b6ad63e722698b410c1c52b8e0b3b004.ppt
- Количество слайдов: 12

Mechanism of Action and Pharmacology of Ezetimibe EZT 2003 -W-166091 -SS Copyright © 2003 MSP Singapore Company, LLC. All rights reserved. Slide 1
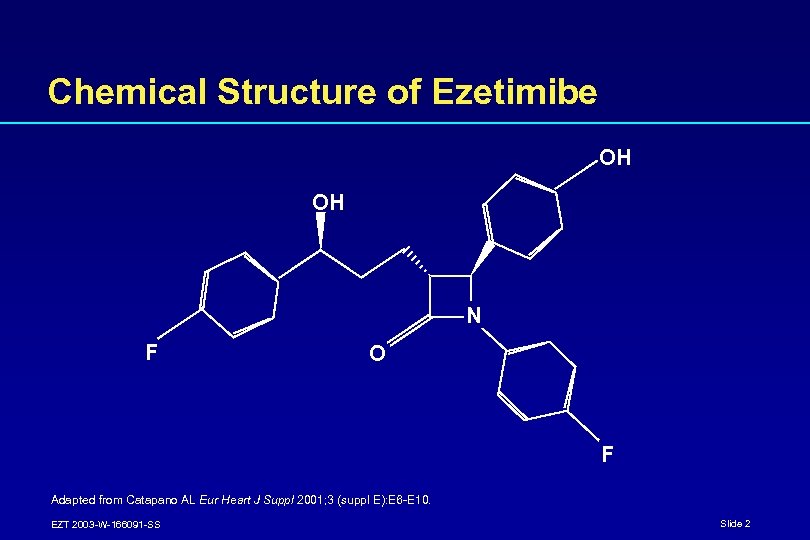
Chemical Structure of Ezetimibe OH OH N F O F Adapted from Catapano AL Eur Heart J Suppl 2001; 3 (suppl E): E 6 -E 10. EZT 2003 -W-166091 -SS Slide 2
Mechanism of Action of Ezetimibe • Localizes at the brush border of the small intestine to prevent and decrease the delivery of intestinal cholesterol to the liver • The reduction of hepatic cholesterol stores leads to an increase in clearance of cholesterol from the blood Adapted from van Heek M et al Br J Pharmacol 2000; 129: 1748 -1754. EZT 2003 -W-166091 -SS Slide 3
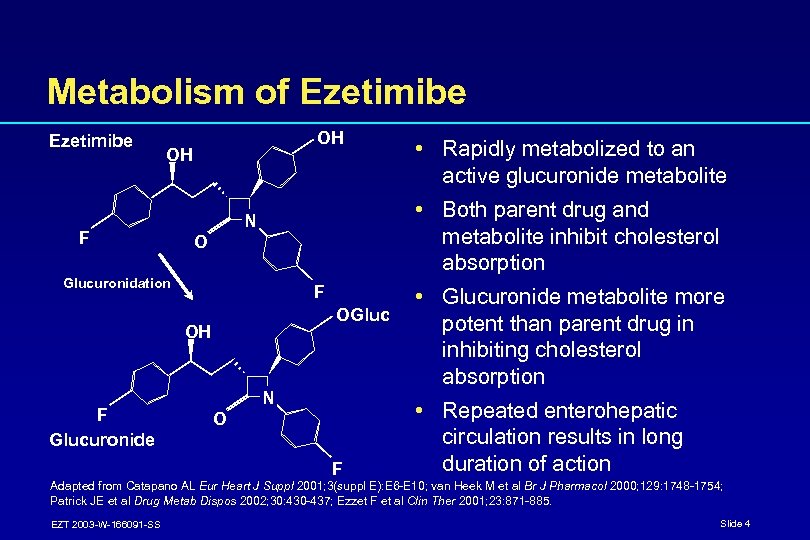
Metabolism of Ezetimibe OH OH F • Both parent drug and metabolite inhibit cholesterol absorption N O Glucuronidation F OGluc OH F Glucuronide • Rapidly metabolized to an active glucuronide metabolite N O F • Glucuronide metabolite more potent than parent drug in inhibiting cholesterol absorption • Repeated enterohepatic circulation results in long duration of action Adapted from Catapano AL Eur Heart J Suppl 2001; 3(suppl E): E 6 -E 10; van Heek M et al Br J Pharmacol 2000; 129: 1748 -1754; Patrick JE et al Drug Metab Dispos 2002; 30: 430 -437; Ezzet F et al Clin Ther 2001; 23: 871 -885. EZT 2003 -W-166091 -SS Slide 4
Pharmacokinetics of Ezetimibe • Elimination half-life of ezetimibe approximately 22 hours • Enterohepatic recirculation of glucuronide metabolite extends duration of action • Long half-life – Permits once-daily dosing – Increases convenience – May improve compliance Adapted from Bays HE et al Clin Ther 2001; 23: 1209 -1230; Kirsten R et al Clin Pharmacokinet 1998; 34: 457 -482. EZT 2003 -W-166091 -SS Slide 5
Ezetimibe: Summary of Pharmacokinetic Parameters • Absorption – Rapid after oral administration – Peak plasma concentration in an average of 2– 3 hours • Distribution – Relative volume of distribution 107. 5 L – 20% reabsorbed due to enterohepatic recirculation • Elimination – Primarily in feces after extensive enterohepatic recirculation – Half-life 22 hours Adapted from Patrick JE et al Drug Metab Dispos 2002; 30: 430 -437; Ezzet F et al Clin Ther 2001; 23: 871 -885. EZT 2003 -W-166091 -SS Slide 6
Factors Influencing Pharmacokinetics of Ezetimibe • Food – No significant effect on oral bioavailability of ezetimibe • Elderly – Plasma concentration of ezetimibe in elderly ( 65 years) twofold higher than in young (18– 45 years) – Differences observed with age not clinically significant – Dosage adjustment not necessary • Gender – Plasma concentration of ezetimibe slightly higher (<20%) in women than in men – LDL-C reduction and safety profile comparable between men and women – Dosage adjustment not necessary Adapted from Data on file, MSD. EZT 2003 -W-166091 -SS Slide 7
Drug Interactions of Ezetimibe • Ezetimibe does not induce cytochrome P 450 enzymes • Statins: no significant pharmacokinetic interactions with atorvastatin, simvastatin, pravastatin, lovastatin, or fluvastatin • Other drugs: no effect on pharmacokinetics of dapsone, dextromethorphan, digoxin, oral contraceptives, glipizide, tolbutamide, midazolam, or warfarin • Cimetidine: no effect on bioavailability of ezetimibe • Antacids: decreased absorption rate of ezetimibe—not clinically significant • Cholestyramine: decreased mean AUC of ezetimibe ~55% – May lessen incremental LDL-C reduction • Fibrates: safety and efficacy of fibrate co-administration not established EZT 2003 -W-166091 -SS Slide 8
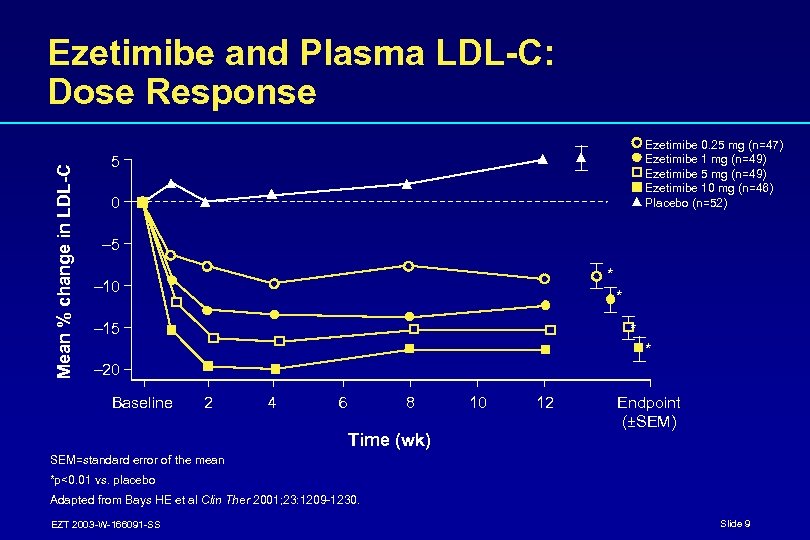
Mean % change in LDL-C Ezetimibe and Plasma LDL-C: Dose Response Ezetimibe 0. 25 mg (n=47) Ezetimibe 1 mg (n=49) Ezetimibe 5 mg (n=49) Ezetimibe 10 mg (n=46) Placebo (n=52) 5 0 – 5 * – 10 * – 15 * * – 20 Baseline 2 4 6 8 Time (wk) 10 12 Endpoint (±SEM) SEM=standard error of the mean *p<0. 01 vs. placebo Adapted from Bays HE et al Clin Ther 2001; 23: 1209 -1230. EZT 2003 -W-166091 -SS Slide 9
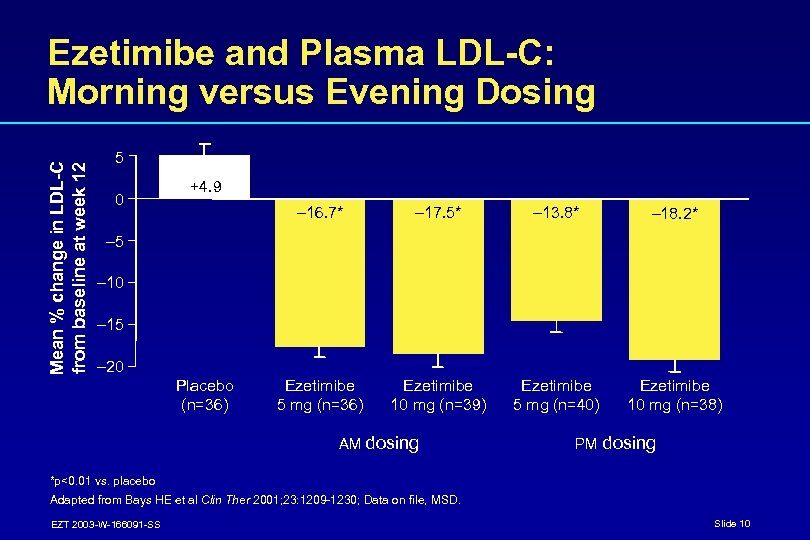
Mean % change in LDL-C from baseline at week 12 Ezetimibe and Plasma LDL-C: Morning versus Evening Dosing 5 0 +4. 9 – 16. 7* – 17. 5* – 13. 8* – 18. 2* Ezetimibe 5 mg (n=36) Ezetimibe 10 mg (n=39) Ezetimibe 5 mg (n=40) Ezetimibe 10 mg (n=38) – 5 – 10 – 15 – 20 Placebo (n=36) AM dosing PM dosing *p<0. 01 vs. placebo Adapted from Bays HE et al Clin Ther 2001; 23: 1209 -1230; Data on file, MSD. EZT 2003 -W-166091 -SS Slide 10
Key Benefits of Ezetimibe: Summary • Unique mechanism of action inhibits absorption of dietary and biliary cholesterol • Complements mechanism of action of cholesterol synthesis inhibitors (statins) • Has additive LDL-C lowering effects with statins • Pharmacokinetics – Long half-life permits once-daily dosing – No known clinically significant pharmacokinetic interactions were seen with statins • Provides greater lipid control when used in co-administration with statins EZT 2003 -W-166091 -SS Slide 11

Before prescribing any of the products mentioned in this presentation, please consult the manufacturers’ full prescribing information. EZT 2003 -W-166091 -SS Slide 12
b6ad63e722698b410c1c52b8e0b3b004.ppt