f52fbed5b08a733b81c62bdd8e9b8ff1.ppt
- Количество слайдов: 11
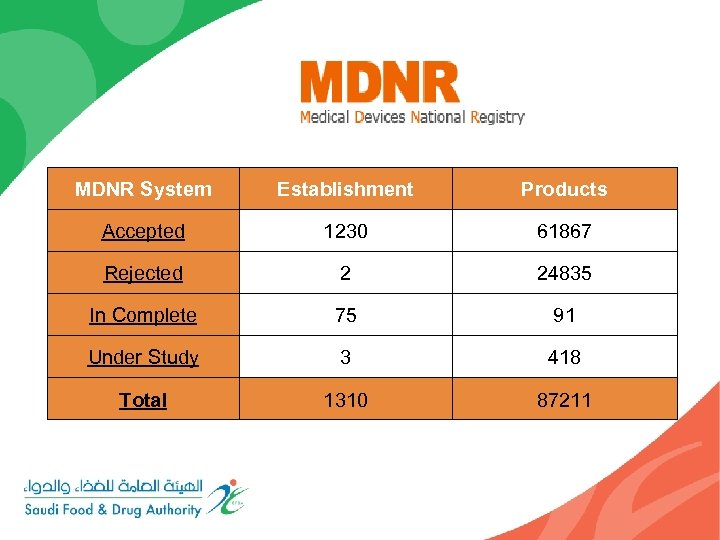
MDNR System Establishment Products Accepted 1230 61867 Rejected 2 24835 In Complete 75 91 Under Study 3 418 Total 1310 87211
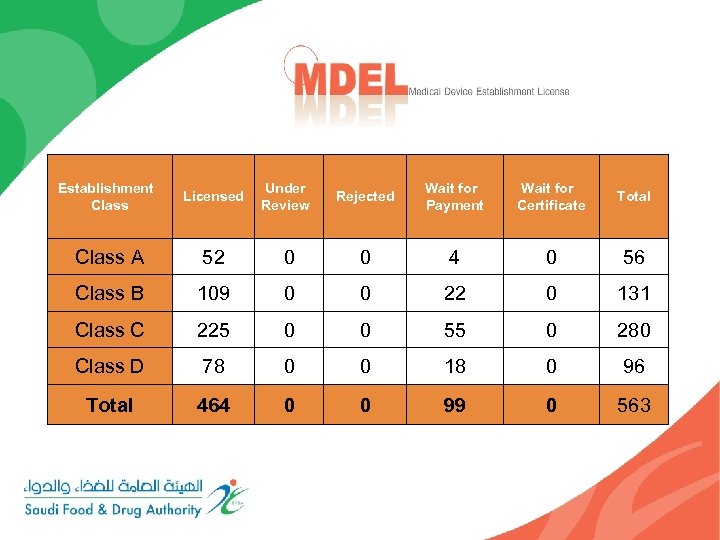
Establishment Class Licensed Under Review Rejected Wait for Payment Wait for Certificate Total Class A 52 0 0 4 0 56 Class B 109 0 0 22 0 131 Class C 225 0 0 55 0 280 Class D 78 0 0 18 0 96 Total 464 0 0 99 0 563
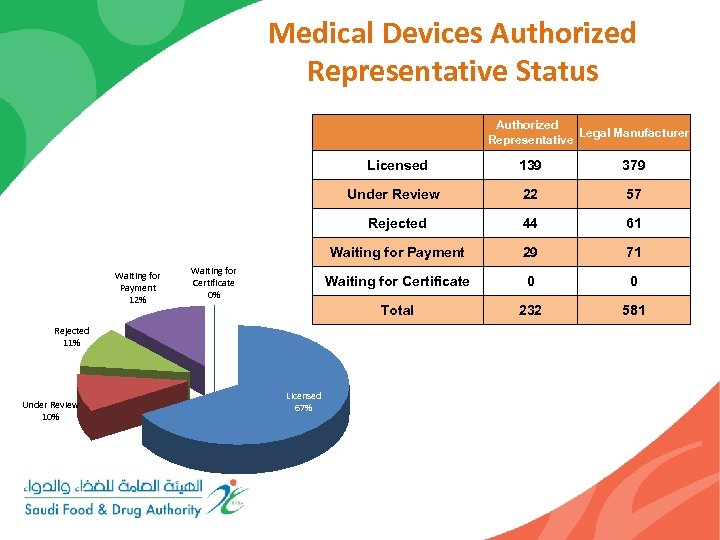
Medical Devices Authorized Representative Status Authorized Legal Manufacturer Representative Licensed Under Review 10% 44 61 29 71 Waiting for Certificate 0 0 Total Licensed 67% 57 Waiting for Payment Rejected 11% 22 Rejected Waiting for Certificate 0% 379 Under Review Waiting for Payment 12% 139 232 581
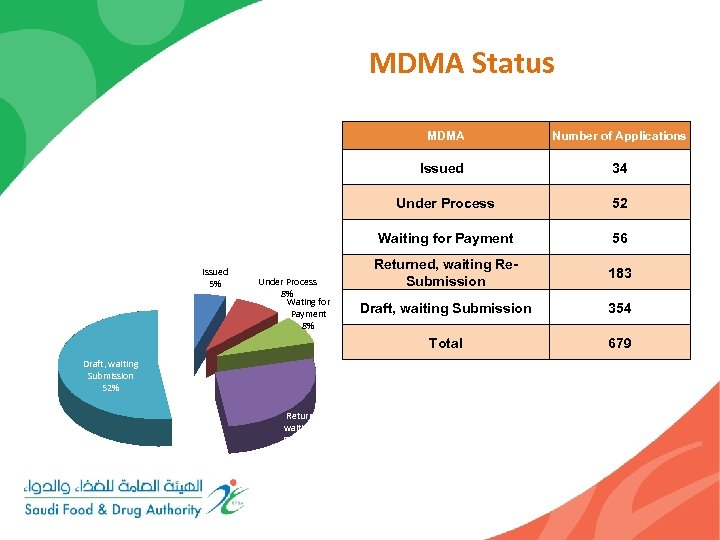
MDMA Status MDMA Number of Applications Issued Under Process Draft, waiting Submission 52% Returned, waiting Re. Submission 27% 56 Returned, waiting Re. Submission 183 Draft, waiting Submission 354 Total Under Process 8% Wating for Payment 8% 52 Waiting for Payment Issued 5% 34 679
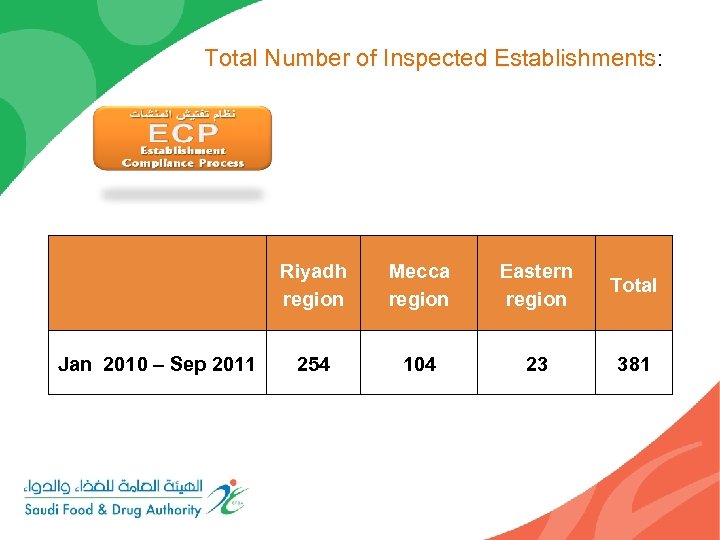
Total Number of Inspected Establishments: Riyadh region Jan 2010 – Sep 2011 Mecca region Eastern region Total 254 104 23 381

Nonconforming products observed during inspection visits Product type Medical Bandages Medical Braces IVD Tests Catheter Tubes Surgical Blades Disposable Syringe Thermometers Surgical Gloves Surgical Bandages Electro Cardio Grave Pads Quantity 1, 890, 685
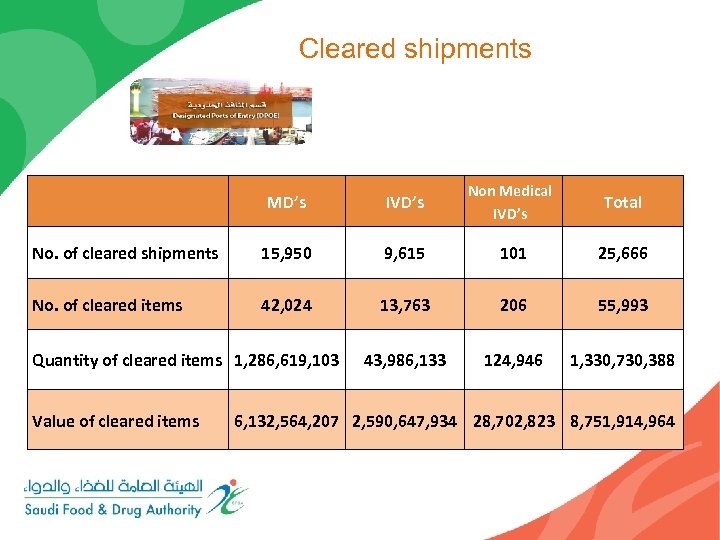
Cleared shipments MD’s IVD’s Non Medical IVD’s Total No. of cleared shipments 15, 950 9, 615 101 25, 666 No. of cleared items 42, 024 13, 763 206 55, 993 43, 986, 133 124, 946 1, 330, 730, 388 Quantity of cleared items 1, 286, 619, 103 Value of cleared items 6, 132, 564, 207 2, 590, 647, 934 28, 702, 823 8, 751, 914, 964
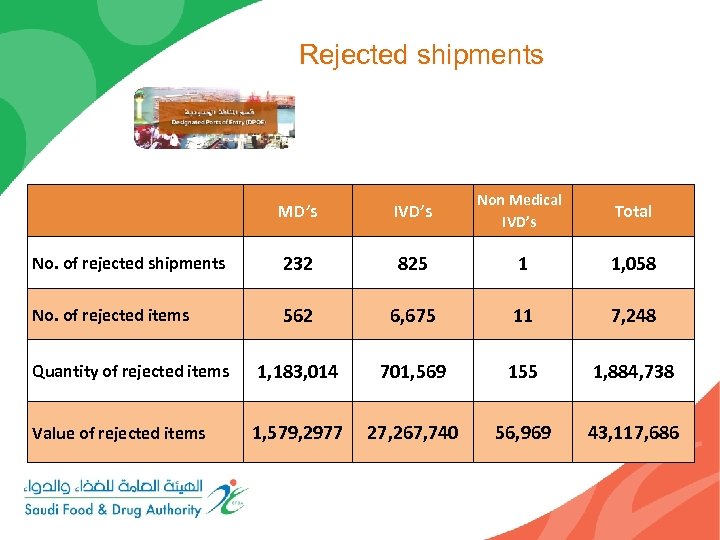
Rejected shipments MD’s IVD’s Non Medical IVD’s Total No. of rejected shipments 232 825 1 1, 058 No. of rejected items 562 6, 675 11 7, 248 Quantity of rejected items 1, 183, 014 701, 569 155 1, 884, 738 Value of rejected items 1, 579, 2977 27, 267, 740 56, 969 43, 117, 686
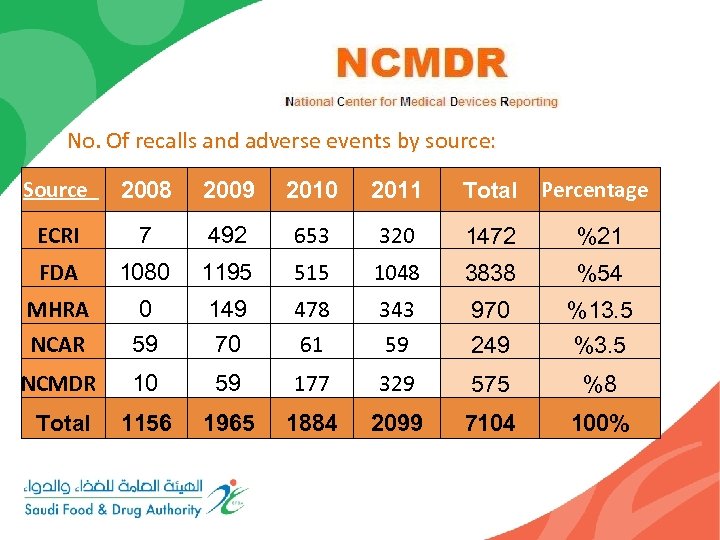
No. Of recalls and adverse events by source: Source 2008 2009 2010 2011 Total Percentage ECRI 7 492 653 320 1472 %21 FDA 1080 1195 515 1048 3838 %54 MHRA 0 149 478 343 970 %13. 5 NCAR 59 70 61 59 249 %3. 5 NCMDR 10 59 177 329 575 %8 Total 1156 1965 1884 2099 7104 100%
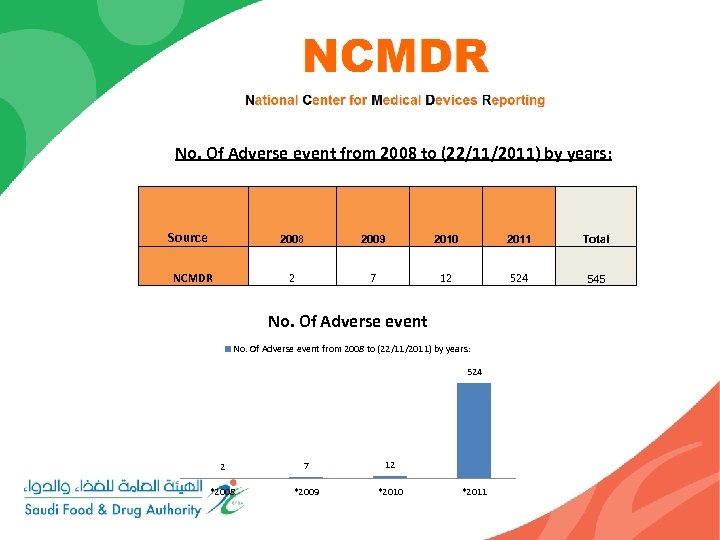
No. Of Adverse event from 2008 to (22/11/2011) by years: Source 2008 2009 2010 2011 Total NCMDR 2 7 12 524 545 No. Of Adverse event from 2008 to (22/11/2011) by years: 524 2 7 12 *2008 *2009 *2010 *2011

Thank You
f52fbed5b08a733b81c62bdd8e9b8ff1.ppt