f75ee98c69707fe197f2eff4454549f2.ppt
- Количество слайдов: 43

Management of Chronic Hepatitis B & C Virus Infection in Pregnancy Masomeh Bayani MD Infectious Diseases and Tropical Medicine Research. Center, Babol University of Medical Sciences, Babol, Iran.

Chronic HBV Infection in Women of Reproductive Age Management of Chronic Hepatitis B Virus Infection in Women of Reproductive Age clinicaloptions. com/hepatitis § Women who contemplate starting a family in the foreseeable future § Women who become pregnant while receiving antiviral therapy § Women who are pregnant, have high HBV DNA, and not currently on treatment § Women with newly diagnosed HBV infection during pregnancy

Chronic HBV Infection in Women Considering Starting a Family Management of Chronic Hepatitis B Virus Infection in Women of Reproductive Age clinicaloptions. com/hepatitis § Should plans to start a family influence management decisions? – Depends on immediacy of plan to become pregnant – Uncertainty regarding safety of antiviral medications in pregnancy – Careful discussion with patient and spouse regarding benefits vs risks § Indications for treatment – Start now: advanced fibrosis/cirrhosis, severe flares/persistently high ALT – Defer: no/mild fibrosis, normal/minimally elevated ALT

Management of Chronic Hepatitis B Virus Infection in Women of Reproductive Age HBV Infection in Women Considering Starting a Family: Which Drug? clinicaloptions. com/hepatitis § FDA classification: based on in vitro and animal studies – Pregnancy class B: telbivudine and tenofovir DF – Pregnancy class C: interferon, adefovir, entecavir, and lamivudine § Human data: – Antiretroviral pregnancy registry: safety established for lamivudine and tenofovir, including exposure in first trimester[1] – Clinical studies of antiviral therapy to prevent perinatal transmission: safety established for lamivudine and telbivudine, mainly exposure in third trimester[2 -5] 1. Antiretroviral Pregnancy Registry. December 2012. 2. Xu WM, et al. J Viral Hepat. 2009; 16: 94 -103. 3. Shi Z, et al. Obstet Gynecol. 2010; 116: 147 -159. 4. Han GR, et al. J Hepatology. 2011; 55: 1215 -1221. 5. Pan CQ, et al. Clin Gastroenterol Hepatol. 2012; 10: 520 -526.

Incidence of Birth Defects With in Utero Exposure to HBV Nucleos(t)ide Analogues Management of Chronic Hepatitis B Virus Infection in Women of Reproductive Age clinicaloptions. com/hepatitis § Data derived from Antiretroviral Pregnancy Registry, 1/1989 - 7/2012[6] – International, voluntary, prospective, exposure-registration cohort – Data on exposure in HBV-monoinfected mothers began in 1/2003 First Trimester Second or Third Trimester Regimen Containing Exposed, n Birth Defects, % (95% CI) Lamivudine 4185 3. 2 (2. 7 -3. 8) 6843 2. 8 (2. 4 -3. 2) Tenofovir 1612 2. 4 (1. 7 -3. 3) 838 2. 3 (1. 4 -3. 5) § Metropolitan Atlanta Congenital Defects Program, a population-based birth defects surveillance program administered by CDC[6, 7] – Overall birth defects: 2. 72% (95% CI: 2. 68 -2. 76) 6. Antiretroviral Pregnancy Registry. December 2012. 7. Correa A, et al. Birth Defects Res A Clin Mol Teratol. 2007; 79: 65 -186.

Management of Chronic Hepatitis B Virus Infection in Women of Reproductive Age Limitations of the Antiretroviral Pregnancy Registry clinicaloptions. com/hepatitis § Depends on voluntary reporting § Information is reviewed but not verified § Long-term follow-up is limited § Data available for live births only § No data on miscarriages or subsequent developmental delay § Limited data on antivirals used for HBV monoinfection – < 100 pregnancies reported for exposure to adefovir, entecavir, or telbivudine 8. Antiretroviral Pregnancy Registry. December 2012.
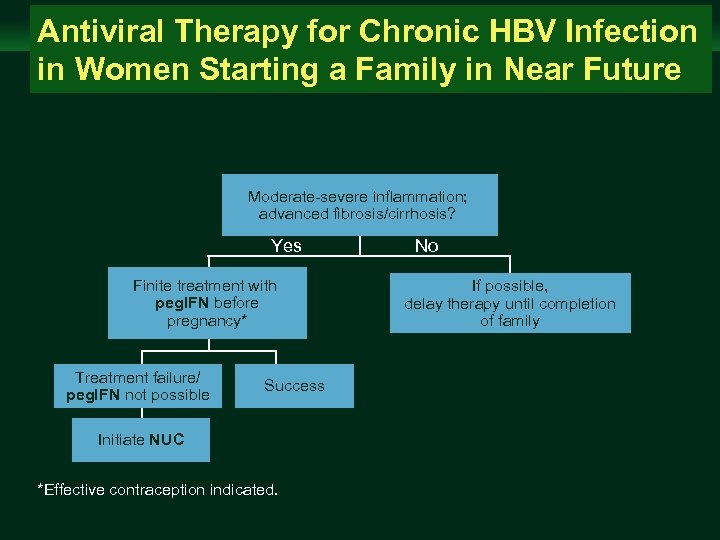
Management of Chronic Hepatitis B Virus Infection in Women of Reproductive Age Antiviral Therapy for Chronic HBV Infection in Women Starting a Family in Near Future clinicaloptions. com/hepatitis Moderate-severe inflammation; advanced fibrosis/cirrhosis? Yes Finite treatment with peg. IFN before pregnancy* Treatment failure/ peg. IFN not possible Success Initiate NUC *Effective contraception indicated. No If possible, delay therapy until completion of family

Antiviral Therapy for Chronic HBV Infection in Women Considering Starting a Family Management of Chronic Hepatitis B Virus Infection in Women of Reproductive Age clinicaloptions. com/hepatitis Which nucleos(t)ide analogue? § Safety to fetus, including exposure during first trimester – Lamivudine, tenofovir, telbivudine § Risk of drug resistance – Lamivudine > telbivudine > tenofovir § Preferred drug: tenofovir – Established safety; potent; low risk of drug resistance § Benefit vs risk discussed with patient and spouse – Inform if become pregnant

Women Who Become Pregnant While Receiving Antiviral Therapy Management of Chronic Hepatitis B Virus Infection in Women of Reproductive Age clinicaloptions. com/hepatitis Continue, switch, or stop? § Review indications for treatment – Advanced fibrosis or cirrhosis: continue – Early/mild disease or uncertain indications: stop? § Assess whetherapeutic endpoint has been reached – HBe. Ag seroconversion: stop? § Discuss potential risks/benefits to mother and fetus

Women Who Become Pregnant While Receiving Antiviral Therapy Management of Chronic Hepatitis B Virus Infection in Women of Reproductive Age clinicaloptions. com/hepatitis § When continuing treatment, evaluate for safety – Tenofovir: continue – Lamivudine or telbivudine: continue if HBV DNA is undetectable – Consider switching to tenofovir if HBV DNA remains detectable to prevent breakthrough during pregnancy – Adefovir, entecavir, or peg. IFN: stop and switch to tenofovir § When stopping or switching, monitor for hepatic flares
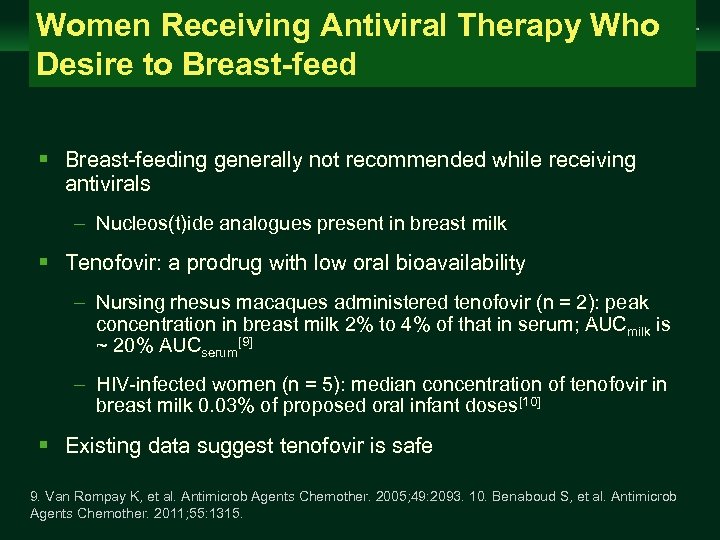
Women Receiving Antiviral Therapy Who Desire to Breast-feed Management of Chronic Hepatitis B Virus Infection in Women of Reproductive Age clinicaloptions. com/hepatitis § Breast-feeding generally not recommended while receiving antivirals – Nucleos(t)ide analogues present in breast milk § Tenofovir: a prodrug with low oral bioavailability – Nursing rhesus macaques administered tenofovir (n = 2): peak concentration in breast milk 2% to 4% of that in serum; AUCmilk is ~ 20% AUCserum[9] – HIV-infected women (n = 5): median concentration of tenofovir in breast milk 0. 03% of proposed oral infant doses[10] § Existing data suggest tenofovir is safe 9. Van Rompay K, et al. Antimicrob Agents Chemother. 2005; 49: 2093. 10. Benaboud S, et al. Antimicrob Agents Chemother. 2011; 55: 1315.

Management of Chronic Hepatitis B Virus Infection in Women of Reproductive Age Preventing Perinatal HBV Transmission: Why Is It So Important? clinicaloptions. com/hepatitis § Risk of progression to chronic infection is inversely related to age at infection Progression to Chronic Infection (%) 100 80 60 40 20 0 Newborns 11. Lok AS, et al. Hepatology. 2009; 50: 661 -662. Infants/Children Immunocompetent Adults
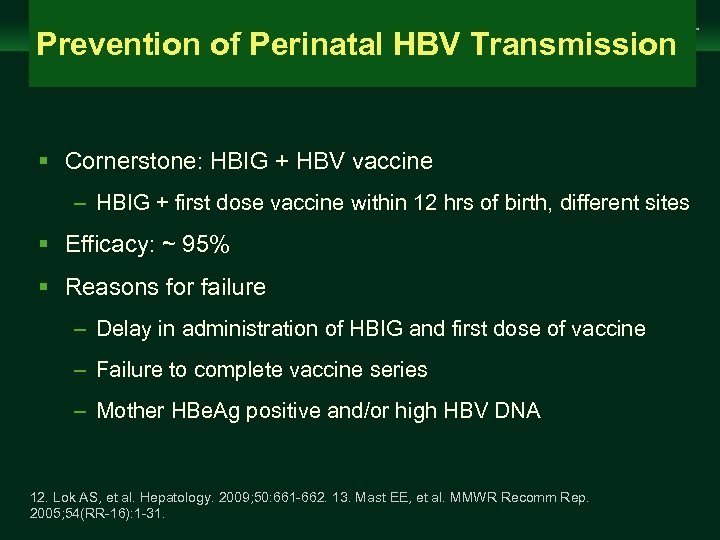
Management of Chronic Hepatitis B Virus Infection in Women of Reproductive Age Prevention of Perinatal HBV Transmission clinicaloptions. com/hepatitis § Cornerstone: HBIG + HBV vaccine – HBIG + first dose vaccine within 12 hrs of birth, different sites § Efficacy: ~ 95% § Reasons for failure – Delay in administration of HBIG and first dose of vaccine – Failure to complete vaccine series – Mother HBe. Ag positive and/or high HBV DNA 12. Lok AS, et al. Hepatology. 2009; 50: 661 -662. 13. Mast EE, et al. MMWR Recomm Rep. 2005; 54(RR-16): 1 -31.
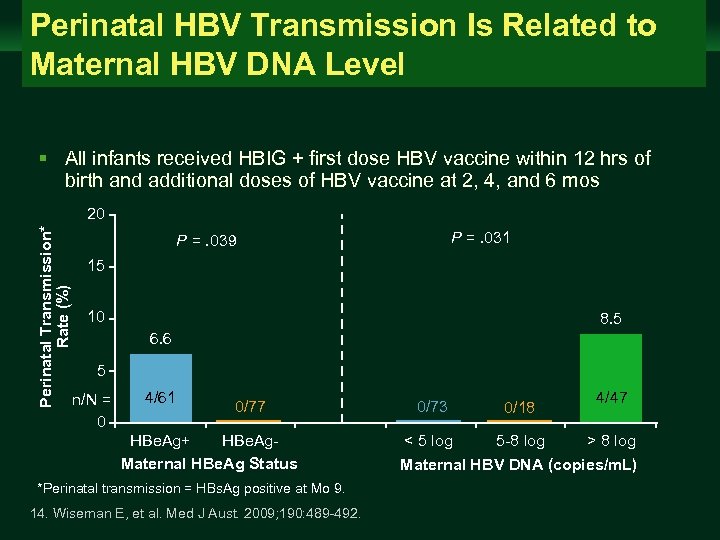
Perinatal HBV Transmission Is Related to Maternal HBV DNA Level Management of Chronic Hepatitis B Virus Infection in Women of Reproductive Age clinicaloptions. com/hepatitis § All infants received HBIG + first dose HBV vaccine within 12 hrs of birth and additional doses of HBV vaccine at 2, 4, and 6 mos Perinatal Transmission* Rate (%) 20 P =. 031 P =. 039 15 10 8. 5 6. 6 5 n/N = 0 4/61 0/77 HBe. Ag+ HBe. Ag. Maternal HBe. Ag Status *Perinatal transmission = HBs. Ag positive at Mo 9. 14. Wiseman E, et al. Med J Aust. 2009; 190: 489 -492. 0/73 0/18 < 5 log 5 -8 log 4/47 > 8 log Maternal HBV DNA (copies/m. L)

Management of Chronic Hepatitis B Virus Infection in Women of Reproductive Age Pregnant Women With High HBV DNA and Not Currently on Antiviral Therapy clinicaloptions. com/hepatitis § Should antiviral therapy be recommended to reduce risk of perinatal transmission? § What should be the cutoff maternal HBV DNA level for initiation of antiviral therapy? § When to start? § Which antiviral drug? § When to stop? § What is the risk of posttreatment flares?
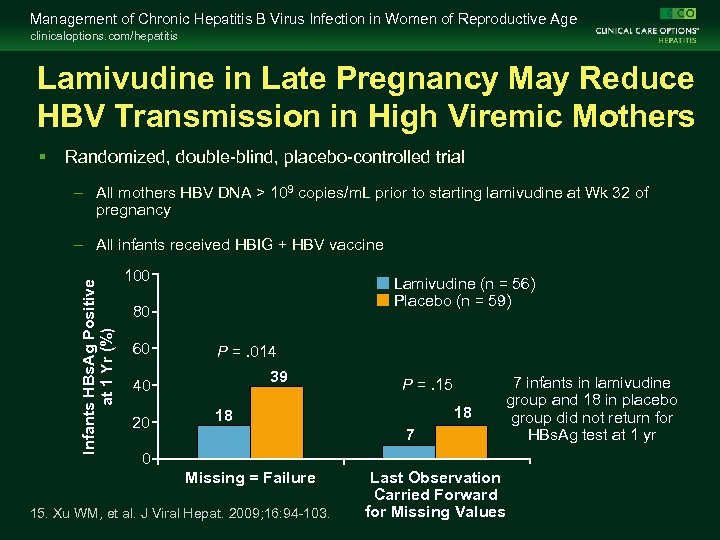
Management of Chronic Hepatitis B Virus Infection in Women of Reproductive Age clinicaloptions. com/hepatitis Lamivudine in Late Pregnancy May Reduce HBV Transmission in High Viremic Mothers § Randomized, double-blind, placebo-controlled trial – All mothers HBV DNA > 109 copies/m. L prior to starting lamivudine at Wk 32 of pregnancy Infants HBs. Ag Positive at 1 Yr (%) – All infants received HBIG + HBV vaccine 100 Lamivudine (n = 56) Placebo (n = 59) 80 60 P =. 014 39 40 20 P =. 15 18 18 7 0 Missing = Failure 15. Xu WM, et al. J Viral Hepat. 2009; 16: 94 -103. Last Observation Carried Forward for Missing Values 7 infants in lamivudine group and 18 in placebo group did not return for HBs. Ag test at 1 yr
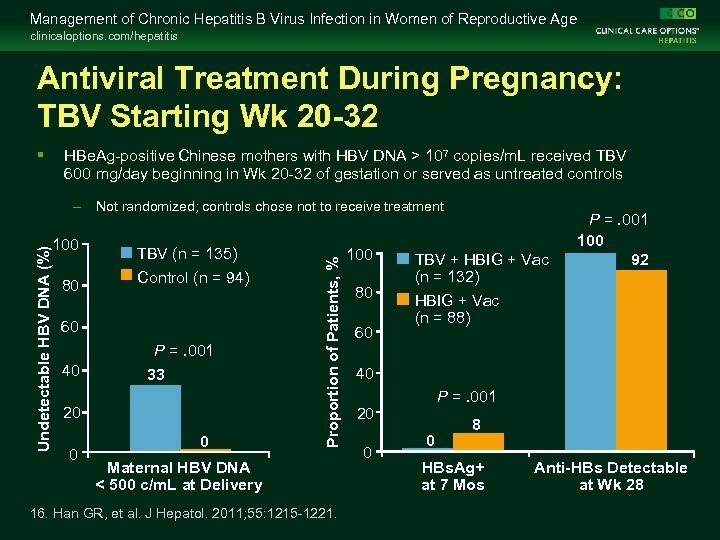
Management of Chronic Hepatitis B Virus Infection in Women of Reproductive Age clinicaloptions. com/hepatitis Antiviral Treatment During Pregnancy: TBV Starting Wk 20 -32 § HBe. Ag-positive Chinese mothers with HBV DNA > 107 copies/m. L received TBV 600 mg/day beginning in Wk 20 -32 of gestation or served as untreated controls 100 80 TBV (n = 135) Control (n = 94) 60 40 P =. 001 33 20 0 0 Proportion of Patients, % Undetectable HBV DNA (%) – Not randomized; controls chose not to receive treatment Maternal HBV DNA < 500 c/m. L at Delivery 16. Han GR, et al. J Hepatol. 2011; 55: 1215 -1221. 100 80 60 TBV + HBIG + Vac (n = 132) HBIG + Vac (n = 88) P =. 001 100 92 40 P =. 001 20 0 0 8 HBs. Ag+ at 7 Mos Anti-HBs Detectable at Wk 28
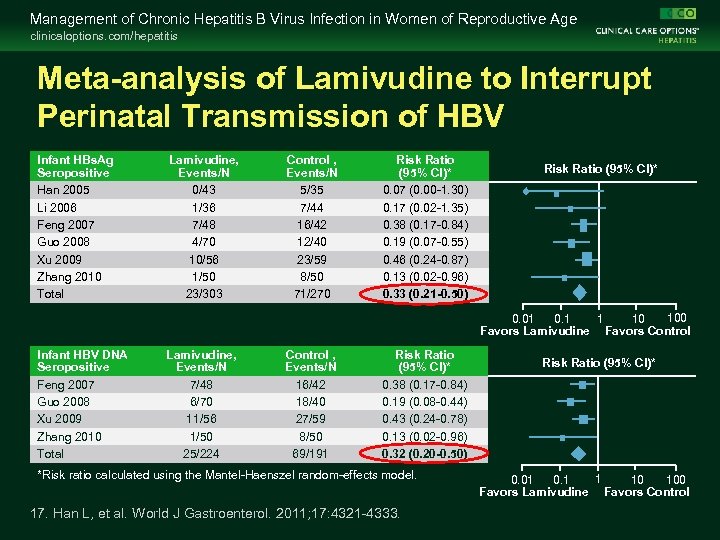
Management of Chronic Hepatitis B Virus Infection in Women of Reproductive Age clinicaloptions. com/hepatitis Meta-analysis of Lamivudine to Interrupt Perinatal Transmission of HBV Infant HBs. Ag Seropositive Han 2005 Li 2006 Feng 2007 Guo 2008 Xu 2009 Zhang 2010 Total Lamivudine, Events/N 0/43 1/36 7/48 4/70 10/56 1/50 23/303 Control , Events/N 5/35 7/44 16/42 12/40 23/59 8/50 71/270 Risk Ratio (95% CI)* 0. 07 (0. 00 -1. 30) 0. 17 (0. 02 -1. 35) 0. 38 (0. 17 -0. 84) 0. 19 (0. 07 -0. 55) 0. 46 (0. 24 -0. 87) 0. 13 (0. 02 -0. 96) 0. 33 (0. 21 -0. 50) Risk Ratio (95% CI)* 100 1 0. 01 10 Favors Lamivudine Favors Control Infant HBV DNA Seropositive Feng 2007 Guo 2008 Xu 2009 Zhang 2010 Total Lamivudine, Events/N 7/48 6/70 11/56 1/50 25/224 Control , Events/N 16/42 18/40 27/59 8/50 69/191 Risk Ratio (95% CI)* 0. 38 (0. 17 -0. 84) 0. 19 (0. 08 -0. 44) 0. 43 (0. 24 -0. 78) 0. 13 (0. 02 -0. 96) 0. 32 (0. 20 -0. 50) *Risk ratio calculated using the Mantel-Haenszel random-effects model. 17. Han L, et al. World J Gastroenterol. 2011; 17: 4321 -4333. Risk Ratio (95% CI)* 1 0. 1 100 0. 01 10 Favors Lamivudine Favors Control

All Women With Newly Diagnosed HBV Infection During Pregnancy Management of Chronic Hepatitis B Virus Infection in Women of Reproductive Age clinicaloptions. com/hepatitis § Register HBV status in OB record – HBIG + first dose of vaccine to baby within 12 hrs of birth – Complete full course of vaccine – Check baby for HBs. Ag and anti-HBs at 9 -15 mos § Counseling on precautions to prevent HBV transmission § Screening and vaccination of family members 18. Lok AS, et al. Hepatology. 2009; 50: 661 -662.

All Women With Newly Diagnosed HBV Infection During Pregnancy Management of Chronic Hepatitis B Virus Infection in Women of Reproductive Age clinicaloptions. com/hepatitis § Refer for further evaluation § Assess HBV replication and liver disease – HBe. Ag/anti-HBe, HBV DNA – Blood counts, liver panel ± ultrasound § Evaluate need for antiviral therapy – For control of liver disease in mother – For prevention of transmission to baby § Emphasize importance of long-term follow-up

Management of Chronic Hepatitis B Virus Infection in Women of Reproductive Age Pregnant Women With High HBV DNA and Not Currently on Antiviral Therapy clinicaloptions. com/hepatitis § Should antiviral be recommended to reduce risk of perinatal transmission? – Yes; although quality of evidence is low, all studies showed benefit and no harm § What should be the cutoff maternal HBV DNA level for initiation of antiviral therapy? – > 8 log 10 IU/m. L: Yes – 6 -8 log 10 IU/m. L: Maybe – < 6 log 10 IU/m. L: No

Pregnant Women With High HBV DNA and Not Currently on Antiviral Therapy Management of Chronic Hepatitis B Virus Infection in Women of Reproductive Age clinicaloptions. com/hepatitis § When to start antiviral? – Late second/early third trimester – Allow at least 4 -6 wks for an effect § Which antiviral drug? – Lamivudine, telbivudine, or tenofovir – Tenofovir preferred: low risk of drug resistance, baseline HBV DNA high, and some mothers may need treatment for their liver disease in the future

Pregnant Women With High HBV DNA and Not Initially on Antiviral Therapy Management of Chronic Hepatitis B Virus Infection in Women of Reproductive Age clinicaloptions. com/hepatitis § When to stop antiviral after delivery? – To prevent perinatal transmission: immediately, especially if mother plans to breast-feed, or up to 3 mos postdelivery – To treat liver disease: continue until therapeutic endpoint § What is the risk of posttreatment flare? – Seemingly rare, but mild ALT elevation common; also seen in postpartum period for women not receiving antiviral – Decompensation not reported in clinical trials; likelihood low because most pregnant women have early-stage liver disease – Important to closely monitor ALT after antiviral therapy is discontinued (eg, 1, 3, and 6 mos posttreatment) 19. Ter Borg MJ, et al. J Viral Hepat. 2008; 15: 37 -41.
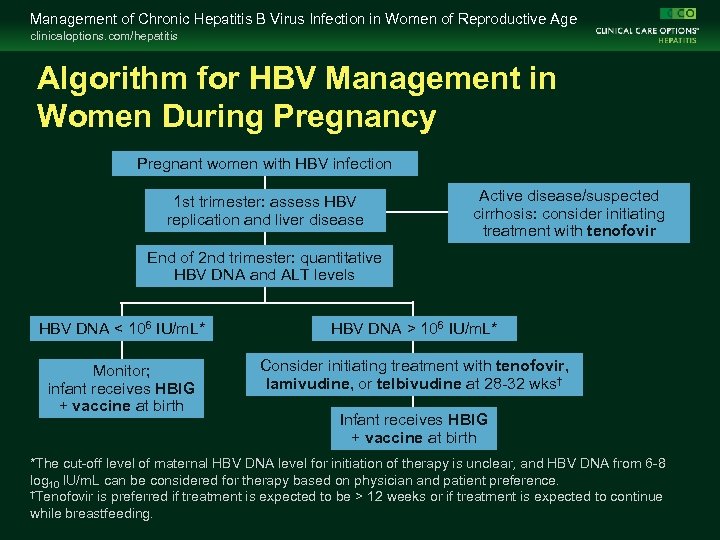
Management of Chronic Hepatitis B Virus Infection in Women of Reproductive Age clinicaloptions. com/hepatitis Algorithm for HBV Management in Women During Pregnancy Pregnant women with HBV infection 1 st trimester: assess HBV replication and liver disease Active disease/suspected cirrhosis: consider initiating treatment with tenofovir End of 2 nd trimester: quantitative HBV DNA and ALT levels HBV DNA < 106 IU/m. L* HBV DNA > 106 IU/m. L* Monitor; infant receives HBIG + vaccine at birth Consider initiating treatment with tenofovir, lamivudine, or telbivudine at 28 -32 wks† Infant receives HBIG + vaccine at birth *The cut-off level of maternal HBV DNA level for initiation of therapy is unclear, and HBV DNA from 6 -8 log 10 IU/m. L can be considered for therapy based on physician and patient preference. †Tenofovir is preferred if treatment is expected to be > 12 weeks or if treatment is expected to continue while breastfeeding.

Management of Chronic Hepatitis B Virus Infection in Women of Reproductive Age HCV Management in Women During Pregnancy clinicaloptions. com/hepatitis § Worldwide, the seroprevalance of HCV in pregnant women is thought to be anywhere from 0. 15% to 2. 4% in the United States and European countries and much higher in countries like Egypt where it is estimated to be as high as 8. 6%. § The prevalence of HCV infection in children ranges from 0. 05% to 0. 36% in studies carried out in the United States and Europe [9– 11] and is much higher in developing countries where it can range from 1. 8% to 5%.

Management of Chronic Hepatitis B Virus Infection in Women of Reproductive Age clinicaloptions. com/hepatitis § The intensity of HCV infection varies during pregnancy as demonstrated by the varying level of viral loads an HCV specific T-cell responses that correspond with the progression of pregnancy. Some studies have shown that there is a decrease in serum alanine transferase levels (ALT) in the 2 nd and 3 nd trimesters of pregnancy, along with a corresponding increase in HCV RNA during these trimesters. It is hypothesized that this may be seen because of a relative suppression of immunity as pregnancy proceeds.
Management of Chronic Hepatitis B Virus Infection in Women of Reproductive Age clinicaloptions. com/hepatitis § Estrogen has shown to suppress intrathymic T-cell differentiation while activating the extrathymic pathways a phenomenon that has also been noted during pregnancy. § The modulation of cytokines as a result of this is important in maintaining tolerance of the paternal antigens in the foetus. This may also increase the proliferation of HCV resulting in higher HCV RNA titres in the 2 nd and 3 rd trimesters.
Management of Chronic Hepatitis B Virus Infection in Women of Reproductive Age clinicaloptions. com/hepatitis § Conversely, HCV RNA titres tend to decrease in the postpartum period. Rates of clearance compared to the nonpregnant control group. In addition, the HCV core protein levels at 3 months postpartum were much lower among patients who cleared their viremia versus those who had persistent HCV elevation.

Management of Chronic Hepatitis B Virus Infection in Women of Reproductive Age clinicaloptions. com/hepatitis § Risks of obstetrical complications in HCV infected pregnant women are varied. § Low in birth weight, small for gestational age, be admitted to the intensive care nursery or require assisted ventilation of some sort. § The risk of cholestasis increased in pregnant women who are also HCV antibody positive, and this tends to occur earlier in the gestation compared to HCV-negative mothers.
Management of Chronic Hepatitis B Virus Infection in Women of Reproductive Age MODIFIERS OF AND RISK FACTORS FOR HCV VERTICAL TRANSMISSION clinicaloptions. com/hepatitis § Both intrauterine and intrapartum transmission are possible. § It is estimated that up to one-third of the infected children acquire the infection in utero, as evidenced by the positive PCR testing within the first 3 days of life, and up to onehalf as late intrauterine or intrapartum and are PCR positive after 3 days of life.
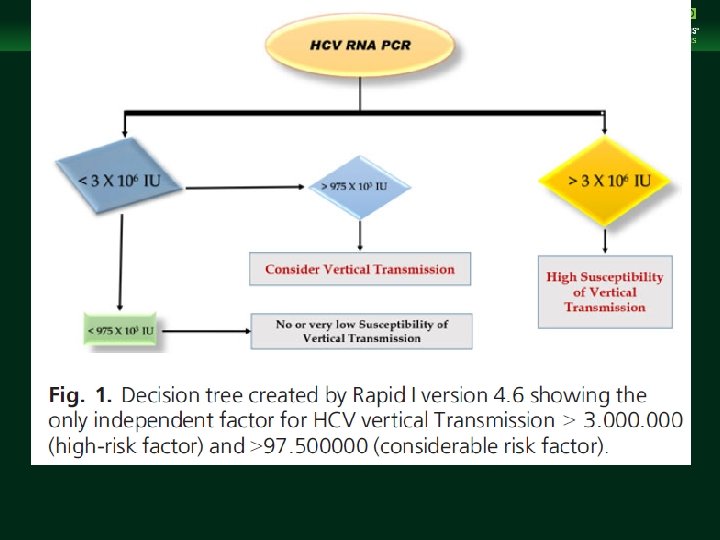
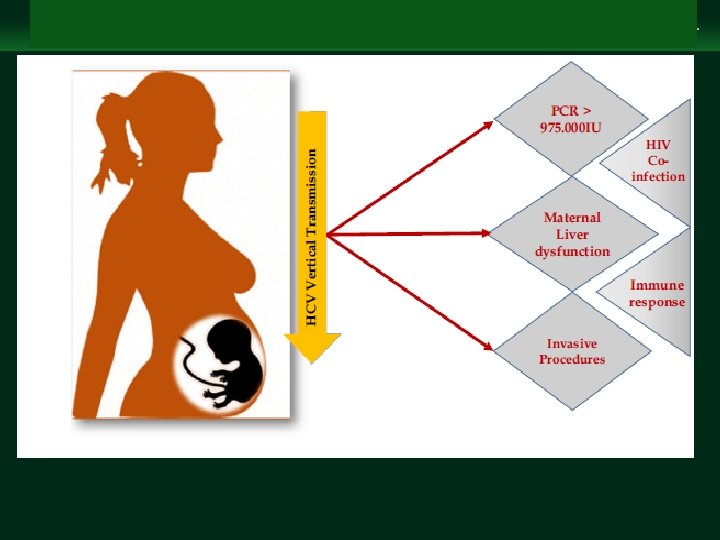
Management of Chronic Hepatitis B Virus Infection in Women of Reproductive Age clinicaloptions. com/hepatitis § Several factors have been studied as potential modifiers of the frequency of HCV vertical transmission. Multiple obstetric, immunological and virologic factors may influence perinatal transmission of HCV. These include maternal HCV RNA levels (at viral titres beyond 105 to 106 copies/m. L), HIV coinfection, HCV genotype, neutralizing antibodies , cytokine modulation, amniocentesis, foetal blood monitoring , prolonged membrane rupture and type of delivery.
Management of Chronic Hepatitis B Virus Infection in Women of Reproductive Age clinicaloptions. com/hepatitis § Maternal HCV and HIV co-infection has consistently been shown to be associated with higher transmission rates. § The incidence of HCV vertical transmission is approximately 3– 5% in HCV RNA-positive-monoinfected mothers, but can be as high as 19% in HIV co-infected mothers. Even when controlling for HIV, presence of HCV viremia increases the odds of vertical transmission by 2. 82 -fold.

Management of Chronic Hepatitis B Virus Infection in Women of Reproductive Age clinicaloptions. com/hepatitis § Rupture of membranes for more than 6 h has been significantly associated with viral transmission. § Use of scalp electrodes may lead to HCV exposure. § Increase in HCV infection in infants following amniocentesis. § Benefits of doing elective C-section in mothers with HCV viremia are controversial.

Management of Chronic Hepatitis B Virus Infection in Women of Reproductive Age clinicaloptions. com/hepatitis § HCV infection of the peripheral blood monocytes have a higher rate of transmission to their infants. § Elevated ALT is also associated with certain HCV genotypes that are more likely to be transmitted like 1 a or 1 b. § Increase in the natural killer (NK) cells in the placenta of HCV-positive mothers may be an explanation for the relatively low rates of vertical transmission; though, the increased cytotoxicity of the NK cells may also lead to a higher risk of preterm delivery in HCV-positive mothers.

Management of Chronic Hepatitis B Virus Infection in Women of Reproductive Age Effect of pregnancy on HCV clinicaloptions. com/hepatitis § Most women are asymptomatic § No deleteriouse effect of pregnancy on the course of HCV infection

Management of Chronic Hepatitis B Virus Infection in Women of Reproductive Age clinicaloptions. com/hepatitis Effect of HCV on pregnancy § Hepatitis C infection dose not affect pregnancy complications and outcomes. § Hepatitis in pregnancy is not associated with increased abortion rate , LBW, stillbrith or congenital malformation. § Prematurity Increased if hepatitis is aqueired in the last trimester Increased risk of obstetric cholestasis

Management of Chronic Hepatitis B Virus Infection in Women of Reproductive Age clinicaloptions. com/hepatitis Managment § Antenatal § Intrapartum § Postnatal

Management of Chronic Hepatitis B Virus Infection in Women of Reproductive Age Antenatal clinicaloptions. com/hepatitis § Screening § High risk groups § Immunization against HBV & HAV

Intrapartum Management of Chronic Hepatitis B Virus Infection in Women of Reproductive Age clinicaloptions. com/hepatitis § Treatment § Elective C/S § Not recommended § Vaginal delivery: § Fetal scalp electrodes and fetal blood sampling should be avoided

Management of Chronic Hepatitis B Virus Infection in Women of Reproductive Age Postpartum clinicaloptions. com/hepatitis § Breast feeding § Although HCV RNA can be found in breast milk, infection through Breast feeding has not been demonstrated. § If nipples are not traumatized Breast feeding is not cotraindicated.
Management of Chronic Hepatitis B Virus Infection in Women of Reproductive Age clinicaloptions. com/hepatitis
Management of Chronic Hepatitis B Virus Infection in Women of Reproductive Age clinicaloptions. com/hepatitis

Management of Chronic Hepatitis B Virus Infection in Women of Reproductive Age clinicaloptions. com/hepatitis § HCV infection during pregnancy and the post-partum period appears to be a highly unique period in the interaction between virus and host. These periods of intense physiological change appear to force some adaptation of the virus that may offer a therapeutic window when more suitable agents come into use. Further studies of how and when the virus is transmitted from mother to child will only enhance the ability for prevention and treatment in the future.
f75ee98c69707fe197f2eff4454549f2.ppt