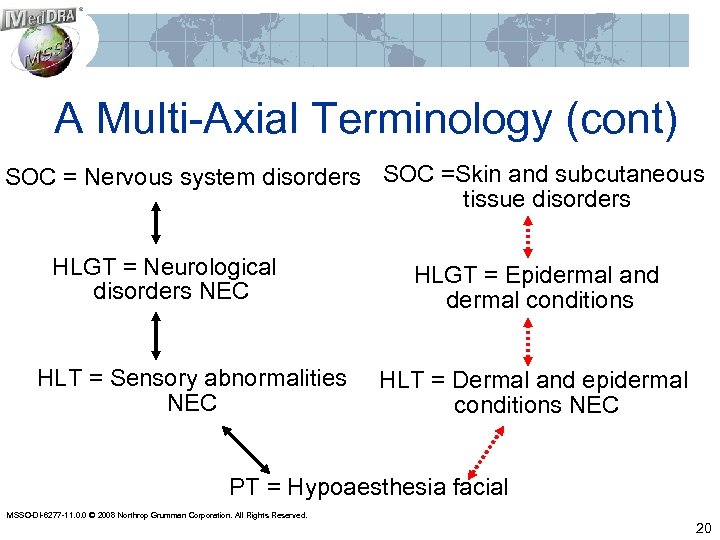 A Multi-Axial Terminology (cont) SOC = Nervous system disorders SOC =Skin and subcutaneous tissue disorders HLGT = Neurological disorders NEC HLT = Sensory abnormalities NEC HLGT = Epidermal and dermal conditions HLT = Dermal and epidermal conditions NEC PT = Hypoaesthesia facial MSSO-DI-6277 -11. 0. 0 © 2008 Northrop Grumman Corporation. All Rights Reserved. 20
A Multi-Axial Terminology (cont) SOC = Nervous system disorders SOC =Skin and subcutaneous tissue disorders HLGT = Neurological disorders NEC HLT = Sensory abnormalities NEC HLGT = Epidermal and dermal conditions HLT = Dermal and epidermal conditions NEC PT = Hypoaesthesia facial MSSO-DI-6277 -11. 0. 0 © 2008 Northrop Grumman Corporation. All Rights Reserved. 20
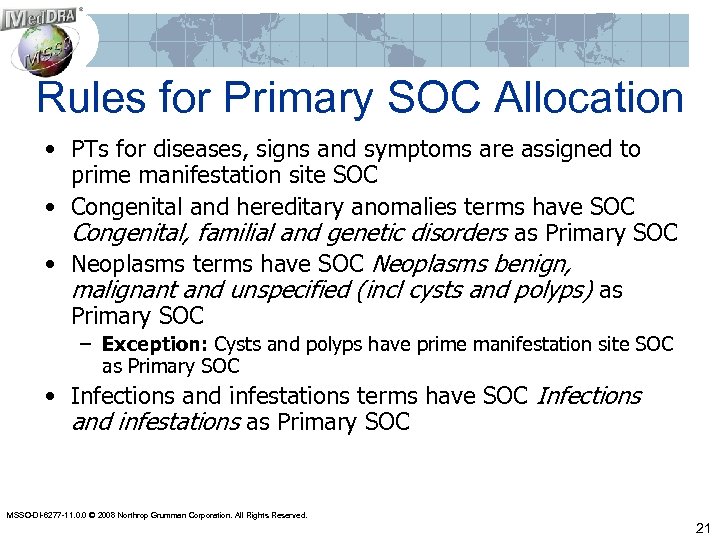 Rules for Primary SOC Allocation • PTs for diseases, signs and symptoms are assigned to prime manifestation site SOC • Congenital and hereditary anomalies terms have SOC Congenital, familial and genetic disorders as Primary SOC • Neoplasms terms have SOC Neoplasms benign, malignant and unspecified (incl cysts and polyps) as Primary SOC – Exception: Cysts and polyps have prime manifestation site SOC as Primary SOC • Infections and infestations terms have SOC Infections and infestations as Primary SOC MSSO-DI-6277 -11. 0. 0 © 2008 Northrop Grumman Corporation. All Rights Reserved. 21
Rules for Primary SOC Allocation • PTs for diseases, signs and symptoms are assigned to prime manifestation site SOC • Congenital and hereditary anomalies terms have SOC Congenital, familial and genetic disorders as Primary SOC • Neoplasms terms have SOC Neoplasms benign, malignant and unspecified (incl cysts and polyps) as Primary SOC – Exception: Cysts and polyps have prime manifestation site SOC as Primary SOC • Infections and infestations terms have SOC Infections and infestations as Primary SOC MSSO-DI-6277 -11. 0. 0 © 2008 Northrop Grumman Corporation. All Rights Reserved. 21
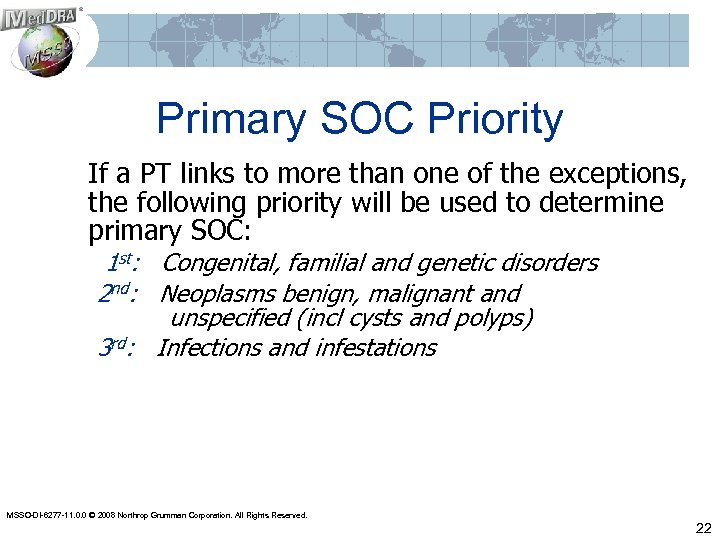 Primary SOC Priority If a PT links to more than one of the exceptions, the following priority will be used to determine primary SOC: 1 st: Congenital, familial and genetic disorders 2 nd: Neoplasms benign, malignant and unspecified (incl cysts and polyps) 3 rd: Infections and infestations MSSO-DI-6277 -11. 0. 0 © 2008 Northrop Grumman Corporation. All Rights Reserved. 22
Primary SOC Priority If a PT links to more than one of the exceptions, the following priority will be used to determine primary SOC: 1 st: Congenital, familial and genetic disorders 2 nd: Neoplasms benign, malignant and unspecified (incl cysts and polyps) 3 rd: Infections and infestations MSSO-DI-6277 -11. 0. 0 © 2008 Northrop Grumman Corporation. All Rights Reserved. 22
 Rules for Primary SOC Allocation (cont) PTs in the following SOCs only appear in that particular SOC and not in others; i. e. , they are not multi-axial • Investigations • Surgical and medical procedures • Social circumstances MSSO-DI-6277 -11. 0. 0 © 2008 Northrop Grumman Corporation. All Rights Reserved. 23
Rules for Primary SOC Allocation (cont) PTs in the following SOCs only appear in that particular SOC and not in others; i. e. , they are not multi-axial • Investigations • Surgical and medical procedures • Social circumstances MSSO-DI-6277 -11. 0. 0 © 2008 Northrop Grumman Corporation. All Rights Reserved. 23
 Regulatory Considerations
Regulatory Considerations
 Regulatory Status of Mandate • US FDA – Med. DRA used in FDA’s internal adverse event database (AERS) since 1997 – Proposed Rule for Safety Reporting Requirements (2003): FDA proposes to use Med. DRA for postmarketing safety reports • Japanese Ministry of Health, Labour and Welfare – Electronic reports mandatory from October 2003 – Med. DRA to be used in Periodic Infection and Safety Reports from April 2004 – For medical devices with biological components, infections to be described with Med. DRA terms MSSO-DI-6277 -11. 0. 0 © 2008 Northrop Grumman Corporation. All Rights Reserved. 25
Regulatory Status of Mandate • US FDA – Med. DRA used in FDA’s internal adverse event database (AERS) since 1997 – Proposed Rule for Safety Reporting Requirements (2003): FDA proposes to use Med. DRA for postmarketing safety reports • Japanese Ministry of Health, Labour and Welfare – Electronic reports mandatory from October 2003 – Med. DRA to be used in Periodic Infection and Safety Reports from April 2004 – For medical devices with biological components, infections to be described with Med. DRA terms MSSO-DI-6277 -11. 0. 0 © 2008 Northrop Grumman Corporation. All Rights Reserved. 25
 Regulatory Status of Mandate (cont) • European Union – Clinical trials • SUSARs (Suspected Unexpected Serious Adverse Reactions) reported to Eudra. Vigilance database require Med. DRA (LLT codes, current, or previous version) – Volume 9 A (Applies to all authorized medicinal products, both prescription and OTC) • Individual Case Safety Reports (ICSRs) reported to Eudra. Vigilance database require Med. DRA (LLT codes, current, or previous version) • Med. DRA required for adverse reaction terms in Periodic Safety Update Report • Standardised Med. DRA Queries (SMQs) recommended for signal detection MSSO-DI-6277 -11. 0. 0 © 2008 Northrop Grumman Corporation. All Rights Reserved. 26
Regulatory Status of Mandate (cont) • European Union – Clinical trials • SUSARs (Suspected Unexpected Serious Adverse Reactions) reported to Eudra. Vigilance database require Med. DRA (LLT codes, current, or previous version) – Volume 9 A (Applies to all authorized medicinal products, both prescription and OTC) • Individual Case Safety Reports (ICSRs) reported to Eudra. Vigilance database require Med. DRA (LLT codes, current, or previous version) • Med. DRA required for adverse reaction terms in Periodic Safety Update Report • Standardised Med. DRA Queries (SMQs) recommended for signal detection MSSO-DI-6277 -11. 0. 0 © 2008 Northrop Grumman Corporation. All Rights Reserved. 26
 Regulatory Status of Mandate (cont) • European Union (cont) – Interface between Eudra. Vigilance and EU Risk Management Plan: Med. DRA to be used to code indications, and risks and interactions (potential and identified) – Summary of Product Characteristics Guideline (Draft Revision, December 2007): Med. DRA to be used, particularly in Contraindications, Special Warnings and Precautions for Use, and Undesirable Effects sections MSSO-DI-6277 -11. 0. 0 © 2008 Northrop Grumman Corporation. All Rights Reserved. 27
Regulatory Status of Mandate (cont) • European Union (cont) – Interface between Eudra. Vigilance and EU Risk Management Plan: Med. DRA to be used to code indications, and risks and interactions (potential and identified) – Summary of Product Characteristics Guideline (Draft Revision, December 2007): Med. DRA to be used, particularly in Contraindications, Special Warnings and Precautions for Use, and Undesirable Effects sections MSSO-DI-6277 -11. 0. 0 © 2008 Northrop Grumman Corporation. All Rights Reserved. 27
 Regulatory Status of Mandate (cont) • ICH M 4 E Guideline on Common Technical Document – Med. DRA recommended in adverse event summary tables • Canada – Draft Guidance Document for Industry, Reporting Adverse Reactions to Marketed Health Products: Med. DRA recommended as standard for adverse reaction reports – Product Monograph (product labeling): Med. DRA is the preferred terminology to describe adverse drug reactions MSSO-DI-6277 -11. 0. 0 © 2008 Northrop Grumman Corporation. All Rights Reserved. 28
Regulatory Status of Mandate (cont) • ICH M 4 E Guideline on Common Technical Document – Med. DRA recommended in adverse event summary tables • Canada – Draft Guidance Document for Industry, Reporting Adverse Reactions to Marketed Health Products: Med. DRA recommended as standard for adverse reaction reports – Product Monograph (product labeling): Med. DRA is the preferred terminology to describe adverse drug reactions MSSO-DI-6277 -11. 0. 0 © 2008 Northrop Grumman Corporation. All Rights Reserved. 28
 CTCAE and Med. DRA • National Cancer Institute’s Common Terminology Criteria for Adverse Events (CTCAE) is a descriptive terminology used for AE reporting in oncology and HIV clinical trials • Need to convert CTCAE terms to Med. DRA terms in clinical trial databases for purposes of analysis and reporting • Blue Ribbon Panel Meeting (BRP) on CTCAE to Med. DRA Mapping held on 6 April 2006 (http: //www. meddramsso. com/MSSOWeb/activities/ archive_brp. htm) MSSO-DI-6277 -11. 0. 0 © 2008 Northrop Grumman Corporation. All Rights Reserved. 29
CTCAE and Med. DRA • National Cancer Institute’s Common Terminology Criteria for Adverse Events (CTCAE) is a descriptive terminology used for AE reporting in oncology and HIV clinical trials • Need to convert CTCAE terms to Med. DRA terms in clinical trial databases for purposes of analysis and reporting • Blue Ribbon Panel Meeting (BRP) on CTCAE to Med. DRA Mapping held on 6 April 2006 (http: //www. meddramsso. com/MSSOWeb/activities/ archive_brp. htm) MSSO-DI-6277 -11. 0. 0 © 2008 Northrop Grumman Corporation. All Rights Reserved. 29
 CTCAE and Med. DRA (cont) BRP Recommendations • A standardized mapping of grades is important for consistency – A guidance document for consistent use is also needed • The stakeholders involved (industry, regulators, cooperative groups, CTEP, MSSO and others) should begin a dialogue to address the optimal use of both terminologies – A collaborative working group should be formed – Optimal data collection practices and conventions could also be addressed – If needed, both terminologies could be modified to achieve harmonization and create an optimal mapping – Provide guidance for dealing with legacy data MSSO-DI-6277 -11. 0. 0 © 2008 Northrop Grumman Corporation. All Rights Reserved. 30
CTCAE and Med. DRA (cont) BRP Recommendations • A standardized mapping of grades is important for consistency – A guidance document for consistent use is also needed • The stakeholders involved (industry, regulators, cooperative groups, CTEP, MSSO and others) should begin a dialogue to address the optimal use of both terminologies – A collaborative working group should be formed – Optimal data collection practices and conventions could also be addressed – If needed, both terminologies could be modified to achieve harmonization and create an optimal mapping – Provide guidance for dealing with legacy data MSSO-DI-6277 -11. 0. 0 © 2008 Northrop Grumman Corporation. All Rights Reserved. 30
 CTCAE and Med. DRA (cont) • BRP recommendation to map CTCAE laboratory terms consistently to Med. DRA investigation terms – E. g. , CTCAE Term “Potassium, serum low (hypokalemia)” to LLT Serum potassium decreased (not LLT Hypokalemia) – E. g. , CTCAE Term “Neutrophils/granulocytes (ANC/AGC) to LLT Neutrophil count decreased • CTCAE v 3. 0 to Med. DRA Version 11. 0 mapping is on MSSO Web site (addresses laboratory term issue but not grade mapping issue) MSSO-DI-6277 -11. 0. 0 © 2008 Northrop Grumman Corporation. All Rights Reserved. 31
CTCAE and Med. DRA (cont) • BRP recommendation to map CTCAE laboratory terms consistently to Med. DRA investigation terms – E. g. , CTCAE Term “Potassium, serum low (hypokalemia)” to LLT Serum potassium decreased (not LLT Hypokalemia) – E. g. , CTCAE Term “Neutrophils/granulocytes (ANC/AGC) to LLT Neutrophil count decreased • CTCAE v 3. 0 to Med. DRA Version 11. 0 mapping is on MSSO Web site (addresses laboratory term issue but not grade mapping issue) MSSO-DI-6277 -11. 0. 0 © 2008 Northrop Grumman Corporation. All Rights Reserved. 31
 CTCAE and Med. DRA (cont) • Eisai encourages sites to report the exact medical condition as the AE • Monitors should ensure that stand alone AE term describes event • When CTCAE laboratory term plus grade is reported, site is queried to provide actual diagnosis – E. g. , Reported term “Neutrophils low” with “CTCAE Grade 3” will be queried to change to diagnosis term “Neutropenia” – “Neutropenia” (PT “Neutropenia”, SOC “Blood and lymphatic system disorders”) – “Neutrophils low” “Neutrophil count low” (PT “Neutrophil count decreased”, SOC “Investigations”) MSSO-DI-6277 -11. 0. 0 © 2008 Northrop Grumman Corporation. All Rights Reserved. 32
CTCAE and Med. DRA (cont) • Eisai encourages sites to report the exact medical condition as the AE • Monitors should ensure that stand alone AE term describes event • When CTCAE laboratory term plus grade is reported, site is queried to provide actual diagnosis – E. g. , Reported term “Neutrophils low” with “CTCAE Grade 3” will be queried to change to diagnosis term “Neutropenia” – “Neutropenia” (PT “Neutropenia”, SOC “Blood and lymphatic system disorders”) – “Neutrophils low” “Neutrophil count low” (PT “Neutrophil count decreased”, SOC “Investigations”) MSSO-DI-6277 -11. 0. 0 © 2008 Northrop Grumman Corporation. All Rights Reserved. 32
 Overview of “Med. DRA Term Selection: Points to Consider” Document
Overview of “Med. DRA Term Selection: Points to Consider” Document
 “Med. DRA Term Selection: Points to Consider” • An ICH-endorsed guide for Med. DRA users • Developed to promote medically accurate and consistent use of Med. DRA in exchange of data (ultimately, for “medically meaningful” retrieval and analysis) • In some cases with more than one option for selecting terms, a “preferred option” is identified but this does not limit Med. DRA users to using that option MSSO-DI-6277 -11. 0. 0 © 2008 Northrop Grumman Corporation. All Rights Reserved. 34
“Med. DRA Term Selection: Points to Consider” • An ICH-endorsed guide for Med. DRA users • Developed to promote medically accurate and consistent use of Med. DRA in exchange of data (ultimately, for “medically meaningful” retrieval and analysis) • In some cases with more than one option for selecting terms, a “preferred option” is identified but this does not limit Med. DRA users to using that option MSSO-DI-6277 -11. 0. 0 © 2008 Northrop Grumman Corporation. All Rights Reserved. 34
 “Points to Consider” Term Selection Document (cont) • Developed by a working group of the ICH Steering Committee – Regulators and industry representatives – EU, Japan, USA – Canadian observer, MSSO, JMO • Current version available on Med. DRA MSSO website http: //www. meddramsso. com/MSSOWeb/activities/PTC. htm MSSO-DI-6277 -11. 0. 0 © 2008 Northrop Grumman Corporation. All Rights Reserved. 35
“Points to Consider” Term Selection Document (cont) • Developed by a working group of the ICH Steering Committee – Regulators and industry representatives – EU, Japan, USA – Canadian observer, MSSO, JMO • Current version available on Med. DRA MSSO website http: //www. meddramsso. com/MSSOWeb/activities/PTC. htm MSSO-DI-6277 -11. 0. 0 © 2008 Northrop Grumman Corporation. All Rights Reserved. 35
 “Points to Consider” Term Selection Document (cont) Document addresses: • Quality of source data • Level of term selected • Use of “Current”/”Non-current” Lowest Level Terms (LLTs) • Choice of term • “Do not subtract or add information” • Quality assurance – Human oversight of automated coding results – Qualification of coder/review staff – Errors in Med. DRA should be addressed by submissions of Change Requests to MSSO; no ad hoc structural alterations to Med. DRA MSSO-DI-6277 -11. 0. 0 © 2008 Northrop Grumman Corporation. All Rights Reserved. 36
“Points to Consider” Term Selection Document (cont) Document addresses: • Quality of source data • Level of term selected • Use of “Current”/”Non-current” Lowest Level Terms (LLTs) • Choice of term • “Do not subtract or add information” • Quality assurance – Human oversight of automated coding results – Qualification of coder/review staff – Errors in Med. DRA should be addressed by submissions of Change Requests to MSSO; no ad hoc structural alterations to Med. DRA MSSO-DI-6277 -11. 0. 0 © 2008 Northrop Grumman Corporation. All Rights Reserved. 36
 “Points to Consider” Document Term Selection Points • Specific Term Selection Points: – Diagnoses and provisional diagnoses with signs and symptoms – Death and other patient outcomes – Suicide and self-harm – Conflicting/ambiguous/vague information – Combination terms – Body site vs. Event specificity – Location vs. Infectious agent – Pre-existing medical conditions – Congenital terms – Neoplasms – Medical/surgical procedures MSSO-DI-6277 -11. 0. 0 © 2008 Northrop Grumman Corporation. All Rights Reserved. 37
“Points to Consider” Document Term Selection Points • Specific Term Selection Points: – Diagnoses and provisional diagnoses with signs and symptoms – Death and other patient outcomes – Suicide and self-harm – Conflicting/ambiguous/vague information – Combination terms – Body site vs. Event specificity – Location vs. Infectious agent – Pre-existing medical conditions – Congenital terms – Neoplasms – Medical/surgical procedures MSSO-DI-6277 -11. 0. 0 © 2008 Northrop Grumman Corporation. All Rights Reserved. 37
 “Points to Consider” Document Term Selection Points (cont) • Specific Term Selection Points (cont): – – – – – Investigations Medication/administration errors and accidental exposures Overdose/Toxicity/Poisonings Drug interactions No adverse effect Unexpected therapeutic effect Modification of effect Use of SOC Social circumstances Medical and/or social history Indication for product use MSSO-DI-6277 -11. 0. 0 © 2008 Northrop Grumman Corporation. All Rights Reserved. 38
“Points to Consider” Document Term Selection Points (cont) • Specific Term Selection Points (cont): – – – – – Investigations Medication/administration errors and accidental exposures Overdose/Toxicity/Poisonings Drug interactions No adverse effect Unexpected therapeutic effect Modification of effect Use of SOC Social circumstances Medical and/or social history Indication for product use MSSO-DI-6277 -11. 0. 0 © 2008 Northrop Grumman Corporation. All Rights Reserved. 38
 Overview of “Med. DRA Data Retrieval and Presentation: Points to Consider” Document
Overview of “Med. DRA Data Retrieval and Presentation: Points to Consider” Document
 Med. DRA Data Retrieval and Presentation: Points to Consider • An ICH-Endorsed Guide for Med. DRA users on Data Output • Developed by an ICH Expert Working Group • Provides data retrieval and presentation options for industry or regulatory purposes • Objective is to promote understanding of implications that various options for data retrieval have on accuracy and consistency of final output • Current version available on Med. DRA MSSO Web site (http: //www. meddramsso. com/MSSOWeb/activities/PTC. htm) MSSO-DI-6277 -11. 0. 0 © 2008 Northrop Grumman Corporation. All Rights Reserved. 40
Med. DRA Data Retrieval and Presentation: Points to Consider • An ICH-Endorsed Guide for Med. DRA users on Data Output • Developed by an ICH Expert Working Group • Provides data retrieval and presentation options for industry or regulatory purposes • Objective is to promote understanding of implications that various options for data retrieval have on accuracy and consistency of final output • Current version available on Med. DRA MSSO Web site (http: //www. meddramsso. com/MSSOWeb/activities/PTC. htm) MSSO-DI-6277 -11. 0. 0 © 2008 Northrop Grumman Corporation. All Rights Reserved. 40
 Data Retrieval PTC • • Points Addressed Quality of Source Data Quality Assurance Organization-Specific Data Characteristics Impact of Med. DRA’s Characteristics on Data Retrieval and Presentation – Grouping terms – Granularity – Multi-axiality • Standardised Med. DRA Queries • Med. DRA Versioning • Queries and Retrieval Examples MSSO-DI-6277 -11. 0. 0 © 2008 Northrop Grumman Corporation. All Rights Reserved. 41
Data Retrieval PTC • • Points Addressed Quality of Source Data Quality Assurance Organization-Specific Data Characteristics Impact of Med. DRA’s Characteristics on Data Retrieval and Presentation – Grouping terms – Granularity – Multi-axiality • Standardised Med. DRA Queries • Med. DRA Versioning • Queries and Retrieval Examples MSSO-DI-6277 -11. 0. 0 © 2008 Northrop Grumman Corporation. All Rights Reserved. 41
 Introduction to Standardised Med. DRA Queries (SMQs)
Introduction to Standardised Med. DRA Queries (SMQs)
 Definition of SMQ • Result of cooperative effort between CIOMS and ICH (MSSO) • Groupings of terms from one or more Med. DRA System Organ Classes (SOCs) related to defined medical condition or area of interest • Included terms may relate to signs, symptoms, diagnoses, syndromes, physical findings, laboratory and other physiologic test data, etc. , related to medical condition or area of interest • Intended to aid in case identification MSSO-DI-6277 -11. 0. 0 © 2008 Northrop Grumman Corporation. All Rights Reserved. 43
Definition of SMQ • Result of cooperative effort between CIOMS and ICH (MSSO) • Groupings of terms from one or more Med. DRA System Organ Classes (SOCs) related to defined medical condition or area of interest • Included terms may relate to signs, symptoms, diagnoses, syndromes, physical findings, laboratory and other physiologic test data, etc. , related to medical condition or area of interest • Intended to aid in case identification MSSO-DI-6277 -11. 0. 0 © 2008 Northrop Grumman Corporation. All Rights Reserved. 43
 SMQs in Production - Examples • As of Version 11. 0, a total of 58 in production (Many other SMQs in development) • • • Adverse pregnancy outcome/reproductive toxicity (incl neonatal disorders) Agranulocytosis Anaphylactic reaction Cerebrovascular disorders Convulsions Depression and suicide/self-injury • • Hepatic disorders Ischaemic heart disease Lack of efficacy/effect Peripheral neuropathy Pseudomembranous colitis Rhabdomyolysis/myopathy Severe cutaneous adverse reactions • Systemic lupus erythematosus MSSO-DI-6277 -11. 0. 0 © 2008 Northrop Grumman Corporation. All Rights Reserved. 44
SMQs in Production - Examples • As of Version 11. 0, a total of 58 in production (Many other SMQs in development) • • • Adverse pregnancy outcome/reproductive toxicity (incl neonatal disorders) Agranulocytosis Anaphylactic reaction Cerebrovascular disorders Convulsions Depression and suicide/self-injury • • Hepatic disorders Ischaemic heart disease Lack of efficacy/effect Peripheral neuropathy Pseudomembranous colitis Rhabdomyolysis/myopathy Severe cutaneous adverse reactions • Systemic lupus erythematosus MSSO-DI-6277 -11. 0. 0 © 2008 Northrop Grumman Corporation. All Rights Reserved. 44
 SMQ Resources • Refer to MSSO Web site for information on SMQs http: //www. meddramsso. com/MSSOWeb/SMQ/index. htm MSSO-DI-6277 -11. 0. 0 © 2008 Northrop Grumman Corporation. All Rights Reserved. 45
SMQ Resources • Refer to MSSO Web site for information on SMQs http: //www. meddramsso. com/MSSOWeb/SMQ/index. htm MSSO-DI-6277 -11. 0. 0 © 2008 Northrop Grumman Corporation. All Rights Reserved. 45
 Med. DRA Maintenance
Med. DRA Maintenance
 Med. DRA Maintenance • Med. DRA is a user responsive terminology • Subscribers may submit change requests to the MSSO for consideration – Per core subscriber: 100 simple change requests per month • Changes at the PT level and below in the Med. DRA terminology structure • Routine: Notified of supplemental change within 7 -10 working days – Weekly supplemental changes – Complex changes above PT level are received all year round. Only implemented in March release. MSSO-DI-6277 -11. 0. 0 © 2008 Northrop Grumman Corporation. All Rights Reserved. 47
Med. DRA Maintenance • Med. DRA is a user responsive terminology • Subscribers may submit change requests to the MSSO for consideration – Per core subscriber: 100 simple change requests per month • Changes at the PT level and below in the Med. DRA terminology structure • Routine: Notified of supplemental change within 7 -10 working days – Weekly supplemental changes – Complex changes above PT level are received all year round. Only implemented in March release. MSSO-DI-6277 -11. 0. 0 © 2008 Northrop Grumman Corporation. All Rights Reserved. 47
 Med. DRA Maintenance (cont) • Twice yearly official updates – 1 September X. 1 release (Simple changes only) – 1 March X. 0 release (Complex and simple changes) • Current version 11. 0 • Next version 11. 1 in September 2008 MSSO-DI-6277 -11. 0. 0 © 2008 Northrop Grumman Corporation. All Rights Reserved. 48
Med. DRA Maintenance (cont) • Twice yearly official updates – 1 September X. 1 release (Simple changes only) – 1 March X. 0 release (Complex and simple changes) • Current version 11. 0 • Next version 11. 1 in September 2008 MSSO-DI-6277 -11. 0. 0 © 2008 Northrop Grumman Corporation. All Rights Reserved. 48
 Med. DRA Versioning Practices
Med. DRA Versioning Practices
 Med. DRA Versioning • “Best Practice” documents – Recommendations for Med. DRA Implementation and Versioning for Clinical Trials (http: //www. meddramsso. com/MSSOWeb/Docs/clinicaltrialversioning. pdf) – Single Case Reporting using Semi-annual Version Control (http: //www. meddramsso. com/MSSOWeb/Docs/VCGuidesemiannual. pdf) • Old topic with a new twist – Focusing on two aspects of versioning • Date/time of transition • Extent of update MSSO-DI-6277 -11. 0. 0 © 2008 Northrop Grumman Corporation. All Rights Reserved. 50
Med. DRA Versioning • “Best Practice” documents – Recommendations for Med. DRA Implementation and Versioning for Clinical Trials (http: //www. meddramsso. com/MSSOWeb/Docs/clinicaltrialversioning. pdf) – Single Case Reporting using Semi-annual Version Control (http: //www. meddramsso. com/MSSOWeb/Docs/VCGuidesemiannual. pdf) • Old topic with a new twist – Focusing on two aspects of versioning • Date/time of transition • Extent of update MSSO-DI-6277 -11. 0. 0 © 2008 Northrop Grumman Corporation. All Rights Reserved. 50
 Date/Time of Transition • MSSO Versioning “Best Practice” calls for transition within a 60 day window – Implemented by most organizations • “Best Practice” calls for a specific date and time for the transition – “The newly released version of Med. DRA should become the reporting version on the first Monday of the second month after it is released. To synchronize this event over the three ICH regions, the MSSO recommends midnight GMT, Sunday to Monday, for the switchover” MSSO-DI-6277 -11. 0. 0 © 2008 Northrop Grumman Corporation. All Rights Reserved. 51
Date/Time of Transition • MSSO Versioning “Best Practice” calls for transition within a 60 day window – Implemented by most organizations • “Best Practice” calls for a specific date and time for the transition – “The newly released version of Med. DRA should become the reporting version on the first Monday of the second month after it is released. To synchronize this event over the three ICH regions, the MSSO recommends midnight GMT, Sunday to Monday, for the switchover” MSSO-DI-6277 -11. 0. 0 © 2008 Northrop Grumman Corporation. All Rights Reserved. 51
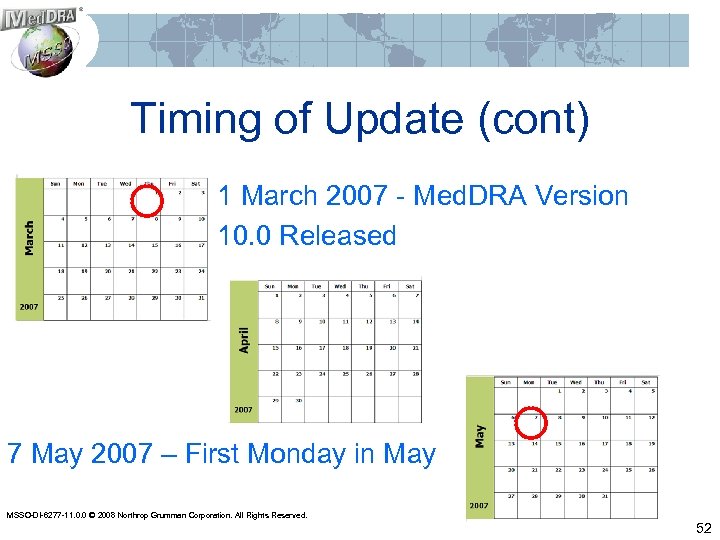 Timing of Update (cont) 1 March 2007 - Med. DRA Version 10. 0 Released 7 May 2007 – First Monday in May MSSO-DI-6277 -11. 0. 0 © 2008 Northrop Grumman Corporation. All Rights Reserved. 52
Timing of Update (cont) 1 March 2007 - Med. DRA Version 10. 0 Released 7 May 2007 – First Monday in May MSSO-DI-6277 -11. 0. 0 © 2008 Northrop Grumman Corporation. All Rights Reserved. 52
 Timing of Update (cont) • Recently, the Danish Medicines Agency implemented the recommendation – “Electronic reports submitted prior to 7 May 2007 using version 10. 0 will be rejected by the Danish Medicines Agency. ” – EMEA working to coordinate within EEA • EMEA and MHLW will accept the current version plus the previous version • FDA has no version requirement MSSO-DI-6277 -11. 0. 0 © 2008 Northrop Grumman Corporation. All Rights Reserved. 53
Timing of Update (cont) • Recently, the Danish Medicines Agency implemented the recommendation – “Electronic reports submitted prior to 7 May 2007 using version 10. 0 will be rejected by the Danish Medicines Agency. ” – EMEA working to coordinate within EEA • EMEA and MHLW will accept the current version plus the previous version • FDA has no version requirement MSSO-DI-6277 -11. 0. 0 © 2008 Northrop Grumman Corporation. All Rights Reserved. 53
 Issues with Timing • Currently, organizations are implementing on different dates 1. On the release date 2. During the implementation period 3. At the end of the implementation period May 7 March 1 2 1 3 60 Day Implementation Period MSSO-DI-6277 -11. 0. 0 © 2008 Northrop Grumman Corporation. All Rights Reserved. 54
Issues with Timing • Currently, organizations are implementing on different dates 1. On the release date 2. During the implementation period 3. At the end of the implementation period May 7 March 1 2 1 3 60 Day Implementation Period MSSO-DI-6277 -11. 0. 0 © 2008 Northrop Grumman Corporation. All Rights Reserved. 54
 Issues with Timing (cont) • The differences in the implementation date negatively impact the communication of ICSRs • MSSO is willing to work with regulators on implementation timing for all Med. DRA users MSSO-DI-6277 -11. 0. 0 © 2008 Northrop Grumman Corporation. All Rights Reserved. 55
Issues with Timing (cont) • The differences in the implementation date negatively impact the communication of ICSRs • MSSO is willing to work with regulators on implementation timing for all Med. DRA users MSSO-DI-6277 -11. 0. 0 © 2008 Northrop Grumman Corporation. All Rights Reserved. 55
 Extent of Update • What does it mean to implement the latest version of Med. DRA? – Since the coding terms in Med. DRA (LLT) are not deleted, an organization could simply apply the latest version with no data changes – What does your organization do? • Re-code data coded to non-current LLTs? • Re-code data with direct matches to new terms? • Re-code data to medically “better” terms available in the new release MSSO-DI-6277 -11. 0. 0 © 2008 Northrop Grumman Corporation. All Rights Reserved. 56
Extent of Update • What does it mean to implement the latest version of Med. DRA? – Since the coding terms in Med. DRA (LLT) are not deleted, an organization could simply apply the latest version with no data changes – What does your organization do? • Re-code data coded to non-current LLTs? • Re-code data with direct matches to new terms? • Re-code data to medically “better” terms available in the new release MSSO-DI-6277 -11. 0. 0 © 2008 Northrop Grumman Corporation. All Rights Reserved. 56
 Extent of Update (cont) • Many approaches to versioning by industry and regulators • No real regulatory mandate • MSSO is considering further developing the versioning “Best Practice” document – Define what it means to update the version – Enhance communication MSSO-DI-6277 -11. 0. 0 © 2008 Northrop Grumman Corporation. All Rights Reserved. 57
Extent of Update (cont) • Many approaches to versioning by industry and regulators • No real regulatory mandate • MSSO is considering further developing the versioning “Best Practice” document – Define what it means to update the version – Enhance communication MSSO-DI-6277 -11. 0. 0 © 2008 Northrop Grumman Corporation. All Rights Reserved. 57
 Versioning Practices at Eisai • Safety Department – Use current version of Med. DRA, driven by EMEA reporting requirements – Med. DRA updated to new version within 60 days of release MSSO-DI-6277 -11. 0. 0 © 2008 Northrop Grumman Corporation. All Rights Reserved. 58
Versioning Practices at Eisai • Safety Department – Use current version of Med. DRA, driven by EMEA reporting requirements – Med. DRA updated to new version within 60 days of release MSSO-DI-6277 -11. 0. 0 © 2008 Northrop Grumman Corporation. All Rights Reserved. 58
 Versioning Practices at Eisai (cont) • Eisai Global Clinical (EGC) – Use current version of Med. DRA, driven by EMEA reporting requirements – Med. DRA updated to new version within 60 days of release – Coding done with current version as data received; re-coded to current version at end of trial – Data in Integrated Safety Summary re-coded from AE verbatim terms to current version • Practices are consistent with the MSSO’s “Recommendations for Med. DRA Implementation and Versioning for Clinical Trials” (http: //www. meddramsso. com/MSSOWeb/Docs/clinicaltrialversioning. pdf) MSSO-DI-6277 -11. 0. 0 © 2008 Northrop Grumman Corporation. All Rights Reserved. 59
Versioning Practices at Eisai (cont) • Eisai Global Clinical (EGC) – Use current version of Med. DRA, driven by EMEA reporting requirements – Med. DRA updated to new version within 60 days of release – Coding done with current version as data received; re-coded to current version at end of trial – Data in Integrated Safety Summary re-coded from AE verbatim terms to current version • Practices are consistent with the MSSO’s “Recommendations for Med. DRA Implementation and Versioning for Clinical Trials” (http: //www. meddramsso. com/MSSOWeb/Docs/clinicaltrialversioning. pdf) MSSO-DI-6277 -11. 0. 0 © 2008 Northrop Grumman Corporation. All Rights Reserved. 59
 Browser Demonstration
Browser Demonstration
 Coding with Med. DRA
Coding with Med. DRA
 What Is “Coding”? Code 1 : a systematic statement of a body of law; especially one given statutory force 2 : a system of principles or rules
What Is “Coding”? Code 1 : a systematic statement of a body of law; especially one given statutory force 2 : a system of principles or rules
 Why Do We Code? • Retrieve • Present • Analyze • Communicate MSSO-DI-6277 -11. 0. 0 © 2008 Northrop Grumman Corporation. All Rights Reserved. 63
Why Do We Code? • Retrieve • Present • Analyze • Communicate MSSO-DI-6277 -11. 0. 0 © 2008 Northrop Grumman Corporation. All Rights Reserved. 63
 What Does Med. DRA Offer? • Size and specificity (“granularity”) • Hierarchy/grouping terms • “Support” SOCs widen data collection/analysis options • Up-to-date and medically rigorous • User-responsive • STANDARDIZATION MSSO-DI-6277 -11. 0. 0 © 2008 Northrop Grumman Corporation. All Rights Reserved. 64
What Does Med. DRA Offer? • Size and specificity (“granularity”) • Hierarchy/grouping terms • “Support” SOCs widen data collection/analysis options • Up-to-date and medically rigorous • User-responsive • STANDARDIZATION MSSO-DI-6277 -11. 0. 0 © 2008 Northrop Grumman Corporation. All Rights Reserved. 64
 Overview of “Med. DRA Term Selection: Points to Consider” Document
Overview of “Med. DRA Term Selection: Points to Consider” Document
 “Points to Consider” Term Selection Document (cont) Document addresses: • Quality of source data • Level of term selected • Use of “Current”/”Non-current” Lowest Level Terms (LLTs) • Choice of term • “Do not subtract or add information” • Quality assurance – Human oversight of automated coding results – Qualification of coder/review staff – Errors in Med. DRA should be addressed by submissions of Change Requests to MSSO; no ad hoc structural alterations to Med. DRA MSSO-DI-6277 -11. 0. 0 © 2008 Northrop Grumman Corporation. All Rights Reserved. 66
“Points to Consider” Term Selection Document (cont) Document addresses: • Quality of source data • Level of term selected • Use of “Current”/”Non-current” Lowest Level Terms (LLTs) • Choice of term • “Do not subtract or add information” • Quality assurance – Human oversight of automated coding results – Qualification of coder/review staff – Errors in Med. DRA should be addressed by submissions of Change Requests to MSSO; no ad hoc structural alterations to Med. DRA MSSO-DI-6277 -11. 0. 0 © 2008 Northrop Grumman Corporation. All Rights Reserved. 66
 “Points to Consider” Document Term Selection Points • Specific Term Selection Points: – Diagnoses and provisional diagnoses with signs and symptoms – Death and other patient outcomes – Suicide and self-harm – Conflicting/ambiguous/vague information – Combination terms – Body site vs. Event specificity – Location vs. Infectious agent – Pre-existing medical conditions – Congenital terms – Neoplasms – Medical/surgical procedures MSSO-DI-6277 -11. 0. 0 © 2008 Northrop Grumman Corporation. All Rights Reserved. 67
“Points to Consider” Document Term Selection Points • Specific Term Selection Points: – Diagnoses and provisional diagnoses with signs and symptoms – Death and other patient outcomes – Suicide and self-harm – Conflicting/ambiguous/vague information – Combination terms – Body site vs. Event specificity – Location vs. Infectious agent – Pre-existing medical conditions – Congenital terms – Neoplasms – Medical/surgical procedures MSSO-DI-6277 -11. 0. 0 © 2008 Northrop Grumman Corporation. All Rights Reserved. 67
 “Points to Consider” Document Term Selection Points (cont) • Specific Term Selection Points (cont): – – – – – Investigations Medication/administration errors and accidental exposures Overdose/Toxicity/Poisonings Drug interactions No adverse effect Unexpected therapeutic effect Modification of effect Use of SOC Social circumstances Medical and/or social history Indication for product use MSSO-DI-6277 -11. 0. 0 © 2008 Northrop Grumman Corporation. All Rights Reserved. 68
“Points to Consider” Document Term Selection Points (cont) • Specific Term Selection Points (cont): – – – – – Investigations Medication/administration errors and accidental exposures Overdose/Toxicity/Poisonings Drug interactions No adverse effect Unexpected therapeutic effect Modification of effect Use of SOC Social circumstances Medical and/or social history Indication for product use MSSO-DI-6277 -11. 0. 0 © 2008 Northrop Grumman Corporation. All Rights Reserved. 68
 “Points to Consider” Term Selection Document (cont) • Obtain clarification of data that are confusing, ambiguous, or unintelligible • Can be optimized by careful design of data collection forms and proper training of relevant staff • REMEMBER: No terminology (including Med. DRA) will help in understanding data that is of poor quality; best to get good information at the beginning MSSO-DI-6277 -11. 0. 0 © 2008 Northrop Grumman Corporation. All Rights Reserved. 69
“Points to Consider” Term Selection Document (cont) • Obtain clarification of data that are confusing, ambiguous, or unintelligible • Can be optimized by careful design of data collection forms and proper training of relevant staff • REMEMBER: No terminology (including Med. DRA) will help in understanding data that is of poor quality; best to get good information at the beginning MSSO-DI-6277 -11. 0. 0 © 2008 Northrop Grumman Corporation. All Rights Reserved. 69
 “Points to Consider” Term Selection Document (cont) • Level of term selection – Lowest level term(s) that “most accurately reflects the reporter’s words ” should be selected • Example: “Mouth sore” select “Sore mouth” (PT “Oral pain”) • Example: “Sores in mouth” select “Sores mouth” (PT “Stomatitis”) MSSO-DI-6277 -11. 0. 0 © 2008 Northrop Grumman Corporation. All Rights Reserved. 70
“Points to Consider” Term Selection Document (cont) • Level of term selection – Lowest level term(s) that “most accurately reflects the reporter’s words ” should be selected • Example: “Mouth sore” select “Sore mouth” (PT “Oral pain”) • Example: “Sores in mouth” select “Sores mouth” (PT “Stomatitis”) MSSO-DI-6277 -11. 0. 0 © 2008 Northrop Grumman Corporation. All Rights Reserved. 70
 “Points to Consider” Term Selection Document (cont) • Level of term selection (cont) – Lowest level term(s) that “most accurately reflects the reporter’s words ” should be selected • Example: “Phlebitis right arm” select “Phlebitis arm” (PT “Phlebitis”, SOC “Vascular disorders”) • Example: “Phlebitis right arm injection site” select “Phlebitis injection site” (PT “Injection site phlebitis”, SOC “General disorders and administration site conditions”) MSSO-DI-6277 -11. 0. 0 © 2008 Northrop Grumman Corporation. All Rights Reserved. 71
“Points to Consider” Term Selection Document (cont) • Level of term selection (cont) – Lowest level term(s) that “most accurately reflects the reporter’s words ” should be selected • Example: “Phlebitis right arm” select “Phlebitis arm” (PT “Phlebitis”, SOC “Vascular disorders”) • Example: “Phlebitis right arm injection site” select “Phlebitis injection site” (PT “Injection site phlebitis”, SOC “General disorders and administration site conditions”) MSSO-DI-6277 -11. 0. 0 © 2008 Northrop Grumman Corporation. All Rights Reserved. 71
 “Points to Consider” Term Selection Document (cont) • Currency – Select current LLTs only – Non-current terms for legacy conversion/ historical purposes MSSO-DI-6277 -11. 0. 0 © 2008 Northrop Grumman Corporation. All Rights Reserved. 72
“Points to Consider” Term Selection Document (cont) • Currency – Select current LLTs only – Non-current terms for legacy conversion/ historical purposes MSSO-DI-6277 -11. 0. 0 © 2008 Northrop Grumman Corporation. All Rights Reserved. 72
 “Points to Consider” Term Selection Document (cont) • Choice of term – Avoid company-specific “work-arounds” for Med. DRA deficiencies. If there is no concept in Med. DRA to represent your term, submit Change Request to MSSO – There may be no exact match for your term in Med. DRA, but use medical judgment to match to an existing Med. DRA term that adequately represents the concept MSSO-DI-6277 -11. 0. 0 © 2008 Northrop Grumman Corporation. All Rights Reserved. 73
“Points to Consider” Term Selection Document (cont) • Choice of term – Avoid company-specific “work-arounds” for Med. DRA deficiencies. If there is no concept in Med. DRA to represent your term, submit Change Request to MSSO – There may be no exact match for your term in Med. DRA, but use medical judgment to match to an existing Med. DRA term that adequately represents the concept MSSO-DI-6277 -11. 0. 0 © 2008 Northrop Grumman Corporation. All Rights Reserved. 73
 “Points to Consider” Term Selection Document “Do not subtract or add information” • Do not make a diagnosis if only signs/symptoms are reported – Example: “Abdominal pain” + “Increased serum amylase” + “Increased serum lipase” inappropriate to select “Pancreatitis” • If have both signs/symptoms and diagnosis, may code both or just diagnosis – Example: “Anaphylactic reaction” also reported with “rash, dyspnea, hypotension and laryngospasm” “Anaphylactic reaction” and “Rash, ” “Dyspnea, ” “Hypotension, ” and “Laryngospasm” can be selected OR, only “Anaphylactic reaction” (preferable) can be selected MSSO-DI-6277 -11. 0. 0 © 2008 Northrop Grumman Corporation. All Rights Reserved. 74
“Points to Consider” Term Selection Document “Do not subtract or add information” • Do not make a diagnosis if only signs/symptoms are reported – Example: “Abdominal pain” + “Increased serum amylase” + “Increased serum lipase” inappropriate to select “Pancreatitis” • If have both signs/symptoms and diagnosis, may code both or just diagnosis – Example: “Anaphylactic reaction” also reported with “rash, dyspnea, hypotension and laryngospasm” “Anaphylactic reaction” and “Rash, ” “Dyspnea, ” “Hypotension, ” and “Laryngospasm” can be selected OR, only “Anaphylactic reaction” (preferable) can be selected MSSO-DI-6277 -11. 0. 0 © 2008 Northrop Grumman Corporation. All Rights Reserved. 74
 “Points to Consider” Document Term Selection Points (cont) • Conflicting information: – e. g. , if “Hyperkalemia with a serum potassium of 1. 6 m. Eq/L” “Serum potassium abnormal” can be selected • Ambiguous information: – e. g. , “GU pain” “Pain” can be selected (“GU” could be “genito-urinary” or “gastric ulcer”) • Vague information: – e. g. , “Patient experienced every listed adverse event” “Unevaluable event” can be selected MSSO-DI-6277 -11. 0. 0 © 2008 Northrop Grumman Corporation. All Rights Reserved. 75
“Points to Consider” Document Term Selection Points (cont) • Conflicting information: – e. g. , if “Hyperkalemia with a serum potassium of 1. 6 m. Eq/L” “Serum potassium abnormal” can be selected • Ambiguous information: – e. g. , “GU pain” “Pain” can be selected (“GU” could be “genito-urinary” or “gastric ulcer”) • Vague information: – e. g. , “Patient experienced every listed adverse event” “Unevaluable event” can be selected MSSO-DI-6277 -11. 0. 0 © 2008 Northrop Grumman Corporation. All Rights Reserved. 75
 “Points to Consider” Document Term Selection Points (cont) • Combination terms – If one term is more specific than the other(s), the most specific term should be selected. E. g. , “Arrhythmia due to atrial fibrillation” “Atrial fibrillation” can be selected – If a Med. DRA term exists that adequately describes the combination, that term should be used. E. g. , “Retinopathy due to diabetes” “Diabetic retinopathy” can be selected MSSO-DI-6277 -11. 0. 0 © 2008 Northrop Grumman Corporation. All Rights Reserved. 76
“Points to Consider” Document Term Selection Points (cont) • Combination terms – If one term is more specific than the other(s), the most specific term should be selected. E. g. , “Arrhythmia due to atrial fibrillation” “Atrial fibrillation” can be selected – If a Med. DRA term exists that adequately describes the combination, that term should be used. E. g. , “Retinopathy due to diabetes” “Diabetic retinopathy” can be selected MSSO-DI-6277 -11. 0. 0 © 2008 Northrop Grumman Corporation. All Rights Reserved. 76
 “Points to Consider” Document Term Selection Points (cont) • Combination terms (cont) – If “splitting” provides more information, it is appropriate to select more than one term • “DIC due to sepsis” “DIC” and “Sepsis” can be selected • “Wrist fracture due to fall” “Wrist fracture” and “Fall” can be selected – In all cases of combination terms, apply medical judgment MSSO-DI-6277 -11. 0. 0 © 2008 Northrop Grumman Corporation. All Rights Reserved. 77
“Points to Consider” Document Term Selection Points (cont) • Combination terms (cont) – If “splitting” provides more information, it is appropriate to select more than one term • “DIC due to sepsis” “DIC” and “Sepsis” can be selected • “Wrist fracture due to fall” “Wrist fracture” and “Fall” can be selected – In all cases of combination terms, apply medical judgment MSSO-DI-6277 -11. 0. 0 © 2008 Northrop Grumman Corporation. All Rights Reserved. 77
 “Points to Consider” Document Term Selection Points (cont) • Investigations – Medical condition vs. laboratory result • “Hypoglycemia” (PT “Hypoglycaemia”, SOC “Metabolism and nutrition disorders”) can be selected • “Decreased glucose” “Glucose decreased” (PT “Blood glucose decreased”, SOC “Investigations”) can be selected MSSO-DI-6277 -11. 0. 0 © 2008 Northrop Grumman Corporation. All Rights Reserved. 78
“Points to Consider” Document Term Selection Points (cont) • Investigations – Medical condition vs. laboratory result • “Hypoglycemia” (PT “Hypoglycaemia”, SOC “Metabolism and nutrition disorders”) can be selected • “Decreased glucose” “Glucose decreased” (PT “Blood glucose decreased”, SOC “Investigations”) can be selected MSSO-DI-6277 -11. 0. 0 © 2008 Northrop Grumman Corporation. All Rights Reserved. 78
 “Points to Consider” Document Term Selection Points (cont) • Investigations – Medical condition vs. laboratory result (cont) • “Fever” (PT “Pyrexia”, SOC “General disorders and administration site conditions”) can be selected • “Temperature increased” “Body temperature increased” (PT “Body temperature increased”, SOC “Investigations”) can be selected MSSO-DI-6277 -11. 0. 0 © 2008 Northrop Grumman Corporation. All Rights Reserved. 79
“Points to Consider” Document Term Selection Points (cont) • Investigations – Medical condition vs. laboratory result (cont) • “Fever” (PT “Pyrexia”, SOC “General disorders and administration site conditions”) can be selected • “Temperature increased” “Body temperature increased” (PT “Body temperature increased”, SOC “Investigations”) can be selected MSSO-DI-6277 -11. 0. 0 © 2008 Northrop Grumman Corporation. All Rights Reserved. 79
 Data Quality
Data Quality
 Importance of Good Quality Data • Quality of initial data has a direct impact on quality of data output: – Recording and coding – Retrieval and analysis MSSO-DI-6277 -11. 0. 0 © 2008 Northrop Grumman Corporation. All Rights Reserved. 81
Importance of Good Quality Data • Quality of initial data has a direct impact on quality of data output: – Recording and coding – Retrieval and analysis MSSO-DI-6277 -11. 0. 0 © 2008 Northrop Grumman Corporation. All Rights Reserved. 81
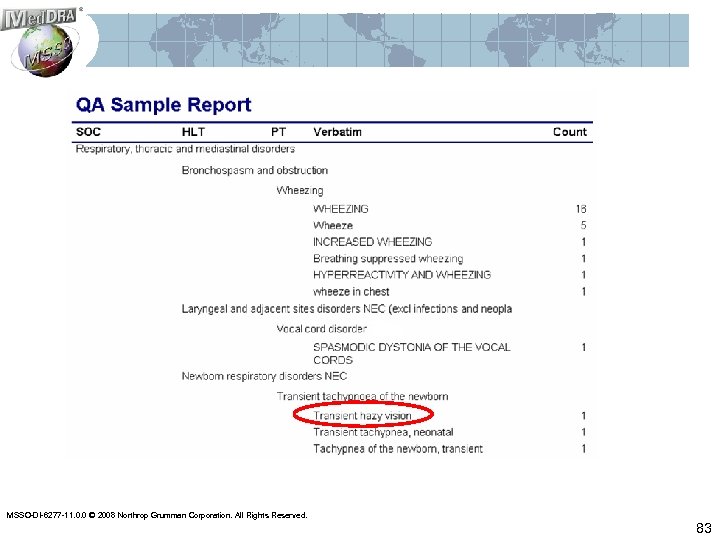
 QA Reports • Allows reviewers to check for consistency (both autoencoded and human-coded terms) • Check for adherence to/deviation from coding conventions • Check for emerging drifts/biases • Multiple data views (verbatim terms to coded terms; coded term to verbatims; by SOC, etc. ) MSSO-DI-6277 -11. 0. 0 © 2008 Northrop Grumman Corporation. All Rights Reserved. 82
QA Reports • Allows reviewers to check for consistency (both autoencoded and human-coded terms) • Check for adherence to/deviation from coding conventions • Check for emerging drifts/biases • Multiple data views (verbatim terms to coded terms; coded term to verbatims; by SOC, etc. ) MSSO-DI-6277 -11. 0. 0 © 2008 Northrop Grumman Corporation. All Rights Reserved. 82
 MSSO-DI-6277 -11. 0. 0 © 2008 Northrop Grumman Corporation. All Rights Reserved. 83
MSSO-DI-6277 -11. 0. 0 © 2008 Northrop Grumman Corporation. All Rights Reserved. 83
 Data Quality, Coding, and Med. DRA Training Module • Target audience: Investigators, study coordinators, company, and CRO personnel • Content includes: – Clinical trial regulations, emphasis on safety – Med. DRA overview – Coding examples - do’s and don’t’s for reporting data – Space for company’s own conventions MSSO-DI-6277 -11. 0. 0 © 2008 Northrop Grumman Corporation. All Rights Reserved. 84
Data Quality, Coding, and Med. DRA Training Module • Target audience: Investigators, study coordinators, company, and CRO personnel • Content includes: – Clinical trial regulations, emphasis on safety – Med. DRA overview – Coding examples - do’s and don’t’s for reporting data – Space for company’s own conventions MSSO-DI-6277 -11. 0. 0 © 2008 Northrop Grumman Corporation. All Rights Reserved. 84
 Data Quality, Coding, and Med. DRA Training Module (cont) • Approx. 1, 000 unique downloads since February 2005 (MSSO Web site -free to subscribers) • Applications: – Customized by company and used at investigators’ meetings and internal web-based training – Data do’s and don’t’s on laminated card as a reminder for coordinators and CRAs MSSO-DI-6277 -11. 0. 0 © 2008 Northrop Grumman Corporation. All Rights Reserved. 85
Data Quality, Coding, and Med. DRA Training Module (cont) • Approx. 1, 000 unique downloads since February 2005 (MSSO Web site -free to subscribers) • Applications: – Customized by company and used at investigators’ meetings and internal web-based training – Data do’s and don’t’s on laminated card as a reminder for coordinators and CRAs MSSO-DI-6277 -11. 0. 0 © 2008 Northrop Grumman Corporation. All Rights Reserved. 85
 What were they thinking? – – – – (Actual verbatim terms) Went to hell Recurrent fatal stroke Hears New Age music when the furnace turns on LK RTCTL UNSP XTRNDL Charcoal-like, gritty granules in his underwear Can’t control patient during menses His nodule is sticking out Normally normal after drinking coffee Died of cancer of the placebo Superior members fornication Barely visible posterior Seeing people in room, seeing chickens at window Seeing stars and chicken farting Patient recently began new job where he works around chicken wings and barbecue sauce MSSO-DI-6277 -11. 0. 0 © 2008 Northrop Grumman Corporation. All Rights Reserved. 86
What were they thinking? – – – – (Actual verbatim terms) Went to hell Recurrent fatal stroke Hears New Age music when the furnace turns on LK RTCTL UNSP XTRNDL Charcoal-like, gritty granules in his underwear Can’t control patient during menses His nodule is sticking out Normally normal after drinking coffee Died of cancer of the placebo Superior members fornication Barely visible posterior Seeing people in room, seeing chickens at window Seeing stars and chicken farting Patient recently began new job where he works around chicken wings and barbecue sauce MSSO-DI-6277 -11. 0. 0 © 2008 Northrop Grumman Corporation. All Rights Reserved. 86
 Overview of “Med. DRA Data Retrieval and Presentation: Points to Consider” Document
Overview of “Med. DRA Data Retrieval and Presentation: Points to Consider” Document
 Data Retrieval PTC • • Points Addressed Quality of Source Data Quality Assurance Organization-Specific Data Characteristics Impact of Med. DRA’s Characteristics on Data Retrieval and Presentation – Grouping terms – Granularity – Multi-axiality • Standardised Med. DRA Queries • Med. DRA Versioning • Queries and Retrieval Examples MSSO-DI-6277 -11. 0. 0 © 2008 Northrop Grumman Corporation. All Rights Reserved. 88
Data Retrieval PTC • • Points Addressed Quality of Source Data Quality Assurance Organization-Specific Data Characteristics Impact of Med. DRA’s Characteristics on Data Retrieval and Presentation – Grouping terms – Granularity – Multi-axiality • Standardised Med. DRA Queries • Med. DRA Versioning • Queries and Retrieval Examples MSSO-DI-6277 -11. 0. 0 © 2008 Northrop Grumman Corporation. All Rights Reserved. 88
 Data Retrieval PTC Quality of Source Data • High quality data output is dependent on maintaining quality of original information reported by using consistent and appropriate term selection (Refer to “Med. DRA Term Selection: Points to Consider” document) • Method of conversion of data into Med. DRA might impact retrieval and presentation legacy data conversion using verbatims or coded terms MSSO-DI-6277 -11. 0. 0 © 2008 Northrop Grumman Corporation. All Rights Reserved. 89
Data Retrieval PTC Quality of Source Data • High quality data output is dependent on maintaining quality of original information reported by using consistent and appropriate term selection (Refer to “Med. DRA Term Selection: Points to Consider” document) • Method of conversion of data into Med. DRA might impact retrieval and presentation legacy data conversion using verbatims or coded terms MSSO-DI-6277 -11. 0. 0 © 2008 Northrop Grumman Corporation. All Rights Reserved. 89
 Data Retrieval PTC Quality Assurance • Documentation of coding conventions, data retrieval and presentation strategies, methods and QA procedures in organization-specific guidelines • Review by individuals with medical background and Med. DRA training • Errors in Med. DRA should be addressed by submissions of Change Requests to MSSO; no ad hoc structural alterations to Med. DRA MSSO-DI-6277 -11. 0. 0 © 2008 Northrop Grumman Corporation. All Rights Reserved. 90
Data Retrieval PTC Quality Assurance • Documentation of coding conventions, data retrieval and presentation strategies, methods and QA procedures in organization-specific guidelines • Review by individuals with medical background and Med. DRA training • Errors in Med. DRA should be addressed by submissions of Change Requests to MSSO; no ad hoc structural alterations to Med. DRA MSSO-DI-6277 -11. 0. 0 © 2008 Northrop Grumman Corporation. All Rights Reserved. 90
 Data Retrieval PTC Impact of Med. DRA’s Characteristics – Grouping Terms • HLGTs and HLTs provide clinically relevant groupings – HLGT Cardiac arrhythmias • HLT Cardiac conduction disorders • HLT Rate and rhythm disorders NEC • HLT Supraventricular arrhythmias • HLT Ventricular arrhythmias and cardiac arrest MSSO-DI-6277 -11. 0. 0 © 2008 Northrop Grumman Corporation. All Rights Reserved. 91
Data Retrieval PTC Impact of Med. DRA’s Characteristics – Grouping Terms • HLGTs and HLTs provide clinically relevant groupings – HLGT Cardiac arrhythmias • HLT Cardiac conduction disorders • HLT Rate and rhythm disorders NEC • HLT Supraventricular arrhythmias • HLT Ventricular arrhythmias and cardiac arrest MSSO-DI-6277 -11. 0. 0 © 2008 Northrop Grumman Corporation. All Rights Reserved. 91
 Data Retrieval PTC Impact of Med. DRA’s Characteristics – Grouping Terms • Caution - ensure all terms are relevant to output – HLT Vascular tests NEC (incl blood pressure) • PT Blood pressure decreased • PT Blood pressure increased • Caution - related PTs in different locations in SOC – HLT Bullous conditions • PT Stevens-Johnson syndrome – HLT Exfoliative conditions • PT Dermatitis exfoliative MSSO-DI-6277 -11. 0. 0 © 2008 Northrop Grumman Corporation. All Rights Reserved. 92
Data Retrieval PTC Impact of Med. DRA’s Characteristics – Grouping Terms • Caution - ensure all terms are relevant to output – HLT Vascular tests NEC (incl blood pressure) • PT Blood pressure decreased • PT Blood pressure increased • Caution - related PTs in different locations in SOC – HLT Bullous conditions • PT Stevens-Johnson syndrome – HLT Exfoliative conditions • PT Dermatitis exfoliative MSSO-DI-6277 -11. 0. 0 © 2008 Northrop Grumman Corporation. All Rights Reserved. 92
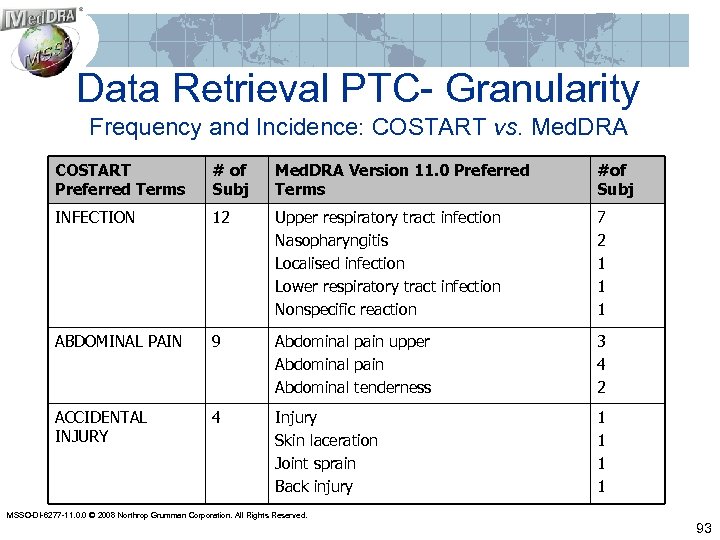 Data Retrieval PTC- Granularity Frequency and Incidence: COSTART vs. Med. DRA COSTART Preferred Terms # of Subj Med. DRA Version 11. 0 Preferred Terms #of Subj INFECTION 12 Upper respiratory tract infection Nasopharyngitis Localised infection Lower respiratory tract infection Nonspecific reaction 7 2 1 1 1 ABDOMINAL PAIN 9 Abdominal pain upper Abdominal pain Abdominal tenderness 3 4 2 ACCIDENTAL INJURY 4 Injury Skin laceration Joint sprain Back injury 1 1 MSSO-DI-6277 -11. 0. 0 © 2008 Northrop Grumman Corporation. All Rights Reserved. 93
Data Retrieval PTC- Granularity Frequency and Incidence: COSTART vs. Med. DRA COSTART Preferred Terms # of Subj Med. DRA Version 11. 0 Preferred Terms #of Subj INFECTION 12 Upper respiratory tract infection Nasopharyngitis Localised infection Lower respiratory tract infection Nonspecific reaction 7 2 1 1 1 ABDOMINAL PAIN 9 Abdominal pain upper Abdominal pain Abdominal tenderness 3 4 2 ACCIDENTAL INJURY 4 Injury Skin laceration Joint sprain Back injury 1 1 MSSO-DI-6277 -11. 0. 0 © 2008 Northrop Grumman Corporation. All Rights Reserved. 93
 Data Retrieval PTC Multi-Axiality • Primary SOC allocation rules affect the way data are distributed across the terminology • Impact on frequencies of medical condition of interest should be considered • Example: for hepatic abnormality search in SOC Hepatobiliary disorders, SOC Investigations (laboratory test terms), SOC Surgical and medical procedures (e. g. PT Liver transplant) MSSO-DI-6277 -11. 0. 0 © 2008 Northrop Grumman Corporation. All Rights Reserved. 94
Data Retrieval PTC Multi-Axiality • Primary SOC allocation rules affect the way data are distributed across the terminology • Impact on frequencies of medical condition of interest should be considered • Example: for hepatic abnormality search in SOC Hepatobiliary disorders, SOC Investigations (laboratory test terms), SOC Surgical and medical procedures (e. g. PT Liver transplant) MSSO-DI-6277 -11. 0. 0 © 2008 Northrop Grumman Corporation. All Rights Reserved. 94
 Data Retrieval PTC Multi-Axiality (cont) • Main presentation is by Primary SOC • Secondary SOCs used for alternate views and presentation of data MSSO-DI-6277 -11. 0. 0 © 2008 Northrop Grumman Corporation. All Rights Reserved. 95
Data Retrieval PTC Multi-Axiality (cont) • Main presentation is by Primary SOC • Secondary SOCs used for alternate views and presentation of data MSSO-DI-6277 -11. 0. 0 © 2008 Northrop Grumman Corporation. All Rights Reserved. 95
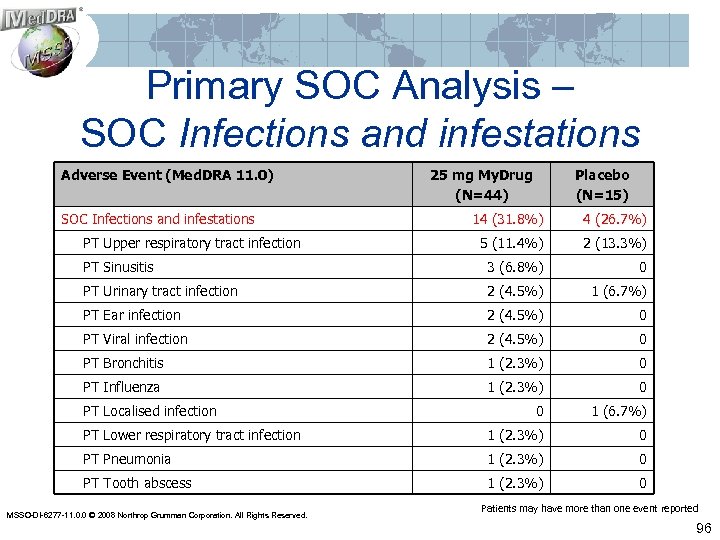 Primary SOC Analysis – SOC Infections and infestations Adverse Event (Med. DRA 11. 0) SOC Infections and infestations 25 mg My. Drug (N=44) Placebo (N=15) 14 (31. 8%) 4 (26. 7%) 5 (11. 4%) 2 (13. 3%) PT Sinusitis 3 (6. 8%) 0 PT Urinary tract infection 2 (4. 5%) 1 (6. 7%) PT Ear infection 2 (4. 5%) 0 PT Viral infection 2 (4. 5%) 0 PT Bronchitis 1 (2. 3%) 0 PT Influenza 1 (2. 3%) 0 0 1 (6. 7%) PT Lower respiratory tract infection 1 (2. 3%) 0 PT Pneumonia 1 (2. 3%) 0 PT Tooth abscess 1 (2. 3%) 0 PT Upper respiratory tract infection PT Localised infection MSSO-DI-6277 -11. 0. 0 © 2008 Northrop Grumman Corporation. All Rights Reserved. Patients may have more than one event reported 96
Primary SOC Analysis – SOC Infections and infestations Adverse Event (Med. DRA 11. 0) SOC Infections and infestations 25 mg My. Drug (N=44) Placebo (N=15) 14 (31. 8%) 4 (26. 7%) 5 (11. 4%) 2 (13. 3%) PT Sinusitis 3 (6. 8%) 0 PT Urinary tract infection 2 (4. 5%) 1 (6. 7%) PT Ear infection 2 (4. 5%) 0 PT Viral infection 2 (4. 5%) 0 PT Bronchitis 1 (2. 3%) 0 PT Influenza 1 (2. 3%) 0 0 1 (6. 7%) PT Lower respiratory tract infection 1 (2. 3%) 0 PT Pneumonia 1 (2. 3%) 0 PT Tooth abscess 1 (2. 3%) 0 PT Upper respiratory tract infection PT Localised infection MSSO-DI-6277 -11. 0. 0 © 2008 Northrop Grumman Corporation. All Rights Reserved. Patients may have more than one event reported 96
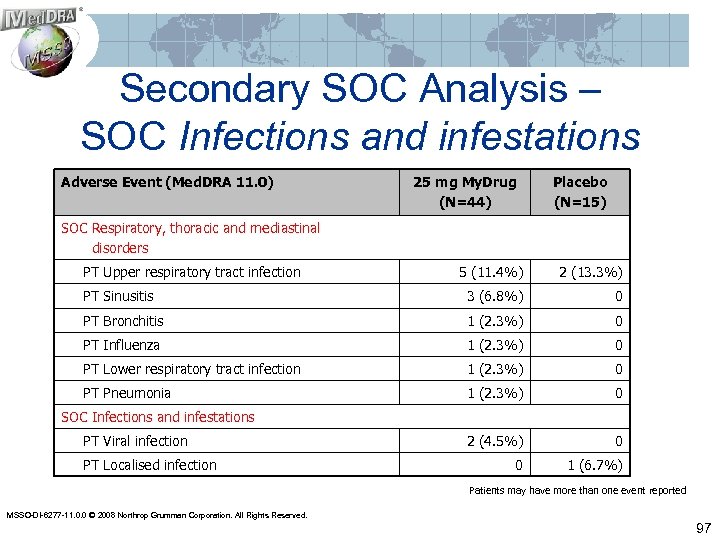 Secondary SOC Analysis – SOC Infections and infestations Adverse Event (Med. DRA 11. 0) 25 mg My. Drug (N=44) Placebo (N=15) SOC Respiratory, thoracic and mediastinal disorders PT Upper respiratory tract infection 5 (11. 4%) 2 (13. 3%) PT Sinusitis 3 (6. 8%) 0 PT Bronchitis 1 (2. 3%) 0 PT Influenza 1 (2. 3%) 0 PT Lower respiratory tract infection 1 (2. 3%) 0 PT Pneumonia 1 (2. 3%) 0 2 (4. 5%) 0 0 1 (6. 7%) SOC Infections and infestations PT Viral infection PT Localised infection Patients may have more than one event reported MSSO-DI-6277 -11. 0. 0 © 2008 Northrop Grumman Corporation. All Rights Reserved. 97
Secondary SOC Analysis – SOC Infections and infestations Adverse Event (Med. DRA 11. 0) 25 mg My. Drug (N=44) Placebo (N=15) SOC Respiratory, thoracic and mediastinal disorders PT Upper respiratory tract infection 5 (11. 4%) 2 (13. 3%) PT Sinusitis 3 (6. 8%) 0 PT Bronchitis 1 (2. 3%) 0 PT Influenza 1 (2. 3%) 0 PT Lower respiratory tract infection 1 (2. 3%) 0 PT Pneumonia 1 (2. 3%) 0 2 (4. 5%) 0 0 1 (6. 7%) SOC Infections and infestations PT Viral infection PT Localised infection Patients may have more than one event reported MSSO-DI-6277 -11. 0. 0 © 2008 Northrop Grumman Corporation. All Rights Reserved. 97
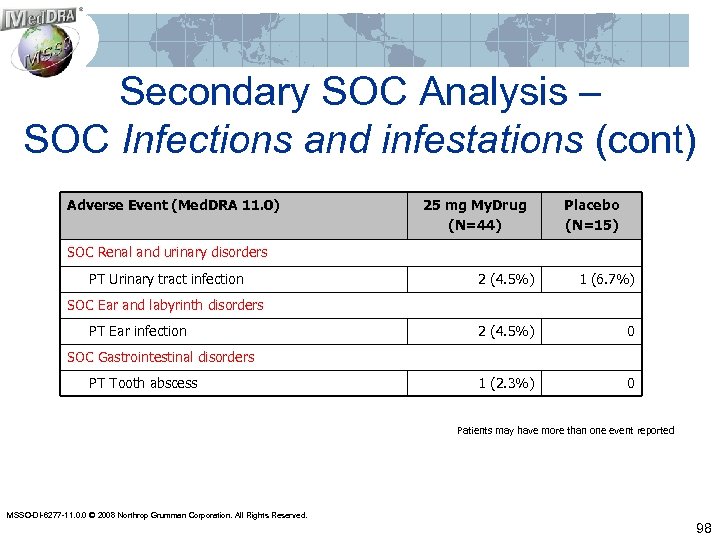 Secondary SOC Analysis – SOC Infections and infestations (cont) Adverse Event (Med. DRA 11. 0) 25 mg My. Drug (N=44) Placebo (N=15) SOC Renal and urinary disorders PT Urinary tract infection 2 (4. 5%) 1 (6. 7%) 2 (4. 5%) 0 1 (2. 3%) 0 SOC Ear and labyrinth disorders PT Ear infection SOC Gastrointestinal disorders PT Tooth abscess Patients may have more than one event reported MSSO-DI-6277 -11. 0. 0 © 2008 Northrop Grumman Corporation. All Rights Reserved. 98
Secondary SOC Analysis – SOC Infections and infestations (cont) Adverse Event (Med. DRA 11. 0) 25 mg My. Drug (N=44) Placebo (N=15) SOC Renal and urinary disorders PT Urinary tract infection 2 (4. 5%) 1 (6. 7%) 2 (4. 5%) 0 1 (2. 3%) 0 SOC Ear and labyrinth disorders PT Ear infection SOC Gastrointestinal disorders PT Tooth abscess Patients may have more than one event reported MSSO-DI-6277 -11. 0. 0 © 2008 Northrop Grumman Corporation. All Rights Reserved. 98
 Data Retrieval PTC Med. DRA Versioning • Med. DRA is updated twice a year • Version used in data retrieval and presentation should be documented • Terms used for queries should be in same version as data being queried MSSO-DI-6277 -11. 0. 0 © 2008 Northrop Grumman Corporation. All Rights Reserved. 99
Data Retrieval PTC Med. DRA Versioning • Med. DRA is updated twice a year • Version used in data retrieval and presentation should be documented • Terms used for queries should be in same version as data being queried MSSO-DI-6277 -11. 0. 0 © 2008 Northrop Grumman Corporation. All Rights Reserved. 99
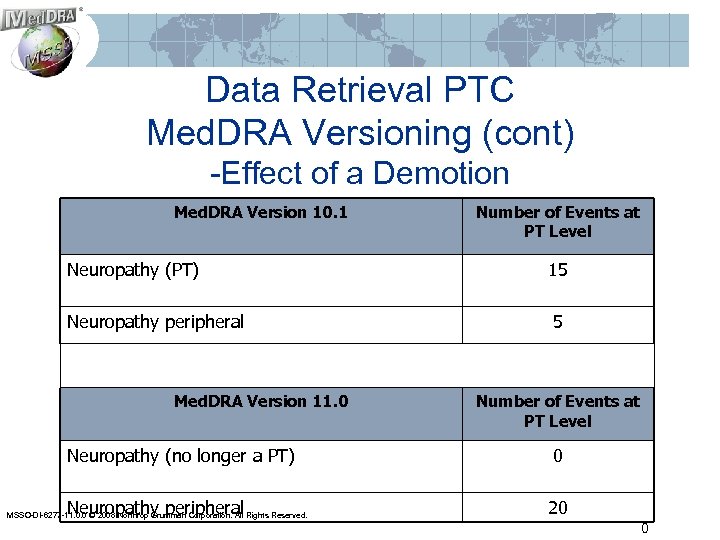 Data Retrieval PTC Med. DRA Versioning (cont) -Effect of a Demotion Med. DRA Version 10. 1 Number of Events at PT Level Neuropathy (PT) 15 Neuropathy peripheral 5 Med. DRA Version 11. 0 Number of Events at PT Level Neuropathy (no longer a PT) 0 Neuropathy peripheral 20 MSSO-DI-6277 -11. 0. 0 © 2008 Northrop Grumman Corporation. All Rights Reserved. 0
Data Retrieval PTC Med. DRA Versioning (cont) -Effect of a Demotion Med. DRA Version 10. 1 Number of Events at PT Level Neuropathy (PT) 15 Neuropathy peripheral 5 Med. DRA Version 11. 0 Number of Events at PT Level Neuropathy (no longer a PT) 0 Neuropathy peripheral 20 MSSO-DI-6277 -11. 0. 0 © 2008 Northrop Grumman Corporation. All Rights Reserved. 0
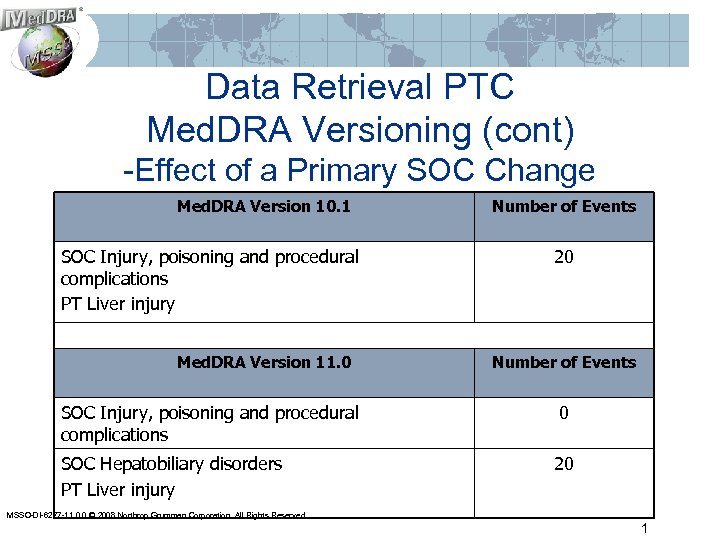 Data Retrieval PTC Med. DRA Versioning (cont) -Effect of a Primary SOC Change Med. DRA Version 10. 1 SOC Injury, poisoning and procedural complications PT Liver injury Med. DRA Version 11. 0 Number of Events 20 Number of Events SOC Injury, poisoning and procedural complications 0 SOC Hepatobiliary disorders PT Liver injury 20 MSSO-DI-6277 -11. 0. 0 © 2008 Northrop Grumman Corporation. All Rights Reserved. 1
Data Retrieval PTC Med. DRA Versioning (cont) -Effect of a Primary SOC Change Med. DRA Version 10. 1 SOC Injury, poisoning and procedural complications PT Liver injury Med. DRA Version 11. 0 Number of Events 20 Number of Events SOC Injury, poisoning and procedural complications 0 SOC Hepatobiliary disorders PT Liver injury 20 MSSO-DI-6277 -11. 0. 0 © 2008 Northrop Grumman Corporation. All Rights Reserved. 1
 Data Retrieval PTC Queries and Retrieval Summary • General Principles – Data retrieval is performed for analysis of clinical trial data, pharmacovigilance, etc. – Strategies, methods and tools used depend on intended use of output – Update previously used searches – Identify safety issues prior to retrieval - information from preclinical & clinical trials, postmarketing surveillance, similar products, regulatory queries – Group related events – Evaluate search results against original question MSSO-DI-6277 -11. 0. 0 © 2008 Northrop Grumman Corporation. All Rights Reserved. 2
Data Retrieval PTC Queries and Retrieval Summary • General Principles – Data retrieval is performed for analysis of clinical trial data, pharmacovigilance, etc. – Strategies, methods and tools used depend on intended use of output – Update previously used searches – Identify safety issues prior to retrieval - information from preclinical & clinical trials, postmarketing surveillance, similar products, regulatory queries – Group related events – Evaluate search results against original question MSSO-DI-6277 -11. 0. 0 © 2008 Northrop Grumman Corporation. All Rights Reserved. 2
 Data Retrieval PTC Queries and Retrieval Summary (cont) • Examples of searches – Overview of safety profile in summary reports – Comparison of frequency of ADR/AE (spontaneous reports or incidence for studies) – Analysis of a specific safety concern – Identification of patient subpopulations at risk (e. g. searching medical history information) MSSO-DI-6277 -11. 0. 0 © 2008 Northrop Grumman Corporation. All Rights Reserved. 3
Data Retrieval PTC Queries and Retrieval Summary (cont) • Examples of searches – Overview of safety profile in summary reports – Comparison of frequency of ADR/AE (spontaneous reports or incidence for studies) – Analysis of a specific safety concern – Identification of patient subpopulations at risk (e. g. searching medical history information) MSSO-DI-6277 -11. 0. 0 © 2008 Northrop Grumman Corporation. All Rights Reserved. 3
 Data Retrieval PTC Queries and Retrieval Examples • Overall presentation of Safety Profiles – Highlight overall distribution of ADRs/AEs – Identify areas for in-depth analysis (focused searches) MSSO-DI-6277 -11. 0. 0 © 2008 Northrop Grumman Corporation. All Rights Reserved. 4
Data Retrieval PTC Queries and Retrieval Examples • Overall presentation of Safety Profiles – Highlight overall distribution of ADRs/AEs – Identify areas for in-depth analysis (focused searches) MSSO-DI-6277 -11. 0. 0 © 2008 Northrop Grumman Corporation. All Rights Reserved. 4
 Data Retrieval PTC Queries and Retrieval Examples (cont) • Overview by Primary SOC – Internationally Agreed Order of SOCs (see PTC or Med. DRA Introductory Guide) – Consider use of HLTs and HLGTs for large data sets – Line listings, tables, graphical displays MSSO-DI-6277 -11. 0. 0 © 2008 Northrop Grumman Corporation. All Rights Reserved. 5
Data Retrieval PTC Queries and Retrieval Examples (cont) • Overview by Primary SOC – Internationally Agreed Order of SOCs (see PTC or Med. DRA Introductory Guide) – Consider use of HLTs and HLGTs for large data sets – Line listings, tables, graphical displays MSSO-DI-6277 -11. 0. 0 © 2008 Northrop Grumman Corporation. All Rights Reserved. 5
 Data Retrieval PTC Queries and Retrieval Examples (cont) • Focused searches: – Secondary SOC assignments – Standardised Med. DRA Queries (SMQs) – Ad hoc queries • Organization-specific or project-specific queries • Send Change Request to MSSO for consideration for SMQ development? • Store for future use and update MSSO-DI-6277 -11. 0. 0 © 2008 Northrop Grumman Corporation. All Rights Reserved. 6
Data Retrieval PTC Queries and Retrieval Examples (cont) • Focused searches: – Secondary SOC assignments – Standardised Med. DRA Queries (SMQs) – Ad hoc queries • Organization-specific or project-specific queries • Send Change Request to MSSO for consideration for SMQ development? • Store for future use and update MSSO-DI-6277 -11. 0. 0 © 2008 Northrop Grumman Corporation. All Rights Reserved. 6
 Browser Demonstration SMQ View
Browser Demonstration SMQ View
 SMQs as a Starting Point • Standard query as starting point promotes commonality of use and better communication • In limited circumstances, SMQs could be customized according to product and patient population characteristics. STRATEGY MUST BE DOCUMENTED. • Cases identified by SMQs should be reviewed for relevance (Case report form, Med. Watch form, etc. ) • May need to develop organization specific or project specific (i. e. , ad hoc) queries. Consider for development as SMQs. MSSO-DI-6277 -11. 0. 0 © 2008 Northrop Grumman Corporation. All Rights Reserved. 8
SMQs as a Starting Point • Standard query as starting point promotes commonality of use and better communication • In limited circumstances, SMQs could be customized according to product and patient population characteristics. STRATEGY MUST BE DOCUMENTED. • Cases identified by SMQs should be reviewed for relevance (Case report form, Med. Watch form, etc. ) • May need to develop organization specific or project specific (i. e. , ad hoc) queries. Consider for development as SMQs. MSSO-DI-6277 -11. 0. 0 © 2008 Northrop Grumman Corporation. All Rights Reserved. 8
 Summary In this course, we: • Reviewed the structure, scope, and characteristics of Med. DRA • Were introduced to the “Med. DRA Term Selection: Points to Consider” document and some of its specific principles • Considered data quality issues and learned about the “Data Quality, Coding and Med. DRA” presentation • Were introduced to the “Med. DRA Data Retrieval and Presentation: Points to Consider” document and some specific retrieval and query examples • Learned about Standardised Med. DRA Queries (SMQs) • Discussed Med. DRA maintenance and versioning MSSO-DI-6277 -11. 0. 0 © 2008 Northrop Grumman Corporation. All Rights Reserved. 9
Summary In this course, we: • Reviewed the structure, scope, and characteristics of Med. DRA • Were introduced to the “Med. DRA Term Selection: Points to Consider” document and some of its specific principles • Considered data quality issues and learned about the “Data Quality, Coding and Med. DRA” presentation • Were introduced to the “Med. DRA Data Retrieval and Presentation: Points to Consider” document and some specific retrieval and query examples • Learned about Standardised Med. DRA Queries (SMQs) • Discussed Med. DRA maintenance and versioning MSSO-DI-6277 -11. 0. 0 © 2008 Northrop Grumman Corporation. All Rights Reserved. 9
 • Mail MSSO Contacts Med. DRA MSSO 3975 Virginia Mallory Drive Chantilly, VA, USA 20151 • Telephone – Toll-free Worldwide 877. 258. 8280 (AT&T) • Fax – 703. 272. 5635 • Products and Services – Toll-free Worldwide 877. 258. 8280 (AT&T) MSSO-DI-6277 -11. 0. 0 © 2008 Northrop Grumman Corporation. All Rights Reserved. 0
• Mail MSSO Contacts Med. DRA MSSO 3975 Virginia Mallory Drive Chantilly, VA, USA 20151 • Telephone – Toll-free Worldwide 877. 258. 8280 (AT&T) • Fax – 703. 272. 5635 • Products and Services – Toll-free Worldwide 877. 258. 8280 (AT&T) MSSO-DI-6277 -11. 0. 0 © 2008 Northrop Grumman Corporation. All Rights Reserved. 0
 MSSO Contacts (cont) • To Subscribe by – E-mail • mssosubscribe@ngc. com – Web site • www. meddramsso. com click on “Subscribe to Med. DRA” • Help desk – Phone • International AT&T Toll Free: 877. 258. 8280 • Direct Dial (USA): 703. 272. 5849 – E-mail • mssohelp@ngc. com MSSO-DI-6277 -11. 0. 0 © 2008 Northrop Grumman Corporation. All Rights Reserved. 1
MSSO Contacts (cont) • To Subscribe by – E-mail • mssosubscribe@ngc. com – Web site • www. meddramsso. com click on “Subscribe to Med. DRA” • Help desk – Phone • International AT&T Toll Free: 877. 258. 8280 • Direct Dial (USA): 703. 272. 5849 – E-mail • mssohelp@ngc. com MSSO-DI-6277 -11. 0. 0 © 2008 Northrop Grumman Corporation. All Rights Reserved. 1
 Acknowledgements • Med. DRA® is a registered trademark of the International Federation of Pharmaceutical Manufacturers and Associations (IFPMA) • COSTART Thesaurus Fifth Edition Food and Drug Administration (FDA) Copyright 1995 US • Hoechst Adverse Reaction Terminology System (HARTS)© 1992 Aventis Pharma • ICD-9 -CM International Classification of Diseases, Ninth Revision, Clinical Modification, Copyright© 1998 Medicode, Inc. • Japanese Adverse Reaction Terminology (J-ART), is a product of the Japanese Ministry of Health, Labour and Welfare (MHLW) • WHO Adverse Reaction Terminology (WHO-ART), Copyright World Health Organization Collaborating Centre for International Drug Monitoring MSSO-DI-6277 -11. 0. 0 © 2008 Northrop Grumman Corporation. All Rights Reserved. 2
Acknowledgements • Med. DRA® is a registered trademark of the International Federation of Pharmaceutical Manufacturers and Associations (IFPMA) • COSTART Thesaurus Fifth Edition Food and Drug Administration (FDA) Copyright 1995 US • Hoechst Adverse Reaction Terminology System (HARTS)© 1992 Aventis Pharma • ICD-9 -CM International Classification of Diseases, Ninth Revision, Clinical Modification, Copyright© 1998 Medicode, Inc. • Japanese Adverse Reaction Terminology (J-ART), is a product of the Japanese Ministry of Health, Labour and Welfare (MHLW) • WHO Adverse Reaction Terminology (WHO-ART), Copyright World Health Organization Collaborating Centre for International Drug Monitoring MSSO-DI-6277 -11. 0. 0 © 2008 Northrop Grumman Corporation. All Rights Reserved. 2


