4a2281ec88098dd7a3f6d824a3d71a95.ppt
- Количество слайдов: 12
 Marshaling Data to Improve Patient Safety Michelle Mello, JD, Ph. D Harvard School of Public Health
Marshaling Data to Improve Patient Safety Michelle Mello, JD, Ph. D Harvard School of Public Health
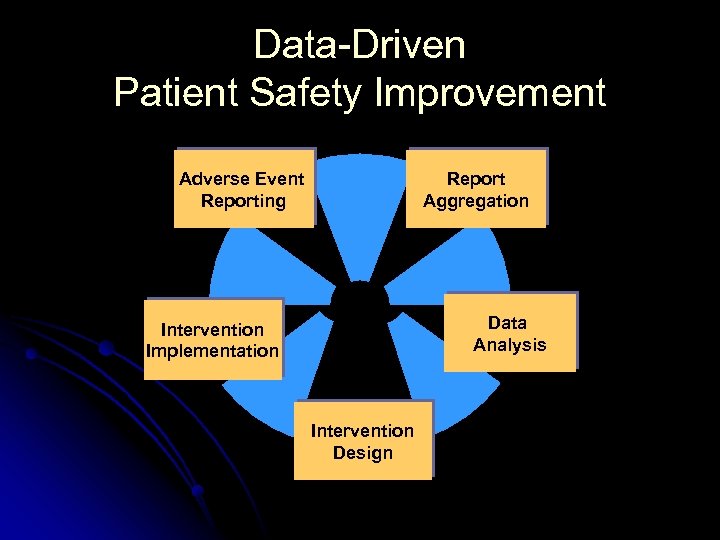 Data-Driven Patient Safety Improvement Adverse Event Reporting Report Aggregation Data Analysis Intervention Implementation Intervention Design
Data-Driven Patient Safety Improvement Adverse Event Reporting Report Aggregation Data Analysis Intervention Implementation Intervention Design
 Major Private Sector Data Collection Efforts l University Health. System Consortium’s Patient Safety Net l l 14 academic medical centers active, +5 by year end ~ 250 reports/site/month across a broad range of incidents (total n≈22, 000) Online reports submitted by clinical staff, risk managers Doctor. Quality, Inc. ’s Risk Prevention & Management System l l l Several dozen participating institutions ~ 70, 000 reports to date Online reports submitted by clinical staff, risk managers
Major Private Sector Data Collection Efforts l University Health. System Consortium’s Patient Safety Net l l 14 academic medical centers active, +5 by year end ~ 250 reports/site/month across a broad range of incidents (total n≈22, 000) Online reports submitted by clinical staff, risk managers Doctor. Quality, Inc. ’s Risk Prevention & Management System l l l Several dozen participating institutions ~ 70, 000 reports to date Online reports submitted by clinical staff, risk managers
 Private Sector Data Collection, continued l Harvard’s Malpractice Insurers Medical Error Prevention and Surveillance Study l l Funded by AHRQ (David Studdert, Principal Investigator) 6 multi-hospital insurers nationwide, including CRICO “Reports” are closed malpractice claims (n≈2, 040) in 4 clinical areas Record reviews conducted by specialist physicians
Private Sector Data Collection, continued l Harvard’s Malpractice Insurers Medical Error Prevention and Surveillance Study l l Funded by AHRQ (David Studdert, Principal Investigator) 6 multi-hospital insurers nationwide, including CRICO “Reports” are closed malpractice claims (n≈2, 040) in 4 clinical areas Record reviews conducted by specialist physicians
 1. Adverse Event Reporting l Reporters: l Risk managers (difficult) l Nurses (good – 60% in UHC) l Pharmacists (good – 29% in UHC) l Physicians (very difficult – 2% in UHC) l What to collect? l Medical injuries l Near-misses and unsafe conditions l Other “adverse events” – falls, fires, suicides, etc. l Contributing factors
1. Adverse Event Reporting l Reporters: l Risk managers (difficult) l Nurses (good – 60% in UHC) l Pharmacists (good – 29% in UHC) l Physicians (very difficult – 2% in UHC) l What to collect? l Medical injuries l Near-misses and unsafe conditions l Other “adverse events” – falls, fires, suicides, etc. l Contributing factors
 Barriers to Reporting l Legal: l Tort fears – confidentiality of report data l HIPAA l Practical: l Cultural norms l Time / hassle factor l Reporting overload: JCAHO, FDA, Department of Health, Board of Medicine, risk management, insurer, peer review committee, UHC or Doctor. Quality
Barriers to Reporting l Legal: l Tort fears – confidentiality of report data l HIPAA l Practical: l Cultural norms l Time / hassle factor l Reporting overload: JCAHO, FDA, Department of Health, Board of Medicine, risk management, insurer, peer review committee, UHC or Doctor. Quality
 2. Report Aggregation l Reporting systems vary in: l l l Vocabulary and definition Typologies of adverse events and contributing factors Range of data collected l Private-sector systems collect comprehensive data, but have limited membership l State systems have l l Theoretically universal reporting, but substantial underreporting Limited range of data fields
2. Report Aggregation l Reporting systems vary in: l l l Vocabulary and definition Typologies of adverse events and contributing factors Range of data collected l Private-sector systems collect comprehensive data, but have limited membership l State systems have l l Theoretically universal reporting, but substantial underreporting Limited range of data fields
 3. Data Analysis l Most multi-institutional systems have limited capacity to conduct data analysis l States: lack of human resources, money l UHC: “like that UPS commercial” l Partnerships with researchers emerging, but still limited l OK to share data with researchers? l Who will pay?
3. Data Analysis l Most multi-institutional systems have limited capacity to conduct data analysis l States: lack of human resources, money l UHC: “like that UPS commercial” l Partnerships with researchers emerging, but still limited l OK to share data with researchers? l Who will pay?
 Data Analysis, continued l Moving beyond descriptive analysis is difficult l Heterogeneity of adverse outcomes, errors, clinical conditions, institutions, and patients l Small sample sizes l Case/control designs are expensive, difficult to power, and pose HIPAA issues
Data Analysis, continued l Moving beyond descriptive analysis is difficult l Heterogeneity of adverse outcomes, errors, clinical conditions, institutions, and patients l Small sample sizes l Case/control designs are expensive, difficult to power, and pose HIPAA issues
 4. Intervention Design l Reporting institutions must receive feedback to maintain a stake in reporting l l l Comparative data and benchmarking are of interest Types of interventions: (1) educational, (2) systems change Clinical leadership / buy-in are essential Should include an evaluation component Key issue: How tailored should the intervention be to particular institutions?
4. Intervention Design l Reporting institutions must receive feedback to maintain a stake in reporting l l l Comparative data and benchmarking are of interest Types of interventions: (1) educational, (2) systems change Clinical leadership / buy-in are essential Should include an evaluation component Key issue: How tailored should the intervention be to particular institutions?
 5. Intervention Implementation l Barriers: l Identifying clinical leaders l Gaining buy-in from busy clinicians who lack a strong stake in QI l Demonstrating the value of claims & report data l Crowding-out from other QI initiatives l Outside of captives, no organizational structure to implement interventions through the insurer, or otherwise coordinate institutions/practice groups
5. Intervention Implementation l Barriers: l Identifying clinical leaders l Gaining buy-in from busy clinicians who lack a strong stake in QI l Demonstrating the value of claims & report data l Crowding-out from other QI initiatives l Outside of captives, no organizational structure to implement interventions through the insurer, or otherwise coordinate institutions/practice groups
 Next Steps in Building an Infrastructure for Data-Driven Patient Safety Improvement l l l Standardization of reporting fields and linkage of data from multiple systems (reporting systems + quality datasets) Stronger partnerships for data analysis Merging of institutional risk management and patient safety units Coordinated leadership from insurers, institutional management, and clinical staff Better financial incentives for patient safety improvement (individual- and institution- level)
Next Steps in Building an Infrastructure for Data-Driven Patient Safety Improvement l l l Standardization of reporting fields and linkage of data from multiple systems (reporting systems + quality datasets) Stronger partnerships for data analysis Merging of institutional risk management and patient safety units Coordinated leadership from insurers, institutional management, and clinical staff Better financial incentives for patient safety improvement (individual- and institution- level)


