258da2b309e8af38830840ce798cb4e8.ppt
- Количество слайдов: 30

MBAA-Rocky Mountain District Technical Summit 25 June 2010 Measuring Dissolved Oxygen with Optical Technology Brian Vaillancourt Mettler-Toledo Ingold Bedford MA 2010

Agenda § § § § Introduction Current Technology Challenges with DO measurements Oxygen Measurement in Breweries New Optical Technology Theory of Operation Benefits Summary 1

Introduction Technology Advancements 2
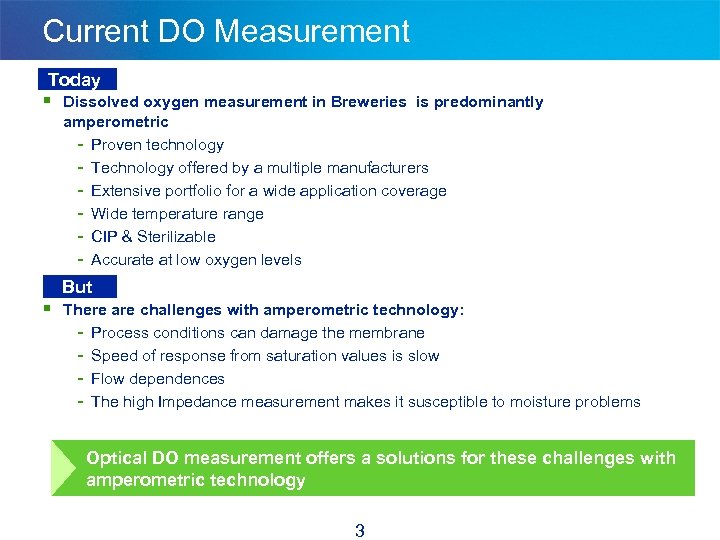
Current DO Measurement Today § Dissolved oxygen measurement in Breweries is predominantly amperometric - Proven technology - Technology offered by a multiple manufacturers - Extensive portfolio for a wide application coverage - Wide temperature range - CIP & Sterilizable - Accurate at low oxygen levels But § There are challenges with amperometric technology: - Process conditions can damage the membrane - Speed of response from saturation values is slow - Flow dependences - The high Impedance measurement makes it susceptible to moisture problems Optical DO measurement offers a solutions for these challenges with amperometric technology 3

Why Measure Oxygen – Key to Quality § Oxygen is considered one of the top beer spoilers § Oxygen in beer reduces the shelf life § The lower the DO when the product is packaged, the longer it will remain “Fresh, Crisp & Clean tasting” § DO in the beer before filling, contributes to nearly 1/3 of the total packaged oxygen “TPO” § Key requirements for successful oxygen control: - Avoid any ingress of oxygen at all process stages Increasing demand for lower oxygen value in water and CO 2 Requirements to oxygen measurement equipment: - Ability to measure in beer Low limit of detection Stable measurement signal No flow dependence Fast response Low maintenance Reduction of oxygen level in beer is directly linked to product quality and shelf life cost savings 4
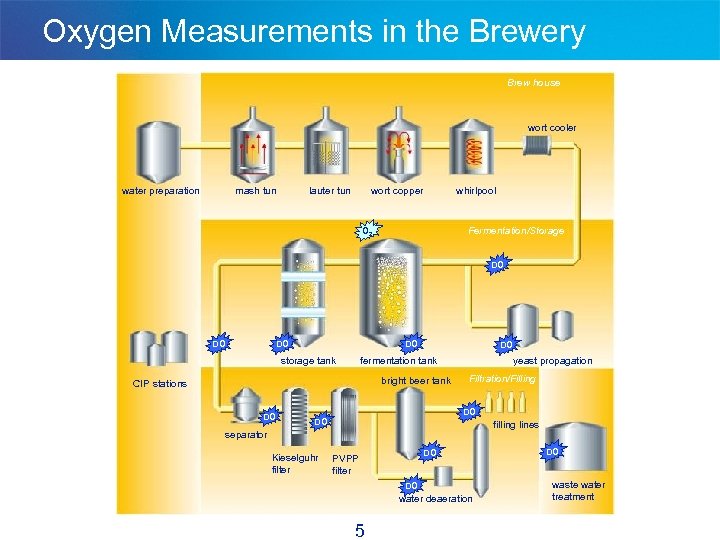
Oxygen Measurements in the Brewery Brew house wort cooler water preparation mash tun lauter tun wort copper whirlpool Fermentation/Storage O 2 DO DO storage tank DO fermentation tank bright beer tank CIP stations DO yeast propagation Filtration/Filling DO DO filling lines separator Kieselguhr filter DO PVPP filter DO water deaeration 5 DO waste water treatment
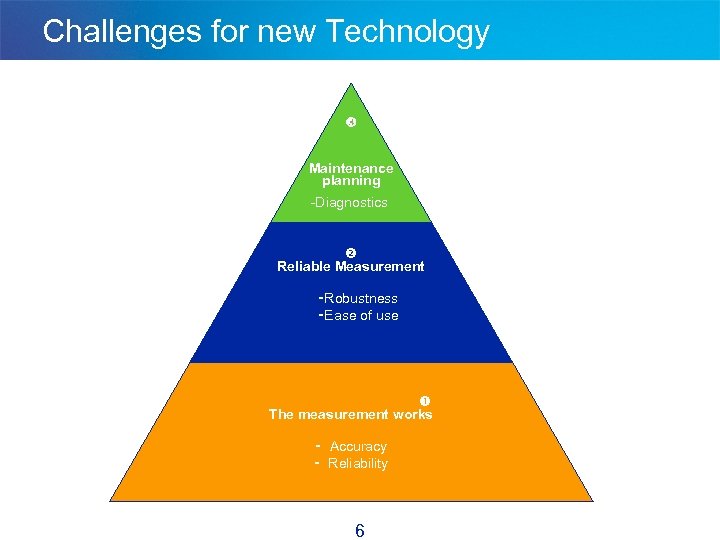
Challenges for new Technology Maintenance planning -Diagnostics Reliable Measurement - Robustness - Ease of use The measurement works - Accuracy - Reliability 6
Keep your focus Optical DO systems allow you to concentrate on your process 7
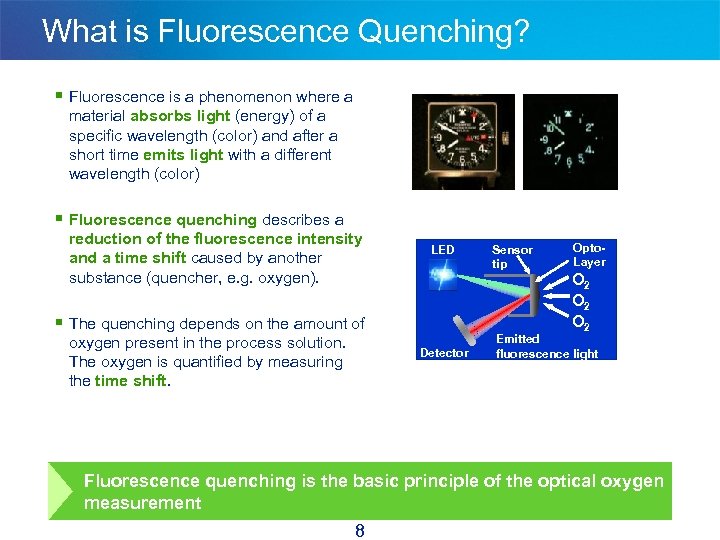
What is Fluorescence Quenching? § Fluorescence is a phenomenon where a material absorbs light (energy) of a specific wavelength (color) and after a short time emits light with a different wavelength (color) § Fluorescence quenching describes a reduction of the fluorescence intensity and a time shift caused by another substance (quencher, e. g. oxygen). LED § The quenching depends on the amount of oxygen present in the process solution. The oxygen is quantified by measuring the time shift. Detector Sensor tip Opto. Layer O 2 O 2 Emitted fluorescence light Fluorescence quenching is the basic principle of the optical oxygen measurement 8
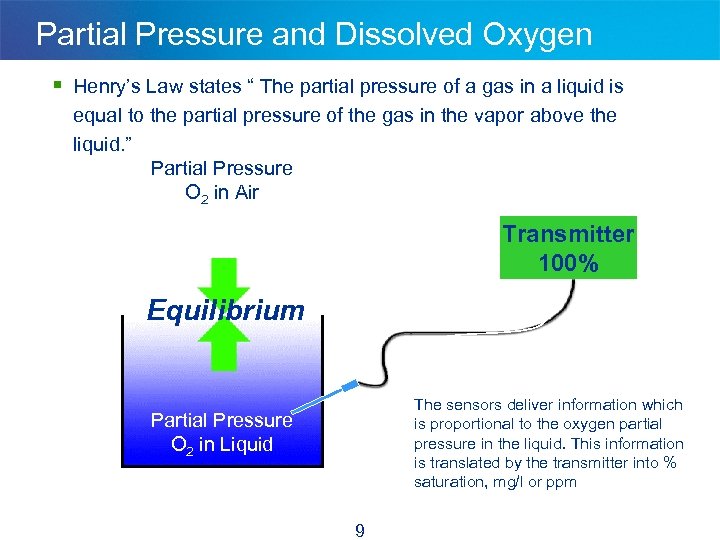
Partial Pressure and Dissolved Oxygen § Henry’s Law states “ The partial pressure of a gas in a liquid is equal to the partial pressure of the gas in the vapor above the liquid. ” Partial Pressure O 2 in Air Transmitter 100% Equilibrium The sensors deliver information which is proportional to the oxygen partial pressure in the liquid. This information is translated by the transmitter into % saturation, mg/l or ppm Partial Pressure O 2 in Liquid 9
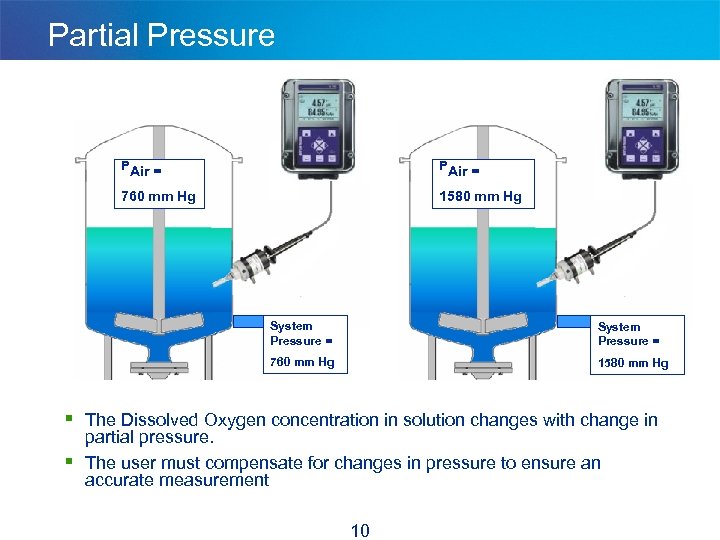
Partial Pressure PAir = 760 mm Hg 1580 mm Hg System Pressure = 760 mm Hg 1580 mm Hg § The Dissolved Oxygen concentration in solution changes with change in § partial pressure. The user must compensate for changes in pressure to ensure an accurate measurement 10
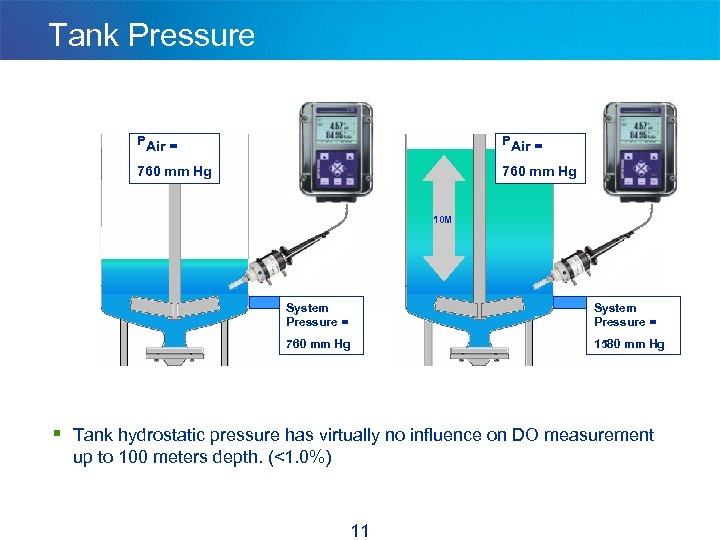
Tank Pressure PAir = 760 mm Hg 10 M System Pressure = 760 mm Hg 1580 mm Hg § Tank hydrostatic pressure has virtually no influence on DO measurement up to 100 meters depth. (<1. 0%) 11

Partial Pressure § Partial Pressure is the pressure that a single gas exerts in a mixture of gases - Oxygen is 160 mm or 212. 2 m. Bar at saturation § Humid Air displaces the Partial Pressure of Oxygen - Example At 20 o. C § 0% Humidity the Partial Pressure of Oxygen is 212. 2 mbar § 23. 3 mbar Humidity the Partial Pressure of Oxygen is 207. 4 mbar § 4. 8 mbar or 2. 26% Difference between the Partial Pressure of Oxygen in Dry Air vs Humid Air 12
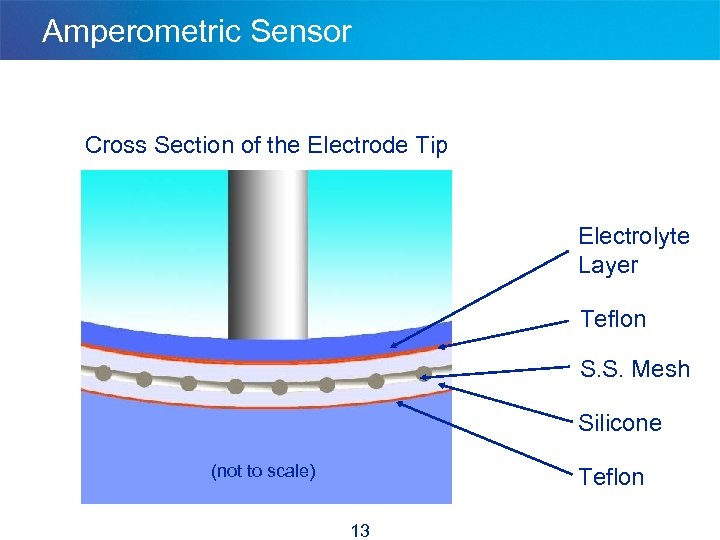
Amperometric Sensor Cross Section of the Electrode Tip Electrolyte Layer Teflon S. S. Mesh Silicone (not to scale) Teflon 13
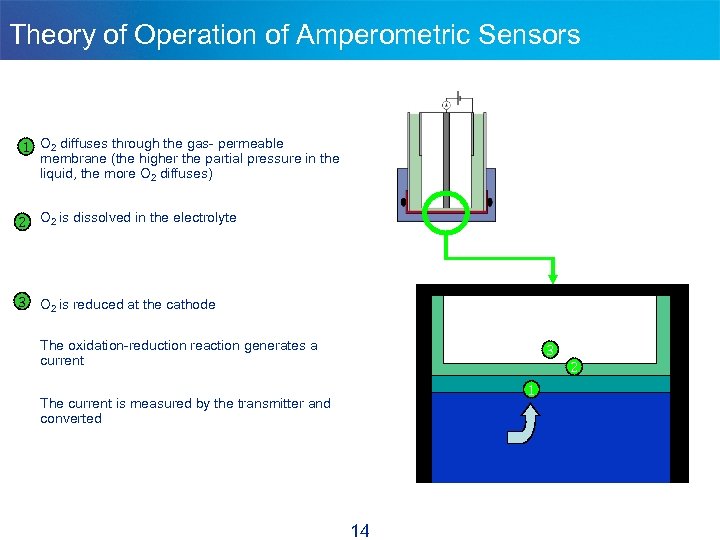
Theory of Operation of Amperometric Sensors 1 O 2 diffuses through the gas- permeable membrane (the higher the partial pressure in the liquid, the more O 2 diffuses) 2 O 2 is dissolved in the electrolyte 3 O 2 is reduced at the cathode The oxidation-reduction reaction generates a current 3 1 The current is measured by the transmitter and converted 14 2

17
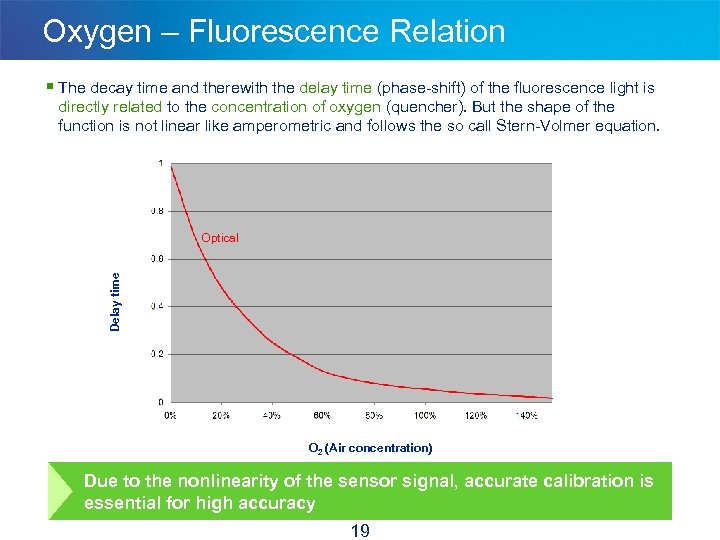
Oxygen – Fluorescence Relation § The decay time and therewith the delay time (phase-shift) of the fluorescence light is directly related to the concentration of oxygen (quencher). But the shape of the function is not linear like amperometric and follows the so call Stern-Volmer equation. Delay time Optical O 2 (Air concentration) Due to the nonlinearity of the sensor signal, accurate calibration is essential for high accuracy 19
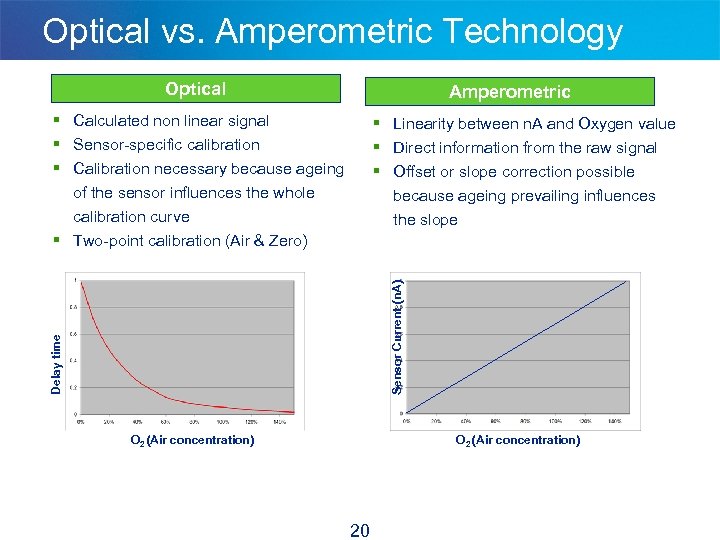
Optical vs. Amperometric Technology Optical Amperometric § Calculated non linear signal § Sensor-specific calibration § Calibration necessary because ageing § Linearity between n. A and Oxygen value § Direct information from the raw signal § Offset or slope correction possible of the sensor influences the whole calibration curve § Two-point calibration (Air & Zero) Delay time Sensor Current (n. A) because ageing prevailing influences the slope O 2 (Air concentration) 20
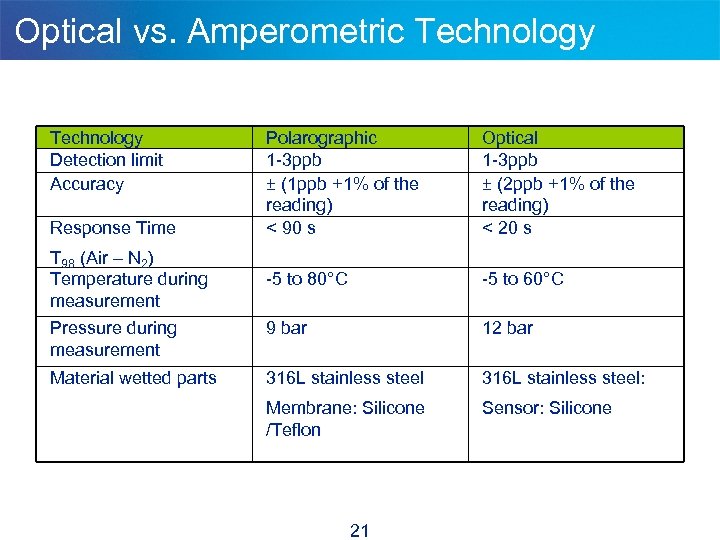
Optical vs. Amperometric Technology Detection limit Accuracy Polarographic 1 -3 ppb ± (1 ppb +1% of the reading) < 90 s Optical 1 -3 ppb ± (2 ppb +1% of the reading) < 20 s -5 to 80°C -5 to 60°C Pressure during measurement 9 bar 12 bar Material wetted parts 316 L stainless steel: Membrane: Silicone /Teflon Sensor: Silicone Response Time T 98 (Air – N 2) Temperature during measurement 21
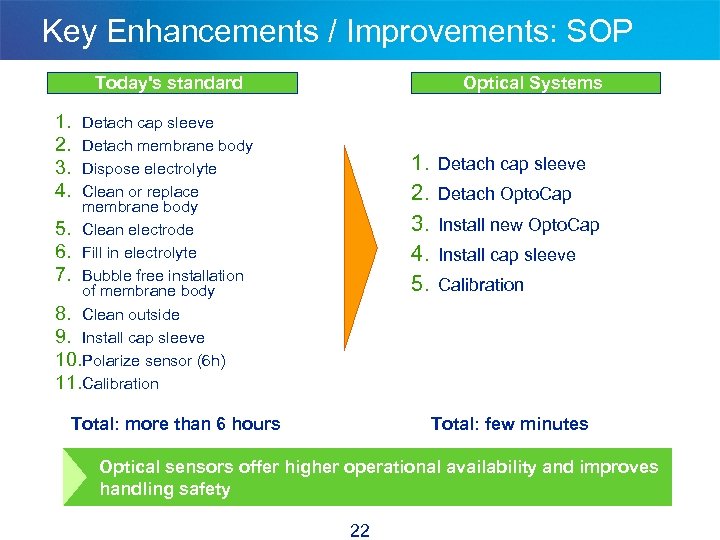
Key Enhancements / Improvements: SOP Today's standard Optical Systems 1. 2. 3. 4. Detach cap sleeve Detach membrane body Dispose electrolyte Clean or replace membrane body 5. Clean electrode 6. Fill in electrolyte 7. Bubble free installation of membrane body 8. Clean outside 9. Install cap sleeve 10. Polarize sensor (6 h) 11. Calibration 1. 2. 3. 4. 5. Total: more than 6 hours Detach cap sleeve Detach Opto. Cap Install new Opto. Cap Install cap sleeve Calibration Total: few minutes Optical sensors offer higher operational availability and improves handling safety 22
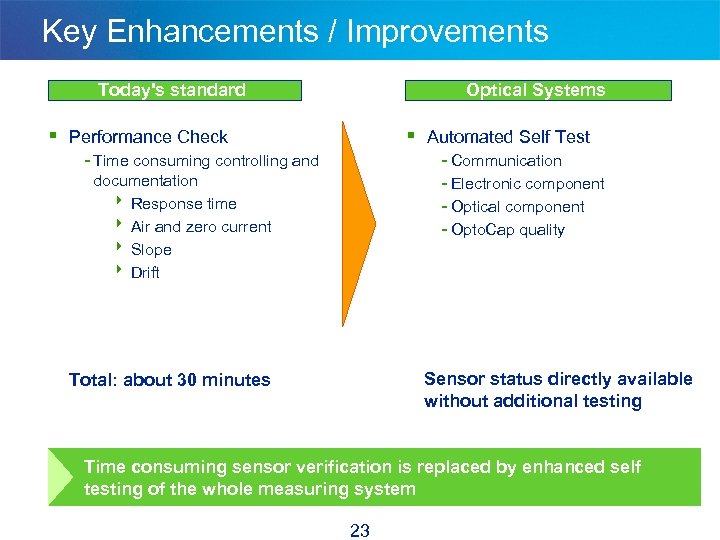
Key Enhancements / Improvements Today's standard Optical Systems § Performance Check § Automated Self Test - Time consuming controlling and - Communication - Electronic component - Optical component - Opto. Cap quality documentation 8 Response time 8 Air and zero current 8 Slope 8 Drift Sensor status directly available without additional testing Total: about 30 minutes Time consuming sensor verification is replaced by enhanced self testing of the whole measuring system 23
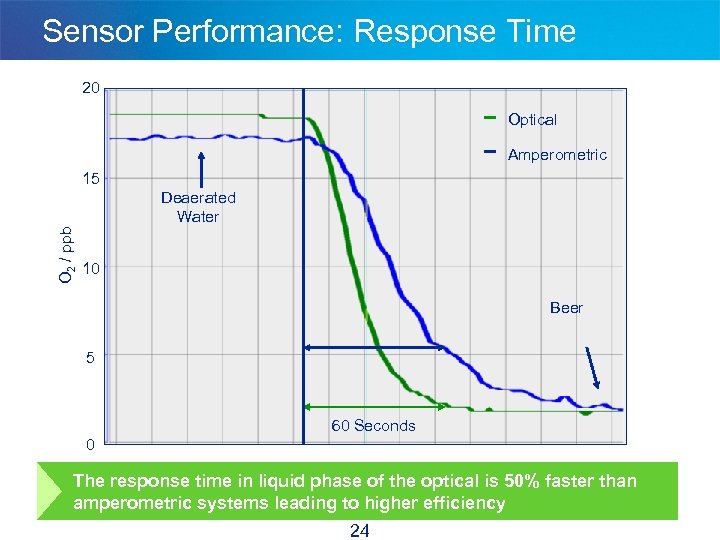
Sensor Performance: Response Time 20 Optical Amperometric 15 O 2 / ppb Deaerated Water 10 Beer 5 60 Seconds 0 The response time in liquid phase of the optical is 50% faster than amperometric systems leading to higher efficiency 24
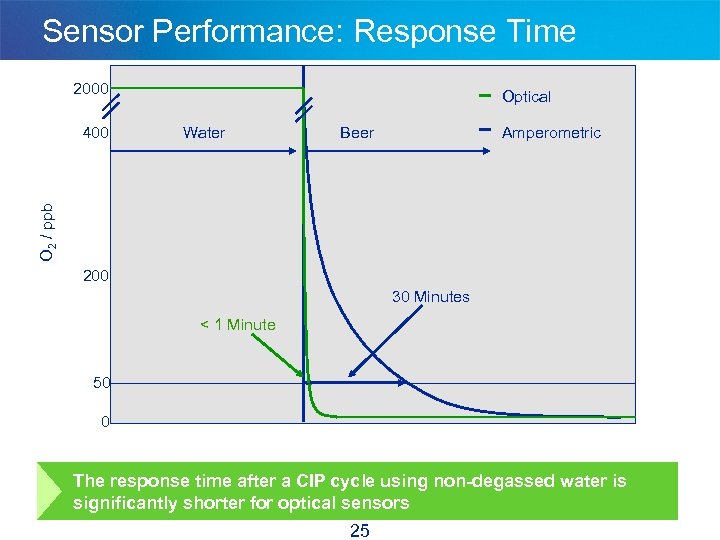
Sensor Performance: Response Time 2000 Water Beer Amperometric O 2 / ppb 400 Optical 200 30 Minutes < 1 Minute 50 0 The response time after a CIP cycle using non-degassed water is significantly shorter for optical sensors 25
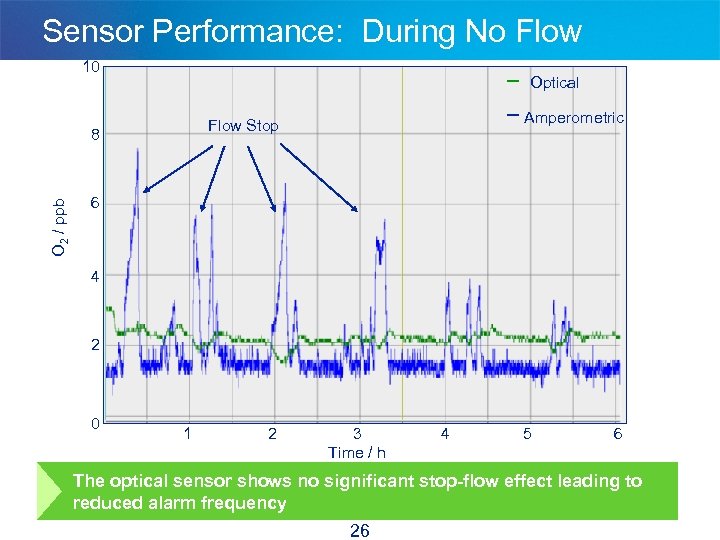
Sensor Performance: During No Flow 10 Optical 8 O 2 / ppb Amperometric Flow Stop 6 4 2 0 1 2 3 Time / h 4 5 6 The optical sensor shows no significant stop-flow effect leading to reduced alarm frequency 26
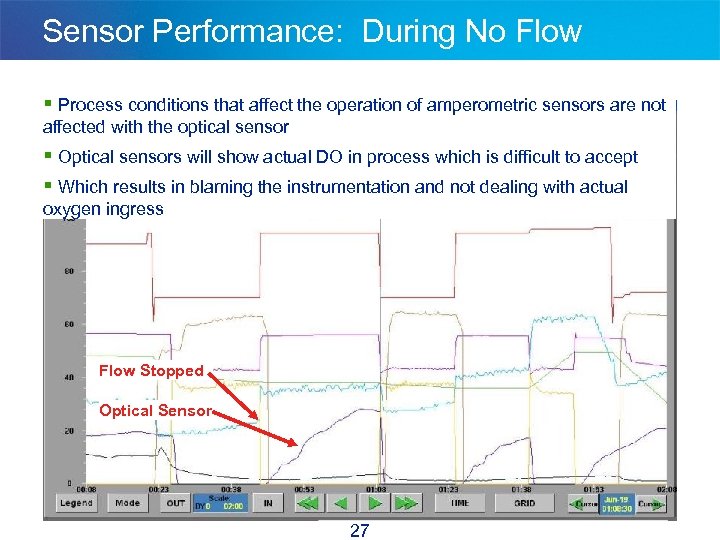
Sensor Performance: During No Flow § Process conditions that affect the operation of amperometric sensors are not affected with the optical sensor § Optical sensors will show actual DO in process which is difficult to accept § Which results in blaming the instrumentation and not dealing with actual oxygen ingress Flow Stopped Optical Sensor 27
Sensor Performance: Extensively Tested § Multiple optical system manufacturers were tested § Test included amperometric technology § The test period lasted 14 months 28
Sensor Performance: Other Benefits § Not susceptible to Hydraulic Shocks (measurement Stable) § No Damage from Hydraulic Spikes (Press-Vac) § Does not see CO 2 bubbles as O 2 - Only responds to the presents of O 2 § Process Orientation of sensor is not important - Does not contain an electrolyte § Opto Cap life expectancy is 12+ Months - Easily replaced onsite and recalibrated § Does not require frequent “calibration”, but only “validation” - Verification has been necessary to become comfortable with this new technology 29

Sensor Performance: Not without issues § Optical spot can not be pulsed during CIP process or at high temperatures - Results in a shift in the calibration values - Most manufacturers deal with this issue by turning off the LED by a temperature shut-off or remote signal to the transmitter § More frequent pulse rate will deplete (bleach out) the optical spot at a faster rate - The pulse rate can be programmed § Multiple/Frequent (weekly) process calibrations will eventually require a two-point calibration be done - Drift rate is less than 1 ppb per month 30
Summary Optical oxygen measurement systems § Provide - Signal stability Faster Response time Extensively less maintenance then amperometric systems Ease of maintenance Process improvement Improved product quality 31

From Brew House to Filler Lines 32
258da2b309e8af38830840ce798cb4e8.ppt